Abstract
Angiopoietin-like 4 (ANGPTL4) is a secreted protein which belongs to the angiopoietin family and is involved in angiogenesis and metabolism regulation. We previously reported the induction of angptl4 by hypoxia in endothelial cells and in human ischemic tissues from peripheral artery disease. We here observed in a mouse model of hindlimb ischemia that the mRNA upregulation in the vessels correlates with the accumulation of the full-length protein in ischemic tissues. We then investigated its functions in endothelial cells. In response to hypoxia, endogenous ANGPTL4 accumulates in the subendothelial extracellular matrix (ECM). Whereas the secreted protein undergoes proteolysis leading to truncated fragments present in the medium, only full-length ANGPTL4 interacts with the ECM. Competition and direct binding assays indicate that the strong interaction of ANGPTL4 with the ECM is heparin/heparan sulfate proteoglycans dependent. The balance between matrix-associated and soluble forms of ANGPTL4 points out the role of the ECM in the regulation of its bioavailability. The angiogenic function of the ECM-bound full-length protein was investigated using either the form associated with the conditioned ECM from ANGPTL4-transfected HEK293 cells, or the purified immobilized protein. We show that matrix-associated as well as immobilized ANGPTL4 limit the formation of actin stress fibers and focal contacts in the adhering endothelial cells and inhibits their adhesion. Immobilized ANGPTL4 also decreases motility of endothelial cells and inhibits the sprouting and tube formation. Altogether, these findings show that hypoxic endothelial cells accumulate ANGPTL4 in the ECM, which in turn negatively regulates their angiogenic capacities through an autocrine pathway.
Keywords: Actins, metabolism, Animals, Anoxia, metabolism, physiopathology, Biological Availability, Blood Proteins, metabolism, pharmacology, Cell Adhesion, drug effects, Cell Movement, drug effects, Cells, Cultured, Cytoskeleton, drug effects, ultrastructure, Endothelial Cells, metabolism, Extracellular Matrix, metabolism, Heparin, analogs & derivatives, metabolism, Hindlimb, blood supply, Ischemia, metabolism, Mice, Mice, Inbred C57BL, Neovascularization, Physiologic, drug effects, Proteoglycans, metabolism
Keywords: Angionenesis, Hypoxia
Cardiovascular disorders such as coronary and peripheral artery diseases lead to a deficient blood supply to tissues and a decrease in oxygen partial pressure, i.e. hypoxia. Because of their location at the interface of circulating blood and peripheral tissues, endothelial cells are exposed to hypoxia. A critical adaptation to hypoxia is angiogenesis which consists in the formation of new blood vessels extending from the pre-existing vasculature.1 This phenomenon occurs through the activation of the endothelial cells by a multistep process including changes of cell-extracellular matrix (ECM) interactions. In ischemic cardiovascular pathologies, reactive angiogenesis is a profitable event. Therefore unraveling the interplay of multiple molecular signals and events which occur in hypoxia and lead to functional new blood vessels is a challenging issue.
We previously identified angiopoietin-like 4 (angptl4) as a hypoxia-induced gene in vitro in human microvascular endothelial cell line (HMEC-1) and in vivo in the vessels of ischemic tissues from peripheral artery disease.2 Human ANGPTL4 is a secreted glycoprotein which belongs to the angiopoietin family.3 It is composed of 406 amino acids and contains an amino-terminal signal sequence, a coiled-coil domain and a carboxy-terminal fibrinogen-like domain. ANGPTL4 oligomerizes and undergoes proteolysis mediated by a cell-associated protease.4
Angiopoietins are major regulators of the balance between destabilization and stabilization of the vasculature occuring during angiogenesis. The angiopoietin-1 tightens vessels by promoting interactions between cells of the vascular wall whereas the angiopoietin-2 loosens these interactions and stimulates the growth of immature vessels.5,6 Angiopoietin-like proteins also play a role in the modulation of angiogenesis, as shown for ANGPTL17,8, ANGPTL28,9, ANGPTL310 and ANGPTL4.2,3,11,12 ANGPTL4, previously known as hepatic fibrinogen/angiopoietin-related protein (HFARP)3, peroxisome proliferator-activated receptor-γ (PPAR-γ) angiopoietin-related gene (PGAR)13 or fasting induced adipose factor (FTAF)14 is also involved in lipid and glucose metabolism.15–17
In the pathological context of ischemic diseases, ANGPTL4 could modulate angiogenesis by modifying the microenvironment in response to hypoxia. A crucial determinant of cell microenvironment is the ECM, whose regulated composition plays a pivotal role in neovessel formation, stability and maturation.18 The ECM is mainly composed of fibrous proteins, like collagen or fibronectin, and interstitial glycosaminoglycans covalently bound to core proteins to form proteoglycans such as heparan sulfate proteoglycans (HSPGs). The interaction of growth factors, including angiopoietin-1 and VEGF, with the ECM in the vicinity of the production site regulates their bioavailability.19,20
In the present study using in vivo and in vitro models, we investigated the expression of ANGPTL4, its interaction with the ECM and its bioactivity on endothelial cells. We report that full-length ANGPTL4 accumulates in the mouse ischemic hindlimb after vascular ligature and in the ECM of hypoxic endothelial cells through heparin/HSPGs. ECM-bound ANGPTL4 reduces endothelial cell adhesion, prevents the organization of focal adhesions and actin stress fibers, decreases cell migration and sprouting. Therefore, ANGPTL4, through its autocrine effect on endothelial cells, participates in the modulation of angiogenesis in a hypoxic microenvironment.
Materials and Methods
Cell culture, expression and purification of recombinant ANGPTL4, immunofluorescence, statistical analysis, antibodies and reagents are described in the online data supplement.
Extraction of ECM-associated proteins
ECM was prepared, according to a protocol adapted from Owensby21, by incubating cells in 5 mmol/L EDTA, 1% Triton, at 4°C. ECM proteins were extracted in Laemmli buffer for Western-blot analysis.
Mouse model of hindlimb ischemia
Unilateral hindlimb ischemia was induced by ligation and excision of the femoral artery in C57BL/6 mice, as previously described.22 Mice were sacrificed at day 2 or 6 (three mice per group). Tissues from both hindlimbs were either fixed in 4% paraformaldehyde (PFA) and paraffin embedded, either snap-frozen and stored at −80°C until used. Probe labelling by in vitro transcription and in situ hybridization were performed as previously described on paraffin sections, with an antisense mouse ANGPTL4 probe.23 Total proteins were extracted from frozen tissues for Western-blot analysis.
Adhesion assay
Conditioned ECM were prepared from Human Embryonic Kidney 293 cells (HEK293) grown to confluence for 48 hours and lifted by 5 mmol/L EDTA. Alternatively, plates were coated overnight at 4°C with increasing concentrations of purified ANGPTL4. Plates were saturated with 1% BSA. Trypsinized endothelial cells were plated in complete medium. Adherent cells were fixed one hour after plating in 4% PFA, stained with 0.1% crystal violet and quantified by measuring the absorbance at 570 nm.
