Abstract
Aim:
(−)-Epicatechin gallate (ECg) modifies the morphology, cell wall architecture and β-lactam antibiotic susceptibility of Staphylococcus aureus. As these effects result primarily from intercalation into the bacterial cytoplasmic membrane, the capacity of ECg to modulate the secretion of two key staphylococcal virulence factors, coagulase and α-toxin, was examined.
Methods and Results:
Bioassays were used to determine coagulase and haemolysin activity in culture supernatants of a number of S. aureus isolates grown in the presence and absence of ECg; α-toxin secretion was also evaluated by immunoblotting. Growth in ECg reduced the levels of activity of both proteins in culture supernatants; the effects could only be partly explained by ECg-mediated inhibition of bioactivity and by induction of secreted proteases.
Conclusion:
ECg suppresses the secretion of coagulase and α-toxin by clinical isolates of S. aureus.
Significance and Impact of the Study:
The observation that secretion of key components of staphylococcal virulence can be compromised by a naturally occurring polyphenol supports the notion that ECg and related compounds may have therapeutic utility for the control of infections that are currently difficult to treat due to the propensity of methicillin-resistant S. aureus to accumulate antibiotic resistance genes.
Keywords: coagulase, epicatechin gallate, exoproteins, Staphylococcus aureus, α-toxin, virulence
Introduction
The leaves of the tea plant, Camellia sinensis, contain abundant quantities of galloyl catechins; (−)-epigallocatechin gallate (EGCg), (−)-epicatechin gallate (ECg) and (−)-catechin gallate (Cg) constitute around 20% of the dry leaf weight (Hara 2001). These polyphenols contribute to the moderate antibacterial activity of green tea and at subinhibitory concentrations exert other, more subtle effects on bacterial cells (Taylor et al 2005). For example, ECg promotes clumping and inhibits separation of dividing Staphylococcus aureus (Hamilton-Miller and Shah 1999; Stapleton et al 2007), EGCg and ECg inhibit staphylococcal biofilm formation (Blanco et al 2005; Stapleton et al 2007) and EGCg, ECg and Cg substantially increase the susceptibility of methicillin-resistant S. aureus (MRSA) to methicillin, oxacillin and other second-generation β-lactam agents (Stapleton et al 2004).
Such phenotypic modifications almost certainly arise as a result of binding of galloyl catechins to membrane bilayers (Caturla et al 2003); the most potent modifier, ECg, interacts with the staphylococcal cytoplasmic membrane by virtue of an exposed hydrophobic domain (Fig. 1) that facilitates intercalation into the phospholipid palisade (Stapleton et al 2006). Galloyl catechins increase the lipid order, producing tightly packed and extended acyl chains in the bilayer (Caturla et al 2003). This perturbation reduces the efficiency of function of a number of membrane proteins, including the penicillin-binding proteins and autolysins, resulting in changes to the architecture and composition of the cell wall (Stapleton et al 2007). The orderly function of the cell is further compromised by release from the cytoplasmic membrane of lipoteichoic acid (Stapleton et al 2007) a polymeric component that, together with peptidoglycan, makes up a polyanionic/polycationic matrix with ion-exchange properties that is involved in the trafficking of ions, nutrients, proteins and antibiotics across the cell wall (Neuhaus and Baddiley 2003).
Figure 1.
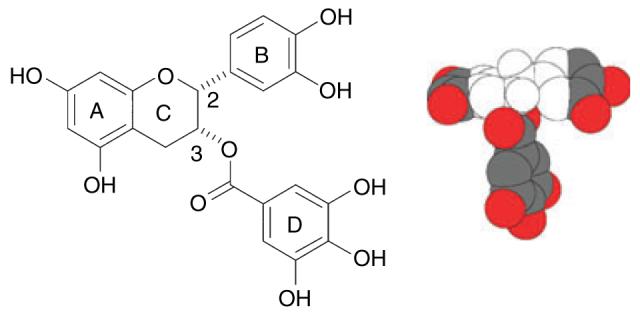
Structure of (−)-epicatechin gallate (ECg), showing the B- and D-ring pharmacophores. The hydrophobic and hydrophilic domains of ECg are indicated in the space-filling model (provided by J.C. Anderson, University of Nottingham, UK) by the colourless and coloured regions, respectively.
