Abstract
Circulating immune complexes containing aberrantly glycosylated IgA1 play a pivotal role in the pathogenesis of IgAN. A portion of IgA1 secreted by IgA1-producing cells in patients with IgAN is galactose-deficient and consequently recognized by anti-glycan IgG or IgA1 antibodies. Some of the resultant immune complexes in the circulation escape normal clearance mechanisms, deposit in the renal mesangium, and induce glomerular injury. Recent studies of the origin of these aberrant molecules, their glycosylation profiles, and mechanisms of biosynthesis have provided new insight into the autoimmune nature of the pathogenesis of this common renal disease. An imbalance in the activities of the pertinent glycosyltransferases in the IgA1-producing cells favors production of molecules with galactose-deficient O-linked glycans at specific sites in the hinge region of the alpha heavy chains. Using sophisticated analytical methods, it may be possible to define biomarkers for diagnostic purposes and identify new therapeutic targets for a future disease-specific therapy.
IgAN - an immune-complex glomerulonephritis
IgA nephropathy (IgAN) is characterized by immune deposits with dominant or co-dominant IgA in the glomerular mesangium (1, 2). The IgA is of the IgA1 subclass (3) and may be accompanied by C3, and IgG or IgM or both (4). Mesangial proliferation and expansion of extracellular matrix is present in biopsies from patients with even mild clinical disease. Glomerular sclerosis and interstitial fibrosis generally signify more serious disease and are frequently associated with progressive disease that leads to end-stage renal failure in 20-40% patients within 20 years of diagnosis (4-6). Hematuria is typical and often includes episodes of macroscopic bleeding that coincide with mucosal infections, including those of upper respiratory tract and digestive system (4, 7-9).
There are substantial data suggesting that the mesangial immune deposits originate from circulating IgA immune complexes. Evidence that the primary cause of IgAN is extra-renal includes recurrence of the disease in 50-60% of patients undergoing renal transplantation (10-14). Moreover, in the few instances in which a kidney from a donor with subclinical IgAN has been engrafted into a patient with end-stage renal failure due to a disorder other than IgAN, the immune deposits cleared from the allograft within several weeks (15). Many patients with IgAN have elevated levels of IgA and IgA-containing immune complexes in the circulation (16-19). Idiotypic determinants are shared between the circulating complexes and the mesangial deposits (20); however, disease-specific idiotypes have not been identified (21). Furthermore, circulating immune complexes in patients with IgAN and in the immune deposits contain IgA1, but not IgA2 (16, 22-25).
IgA1: structure and glycosylation
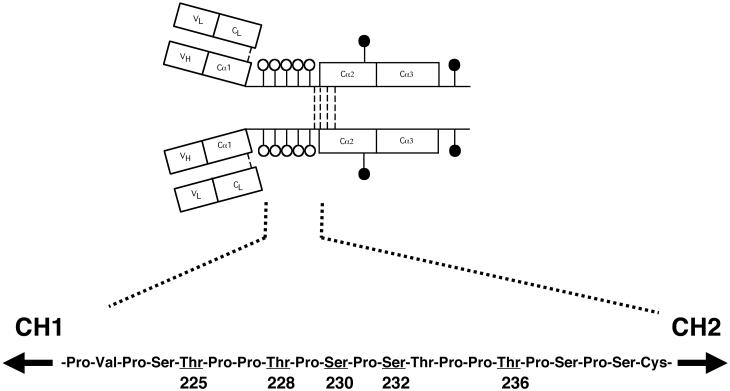
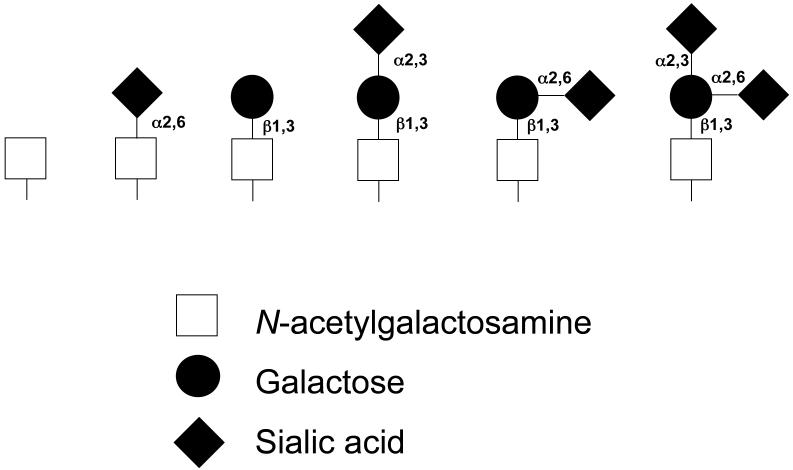
IgA1 represents one of two structurally and functionally distinct subclasses of IgA, the other being IgA2 (26-28). Unlike IgA2, the heavy chains of IgA1 molecules contain a unique insertion in its hinge-region segment between the first and second constant region domains (Figure 1a). This hinge region, which has a high content of proline, serine, and threonine, is the site of attachment of as many as five O-linked glycan chains consisting of N-acetylgalactosamine with a ß1,3-linked galactose that may be sialylated (29-34). Sialic acid may also be attached to N-acetylgalactosamine by an α2,6 linkage. The carbohydrate composition of these O-linked glycans in the hinge region of normal circulatory IgA1 is variable. The prevailing forms include N-acetylgalactosamine-galactose disaccharide, and its mono- and di-sialylated forms (18, 31, 35) (Figure 1b). Galactose-deficient variants with terminal N-acetylgalactosamine or sialylated N-acetylgalactosamine are rarely found in the O-glycans of normal circulatory IgA1 (31), but are much more common in patients with IgAN, predominantly in the glomerular immune deposits and in circulating immune complexes (18, 23, 36-39).
Figure 1.
a) IgA1 and its hinge region with O-linked glycans (white circles) and N-linked glycans (black circles). Underlined and numbered amino acids denote usual sites of attachment of as many as five O-linked glycans (31, 34). b) Variants of O-linked glycans in the hinge region of human circulatory IgA1 (30, 31).