Migration assay
Transwell filters (8 μm pores) were coated on the lower side with fibronectin (10 μg/mL) ± ANGPTL4 at various concentrations. Human Umbilical Vein Endothelial Cells (HUVEC) were seeded on the upper side of the membrane in ECBM2 with 0,2% FCS for 2 hours. Cells from the upper side were mechanically removed and cells from lower side were fixed. Nuclei were stained with 4′-6 Diamidino-2-phenylindole (DAPI) and counted.
Time-lapse videomicroscopy
HUVEC were detached by 10 mmol/L EDTA and seeded in complete medium on wells coated with fibronectin and/or purified ANGPTL4. After 40 min, cells were recorded at a 5 min lapse interval for 5 hours on inverted Leica microscope IRBE equipped with 10x PH 0.40 objective lenses, enclosed in a Life Imaging Services environmental incubator. Images were acquired using a Coolsnap HQ Roper Scientific camera. Motion-analysis was performed with the MetaMorph software.
Tube formation assay
Culture wells were coated on ice with Matrigel (Collaborative Biochemical Products, Bedford, MA) alone or mixed with ANGPTL4 (1 or 5 μg/mL). After 30 min at 37°C, HMEC-1 were seeded on top and further incubated for 24 hours in the presence of 0.5% FCS. The number of tubes was counted from four pictures acquired per condition with a 4x objective.
Sprouting assay
Spheroids of 500 HUVEC were generated as previously described24 and embedded in collagen gels containing ANGPTL4 (1 μg/mL) or not. Endothelial sprouting was stimulated with 20 ng/mL VEGF for 24 hours. Sprouting intensity was quantitated by determining the cumulative sprout length per spheroid using an Olympus IX50 inverted microscope and the Cell P software (Soft Imaging System, Germany). The mean of the cumulative sprout length of 10 randomly selected spheroids was analyzed as an individual data point.
Results
ANGPTL4 accumulates in ischemic hindlimb
Having previously shown that ANGPTL4 mRNA is upregulated in the vessel wall of human ischemic limbs2, we addressed the issue in a mouse model of hindlimb ischemia. In situ hybridization at day 6 after ligature showed an upregulation of ANGPTL4 mRNA in the interstitial capillaries of the ischemic leg compared to the controlateral one (Figure 1A) but not in the muscular fibers. Full-length mouse ANGPTL4 protein was also overexpressed in the ischemic limb (Figure 1B).
Figure 1. Expression of ANGPTL4 in mice ischemic hindlimbs.
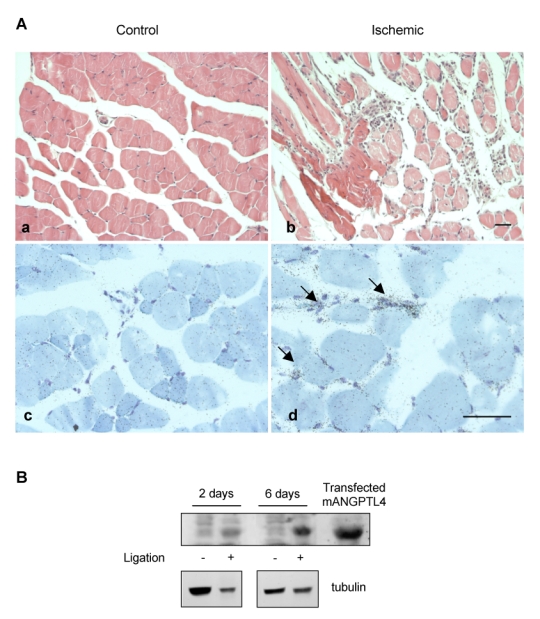
Ischemic and controlateral tibialis anterialis muscles were harvested at day 2 or 6 post ligation of the left femoral artery. A, At day 6, non ischemic muscle shows normal histology with Hematoxylin and Eosin staining (a) and no detectable signal in vessels nor muscle fibers after in situ hybridization with the FIAF antisense probe (c). Ischemic muscle shows fiber necrosis, interstitial oedema and inflammatory infiltrate (b). Signal is detected in small vessels (arrows) in ischemic areas with the FIAF antisense probe (d). Bar = 100 μm. B, Western-blot analysis of muscle protein extracts (100 μg/lane) with anti-mouse ANGPTL4 (top panel) or anti-tubulin (bottom panel). Transfected mouse ANGPTL4 was used as a positive control.
Hypoxia-induced endogenous ANGPTL4 accumulates in the extracellular matrix of endothelial cells
Since the vessels are a site of expression of ANGPTL4 in ischemic tissues, the hypoxic regulation of the protein was further analyzed in primary cultures of HUVEC. The expression of full-length ANGPTL4 (55 kDa) was markedly induced in conditioned medium (CM) from HUVEC grown in hypoxia (1% O2) for 48 hours, as compared to cells grown in normoxia (21% O2) (Figure 2A, top panel). A high level of full-length ANGPTL4 was associated to the ECM and was greatly increased by hypoxia (Figure 2A, bottom panel). The binding properties of ANGPTL4 to the ECM have been compared to those of the matricellular proteins Cyr61/CCN1, which strongly binds the HSPGs, and Nov/CCN3, which displays a weak interaction with the matrix.25 As shown in Figure 2A, Nov was detected in the CM and was absent from the ECM extract whereas Cyr61 was both detected in the CM and the ECM. Immunofluorescence analysis of unpermeabilized HUVEC in hypoxic condition showed that the distribution of ANGPTL4 in the ECM was diffuse without visible fibrillar organization, in a pattern similar to Cyr61 (Figure 2B). Nov was not detected in the ECM.
Figure 2. Interaction of ANGPTL4 with the ECM from endothelial cells in hypoxic conditions.