We have proposed that analogues of ECg, with better pharmacokinetic properties than the natural molecule, administered in combination with β-lactams such as oxacillin and flucloxacillin could restore the therapeutic efficacy of these inexpensive agents (Anderson et al 2005a,b); these antibiotics are currently ineffective against MRSA. The virulence of S. aureus is largely determined by cell wall-associated proteins and secreted toxins that are regulated and expressed according to the nutritional microenvironment (Bronner et al 2004). As cytoplasmic membrane intercalation of galloyl catechins is likely to have a profound effect on the physical properties of this bilayer and will interfere with transmembrane transport processes, we examined the effect of ECg on some of the virulence-associated proteins that are secreted by S. aureus into the surrounding environment.
Materials and methods
Bacteria and reagents
The epidemic MRSA isolates EMRSA-15 and EMRSA-16 were from clinical samples processed at the Royal Free and University College Medical School, London and were kindly provided by J.M.T. Hamilton-Miller, who also donated Australian (AUS-5), Brazilian (BZ-9 & BZ-17) and Danish (DEN-3) MRSA isolates. All were grown in Mueller-Hinton broth (Oxoid, UK) with agitation. Highly purified ECg was a gift from Mitsui Norin, Tokyo, Japan.
Determination of coagulase activity
Supernatants from postexponential phase cultures (OD600 nm of 5) grown in the presence and absence of ECg (25 μg ml−1) were serially diluted twofold and incubated with rabbit plasma (Sigma-Aldrich, Poole, UK). The titre recorded was the reciprocal of the highest dilution that caused clotting after 4 h incubation at 37°C. To determine if any reductions in coagulase activity were due to inhibition by residual ECg in the supernatants, control samples were preincubated with ECg (25 μg ml−1) for 1 h at 37°C and the titre determined as above.
α-Toxin assay
Ten microlitres of supernatants from postexponential phase cultures (OD600 nm of 5) grown in the presence and absence of ECg (25 μg ml−1) were added to 1 ml−1 of 2% rabbit blood (E&O Laboratories, Bonnybridge, UK) in phosphate-buffered saline and incubated for 25 min at 37°C. After incubation for 25 min at 4°C, non-lysed blood cells were removed by centrifugation at 5000 g for 2 min. The haemolytic activity was determined by measuring the optical density (405 nm) of the cell-free supernatant and the values obtained adjusted to reflect the optical densities of the original cultures (measured at 600 nm). To verify that the decrease in haemolytic activity in the supernatants was not due to the inhibitory activity of residual ECg, control supernatants were preincubated with ECg (25 μg ml−1) for 1 h. Secretion of α-toxin into the culture medium was also determined by SDS-PAGE, using 10% polyacrylamide gels, followed by transfer of proteins to polyvinylidene fluoride membranes (Millipore, Watford, UK) by electrotransfer and α-toxin bands detected using a rabbit polyclonal anti-staphylococcal α-toxin primary antibody (Sigma-Aldrich), an anti-rabbit IgG–peroxidase secondary antibody (Sigma-Aldrich) and the chromogenic peroxidase substrate 3,3′,5,5′-tetrazolium chloride (Sigma-Aldrich).
Protease activity
Supernatants from postexponential phase cultures were assayed with casein labelled with N-(resorufin-4-carbonyl)piperidine-4-carbonic acid (Calbiochem, San Diego, CA, USA) following the manufacturer's instructions. Incubation in the presence of resorufin-labelled casein was conducted at 37°C for 3 h. Hydrolysis was measured fluorometrically at 584 nm after excitation of samples at 574 nm.