IgAN - a disease of aberrant glycosylation
Analysis of the glycosylation of IgA1 in patients with IgAN has provided new insights into the mechanisms underlying formation of immune complexes and their deposition in the mesangium (18, 23, 40-44). Specifically, aberrant glycosylation of the O-linked glycans (galactose deficiency) in IgA1 hinge region appears to be a key pathogenetic factor contributing to the development of IgAN (18, 23, 39-41, 45). Notably, galactose-deficient IgA1 is the predominant glycosylation variant of IgA1 in the mesangium (37, 38). A relationship between galactose deficiency and nephritis has been observed in other diseases. Galactose-deficient IgA1 (46) and IgA-IgG circulating complexes (47) are found in sera of patients with Henoch-Schöenlein purpura who develop nephritis but not in sera of patients who do not. Also, patients with IgA1 myeloma may have very high levels of circulatory IgA1, but only those with aberrantly glycosylated IgA1 develop an immune-complex glomerulonephritis (48, 49).
Analysis of O-glycans on IgA1 has been challenging (50). The reasons include the structure of the hinge region (there are nine serine and threonine residues, the potential sites of attachment of five glycan chains (51)) and the heterogeneity of composition of the attached glycans (35). The techniques applied to the analysis include the use of lectins, ELISA, western blotting, mass spectrometry, gas-liquid chromatography, and specific proteases (Table 1). The greatest challenge has been the assignment of the sites of attachment of the glycans, including those that are aberrantly glycosylated. The most promising techniques are based on high-resolution mass spectrometry, such as Fourier transform ion cyclotron resonance mass spectrometry, using electron-capture or electron-transfer fragmentation techniques (34, 52).
Table 1.
Examples of techniques for analysis of IgA1 O-glycans
| Technology | Data generated | References |
|---|---|---|
| Gas-liquid chromatography | Composition of monosaccharides | (18, 39) |
| Lectin ELISA | Terminal sugar | (18, 36, 37, 39, 43, 44) |
| Lectin western blotting | Terminal sugar | (43, 44) |
| Fluorophore-assisted carbohydrate electrophoresis | Profile of released O-glycans | (96) |
| HPLC | Profile of released O-glycans | (97) |
| MALDI-TOF MS of trypsin-digested hinge-region fragment | Profile of hinge-region glycopeptides | (33, 75, 98, 99) |
| ESI MS of trypsin-digested hinge-region fragment | Profile of hinge-region glycopeptides | (38) |
| MALDI-TOF MS of hinge-region fragment generated with two bacterial proteases | Profile of hinge-region glycopeptides | (35) |
| FT-ICR MS of hinge-region proteolytic fragment | Profile of hinge-region glycopeptides | (34, 52, 75) |
| SELDI MS | Profile of hinge-region glycopeptides with aberrant glycans | (100) |
| Lectin western blotting of IgA1 digested with bacterial proteases | Sites of glycan attachment | (44) |
| AI-ECD FT-ICR MS of hinge-region proteolytic fragment | Sites of glycan attachment of individual glycopeptides | (34, 52, 75) |
Abbreviations:
HPLC: high-pressure liquid chromatography
ESI: electrospray ionization
MS: mass spectrometry
MALDI-TOF: matrix-assisted laser desorption ionization-time of flight
FT-ICR: Fourier transform ion cyclotron resonance
AI-ECD: activated-ion electron capture dissociation
SELDI: surface-enhanced laser desorption ionization
Biosynthesis and catabolism of IgA1
When the daily synthesis of all isotypes of immunoglobulins is taken into account, the production of IgA far exceeds the synthesis of IgG, IgM, IgD, and IgE combined. However, more than two-thirds of all IgA finishes its short life-span in the external secretions (half-life of IgA in the circulation is ∼4-5 days) (28). Quantitative studies of IgA production and the distribution of IgA-producing cells in tissues clearly indicate that 90-95% of circulatory IgA is produced in the bone marrow, lymph nodes, and spleen, with a small contribution from the mucosal tissues (28). In contrast, most external secretions, with the exception of those in the urine and the male and female genital tracts, contain IgA originating from local synthesis by the abundant IgA-producing plasma cells. Studies in primates (53) demonstrated that IgA is catabolized by hepatocytes that recognize glycan moieties on IgA heavy chains. In contrast to other immunoglobulin isotypes, human IgA1 occurs in two subclasses and two molecular forms, monomeric and polymeric, with characteristic distributions in various body fluids and in immunoglobulin-producing cells in the systemic and mucosal tissues (28). Typically, most of the IgA-secreting cells in the bone marrow and lymph nodes produce monomeric IgA (>95%) of the IgA1 subclass (∼85%). In mucosal tissues, IgA-secreting cells produce polymeric IgA with J chain. However, the distribution of IgA1- or IgA2-producing cells displays marked differences among mucosal tissues. For example, the upper respiratory and gastrointestinal tracts are populated by IgA1-producing cells while the large intestine and the female genital tract contain equal numbers or a slight excess of IgA2-producing cells. Human salivary, lacrimal, and mammary glands contain approximately equal numbers of IgA1- and IgA2-producing cells (28).
Whether the bone marrow or mucosal tissue is the origin of IgA1 in circulating immune complexes and the mesangial deposits of patients with IgAN has been a matter of controversy. A signature clinical manifestation of the disease, macroscopic hematuria, is often accompanied by a concurrent infection of the upper respiratory tract. Because this site is populated by cells producing polymeric IgA1, this feature favors a mucosal origin of the pathogenic IgA1 (54). However, in other studies, polymeric IgA1- and J chain-producing cells have been detected in the bone marrow of patients with IgAN (55-58). Unfortunately, samples of mucosal tissues, lymph nodes, and bone marrow from patients with IgAN are not easily available to resolve this dispute.