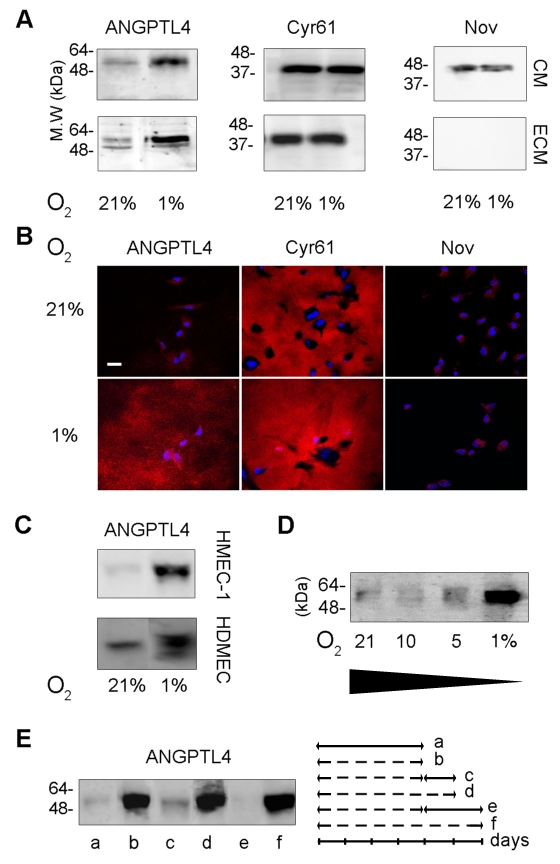
A, B and C, endothelial cells were grown in 21% or 1% O2 for two days. A, Western-blot analysis of the CM or ECM from HUVEC was performed using anti-ANGPTL4, anti-Cyr61 and anti-Nov antibodies. B, Immunofluorescence was performed on unpermeabilized HUVEC, with anti-ANGPTL4 (left panel), anti-Cyr61 (medium panel) and anti-Nov (right panel) antibodies. Bar = 40 μm. C, Western-blot analysis of ECM from microvascular endothelial cells. D and E, Western-blot analysis with anti-ANGPTL4 of ECM from HUVEC. D, Cells were grown in the indicated O2 concentrations for two days. E, Cells were grown under various conditions: 21% O2 (continuous line) for four days (a); 1% O2 (hatched line) for four (b) or five (d) or six (f) days; 1% O2 for four days followed by 21% O2 for one (c) or two days (e). Cells were cultured in presence of 25 μg/mL heparin for the CM analysis and in absence of heparin for the ECM analysis. Results are representative of three independent experiments.
ANGPTL4 was also accumulated in the ECM of hypoxic endothelial cells from microvascular origin i.e. primary culture of Human Dermal Microvascular Cells (HDMEC) and HMEC-1 (Figure 2C). ANGPTL4 accumulation in the matrix is regulated by the level and duration of hypoxia. Whereas 10% O2 for two days failed to up-regulate matrix associated-ANGPTL4 in the HUVEC, a 5% O2 exposure induced a detectable increase of the ANGPTL4 level (Figure 2D). Thus the amount of ECM-bound ANGPTL4 depends on the level of oxygen. Besides, ANGPTL4 accumulation is rapidly reversible. Reoxygenation of the HUVEC for 24 hours reverted the ECM-bound protein amount to its basal level (Figure 2E). Altogether, ANGPTL4 accumulation occurs in the endothelial ECM in mild to severe hypoxic conditions via a dynamic process.
Interaction of full-length ANGPTL4 with ECM regulates its bioavailability
ANGPTL4 undergoes oligomerization and proteolytic processing in the CM of cell culture or in plasma.4,26 To determine which form interacts with the ECM, recombinant ANGPTL4 tagged with either myc-his or flag C-terminal epitope was expressed in dihydrofolate reductase-deficient Chinese Hamster Ovary (CHO-DHFR) cells, HEK293 cells or HUVEC. Full-length ANGPTL4 was detected in the CM and the ECM of the three cell types (Figure 3). In non reducing conditions, two bands of higher molecular weight corresponding to oligomeric forms were detected indicating that ANGPTL4 monomers and oligomers interact with the ECM (Figure 3A).
Figure 3. Accumulation of full-length ANGPTL4 in the ECM.
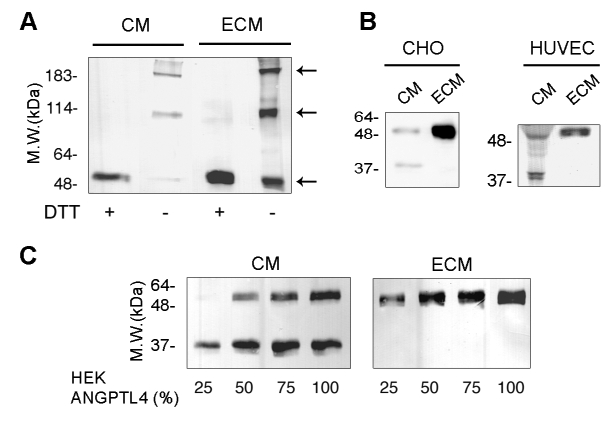
A, CM or ECM from CHO-DHFR-ANGPTL4-myc cells was analyzed by Western-blot using anti-myc antibody on 7.5% SDS-PAGE in reducing (+DTT) or non-reducing (−DTT) conditions. B, CM or ECM from CHO-DHFR-ANGPTL4-myc cells or transfected HUVEC was analyzed by Western-blot using anti-myc antibody. C, Increasing ratios of HEK293-ANGPTL4-flag versus HEK293 cells were plated in the indicated proportions and grown until confluence. CM (left panel) and ECM (right panel) were analyzed by Western-blot using anti-flag antibody. Results are representative of three independent experiments.
To evaluate the ratio of matrix-bound versus soluble ANGPTL4, CHO-DHFR-ANGPTL4-myc cells were seeded in serum-free medium and grown for 24 hours. CM and ECM were prepared from an equal cell number. Most of the full-length ANGPTL4 was accumulated in the ECM (Figure 3B, left panel). A C-terminal proteolytic fragment was identified in the CM. This 35 kDa-fragment was absent from the ECM fraction. Accordingly, HUVEC expressing ANGPTL4-myc displayed two bands of 55 and 35 kDa in the CM, indicating that endothelial cells are also able to process the soluble ANGPTL4. This phenomenon could not be revealed for the endogenous protein since the anti-ANGPTL4 antibody does not allow the recognition of the 35 kDa form. In agreement with the detection of endogenous ANGPTL4 in the ECM, only full-length 55 kDa recombinant ANGPTL4 interacted with the ECM from HUVEC (Figure 3B, right panel).
To further investigate the distribution of ANGPTL4 in the ECM and the medium, varying ratios of HEK293 and HEK293-ANGPTL4-flag cells were seeded in serum-free medium for 24 hours. Using a low ratio of ANGPTL4 expressing cells (Figure 3C, first lane in both panels), the full-length ANGPTL4 was detected in the ECM, whereas the C-terminal fragment alone was detected in the CM. With increasing proportions of HEK293-ANGPTL4, ECM-bound ANGPTL4 still consisted of the full-length protein while increasing amounts of both full-length and processed ANGPTL4 were detected in the CM. These experiments suggest that full-length ANGPTL4 is preferentially accumulated in the ECM until it reaches a saturation level.