Results
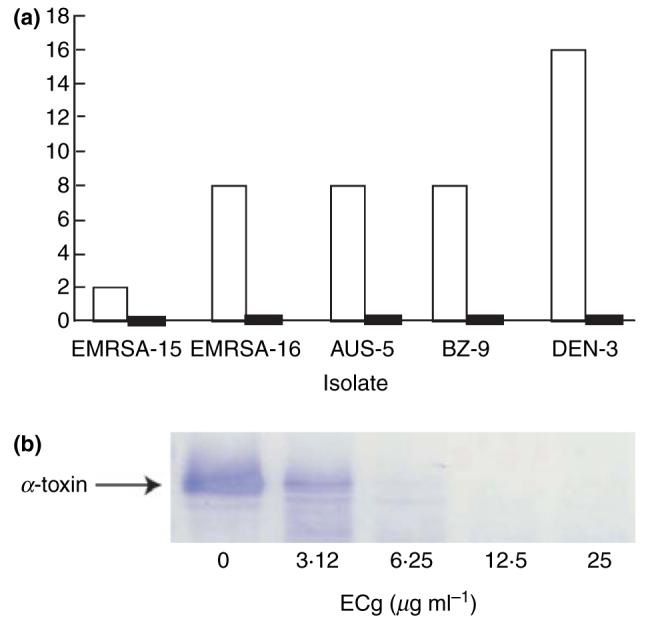
Five strains, EMRSA-15, EMRSA-16, AUS-5, BZ-9 and DEN-3, were grown in ECg-free medium and in the presence of 25 μg ml−1 of ECg: culture supernatants were examined for the presence of coagulase (Fig. 2). There was some variation in the amount of coagulase associated with the strains; for example, the coagulase titre in the growth medium after culture of DEN-3 was eightfold higher than that for EMRSA-15. When ECg was added to the growth medium, there was a large reduction in coagulase activity: no clot was formed after incubation of any of the broth supernatants in the presence of rabbit plasma. Preincubation of culture supernatants with ECg indicated that the compound had no significant effect on coagulase activity.
Figure 2.
Coagulase activity in culture supernatants following growth of methicillin-resistant S. aureus (MRSA) isolates in the absence (□) or presence (■) of 25 μg ml−1 of (−)-epicatechin gallate (ECg). Coagulase activity is expressed as the highest dilution of supernatant that caused clotting of rabbit plasma following incubation at 37°C for 4 h. Differences between pairs were significant: n = 3; Wilcoxon-signed rank test P < 0.05 (Wilcoxon 1945).
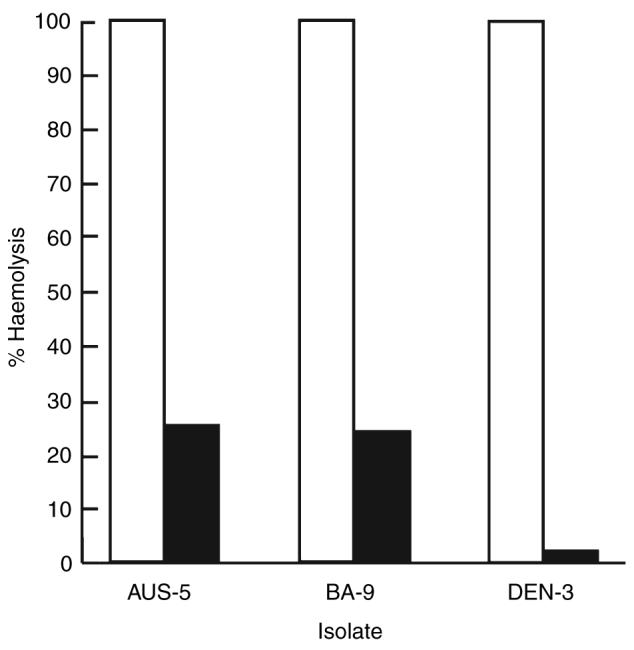
ECg, at a concentration of 25 μg ml−1, also reduced the level of α-toxin secretion: a reduction of 99% was found with DEN-3 and was around 75% for two other strains examined, AUS-5 and BZ-9 (Fig. 3). The effect of increasing concentrations of ECg on the secretion of α-toxin by BZ-17 was investigated by Western immunoblotting (Fig. 3). Growth in the presence of 3·12 g ml−1 of ECg produced a discernable reduction in toxin secretion, at 6·25 μg ml−1 little of the protein could be found in the growth medium and at 12·5 μg ml−1 no immunoreactive protein could be detected.