Biosynthesis of O-linked glycans on IgA1
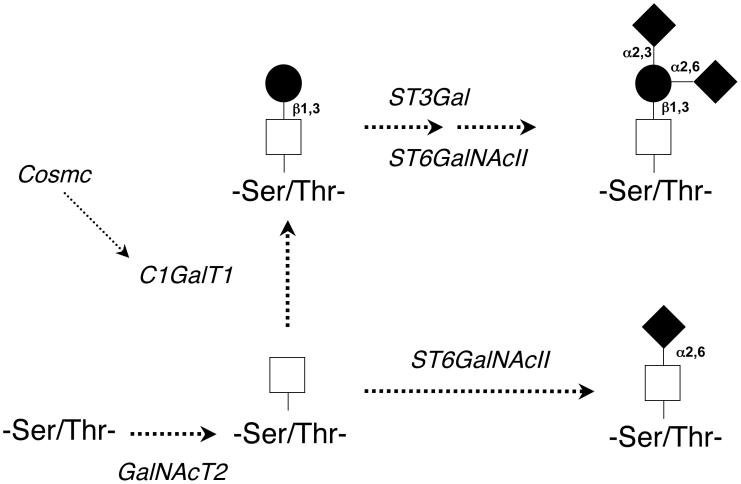
O-linked glycans of IgA1 are synthesized in a step-wise manner, beginning with attachment of N-acetylgalactosamine to serine or threonine, catalyzed by UDP-N-acetylgalactosaminyl-transferase 2 (GalNAcT2) (59, 60) (Figure 2). The O-glycan chain is then extended by sequential attachment of galactose and/or sialic acid residues to the N-acetylgalactosamine. The addition of galactose is mediated by core 1 ß1,3-galactosyltransferase (C1ß3GalT1) that transfers galactose from UDP-galactose to a N-acetylgalactosamine residue (61). The stability of this enzyme depends on its interaction with a chaperone, Cosmc (Core-1-β3-Gal-T Specific Molecular Chaperone) (61-64). In the absence of Cosmc, the C1ß3GalT1 protein is degraded rapidly, thereby resulting in undergalactosylation of N-acetylgalactosamine in O-linked glycans. The glycan structure is completed by the sialyltransferases (specific for α2,3-galactose and α2,6-N-acetylgalactosamine) that attach negatively charged sialic acid to the galactose or N-acetylgalactosamine residues. Sialylation of N-acetylgalactosamine in IgA1-secreting cells is mediated by a N-acetylgalactosamine-specific α2,6-sialyltransferase, ST6GalNAcII (65). If sialic acid is linked to N-acetylgalactosamine prior to attachment of galactose, this “premature” sialylation precludes subsequent attachment of a galactose residue (65-67). Thus, the relative activity of ST6GalNAcII and C1ß3GalT1/Cosmc can directly influence the glycosylation of IgA1. Studies of the variants of the C1ß3GalT1 gene found an association of certain polymorphisms with IgAN (68). Other studies looking at genetic factors contributing to IgAN are ongoing (69).
Figure 2.
Enzymes involved in glycosylation of hinge region of IgA1. Galactose-deficient IgA1 may be generated by decreased activity of C1GalT1/Cosmc, by increased activity of ST6GalNAcII, or both.
Recent studies with Epstein-Barr virus-immortalized cell lines from peripheral blood lymphocytes of patients with IgAN and healthy controls confirmed the above-described pathways in the IgA1-secreting cells (70). Furthermore, detailed analysis of enzymatic activities in the cell lines from patients with IgAN indicated an imbalance in the activities of the pertinent glycosyltransferases. The ß1,3-galactosyltransferase activity was significantly lower and the N-acetylgalactosamine-specific α2,6-sialyltransferase activity was significantly higher (71). Studies of the aberrant glycosylation in IgAN represent a promising field with a potentially great impact on the future care of patients (72).
Anti-IgA1 antibodies as a component of circulating immune complexes
Although IgA1-IgG immune complexes have been detected by many investigators, the true nature of IgA1-IgG interaction was demonstrated only recently (23). Dissociability of circulating immune complexes at acidic pH and inhibition of reformation by N-acetylgalactosamine-bearing glycoproteins implied an antigen-antibody nature of the IgA1-IgG interaction in these complexes (23). The presence of IgG antibodies, and to lesser degree IgA1 or IgM antibodies, to IgA1 in sera of healthy individuals and patients with IgAN has been described (73, 74). However, the antigenic determinants expressed on the IgA1 molecules were unknown. More recently, with the application of bacterial IgA proteases, lectin western blots, and high-resolution mass spectrometry, the antigenic determinants have been shown to consist of a N-acetylgalactosamine residue in the central and C-terminal portions of the hinge region of the heavy chain (75). We have detected and isolated circulating IgG-producing cells that secrete IgG specific for aberrantly glycosylated IgA1 (70).
The stimulus that leads to the formation of serum antibodies specific for N-acetylgalactosamine in the IgA1 hinge-region in healthy individuals and patients with IgAN is unknown. However, some viruses (e.g., respiratory syncitial virus, Epstein-Barr virus) and Gram-positive bacteria (e.g., streptococcus) express GalNAc-containing structures on their surfaces. It is therefore possible that these structures may mimic the glycan epitopes on galactose-deficient IgA1.
The relatively short half-life of normal serum IgA is due to its rapid catabolism by hepatocytes (32, 53, 76, 77). Hepatocytes express the asialoglycoprotein receptor (32, 77) that binds glycoproteins through a terminal galactose or N-acetylgalactosamine residue (32, 77-79). Because of this structural pre-requisite, the absence or enzymatic removal of the otherwise terminal sialic acid is essential for effective binding of IgA1. Indeed, human IgA1 myeloma proteins and polyclonal human IgA1 are removed promptly from the circulation after enzymatic cleavage of terminal sialic acid (32, 80, 81).
Galactose-deficient IgA1 has a longer life-span than normal IgA1 (82). Galactose deficiency by itself should not hinder disposal of IgA1 molecules because the asialoglycoprotein receptor recognizes terminal N-acetylgalactosamine as well as galactose (78). However, if the N-acetylgalactosamine is linked to sialic acid or is occupied by an antibody, the IgA1 cannot be recognized by the asialoglycoprotein receptor and therefore escapes hepatic catabolism (40, 83). Because galactose-deficient circulatory IgA1 is present predominantly in the form of immune complexes, it is plausible to speculate that this IgA1 does not effectively reach the hepatic asialoglycoprotein receptor (Figure 3). The larger size of the complexes, compared to uncomplexed IgA1, precludes binding to this receptor because the relatively small endothelial fenestrae block entry into the space of Disse. Thus, immune complexes containing aberrantly glycosylated IgA1 are not efficiently cleared by the liver and reach the glomerular capillaries where larger endothelial fenestrae permit their entry into the mesangium (25, 45, 67, 84).
Figure 3.