ANGPTL4 strongly binds to the endothelial ECM and interacts with heparin and heparan sulfate proteoglycans
The strength of the interaction of ANGPTL4 with the ECM was then characterized. Recombinant ANGPTL4 was first allowed to interact with the ECM of normoxic HUVEC containing few endogenous ANGPTL4. ECM-associated ANGPTL4 was analyzed after treatment with increasing concentrations of NaCl (Figure 4A and B). Full-length ANGPTL4 displayed a strong association with the ECM as 0.8 mol/L NaCl was required to release more than 50% of the bound protein from the ECM. Furthermore, ANGPTL4 was still detected in presence of 1.2 mol/L NaCl.
Figure 4. Full-length ANGPTL4 strongly interacts with the ECM through heparin and HSPGs.
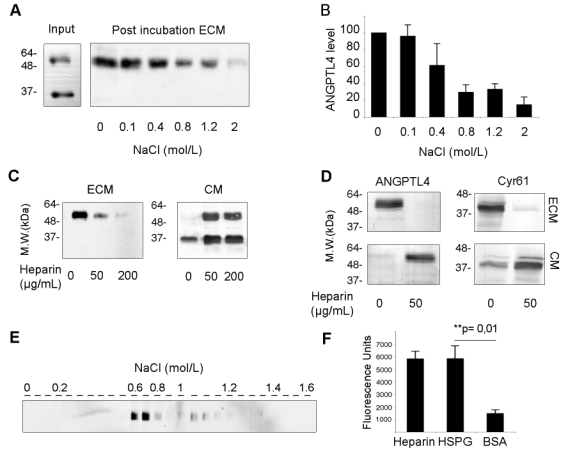
A and B, CM from HEK293-ANGPTL4-flag cells (Input) was incubated on ECM from normoxic HUVEC. ECM was then treated by NaCl at the indicated concentrations for 2 hours. ANGPTL4 in the post-incubation ECM was analyzed by Western-blot (A) using anti-flag antibody and quantified (B) using the Quantity One software (Bio-Rad Laboratories). C and D, HEK293-ANGPTL4 cells (C) or hypoxic HUVEC (D) were grown in the presence of increasing amounts of heparin as indicated. ECM and CM from the same plate were analyzed by Western-blot using anti-flag (C) or anti-ANGPTL4 and anti-Cyr61 antibodies (D). E, The elution profile of ANGPTL4-flag from a heparin-sepharose affinity column with a NaCl gradient was analyzed by Western-blot using anti-flag antibody. F, Direct binding of purified ANGPTL4-myc-his (1 μg/mL) to immobilized HSPGs (60 μg/mL), heparin (50 μg/mL) or BSA (10 mg/mL) was measured in triplicate in a solid phase binding assay. Results are representative of three independent experiments. Quantification indicate mean ± SD of three independent experiments.
ANGPTL4 could accumulate in the ECM through interaction with HSPGs, as previously reported for Cyr61.25 Increasing amounts of heparin added to the culture medium of HEK293-ANGPTL4 cells led to a dose-dependent displacement of the full-length protein from the ECM to the medium, suggesting a competition between soluble heparin and matrix HSPGs for recombinant ANGPTL4 binding (Figure 4C). Moreover in the presence of heparin (50 μg/mL), endogenous ANGPTL4 was shifted from the ECM of hypoxic HUVEC into the CM, as well as Cyr61 (Figure 4D). Similar results have been obtained with microvascular endothelial cells (data not shown). Full-length ANGPTL4 was applied on heparin-sepharose affinity column to further investigate a potential direct interaction. ANGPTL4 efficiently bound to heparin since it was eluted at 0.7 mol/L NaCl (Figure 4E). This strong affinity of ANGPTL4 for heparin was in a range similar to that previously reported for Cyr61.27 Moreover, in a solid phase binding assay, purified full-length ANGPTL4 interacted with immobilized heparin as well as immobilized HSPGs, whereas it weakly interacted with BSA (Figure 4F). Altogether, our results demonstrate that both recombinant and endogenous full-length ANGPTL4 strongly interact with the ECM in a heparin/HSPGs dependent manner.
Matrix-bound ANGPTL4 inhibits endothelial cell adhesion to the ECM
The functional role of the ECM-bound full-length ANGPTL4 was then investigated. Conditioned ECM from HEK293 or HEK293-ANGPTL4-flag cells were used to quantify the adhesion of endothelial cells one hour after plating. As compared to control ECM, HUVEC adhesion was decreased by 32.7 ± 16.7% on ANGPTL4-containing ECM (Figure 5A). To check the direct involvement of ANGPTL4 in the anti-adhesive effect, endothelial adhesion was analyzed on increasing concentrations of purified immobilized protein (Figure 5B). HUVEC adhesion was reduced in a dose-dependent way, reaching 39.5 ± 9.7% of decrease on 5 μg/mL ANGPTL4. Adhesion of Human Umbilical Artery Endothelial Cells (HUAEC) and HMEC-1 on ANGPTL4 were also decreased by 52.4 ± 5.7% and 28.4 ± 8.2% respectively, when compared to control adhesion (Figure 5C).
Figure 5. ANGPTL4 inhibits adhesion of endothelial cells.
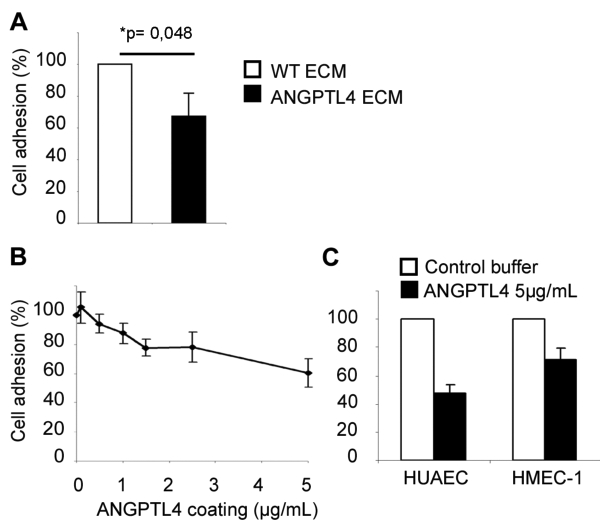
A, HUVEC were plated on conditioned ECM from wild-type HEK293 cells (WT ECM) or from HEK293-ANGPTL4 cells (ANGPTL4 ECM). Basal adhesion (at 100%) was set on control WT ECM. B and C, Human endothelial cells (B: HUVEC, C: HUAEC or HMEC-1) were plated on purified immobilized ANGPTL4. Basal adhesion (at 100%) was set on control buffer. Results are mean ± SD of three independent experiments for HUVEC and of triplicates of one representative experiment for HUAEC and HMEC-1.