Figure 3.
(a) Haemolysis of rabbit erythrocytes by supernatants of cultures of methicillin-resistant S. aureus (MRSA) isolates in the absence (□) or presence (■) of 25 μg ml−1 of (−)-epicatechin gallate (ECg). Haemolysis, indicative of α-toxin activity, is expressed relative to erythrocytes lysed with water (100%); (b) Western blot of SDS-PAGE gel of supernatants from cultures of strain BZ-17 grown in the presence of increasing concentrations of ECg. Differences between pairs were significant: n = 3–8; Wilcoxon-signed rank test 0·05 < P <0·005.
Preincubation of culture supernatants with ECg indicated that the polyphenol had a limited capacity to inhibit haemolytic activity, producing reductions of 17–28% in comparison to culture supernatants that had not been incubated with ECg. This difference could not fully account for the large ECg-mediated reductions observed following growth in the presence of ECg. To investigate if increases in protease secretion by MRSA isolates resulting from growth in ECg-containing medium might contribute to the apparent reductions in α-toxin secretion, extracellular proteases were quantified using resorufin-labelled casein. Growth in ECg had no significant effect on protease secretion by isolates EMRSA-15 and AUS-5 but increased the very low levels of protease activity in BZ-9 culture supernatants by 24-fold. Protease expression in DEN-3 was increased fivefold leading to relatively high-level protease levels in the broth supernatant.
Discussion
Green tea consumption is linked to a low incidence of various pathological conditions, including cardiovascular disease, diabetes, obesity and cancer. EGCg, the most abundant polyphenolic component of green tea, induces apoptotic cell death and cell cycle arrest in tumour cells but not in their normal counterparts; it also favourably affects several signal transduction pathways and is efficacious in animal models of tumour induction (Chen et al 2004). On the strength of such studies, EGCg is undergoing clinical evaluation in chronic lymphocytic leukaemia and has proven to possess a very attractive patient safety profile (Chow et al. 2003). These activities are creating a precedent for therapeutic interventions with natural polyphenols or synthetic structural analogues. There is a growing body of evidence that galloyl catechins, in particular ECg, have the capacity to modify the phenotype of S. aureus in a fashion that suggests that they may have utility in the treatment of staphylococcal (Taylor et al 2005) and other (Song and Seong 2007) infections. To this growing list of advantageous properties we now add the capacity to substantially decrease the secretion of the major virulence determinants, coagulase and α-toxin. We have proposed that analogues of ECg may be useful for the treatment of MRSA infections when used in combination with a β-lactam antibiotic (Anderson et al 2005a,b); the observations reported here raise the possibility that such molecules may have utility as anti-infective agents in their own right.
It is probable that much of the reduction in coagulase and haemolytic activity in supernatants is due to a prevention of secretion of the proteins as a result of changes to the physical nature of the staphylococcal cytoplasmic membrane. Alternatively, intercalation of galloyl catechins into the cytoplasmic membrane may interfere with membrane-associated signal transduction processes. As noted by others (Okubo et al 1989; Ikigai et al 1990), ECg is a low-level inhibitor of α-toxin activity; but the level of inhibition that we observed cannot account for the large reduction in haemolytic activity. Growth in the presence of ECg clearly stimulated the secretion of protease by DEN-3 and protease activity may well account for the large reduction in observed extracellular α-toxin activity, but this did not appear to be the case with the other isolates examined. These differences highlight the need to examine a selection of MRSA isolates in this area before drawing firm conclusions.