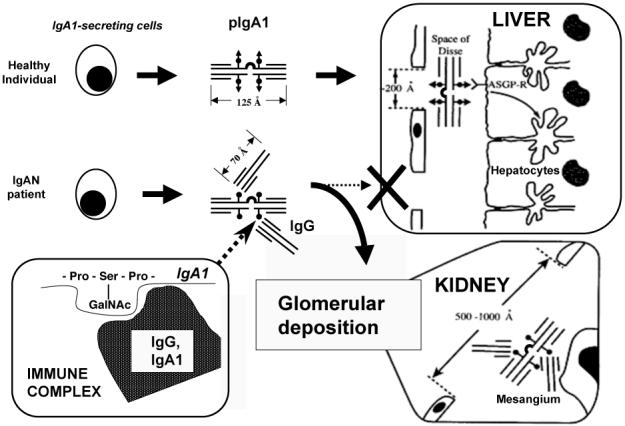
A model of pathogenesis of IgAN. Some polymeric IgA1 (pIgA1) produced by B cells and plasma cells in patients with IgAN is galactose-deficient and is recognized by anti-glycan IgG or IgA1 antibodies. The resultant immune complexes are too bulky to enter the space of Disse to reach the asialoglycoprotein receptor (ASGP-R) on hepatocytes, but are able to pass through the larger fenestrae in glomerular capillaries overlying the mesangium. These deposited complexes induce glomerular injury.
Biological activities of IgA1-containing immune complexes
Cultured human mesangial cells present a convenient model to evaluate biologic activities of IgA complexes (40, 41, 85, 86). Immune complexes from sera of patients with IgAN containing galactose-deficient IgA1 bind to the mesangial cells more efficiently than do uncomplexed IgA1 or immune complexes from healthy controls. Assessment of the biological activity of IgA1 complexes showed that large-molecular-weight IgA1 complexes stimulated cellular proliferation and production of some cytokines (e.g., IL-6, TGF-β). In contrast, IgA1-depleted fractions were devoid of stimulatory activity (41). Further support for the pathogenic role of IgA1 complexes has come from supplementation experiments. Addition of small amounts of desialylated polymeric IgA1 to sera of patients with IgAN led to formation of new immune complexes: the amount of stimulatory complexes of molecular mass 800-900 kDa increased. ELISA indicated that these complexes contained IgG and IgA1 (41). In contrast, uncomplexed IgA1 did not alter cellular proliferation. Complexes in the native sera of patients with IgAN enhanced cellular proliferation more than did complexes of similar mass from healthy volunteers (41). Furthermore, complexes of patients with IgAN collected during an episode of macroscopic hematuria stimulated cellular proliferation more than did complexes obtained during a later quiescent phase. IgA1 complexes with high levels of galactose-deficient IgA1 induced more proliferation than did complexes with low levels of galactose-deficient IgA1. In vivo studies in experimental animals showed that large-molecular-weight immune complexes generally induced more severe glomerular lesions than did small complexes (87).
Several findings point to activation of mesangial cells through an IgA-specific receptor(s) (40, 41, 88). However, none of the known IgA receptors (CD89, asialoglycoprotein receptor, and polymeric immunoglobulin receptor) is expressed on human mesangial cells (24, 67, 89-91). Among the recently identified candidate receptors that may mediate binding of IgA1 and IgA1 complexes are CD71 (transferrin receptor) (88, 91-93) and the Fcα/μ receptor (94). CD71 appears to be the major IgA1 receptor on human mesangial cells (reviewed by Moura in this issue) (88, 92). Notably, the expression of CD71 is enhanced in the mesangium of IgAN patients and it co-localizes with IgA1 deposits (95). Engagement of CD71 by IgA1 induces cellular proliferation and cytokine production. This accentuated cellular proliferation and cytokine production by IgA1 is inhibited completely by anti-CD71 blocking antibody, indicating that CD71 plays a major role in IgA1 binding (93).
Hypothetical model of the pathogenesis of IgAN
Based on published data, a hypothetical model of the pathogenesis of IgAN is emerging. Some IgA1 molecules produced by immunoglobulin-secreting cells in patients with IgAN are galactose-deficient and consequently recognized by anti-glycan IgG (or IgA1) antibodies. The resultant immune complexes are too bulky to enter the space of Disse in the liver. IgA1-containing immune complexes that escape normal clearance mechanisms reach the renal circulation and pass through the larger fenestrae in the glomerular capillaries overlying the mesangium. These complexes bind to mesangial cells and induce glomerular injury (Figure 3). Together, these characteristics classify IgAN as an autoimmune disease, with the aberrantly glycosylated IgA1 being the autoantigen.
Acknowledgments
Supported in part by grants from National Institutes of Health DK78244, DK61525, DK71802, and DK64400, and by a grant from Czech Republic VZ MSM0021620812.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Berger J, Hinglais N. Les depots intercapillaires d’IgA-IgG (Intercapillary deposits of IgA-IgG) J Urol Nephrol. 1968;74:694–695. [PubMed] [Google Scholar]
- 2.Jennette JC. The immunohistology of IgAN. Am J Kidney Dis. 1988;12:348–352. doi: 10.1016/s0272-6386(88)80022-2. [DOI] [PubMed] [Google Scholar]
- 3.Conley ME, Cooper MD, Michael AF. Selective deposition of immunoglobulin A1 in immunoglobulin A nephropathy, anaphylactoid purpura nephritis, and systemic lupus erythematosus. J Clin Invest. 1980;66:1432–1436. doi: 10.1172/JCI109998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Emancipator SN. IgAN and Henoch-Schönlein syndrome. In: Jennette JC, Olson JL, Schwartz MM, Silva FG, editors. Heptinstall’s Pathology of the Kidney. Lippincott-Raven Publishers; Philadelphia: 1998. pp. 479–539. [Google Scholar]
- 5.D’Amico G. Natural history of idiopathic IgAN: role of clinical and histological prognostic factors. Am J Kidney Dis. 2000;36:227–237. doi: 10.1053/ajkd.2000.8966. [DOI] [PubMed] [Google Scholar]