Matrix-bound ANGPTL4 prevents the formation of actin stress fibers and the organization of focal contacts of adhering endothelial cells
Cell adhesion on ECM affects cytoskeletal dynamics. The organization of the actin cytoskeleton of endothelial cells was investigated during the adhesion process on ANGPTL4. HUVEC were plated on conditioned ECM either from HEK293 or HEK293-ANGPTL4-flag cells (Figure 6A). Two hours after plating on the control ECM, cells displayed a well ordered array of transversal actin stress fibers extending to focal adhesions evidenced by vinculin. This organized cytoskeleton was similar to that from HUVEC adhering on fibronectin. In contrast, HUVEC adhering on ECM from HEK293-ANGPTL4 cells were almost devoid of actin stress fibers and focal adhesions. HUVEC displayed a similar disorganized cytoskeleton on purified immobilized ANGPTL4 compared to control coating. On wild-type ECM, 57.3 ± 6.7% HUVEC displayed stress fibers versus 29.7 ± 3.9% on ECM containing ANGPTL4. On control buffer, 67.4 ± 3.7% cells displayed an organized cytoskeleton versus 38.7 ± 5.8% on purified ANGPTL4 (Figure 6B, top panel). The alteration of actin organization and focal adhesion distribution induced by ANGPTL4 was dose-dependent (Figure 6B, bottom panel).
Figure 6. ANGPTL4 inhibits the organization of actin cytoskeleton in HUVEC.
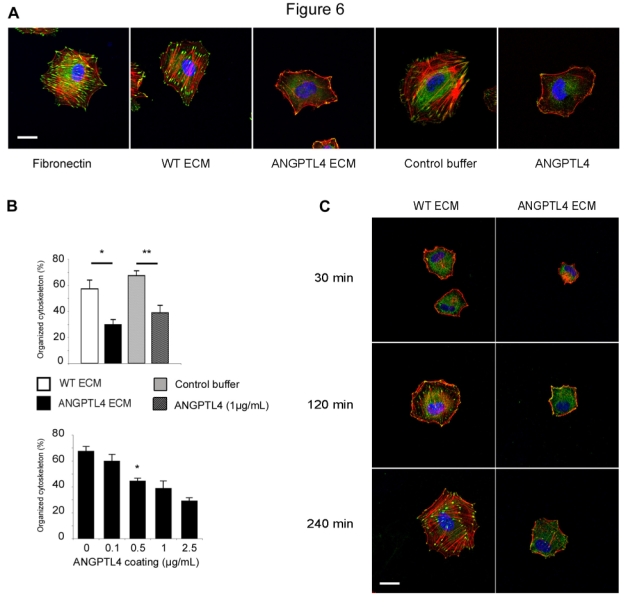
A, Morphology and actin cytoskeleton were analyzed after plating cells either on fibronectin (5 μg/mL), ECM from wild-type HEK293 cells (WT ECM), ECM from HEK293-ANGPTL4 cells (ANGPTL4 ECM), control buffer or immobilized ANGPTL4 (1 μg/mL). Two hours after adhesion, cells were processed for immunofluorescence with Rhodamin-Phalloidin (red), anti-vinculin (green), TO-PRO®-3 (blue). Bar = 20 μm. B, Actin cytoskeleton organization was quantified after two hours adhesion on the indicated coatings (top panel) or on immobilized ANGPTL4 (bottom panel). Data are mean ± SD of three independent experiments, *:p=0.05, **:p=0.02. C, Time course of actin cytoskeleton organization during the adhesion process. Bar = 20 μm. Representative pictures were taken from one out of three independent experiments.
A time course of the effect of ECM-associated ANGPTL4 was performed during the adhesion process (Figure 6C). The dynamic reorganization of actin fibers and focal adhesion progressed over the next 4 hours after adhesion on control ECM. In contrary, stress fibers failed to form up to 4 hours after plating on ANGPTL4-containing ECM.
Immobilized ANGPTL4 inhibits endothelial cell migration
Endothelial cell migration is an essential step of the angiogenic process. A migration assay was performed in a Transwell system where the lower side of the membrane was coated with fibronectin in the absence or presence of ANGPTL4 (Figure 7A). HUVEC showed a decreased locomotion towards fibronectin when immobilized ANGPTL4 was added. Furthermore, this effect was dose-dependent with a maximal reduction of 47 % of the number of cells that had migrated (Figure 7A, right panel).
Figure 7. ANGPTL4 reduces endothelial cell migration.
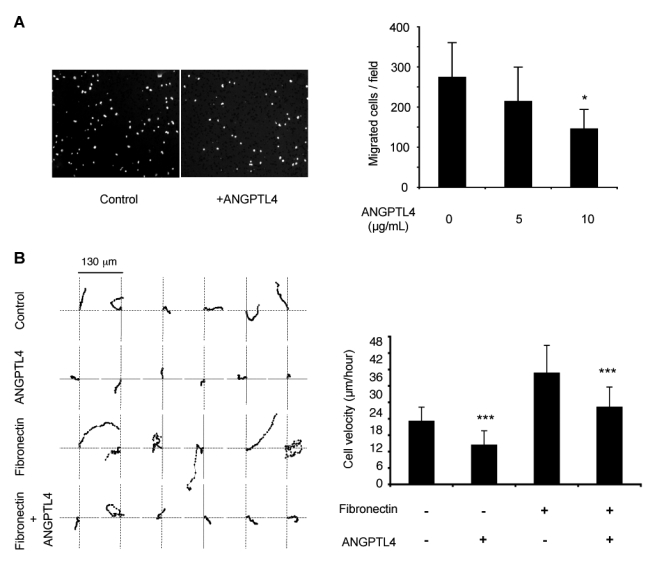
A, HUVEC migrated through the Transwell membrane. Left panel, field (objective X5) of the control (fibronectin alone) and ANGPTL4 (10 μg/mL) bottom side of Transwell using DAPI nuclear staining. Right panel, quantification of cells which migrated towards fibronectin in the absence or presence of ANGPTL4. Results are mean ± SD of three independent experiments, *p=0.05. B, Time-lapse videomicroscopy. Left panel, migration paths of individual HUVEC seeded on plastic (control), fibronectin (5 μg/mL) ± ANGPTL4 (5 μ/mL). Each point indicates the position (XY coordinates) of the cell at 5-min intervals. Position of 62 consecutive intervals are shown. Right panel, quantification of the cell velocity. Results are mean ± SD of 28 to 48 independent cells in a representative experiment out of three independent experiments. ***p=0.001.
Cell migration was further analyzed by time-lapse videomicroscopy. The motility tracking of individual HUVEC plated on various coatings was monitored. Six representative paths from 28 to 48 analyzed cells are shown in Figure 7B, left panel. Immobilized ANGPTL4 decreased HUVEC migration speed when compared to plastic or fibronectin alone (control: 23.08 ± 5.16 μm/hour; ANGPTL4: 14.34 ± 5.22 μm/hour; fibronectin: 40.62 ± 10.18 μm/hour), as quantified in Figure 7B, right panel. Furthermore, ANGPTL4 immobilized in presence of fibronectin was able to counteract the fibronectin-induced high motility (ANGPTL4 + fibronectin: 28.20 ± 7.37 μm/hour). Movies are shown in supplemental Figure I.