Some antibiotics, when used at subinhibitory concentrations, may affect the expression of virulence determinants (Ohlsen et al. 1998). Clindamycin significantly decreases the production of α- and δ-haemolysins, lipase, DNase and TSST-1 by down-regulation of the expression of genes coding for these proteins (Herbert et al 2001). In contrast, some β-lactam agents and aminoglycosides induce expression of increased levels of certain virulence determinants, including α-toxin (Ohlsen et al 1998). Glycerol monolaurate, a compound that modulates β-lactamase-mediated β-lactam resistance, also influences the production of α-toxin and other virulence determinants by interference with signal transduction (Projan et al 1994), a likely membrane-associated effect.
α-Toxin is involved not only in haemolysis but also plays a role in cell-to-cell communication, particularly in relation to biofilm formation (Caiazza and O'Toole 2003), a key event in the pathogenesis of MRSA infections; it is conceivable, therefore, that ECg could play a role in preventing the establishment of MRSA. Further detailed study is required to determine the extent of the modulation of virulence determinants in staphylococci and whether these effects translate into reduce virulence in suitable in vivo models of infection.
Acknowledgements
This work was funded through MRC Strategic Grant G0000996.
References
- Anderson JC, Headley C, Stapleton PD, Taylor PW. Synthesis and antibacterial activity of a hydrolytically stable (−)-epicatechin gallate analogue for the modulation of β-lactam resistance in Staphylococcus aureus. Bioorg Med Chem Lett. 2005a;15:2633–2635. doi: 10.1016/j.bmcl.2005.03.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson JC, Headley C, Stapleton PD, Taylor PW. Asymmetric total synthesis of B-ring modified (−)-epicatechin gallate analogues and their modulation of β-lactam resistance in Staphylococcus aureus. Tetrahedron. 2005b;61:7703–7711. doi: 10.1016/j.tet.2005.05.086. [DOI] [PubMed] [Google Scholar]
- Blanco AR, Sudano-Roccaro A, Spoto GC, Nostro A, Rusciano D. Epigallocatechin gallate inhibits biofilm formation by ocular staphylococcal isolates. Antimicrob Agents Chemother. 2005;49:4339–4343. doi: 10.1128/AAC.49.10.4339-4343.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronner S, Monteil H, Prévost G. Regulation of virulence determinants in Staphylococcus aureus: complexity and applications. FEMS Microbiol Lett. 2004;28:183–200. doi: 10.1016/j.femsre.2003.09.003. [DOI] [PubMed] [Google Scholar]
- Caiazza NC, O'Toole GA. Alpha-toxin is required for biofilm formation by Staphylococcus aureus. J Bacteriol. 2003;185:3214–3217. doi: 10.1128/JB.185.10.3214-3217.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caturla N, Vera-Samper E, Villalain J, Reyes Mateo C, Micol V. The relationship between the antioxidant and antibacterial properties of galloylated catechins and the structure of phospholipid model membranes. Free Radic Biol Med. 2003;34:648–662. doi: 10.1016/s0891-5849(02)01366-7. [DOI] [PubMed] [Google Scholar]
- Chen D, Daniel KG, Kuhn DJ, Kazi A, Bhuiyan M, Li L, Wang Z, Wan SB, et al. Green tea and tea polyphenols in cancer prevention. Front Biosci. 2004;9:2618–2631. doi: 10.2741/1421. [DOI] [PubMed] [Google Scholar]
- Chow SH-H, Cai Y, Hakim IA, Crowell JA, Shahi F, Brooks CA, Dorr RT, Hara Y, et al. Pharmacokinetics and safety of green tea polyphenols after multiple-dose administration of epigallocatechin gallate and Polyphenon E in healthy individuals. Clin Cancer Res. 2003;9:3312–3319. [PubMed] [Google Scholar]