- 6.Yoshikawa N, Ito H, Nakamura H. Prognostic indicators in childhood IgAN. Nephron. 1992;60:60–67. doi: 10.1159/000186706. [DOI] [PubMed] [Google Scholar]
- 7.McCallum D, Smith L, Harley F, et al. IgAN and thin basement membrane disease in association with Crohn disease. Pediat Nephrol. 1997;11:637–640. doi: 10.1007/s004670050355. [DOI] [PubMed] [Google Scholar]
- 8.Bene MC, Hurault De Ligny B, Kessler M, et al. Confirmation of tonsillar anomalies in IgAN: a multicenter study. Nephron. 1991;58:425–428. doi: 10.1159/000186474. [DOI] [PubMed] [Google Scholar]
- 9.Emancipator SN, Mestecky J, Lamm ME. IgAN and related diseases. In: Mestecky J, Bienenstock J, Lamm ME, Mayer L, McGhee JR, Strober W, editors. Mucosal Immunology. 3rd edition Elsevier Academic Press; Amsterdam: 2005. pp. 1579–1600. [Google Scholar]
- 10.Coppo R, Amore A, Cirina P, et al. Characteristics of IgA and macromolecular IgA in sera from IgAN transplanted patients with and without IgAN recurrence. Contrib Nephrol. 1995;111:85–92. doi: 10.1159/000423881. [DOI] [PubMed] [Google Scholar]
- 11.Coppo R, Amore A, Cirina P, et al. IgA serology in recurrent and non-recurrent IgAN after renal transplantation. Nephrol Dial Transplant. 1995;10:2310–2315. doi: 10.1093/ndt/10.12.2310. [DOI] [PubMed] [Google Scholar]
- 12.Odum J, Peh CA, Clarkson AR, et al. Recurrent mesangial IgA nephritis following renal transplantation. Nephrol Dial Transplant. 1994;9:309–312. [PubMed] [Google Scholar]
- 13.Julian BA. Treatment of IgAN. Semin Nephrol. 2000;20:277–285. [PubMed] [Google Scholar]
- 14.Berger J. Recurrence of IgAN in renal allografts. Am J Kidney Dis. 1988;12:371–372. doi: 10.1016/s0272-6386(88)80027-1. [DOI] [PubMed] [Google Scholar]
- 15.Silva FG, Chander P, Pirani CL, et al. Disappearance of glomerular mesangial IgA deposits after renal allograft transplantation. Transplantation. 1982;33:241–246. [PubMed] [Google Scholar]
- 16.Czerkinsky C, Koopman WJ, Jackson S, et al. Circulating immune complexes and immunoglobulin A rheumatoid factor in patients with mesangial immunoglobulin A nephropathies. J Clin Invest. 1986;77:1931–1938. doi: 10.1172/JCI112522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Coppo R, Basolo B, Piccoli G, et al. IgA1 and IgA2 immune complexes in primary IgAN and Henoch-Schönlein nephritis. Clin Exp Immunol. 1984;57:583–590. [PMC free article] [PubMed] [Google Scholar]
- 18.Tomana M, Matousovic K, Julian BA, et al. Galactose-deficient IgA1 in sera of IgAN patients is present in complexes with IgG. Kidney Int. 1997;52:509–516. doi: 10.1038/ki.1997.361. [DOI] [PubMed] [Google Scholar]
- 19.Schena FP, Pastore A, Ludovico N, et al. Increased serum levels of IgA1-IgG immune complexes and anti-F(ab’)2 antibodies in patients with primary IgAN. Clin Exp Immunol. 1989;77:15–20. [PMC free article] [PubMed] [Google Scholar]
- 20.Gonzales-Cabrero J, Egido J, Mampaso F, et al. Characterization of circulating idiotypes containing immune complexes and their presence in the glomerular mesangium in patients with IgAN. Clin Exp Immunol. 1989;76:204–209. [PMC free article] [PubMed] [Google Scholar]
- 21.van den Wall Bake AWL, Bruijn JA, Accavitti MA, et al. Shared idiotypes in mesangial deposits in IgAN are not disease-specific. Kidney Int. 1993;44:65–74. doi: 10.1038/ki.1993.214. [DOI] [PubMed] [Google Scholar]
- 22.Coppo R, Basolo B, Martina G, et al. Circulating immune complexes containing IgA, IgG and IgM in patients with primary IgAN and with Henoch-Schönlein nephritis. Correlation with clinical and histologic signs of activity. Clin Nephrol. 1982;18:230–239. [PubMed] [Google Scholar]
- 23.Tomana M, Novak J, Julian BA, et al. Circulating immune complexes in IgAN consist of IgA1 with galactose-deficient hinge region and antiglycan antibodies. J Clin Invest. 1999;104:73–81. doi: 10.1172/JCI5535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barratt J, Feehally J. IgAN. J Am Soc Nephrol. 2005;16:2088–2097. doi: 10.1681/ASN.2005020134. [DOI] [PubMed] [Google Scholar]
- 25.Coppo R, Amore A. Aberrant glycosylation in IgAN. Kidney Int. 2004;65:1544–1547. doi: 10.1111/j.1523-1755.2004.05407.x. [DOI] [PubMed] [Google Scholar]
- 26.Mestecky J. Immunobiology of IgA. Am J Kidney Dis. 1988;12:378–383. doi: 10.1016/s0272-6386(88)80029-5. [DOI] [PubMed] [Google Scholar]
- 27.Mestecky J, Lue C, Tarkowski A, et al. Comparative studies of the biological properties of human IgA subclasses. Protides Biol Fluids. 1989;36:173–182. [Google Scholar]
- 28.Mestecky J, Moro I, Kerr MA, et al. Mucosal immunoglobulins. In: Mestecky J, Bienenstock J, Lamm ME, Mayer L, McGhee JR, Strober W, editors. Mucosal Immunology. 3rd edition Elsevier Academic Press; Amsterdam: 2005. pp. 153–181. [Google Scholar]
- 29.Baenziger J, Kornfeld S. Structure of the carbohydrate units of IgA1 immunoglobulin II. Structure of the O-glycosidically linked oligosaccharide units. J Biol Chem. 1974;249:7270–7281. [PubMed] [Google Scholar]
- 30.Field MC, Dwek RA, Edge CJ, et al. O-linked oligosaccharides from human serum immunoglobulin A1. Biochem Soc Trans. 1989;17:1034–1035. doi: 10.1042/bst0171034. [DOI] [PubMed] [Google Scholar]