Matrix-bound ANGPTL4 inhibits tube formation and sprouting
The ability of microvascular endothelial cells to form tubes was assessed in Matrigel containing increasing concentrations of full-length ANGPTL4. Tubes formation of HMEC-1 was reduced on ANGPTL4 and Matrigel when compared to Matrigel alone (Figure 8A and B). To further characterize the role of ANGPTL4 in the sprouting process, we used a 3D spheroid model.24 HUVEC spheroids were stimulated to sprout in a collagen gel in the absence or presence of immobilized ANGPTL4. Figure 8C shows representative spheroids for each condition and the sprouting was quantified on panel D. Whereas ANGPTL4 embedded in the collagen gel has no statistically significant effect in basal conditions, it decreased the sprouting of VEGF-stimulated HUVEC.
Figure 8. ANGPTL4 decreases tube formation and sprouting of endothelial cells.
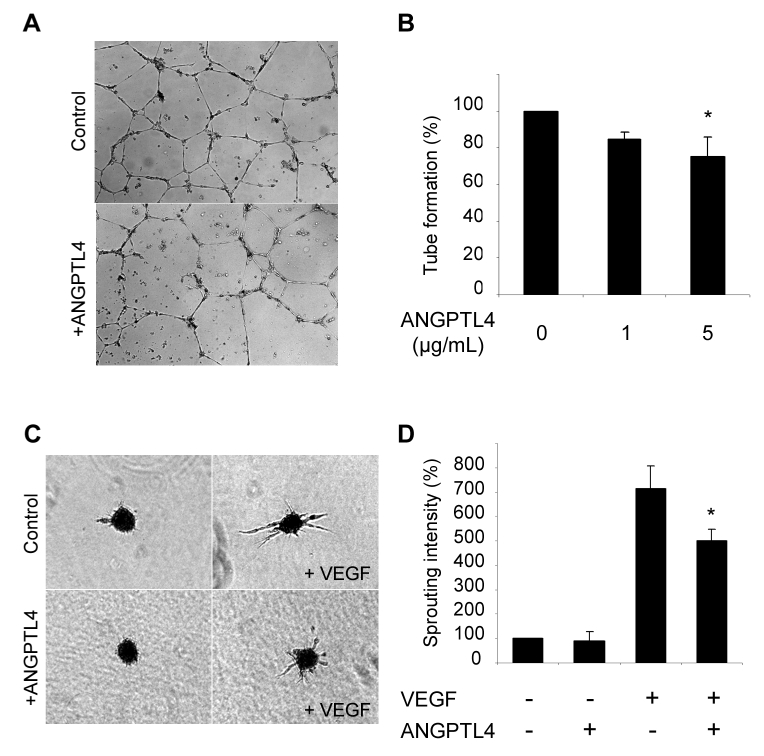
A and B, HMEC-1 were seeded on Matrigel containing various concentrations of ANGPTL4. Tube formation was assessed after 24 hours. A, Tubes formed on Matrigel alone (top) or on Matrigel containing 5 μg/mL ANGPTL4 (bottom). B, Quantification of tube number. Results are mean ± SD of three independent experiments. C and D, The sprouting assay was performed on HUVEC spheroids embedded in collagen ± ANGPTL4. C, Representative spheroids without (top) or with (bottom) ANGPTL4. D, Quantification of sprouting. Results are mean ± SD of three independent experiments, *p=0.05.
Discussion
Previous in vivo studies reported a strong upregulation of angptl4 mRNA in human samples from critical leg ischemia2 and an inhibition of angiogenesis and vascular leakiness.11 In this study, we observed an accumulation of the protein in a mouse model of hindlimb ischemia. Altogether, these data suggest a pathophysiological role of ANGPTL4 in altering efficient neovascularization in ischemic tissues. Thus deciphering the cellular mechanisms of the angiogenic responses to ANGPTL4 is an important issue.
We here demonstrate that ANGPTL4, a member of the angiopoietin family, strongly interacts with the ECM through HSPGs and that matrix-bound ANGPTL4 inhibits endothelial cell adhesion, cytoskeleton organization, migration and sprouting. Angiopoietin-1 also interacts with the ECM whereas angiopoietin-2 is more diffusible.19 These interactions provide different local concentrations, involve other proteins as co-receptors and are thought to account in part for the differences in the biological effect of the angiopoietins. In addition to the transcriptional regulation by hypoxia2,28, PPARs agonists and nutritional factors13,14,26, we here propose a new regulatory mechanism of the bioavailability of ANGPTL4 through ECM binding. This interaction with the ECM participates in concentrating the full-length protein near its production site. Consistently we found an increase in full-length ANGPTL4 in the mouse ischemic muscle while the interstitial vessels appear as a major expression site. Altogether these findings suggest that the ECM from the vascular wall might constitute a dynamic reservoir of ANGPTL4. Its up-regulation by hypoxia could be involved in microenvironment modifications occuring in ischemic events. This hypothesis can not be fully tested in vivo, given the paucity of tools to detect endogenous ANGPTL4.
We show here a direct interaction of ANGPTL4 with heparin. However a higher salt concentration was required to release ANGPTL4 from subendothelial ECM than from the heparin-sepharose. This suggests that other ECM components are likely involved in the interaction between ANGPTL4 and the matrix. A putative heparin-binding motif has been found in ANGPTL3, the closest related known member of the family29, whereas no consensus sequence exists in ANGPTL4. However many heparin-binding proteins do not display a consensus sequence, suggesting rather the implication of a structural heparin-binding motif which remains to be identified for ANGPTL4.
ANGPTL4 is proteolyzed in vitro and in vivo and circulates in the peripheral blood mainly as truncated fragments4,26, suggesting the relative instability of the full-length soluble form. Interestingly, we show here that full-length ANGPTL4 preferentially interacts with the ECM. This binding may protect ANGPTL4 from proteolysis and regulate its processing. Differences in the ratio of the full-length and truncated forms reported in human liver and adipose tissue26 could partly result from a variable ANGPTL4 binding capacity to the ECM in these organs.
Once incorporated in the ECM, full-length ANGPTL4 could be part of the guidance cues provided by the matrix to the endothelial cells and involved in vascular morphogenesis and angiogenesis.18 Cell adhesion on the ECM is a dynamic process involving attachment, spreading and formation of focal adhesions and stress fibers, which generate a strong adhesive state. A state of intermediate adhesion, characterized by a fairly spread shape of the cells with disruption of focal adhesions and disassembly of actin stress fibers, is thought to represent the physiological « de-adhesion » process, allowing tissue remodeling.30 Some ECM-associated proteins, the matricellular proteins, which include thrombospondin-1 (TSP-1), Secreted Protein Acidic and Rich in Cystein (SPARC) and the CCN family of proteins (Cyr61, Connective Tissue Growth Factor, Nov) regulate cell-matrix interactions.31 In the present study, the anti-adhesive activity induced by matrix-associated ANGPTL4 is similar to that observed for TSP-1 or SPARC. The cytoskeleton disorganization could explain the defective migration of endothelial cells on an attractive substratum as fibronectin.
