Abstract
CD4+ T cells play a central role in the induction and persistence of CD8+ T cells in several models of autoimmune and infectious disease. To improve the efficacy of a synthetic peptide vaccine based on the self-Ag, gp100, we sought to provide Ag-specific T cell help. To identify a gp100 epitope restricted by the MHC class II allele with the highest prevalence in patients with malignant melanoma (HLA-DRB1*0401), we immunized mice transgenic for a chimeric human-mouse class II molecule (DR4-IE) with recombinant human gp100 protein. We then searched for the induction of CD4+ T cell reactivity using candidate epitopes predicted to bind to DRB1*0401 by a computer-assisted algorithm. Of the 21 peptides forecasted to bind most avidly, murine CD4+ T cells recognized the epitope (human gp10044–59, WNRQLYPEWTEAQRLD) that was predicted to bind best. Interestingly, the mouse helper T cells also recognized human melanoma cells expressing DRB1*0401. To evaluate whether human CD4+ T cells could be generated from the peripheral blood of patients with melanoma, we used the synthetic peptide h-gp10044–59 to sensitize lymphocytes ex vivo. Resultant human CD4+ T cells specifically recognized melanoma, as measured by tumor cytolysis and the specific release of cytokines and chemokines. HLA class II transgenic mice may be useful in the identification of helper epitopes derived from Ags of potentially great clinical utility.
Therapeutic anti-cancer vaccines can elicit immune responses capable of destroying established tumors (1). Their design was made possible by identification of Ags expressed by human tumor cells (2). Vaccines corresponding to MHC class I-restricted epitopes presented on the tumor cell surface have been administered in clinical trials and can activate cytotoxic CD8+ T cells capable of specifically recognizing tumor cells (3-5).
Despite the emphasis on CD8+ T cell responses, there is growing evidence to support an important role for CD4+ T cells in antitumor immunity. Murine studies have demonstrated that CD4+ T cells exert helper activity through the induction and maintenance of CD8+ T cells and B cells and have direct and indirect effects on tumor cells, including those deficient in MHC class II (reviewed in Ref. 6). In humans, CD4+ T cells play a crucial role in the induction of several autoimmune diseases (7) and in the resistance to pathogens (8, 9). The evidence for their role in human cancer is less substantial and limited to studies that demonstrate both tumor-specific CD4+ and CD8+ T cells at the tumor site (10, 11). In addition, tumor-specific IgG Abs have been detected in cancer patients, thus implying the active role of CD4+ T cells in isotype switching (12).
There is accumulating evidence that the combined application of class I and class II epitopes derived from the same tumor Ag may potentiate antitumor effector function and long-term immunity (12a, 13-16). CD4+ T cells activate dendritic cells (DC),2 principally through the interaction of CD40 and its ligand. “Conditioned” DC can, in turn, cross-present Ag to cognate CD8+ T cells at the tumor site or in remote locations (13, 17-20). Therefore, a need exists for the development of techniques useful in the identification of tumor-associated MHC class II-restricted epitopes in human cancers. While the targets of several CD4+ T cells with potential anti-tumor activity have been identified (21-26), no reliable, repeatable method for class II-restricted epitope identification has been established.
We sought to identify an epitope derived from the melanocyte differentiation Ag, human (h) gp100. More than 75% of melanomas express h-gp100 (27) and CD8+ T cells derived from tumor-infiltrating lymphocytes (TIL) recognize h-gp100 presented on the surface of melanoma cells (28). The adoptive transfer of TIL that have specificity for h-gp100 are significantly more efficacious at mediating tumor regression than TIL with specificities for other Ags (29). Thirteen different MHC class I-restricted epitopes from h-gp100 have now been identified (2). Vaccination with a modified, “anchor-fixed” h-gp100 CD8+ T cell epitope in combination with IL-2 has been reported to result in a 42% response rate in patients with metastatic melanoma (3, 30). However, only a minority of patients responded clinically to this vaccine regimen, and most of the responses observed were transient in nature (3).
To increase the immunogenicity and therapeutic efficacy of vaccines comprised of h-gp100 CD8+ T cell epitopes, we sought to provide Ag-specific CD4+ T cell help. To identify a shared DR-restricted epitope from h-gp100 of potential clinical value, we targeted an MHC class II allele with the highest prevalence in patients with metastatic melanoma (31). Our strategy involves the immunization with recombinant h-gp100 protein of DR4-IE transgenic (Tg) mice that express a chimeric mouse-human MHC class II molecule from HLA-DRB1*0401. We then searched for the induction of CD4+ T cell reactivity using candidate epitopes derived from a computer-assisted prediction algorithm.