- 31.Mattu TS, Pleass RJ, Willis AC, et al. The glycosylation and structure of human serum IgA1, Fab, and Fc regions and the role of N-glycosylation on Fcα receptor interactions. J Biol Chem. 1998;273:2260–2272. doi: 10.1074/jbc.273.4.2260. [DOI] [PubMed] [Google Scholar]
- 32.Tomana M, Kulhavy R, Mestecky J. Receptor-mediated binding and uptake of immunoglobulin A by human liver. Gastroenterology. 1988;94:887–892. doi: 10.1016/0016-5085(88)90252-1. [DOI] [PubMed] [Google Scholar]
- 33.Tarelli E, Smith AC, Hendry BM, et al. Human serum IgA1 is substituted with up to six O-glycans as shown by matrix assisted laser desorption ionisation time-of-flight mass spectrometry. Carbohydr Res. 2004;339:2329–2335. doi: 10.1016/j.carres.2004.07.011. [DOI] [PubMed] [Google Scholar]
- 34.Renfrow MB, Cooper HJ, Tomana M, et al. Determination of aberrant O-glycosylation in the IgA1 hinge region by electron capture dissociation Fourier transform-ion cyclotron resonance mass spectrometry. J Biol Chem. 2005;280:19136–19145. doi: 10.1074/jbc.M411368200. [DOI] [PubMed] [Google Scholar]
- 35.Novak J, Tomana M, Kilian M, et al. Heterogeneity of O-glycosylation in the hinge region of human IgA1. Mol Immunol. 2000;37:1047–1056. doi: 10.1016/s0161-5890(01)00019-0. [DOI] [PubMed] [Google Scholar]
- 36.Allen AC, Harper SJ, Feehally J. Galactosylation of N- and O-linked carbohydrate moieties of IgA1 and IgG in IgAN. Clin Exp Immunol. 1995;100:470–474. doi: 10.1111/j.1365-2249.1995.tb03724.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Allen AC, Bailey EM, Brenchley PEC, et al. Mesangial IgA1 in IgAN exhibits aberrant O-glycosylation: Observations in three patients. Kidney Int. 2001;60:969–973. doi: 10.1046/j.1523-1755.2001.060003969.x. [DOI] [PubMed] [Google Scholar]
- 38.Hiki Y, Odani H, Takahashi M, et al. Mass spectrometry proves under-O-glycosylation of glomerular IgA1 in IgAN. Kidney Int. 2001;59:1077–1085. doi: 10.1046/j.1523-1755.2001.0590031077.x. [DOI] [PubMed] [Google Scholar]
- 39.Mestecky J, Tomana M, Crowley-Nowick PA, et al. Defective galactosylation and clearance of IgA1 molecules as a possible etiopathogenic factor in IgAN. Contrib Nephrol. 1993;104:172–182. doi: 10.1159/000422410. [DOI] [PubMed] [Google Scholar]
- 40.Novak J, Vu HL, Novak L, et al. Interactions of human mesangial cells with IgA and IgA-containing circulating immune complexes. Kidney Int. 2002;62:465–475. doi: 10.1046/j.1523-1755.2002.00477.x. [DOI] [PubMed] [Google Scholar]
- 41.Novak J, Tomana M, Matousovic K, et al. IgA1-containing immune complexes in IgAN differentially affect proliferation of mesangial cells. Kidney Int. 2005;67:504–513. doi: 10.1111/j.1523-1755.2005.67107.x. [DOI] [PubMed] [Google Scholar]
- 42.Barratt J, Smith AC, Feehally J. The pathogenic role of IgA1 O-linked glycosylation in the pathogenesis of IgAN. Nephrology (Carlton) 2007;12:275–284. doi: 10.1111/j.1440-1797.2007.00797.x. [DOI] [PubMed] [Google Scholar]
- 43.Moldoveanu Z, Wyatt RJ, Lee J, et al. Patients with IgAN have increased serum galactose-deficient IgA1 levels. Kidney Int. 2007;71:1148–1154. doi: 10.1038/sj.ki.5002185. [DOI] [PubMed] [Google Scholar]
- 44.Moore JS, Kulhavy R, Tomana M, et al. Reactivities of N-acetylgalactosamine-specific lectins with human IgA1 proteins. Mol Immunol. 2007;44:2598–2604. doi: 10.1016/j.molimm.2006.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Julian BA, Novak J. IgAN: an update. Current Opin Nephrol Hypertens. 2004;13:171–179. doi: 10.1097/00041552-200403000-00005. [DOI] [PubMed] [Google Scholar]
- 46.Allen AC, Willis FR, Beattie TJ, et al. Abnormal IgA glycosylation in Henoch-Schönlein purpura restricted to patients with clinical nephritis. Nephrol Dial Transplant. 1998;13:930–934. doi: 10.1093/ndt/13.4.930. [DOI] [PubMed] [Google Scholar]
- 47.Levinsky RJ, Barratt TM. IgA immune complexes in Henoch-Schönlein purpura. Lancet. 1979;2:1100–1103. doi: 10.1016/s0140-6736(79)92505-4. [DOI] [PubMed] [Google Scholar]
- 48.van der Helm-van Mil AHM, Smith AC, Pouria S, et al. Immunoglobulin A multiple myeloma presenting with Henoch-Schönlein purpura associated with reduced sialylation of IgA1. Br J Haematol. 2003;122:915–917. doi: 10.1046/j.1365-2141.2003.04539.x. [DOI] [PubMed] [Google Scholar]
- 49.Zickerman AM, Allen AC, Talwar V, et al. IgA myeloma presenting as Henoch-Schönlein purpura with nephritis. Am J Kidney Dis. 2000;36:E19. doi: 10.1053/ajkd.2000.16221. [DOI] [PubMed] [Google Scholar]
- 50.Allen AC. Methodological approaches to the analysis of IgA1 O-glycosylation in IgAN. J Nephrol. 1999;12:76–84. [PubMed] [Google Scholar]
- 51.Frangione B, Wolfenstein-Todel C. Partial duplication in the “hinge” region of IgA1 myeloma proteins. Proc Natl Acad Sci USA. 1972;69:3673–3676. doi: 10.1073/pnas.69.12.3673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Renfrow MB, MacKay CL, Chalmers MJ, et al. Analysis of O-glycan heterogeneity in IgA1 myeloma proteins by Fourier transform ion cyclotron resonance mass spectrometry: Implications for IgAN. Anal Bioanal Chem. 2007 doi: 10.1007/s00216-007-1500-z. In Press. [DOI] [PubMed] [Google Scholar]
- 53.Moldoveanu Z, Moro I, Radl J, et al. Site of catabolism of autologous and heterologous IgA in non-human primates. Scand J Immunol. 1990;32:577–583. doi: 10.1111/j.1365-3083.1990.tb03199.x. [DOI] [PubMed] [Google Scholar]