The pathway leading to the inhibition of endothelial cell adhesion, migration and cytoskeleton disruption is likely to involve the integrin family, which are primary ECM receptors. ANGPTL4, similarly to the angiopoietins32, or ANGPTL310 could directly bind an integrin, or inhibit cell-ECM interaction through a downstream impairement of integrin function. ANGPTL4 receptor has not been characterized yet and these hypotheses need further investigation.
In summary, ANGPTL4 is a hypoxia-induced angiogenic modulator, whose bioavailability is regulated by a strong interaction with the ECM and which acts as a chemorepellent and disorganizes the actin cytoskeleton of endothelial cells. Thus, ECM-bound ANGPTL4 may be involved through an autocrine pathway in the highly regulated vascular morphogenesis occuring in response to hypoxia.
Acknowledgments
We thank Corinne Ardidie-Robouant, Alain Barret and Eric Etienne for technical assistance, Florence Ruggiero for helpful discussions and Vassili Tsatsaris for umbilical cord samples. AC was the recipient of a “Groupe de Reflexion sur la Recherche Cardiovasculaire” and a “Fondation Bettencourt Schueller” grant, AG was financially supported by a Lefoulon-Delalande grant, SLJ was a recipient of a “Fondation pour la Recherche Medicale” grant. This study was supported by a fellowship from the “Fondation de France” to SG. SG belongs to the European Vascular Genomics Network (http://www.evgn.org) (Contract No LSHMCT-2003-503254). This work was supported in part by a grant from Novartis.
Footnotes
Disclosures
None
Conflicts of interest
None
References
- 1.Carmeliet P. Angiogenesis in health and disease. Nat Med. 2003;9:653–60. doi: 10.1038/nm0603-653. [DOI] [PubMed] [Google Scholar]
- 2.Le Jan S, Amy C, Cazes A, Monnot C, Lamande N, Favier J, Philippe J, Sibony M, Gasc JM, Corvol P, Germain S. Angiopoietin-like 4 is a proangiogenic factor produced during ischemia and in conventional renal cell carcinoma. Am J Pathol. 2003;162:1521–8. doi: 10.1016/S0002-9440(10)64285-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim I, Kim HG, Kim H, Kim HH, Park SK, Uhm CS, Lee ZH, Koh GY. Hepatic expression, synthesis and secretion of a novel fibrinogen/angiopoietin-related protein that prevents endothelial-cell apoptosis. Biochem J. 2000;346:603–10. [PMC free article] [PubMed] [Google Scholar]
- 4.Ge H, Yang G, Huang L, Motola DL, Pourbahrami T, Li C. Oligomerization and regulated proteolytic processing of angiopoietin-like protein 4. J Biol Chem. 2004;279:2038–45. doi: 10.1074/jbc.M307583200. [DOI] [PubMed] [Google Scholar]
- 5.Suri C, Jones PF, Patan S, Bartunkova S, Maisonpierre PC, Davis S, Sato TN, Yancopoulos GD. Requisite role of angiopoietin-1, a ligand for the TIE2 receptor, during embryonic angiogenesis. Cell. 1996;87:1171–80. doi: 10.1016/s0092-8674(00)81813-9. [DOI] [PubMed] [Google Scholar]
- 6.Maisonpierre PC, Suri C, Jones PF, Bartunkova S, Wiegand SJ, Radziejewski C, Compton D, McClain J, Aldrich TH, Papadopoulos N, Daly TJ, Davis S, Sato TN, Yancopoulos GD. Angiopoietin-2, a natural antagonist for Tie2 that disrupts in vivo angiogenesis. Science. 1997;277:55–60. doi: 10.1126/science.277.5322.55. [DOI] [PubMed] [Google Scholar]
- 7.Dhanabal M, LaRochelle WJ, Jeffers M, Herrmann J, Rastelli L, McDonald WF, Chillakuru RA, Yang M, Boldog FL, Padigaru M, McQueeney KD, Wu F, Minskoff SA, Shimkets RA, Lichenstein HS. Angioarrestin: an antiangiogenic protein with tumor-inhibiting properties. Cancer Res. 2002;62:3834–41. [PubMed] [Google Scholar]
- 8.Kubota Y, Oike Y, Satoh S, Tabata Y, Niikura Y, Morisada T, Akao M, Urano T, Ito Y, Miyamoto T, Nagai N, Koh GY, Watanabe S, Suda T. Cooperative interaction of Angiopoietin-like proteins 1 and 2 in zebrafish vascular development. Proc Natl Acad Sci U S A. 2005;102:13502–7. doi: 10.1073/pnas.0501902102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim I, Moon SO, Koh KN, Kim H, Uhm CS, Kwak HJ, Kim NG, Koh GY. Molecular cloning, expression, and characterization of angiopoietin-related protein. Angiopoietin-related protein induces endothelial cell sprouting. J Biol Chem. 1999;274:26523–8. doi: 10.1074/jbc.274.37.26523. [DOI] [PubMed] [Google Scholar]
- 10.Camenisch G, Pisabarro MT, Sherman D, Kowalski J, Nagel M, Hass P, Xie MH, Gurney A, Bodary S, Liang XH, Clark K, Beresini M, Ferrara N, Gerber HP. ANGPTL3 stimulates endothelial cell adhesion and migration via integrin alpha vbeta 3 and induces blood vessel formation in vivo. J Biol Chem. 2002;277:17281–90. doi: 10.1074/jbc.M109768200. [DOI] [PubMed] [Google Scholar]
- 11.Ito Y, Oike Y, Yasunaga K, Hamada K, Miyata K, Matsumoto S, Sugano S, Tanihara H, Masuho Y, Suda T. Inhibition of angiogenesis and vascular leakiness by angiopoietin-related protein 4. Cancer Res. 2003;63:6651–7. [PubMed] [Google Scholar]
- 12.Hermann LM, Pinkerton M, Jennings K, Yang L, Grom A, Sowders D, Kersten S, Witte DP, Hirsch R, Thornton S. Angiopoietin-like-4 is a potential angiogenic mediator in arthritis. Clin Immunol. 2005;115:93–101. doi: 10.1016/j.clim.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 13.Yoon JC, Chickering TW, Rosen ED, Dussault B, Qin Y, Soukas A, Friedman JM, Holmes WE, Spiegelman BM. Peroxisome proliferator-activated receptor gamma target gene encoding a novel angiopoietin-related protein associated with adipose differentiation. Mol Cell Biol. 2000;20:5343–9. doi: 10.1128/mcb.20.14.5343-5349.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kersten S, Mandard S, Tan NS, Escher P, Metzger D, Chambon P, Gonzalez FJ, Desvergne B, Wahli W. Characterization of the fasting-induced adipose factor FIAF, a novel peroxisome proliferator-activated receptor target gene. J Biol Chem. 2000;275:28488–93. doi: 10.1074/jbc.M004029200. [DOI] [PubMed] [Google Scholar]