- Hamilton-Miller JMT, Shah S. Disorganization of cell division of methicillin-resistant Staphylococcus aureus by a component of tea (Camellia sinensis): a study by electron microscopy. FEMS Microbiol Lett. 1999;176:463–469. doi: 10.1111/j.1574-6968.1999.tb13698.x. [DOI] [PubMed] [Google Scholar]
- Hara Y. Green Tea: Health Benefits and Applications. New York: Marcel Dekker; 2001. [Google Scholar]
- Herbert S, Barry P, Novick RP. Subinhibitory clindamycin differentially inhibits transcription of exoprotein genes in Staphylococcus aureus. Infect Immunol. 2001;69:2996–3003. doi: 10.1128/IAI.69.5.2996-3003.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikigai H, Toda M, Okubo S, Hara Y, Shimamura T. Relationship between the anti-haemolysin activity and the structure of catechins and theaflavins. Nippon Saikingaku Zasshi. 1990;45:913–919. doi: 10.3412/jsb.45.913. [DOI] [PubMed] [Google Scholar]
- Neuhaus FC, Baddiley J. A continuum of anionic charge: structures and functions of d-alanyl-teichoic acids in Gram-positive bacteria. Microbiol Mol Biol Rev. 2003;67:686–723. doi: 10.1128/MMBR.67.4.686-723.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlsen K, Ziebuhr W, Koller K-P, Hell W, Wichelhaus TA, Hacker J. Effects of subinhibitory concentrations of antibiotics on alpha-toxin (hla) gene expression of methicillin-sensitive and methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother. 1998;42:2817–2823. doi: 10.1128/aac.42.11.2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okubo S, Ikigai H, Toda M, Shimamura T. The anti-haemolysin activity of tea and coffee. Lett Appl Microbiol. 1989;9:65–66. [Google Scholar]
- Projan SJ, Brown-Skrobot S, Schlievert PM, Vandenesch F, Novick RP. Glycerol monolaurate inhibits the production of β-lactamase, toxic shock syndrome toxin-1, and other staphylococcal exoproteins by interfering with signal transduction. J Bacteriol. 1994;176:4204–4209. doi: 10.1128/jb.176.14.4204-4209.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song JM, Seong BL. Tea catechins as a potential alternative anti-infectious agent. Future Drugs. 2007;5:497–506. doi: 10.1586/14787210.5.3.497. [DOI] [PubMed] [Google Scholar]
- Stapleton PD, Shah S, Anderson JC, Hara Y, Hamilton-Miller JMT, Taylor PW. Modulation of β-lactam resistance in Staphylococcus aureus by catechins and gallates. Int J Antimicrob Agents. 2004;23:462–467. doi: 10.1016/j.ijantimicag.2003.09.027. [DOI] [PubMed] [Google Scholar]
- Stapleton PD, Shah S, Hara Y, Taylor PW. Potentiation of catechin gallate-mediated sensitization of Staphylococcus aureus to oxacillin by nongalloylated catechins. Antimicrob Agents Chemother. 2006;50:752–755. doi: 10.1128/AAC.50.2.752-755.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stapleton PD, Shah S, Ehlert K, Hara Y, Taylor PW. The β-lactam-resistance modifier (−)-epicatechin gallate alters the architecture of the cell wall of Staphylococcus aureus. Microbiology. 2007;153:2093–2103. doi: 10.1099/mic.0.2007/007807-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor PW, Hamilton-Miller JMT, Stapleton PD. Antimicrobial properties of green tea catechins. Food Sci Technol Bull. 2005;2:71–81. doi: 10.1616/1476-2137.14184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcoxon F. Individual comparisons by ranking methods. Biometrics Bull. 1945;1:80–85. [Google Scholar]