- 54.Smith AC, Molyneux K, Feehally J, et al. O-glycosylation of serum IgA1 antibodies against mucosal and systemic antigens in IgAN. J Am Soc Nephrol. 2006;17:3520–3528. doi: 10.1681/ASN.2006060658. [DOI] [PubMed] [Google Scholar]
- 55.Allen A, Harper S, Feehally J. Origin and structure of pathogenic IgA in IgAN. Biochem Soc Trans. 1997;25:486–490. doi: 10.1042/bst0250486. [DOI] [PubMed] [Google Scholar]
- 56.Harper SJ, Allen AC, Layward L, et al. Increased immunoglobulin A and immunoglobulin A1 cells in bone marrow trephine biopsy specimens in immunoglobulin A nephropathy. Am J Kidney Dis. 1994;24:888–892. doi: 10.1016/s0272-6386(12)81056-0. [DOI] [PubMed] [Google Scholar]
- 57.Harper SJ, Feehally J. The pathogenic role of immunoglobulin A polymers in immunoglobulin A nephropathy. Nephron. 1993;65:337–345. doi: 10.1159/000187509. [DOI] [PubMed] [Google Scholar]
- 58.Harper SJ, Pringle JH, Gillies A, et al. Simultaneous in situ hybridisation of native mRNA and immunoglobulin detection by conventional immunofluorescence in paraffin wax embedded sections. Journal of Clinical Pathology. 1992;45:114–119. doi: 10.1136/jcp.45.2.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Piller V, Piller F, Fukuda M. Biosynthesis of truncated O-glycans in the T cell line Jurkat. Localization of O-glycan initiation. J Biol Chem. 1990;265:9264–9271. [PubMed] [Google Scholar]
- 60.Iwasaki H, Zhang Y, Tachibana K, et al. Initiation of O-glycan synthesis in IgA1 hinge region is determined by a single enzyme, UDP-N-acetyl-alpha-D-galactosamine:polypeptide N-acetylgalactosaminyltransferase 2. J Biol Chem. 2003;278:5613–5621. doi: 10.1074/jbc.M211097200. [DOI] [PubMed] [Google Scholar]
- 61.Ju T, Brewer K, D’Souza A, et al. Cloning and expression of human core 1 β1,3-galactosyltransferase. J Biol Chem. 2002;277:178–186. doi: 10.1074/jbc.M109060200. [DOI] [PubMed] [Google Scholar]
- 62.Ju T, Cummings RD. A unique molecular chaperone Cosmc required for activity of the mammalian core 1 β3-galactosyltransferase. Proc Natl Acad Sci U S A. 2002;99:16613–16618. doi: 10.1073/pnas.262438199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ju T, Cummings RD. Protein glycosylation: chaperone mutation in Tn syndrome. Nature. 2005;437:1252. doi: 10.1038/4371252a. [DOI] [PubMed] [Google Scholar]
- 64.Kudo T, Iwai T, Kubota T, et al. Molecular cloning and characterization of a novel UDP-Gal:GalNAcα peptide β1,3-galactosyltransferase (C1Gal-T2), an enzyme synthesizing a core 1 structure of O-glycan. J Biol Chem. 2002;277:47724–47731. doi: 10.1074/jbc.M205839200. [DOI] [PubMed] [Google Scholar]
- 65.Raska M, Moldoveanu Z, Suzuki H, et al. Identification and characterization of CMP-NeuAc:GalNAc-IgA1 α2,6-sialyltransferase in IgA1-producing cells. J Mol Biol. 2007;369:69–78. doi: 10.1016/j.jmb.2007.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Schachter H, McGuire EJ, Roseman S. Sialic acids. XIII. A uridine diphosphate D-galactose: mucin galactosyltransferase from porcine submaxillary gland. J Biol Chem. 1971;246:5321–5328. [PubMed] [Google Scholar]
- 67.Novak J, Julian BA, Tomana M, et al. Progress in molecular and genetic studies of IgAN. J Clin Immunol. 2001;21:310–327. doi: 10.1023/a:1012284402054. [DOI] [PubMed] [Google Scholar]
- 68.Li GS, Zhang H, Lv JC, et al. Variants of C1GALT1 gene are associated with the genetic susceptibility to IgAN. Kidney Int. 2007 doi: 10.1038/sj.ki.5002088. In Press. [DOI] [PubMed] [Google Scholar]
- 69.Beerman I, Novak J, Wyatt RJ, et al. Genetics of IgAN. Nat Clin Pract Nephrol. 2007;3:325–338. doi: 10.1038/ncpneph0492. [DOI] [PubMed] [Google Scholar]
- 70.Suzuki H, Moldoveanu Z, Hall S, et al. IgAN: characterization of IgG antibodies specific for galactose-deficient IgA1. Contrib Nephrol. 2007;157:129–133. doi: 10.1159/000102454. [DOI] [PubMed] [Google Scholar]
- 71.Suzuki H, Moldoveanu Z, Hall S, et al. IgA1-secreting cell lines from patients with IgAN produce aberrantly glycosylated IgA1. J Clin Invest. 2007 doi: 10.1172/JCI33189. In Revision. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Glassock RJ. Concluding remarks. Contrib Nephrol. 2007;157:169–173. doi: 10.1159/000102463. [DOI] [PubMed] [Google Scholar]
- 73.Jackson S, Montgomery RI, Julian BA, et al. Aberrant synthesis of antibodies directed at the Fab of IgA in patients with IgA nephropathies. Clin Immunol Immunopath. 1987;45:208–213. doi: 10.1016/0090-1229(87)90035-3. [DOI] [PubMed] [Google Scholar]
- 74.Jackson S. Immunoglobulin-antiimmunoglobulin interactions and immune complexes in IgAN. Am J Kidney Dis. 1988;12:425–429. doi: 10.1016/s0272-6386(88)80039-8. [DOI] [PubMed] [Google Scholar]
- 75.Novak J, Moldoveanu Z, Renfrow MB, et al. IgAN and Henoch-Schoenlein Purpura nephritis: aberrant glycosylation of IgA1, formation of IgA1-containing immune complexes, and activation of mesangial cells. Contrib Nephrol. 2007;157:134–138. doi: 10.1159/000102455. [DOI] [PubMed] [Google Scholar]
- 76.Moldoveanu Z, Epps JM, Thorpe SR, et al. The sites of catabolism of murine monomeric IgA. J Immunol. 1988;141:208–213. [PubMed] [Google Scholar]
- 77.Stockert RJ, Kressner MS, Collins JD, et al. IgA interactions with the asialoglycoprotein receptor. Proc Natl Acad Sci USA. 1982;79:6229–6231. doi: 10.1073/pnas.79.20.6229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Baenziger JU, Fiete D. Galactose and N-acetylgalactosamine-specific endocytosis of glycopeptides by isolated rat hepatocytes. Cell. 1980;22:611–620. doi: 10.1016/0092-8674(80)90371-2. [DOI] [PubMed] [Google Scholar]