- 15.Yoshida K, Shimizugawa T, Ono M, Furukawa H. Angiopoietin-like protein 4 is a potent hyperlipidemia-inducing factor in mice and inhibitor of lipoprotein lipase. J Lipid Res. 2002;43:1770–2. doi: 10.1194/jlr.c200010-jlr200. [DOI] [PubMed] [Google Scholar]
- 16.Xu A, Lam MC, Chan KW, Wang Y, Zhang J, Hoo RL, Xu JY, Chen B, Chow WS, Tso AW, Lam KS. Angiopoietin-like protein 4 decreases blood glucose and improves glucose tolerance but induces hyperlipidemia and hepatic steatosis in mice. Proc Natl Acad Sci USA. 2005;102:6086–91. doi: 10.1073/pnas.0408452102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yu X, Burgess SC, Ge H, Wong KK, Nassem RH, Garry DJ, Sherry AD, Malloy CR, Berger JP, Li C. Inhibition of cardiac lipoprotein utilization by transgenic overexpression of Angptl4 in the heart. Proc Natl Acad Sci USA. 2005;102:1767–72. doi: 10.1073/pnas.0409564102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Davis GE, Senger DR. Endothelial extracellular matrix: biosynthesis, remodeling, and functions during vascular morphogenesis and neovessel stabilization. Circ Res. 2005;97:1093–107. doi: 10.1161/01.RES.0000191547.64391.e3. [DOI] [PubMed] [Google Scholar]
- 19.Xu Y, Yu Q. Angiopoietin-1, unlike angiopoietin-2, is incorporated into the extracellular matrix via its linker peptide region. J Biol Chem. 2001;276:34990–8. doi: 10.1074/jbc.M103661200. [DOI] [PubMed] [Google Scholar]
- 20.Hutchings H, Ortega N, Plouet J. Extracellular matrix-bound vascular endothelial growth factor promotes endothelial cell adhesion, migration, and survival through integrin ligation. Faseb J. 2003;17:1520–2. doi: 10.1096/fj.02-0691fje. [DOI] [PubMed] [Google Scholar]
- 21.Owensby DA, Morton PA, Schwartz AL. Interactions between tissue-type plasminogen activator and extracellular matrix-associated plasminogen activator inhibitor type 1 in the human hepatoma cell line HepG2. J Biol Chem. 1989;264:18180–7. [PubMed] [Google Scholar]
- 22.Couffinhal T, Silver M, Zheng LP, Kearney M, Witzenbichler B, Isner JM. Mouse model of angiogenesis. Am J Pathol. 1998;152:1667–79. [PMC free article] [PubMed] [Google Scholar]
- 23.Le Jan S, Le Meur N, Cazes A, Philippe J, Le Cunff M, Leger J, Corvol P, Germain S. Characterization of the expression of the hypoxia-induced genes neuritin, TXNIP and IGFBP3 in cancer. FEBS Lett. 2006;580:3395–400. doi: 10.1016/j.febslet.2006.05.011. [DOI] [PubMed] [Google Scholar]
- 24.Korff T, Augustin HG. Tensional forces in fibrillar extracellular matrices control directional capillary sprouting. J Cell Sci. 1999;112:3249–58. doi: 10.1242/jcs.112.19.3249. [DOI] [PubMed] [Google Scholar]
- 25.Brigstock DR. The connective tissue growth factor/cysteine-rich 61/nephroblastoma overexpressed (CCN) family. Endocr Rev. 1999;20:189–206. doi: 10.1210/edrv.20.2.0360. [DOI] [PubMed] [Google Scholar]
- 26.Mandard S, Zandbergen F, Tan NS, Escher P, Patsouris D, Koenig W, Kleemann R, Bakker A, Veenman F, Wahli W, Muller M, Kersten S. The direct peroxisome proliferator-activated receptor target fasting-induced adipose factor (FIAF/PGAR/ANGPTL4) is present in blood plasma as a truncated protein that is increased by fenofibrate treatment. J Biol Chem. 2004;279:34411–20. doi: 10.1074/jbc.M403058200. [DOI] [PubMed] [Google Scholar]
- 27.Kireeva ML, Latinkic BV, Kolesnikova TV, Chen CC, Yang GP, Abler AS, Lau LF. Cyr61 and Fisp12 are both ECM-associated signaling molecules: activities, metabolism, and localization during development. Exp Cell Res. 1997;233:63–77. doi: 10.1006/excr.1997.3548. [DOI] [PubMed] [Google Scholar]
- 28.Belanger AT, Lu H, Date T, Liu LX, Vincent KA, Akita GY, Cheng SH, Gregory RJ, Jiang C. Hypoxia up-regulates expression of peroxisome proliferator-activated receptor gamma angiopoietin-related gene (PGAR) in cardiomyocytes: role of hypoxia inducible factor 1α. J Mol Cell Cardiol. 2002;34:765–74. doi: 10.1006/jmcc.2002.2021. [DOI] [PubMed] [Google Scholar]
- 29.Ono M, Shimizugawa T, Shimamura M, Yoshida K, Noji-Sakikawa C, Ando Y, Koishi R, Furukawa H. Protein region important for regulation of lipid metabolism in angiopoietin-like 3 (ANGPTL3): ANGPTL3 is cleaved and activated in vivo. J Biol Chem. 2003;278:41804–9. doi: 10.1074/jbc.M302861200. [DOI] [PubMed] [Google Scholar]
- 30.Murphy-Ullrich JE. The de-adhesive activity of matricellular proteins: is intermediate cell adhesion an adaptive state? J Clin Invest. 2001;107:785–90. doi: 10.1172/JCI12609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bornstein P, Sage EH. Matricellular proteins: extracellular modulators of cell function. Curr Opin Cell Biol. 2002;14:608–16. doi: 10.1016/s0955-0674(02)00361-7. [DOI] [PubMed] [Google Scholar]
- 32.Carlson TR, Feng Y, Maisonpierre PC, Mrksich M, Morla AO. Direct cell adhesion to the angiopoietins mediated by integrins. J Biol Chem. 2001;276:26516–25. doi: 10.1074/jbc.M100282200. [DOI] [PubMed] [Google Scholar]