- 79.Baenziger JU, Maynard Y. Human hepatic lectin. Physicochemical properties and specificity. J Biol Chem. 1980;255:4607–4613. [PubMed] [Google Scholar]
- 80.Phillips JO, Russell MW, Brown TA, et al. Selective hepatobiliary transport of human polymeric IgA in mice. Mol Immunol. 1984;21:907–914. doi: 10.1016/0161-5890(84)90147-0. [DOI] [PubMed] [Google Scholar]
- 81.Phillips JO, Stohrer R, Russell MW, et al. Analysis of the hepatobiliary transport of IgA with monoclonal anti-idiotype and anti-allotype antibodies. Mol Immunol. 1986;23:339–346. doi: 10.1016/0161-5890(86)90061-1. [DOI] [PubMed] [Google Scholar]
- 82.Mestecky J, Hashim OH, Tomana M. Alterations in the IgA carbohydrate chains influence the cellular distribution of IgA1. Contrib Nephrol. 1995;111:66–72. doi: 10.1159/000423879. [DOI] [PubMed] [Google Scholar]
- 83.Phillips JO, Komiyama K, Epps JM, et al. Role of hepatocytes in the uptake of IgA and IgA-containing immune complexes in mice. Mol Immunol. 1988;25:873–879. doi: 10.1016/0161-5890(88)90124-1. [DOI] [PubMed] [Google Scholar]
- 84.Couser WG. Glomerulonephritis. Lancet. 1999;353:1509–1515. doi: 10.1016/S0140-6736(98)06195-9. [DOI] [PubMed] [Google Scholar]
- 85.Chen A, Chen WP, Sheu LF, et al. Pathogenesis of IgAN: in vitro activation of human mesangial cells by IgA immune complex leads to cytokine secretion. J Pathol. 1994;173:119–126. doi: 10.1002/path.1711730208. [DOI] [PubMed] [Google Scholar]
- 86.Amore A, Cirina P, Conti G, et al. Glycosylation of circulating IgA in patients with IgAN modulates proliferation and apoptosis of mesangial cells. J Am Soc Nephrol. 2001;12:1862–1871. doi: 10.1681/ASN.V1291862. [DOI] [PubMed] [Google Scholar]
- 87.Haakenstad AO, Mannik M. The biology of immune complexes. In: Talal N, editor. Autoimmunity Genetic, immunologic, virologic, and clinical aspects. Academic Press; New York: 1977. pp. 277–360. [Google Scholar]
- 88.Moura IC, Arcos-Fajardo M, Sadaka C, et al. Glycosylation and size of IgA1 are essential for interaction with mesangial transferrin receptor in IgAN. J Am Soc Nephrol. 2004;15:622–634. doi: 10.1097/01.asn.0000115401.07980.0c. [DOI] [PubMed] [Google Scholar]
- 89.Barratt J, Greer MR, Pawluczyk IZ, et al. Identification of a novel Fcα receptor expressed by mesangial cells. Kidney Int. 2000;57:1936–1948. doi: 10.1046/j.1523-1755.2000.00043.x. [DOI] [PubMed] [Google Scholar]
- 90.Monteiro RC. Pathogenic role of IgA receptors in IgAN. Contrib Nephrol. 2007;157:64–69. doi: 10.1159/000102306. [DOI] [PubMed] [Google Scholar]
- 91.Monteiro RC, Van De Winkel JG. IgA Fc Receptors. Annu Rev Immunol. 2003;21:177–204. doi: 10.1146/annurev.immunol.21.120601.141011. [DOI] [PubMed] [Google Scholar]
- 92.Moura IC, Centelles MN, Arcos-Fajardo M, et al. Identification of the transferrin receptor as a novel immunoglobulin (Ig)A1 receptor and its enhanced expression on mesangial cells in IgAN. J Exp Med. 2001;194:417–425. doi: 10.1084/jem.194.4.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Tamouza H, Vende F, Tiwari M, et al. Transferrin receptor engagement by polymeric IgA1 induces receptor expression and mesangial cell proliferation: role in IgAN. Contrib Nephrol. 2007;157:144–147. doi: 10.1159/000102457. [DOI] [PubMed] [Google Scholar]
- 94.McDonald KJ, Cameron AJM, Allen JM, et al. Expression of Fc α/μ receptor by human mesangial cells: a candidate receptor for immune complex deposition in IgAN. Biochem Biophys Res Commun. 2002;290:438–442. doi: 10.1006/bbrc.2001.6218. [DOI] [PubMed] [Google Scholar]
- 95.Haddad E, Moura IC, Arcos-Fajardo M, et al. Enhanced expression of the CD71 mesangial IgA1 receptor in Berger disease and Henoch-Schönlein nephritis: Association between CD71 expression and IgA deposits. J Am Soc Nephrol. 2003;14:327–337. doi: 10.1097/01.asn.0000046961.04917.83. [DOI] [PubMed] [Google Scholar]
- 96.Allen AC, Bailey EM, Barratt J, et al. Analysis of IgA1 O-glycans in IgAN by fluorophore-assisted carbohydrate electrophoresis. J Am Soc Nephrol. 1999;10:1763–1771. doi: 10.1681/ASN.V1081763. [DOI] [PubMed] [Google Scholar]
- 97.Royle L, Roos A, Harvey DJ, et al. Secretory IgA N- and O-glycans provide a link between the innate and adaptive immune systems. J Biol Chem. 2003;278:20140–20153. doi: 10.1074/jbc.M301436200. [DOI] [PubMed] [Google Scholar]
- 98.Hiki Y, Tanaka A, Kokubo T, et al. Analyses of IgA1 hinge glycopeptides in IgAN by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. J Am Soc Nephrol. 1998;9:577–582. doi: 10.1681/ASN.V94577. [DOI] [PubMed] [Google Scholar]
- 99.Pouria S, Corran PH, Smith AC, et al. Glycoform composition profiling of O-glycopeptides derived from human serum IgA1 by matrix-assisted laser desorption ionization-time of flight-mass spectrometry. Anal Biochem. 2004;330:257–263. doi: 10.1016/j.ab.2004.03.053. [DOI] [PubMed] [Google Scholar]
- 100.Takahashi K, Hiki Y, Odani H, et al. Structural analyses of O-glycan sugar chains on IgA1 hinge region using SELDI-TOFMS with various lectins. Biochem Biophys Res Commun. 2006;350:580–587. doi: 10.1016/j.bbrc.2006.09.075. [DOI] [PubMed] [Google Scholar]