Materials and Methods
Animals
Murine class II-deficient, DR4-IE Tg mice express HLA-DRA-IE-α and HLA-DRB1*0401-IE-β chimeric genes containing the Ag-binding α1 and β1 domains from the human HLA-DRA and HLA-DRB1*0401 molecules, respectively, with the remaining domains comprised of murine IEd-α2 and IEd-β2 chains (32). Female DR4-IE Tg mice 6- to 10-wk-old were used for all experiments. Founder mice were obtained through Paul Lehmann (at Case Western Reserve University, Cleveland, OH) and maintained and bred in a barrier facility (Biocon, Rockville, MD). Mice were confirmed to have two allelic copies of the DR4-IE chimeric transgene using PCR amplification of genomic DNA (oligonucleotide primers previously described (32)). Eighty microsatellite markers (Research Genetics, Huntsville, AL), evenly distributed across the murine genome, were used to assess genetic similarity to C57BL/6n mice (33). Purebred DR4-IE Tg mice were matched at 77 of 80 markers and accepted the normal growth of murine melanoma B16 (H-2b) (data not shown).
HLA typing
HLA serotypes and DNA genotypes of fresh human PBMC and tumor cell lines were determined by the National Institutes of Health HLA Laboratory, as described (34). The MHC class II genotype of patient “Te” was HLA-DRB1*0401, 1501, DQB1*0302, 0602, DRB4*01, DRB5*0101; the genotype of patient “Th” was HLA-DRB1*0401, 1501, DRQB1*0302, 06, DRB4*01, DRB5*0501; and the genotype of patient “Wa” was HLA-DRB1*0401, 1501, DRQB1*0301, 06, DRB4*01, DRB5*0501. The HLA-DRB1* genotypes of tumor lines used in the following experiments included 697 Mel and EBV-B (0401,1501); 888 Mel (1502,1601); 1102 Mel and EBV-B (0401,1502); 1359 Mel and EBV-B (0401,0301); and 1498 Mel (0401,0802).
Peptides
The 21 h-gp100 peptides used in the initial screening of candidate epitopes were synthesized using a solid-phase method based on fluorenylmethoxycarbonyl (F-moc) chemistry on a multiple peptide synthesizer (Model AMS 422; Gilson, Worthington, OH). The molecular masses of peptides were verified by laser desorption mass spectrometry (Biosynthesis, Lewisville, TX). All other peptides were synthesized by Macromolecular Resources (Fort Collins, CO) to a purity >99% as assessed by HPLC and laser desorption mass spectrometry.
Recombinant h-gp100 protein
The gene encoding h-gp100 (28) was amplified by PCR with primers (forward: AGG,CGC,AGA,CTT,ATG,AAG,CA; reverse: CTG,CCC,AAG,GCC,TGC,TTC,TTG) designed to delete the N-terminal 23 amino acids (probable signal sequence) and the C-terminal 66 amino acids (probable transmembrane region). The truncated gene was then cloned into the PET28a+ expression vector (Novagen, Madison, WI) and transformed into BL21(DE3) Escherichia coli (Novagen). E. coli were grown to OD600 0.6, then protein expression was induced with isopropyl β-d-thiogalactoside 1 μg/ml for 3 h. The bacteria were harvested; inclusion bodies were isolated and lysed in 6 M urea, then proteins were purified by preparative scale SDS-PAGE in a Prep cell (Bio-Rad, Hercules, CA) electrophoresis chamber. Protein fractions at 61.5 kDa (theoretical molecular mass of truncated h-gp100) were collected, dialyzed, and precipitated, and a purity of >80% was estimated based on SDS-PAGE with Colloidal Blue (Novex, San Diego, CA) staining.
DRB1*0401 computer-assisted algorithm
To generate a list of candidate h-gp100 epitopes capable of binding HLA-DRB1*0401, we selected an algorithm based on work by Alessandro Sette and his colleagues (35), which uses numerical values corresponding to the relative strength of individual amino acids along a nine-residue core region critical for MHC binding. By multiplying each value from P1 through P9, an algorithm value is obtained. A computer program was written that would permit the input of protein sequences of any length, generating a list of nine-residue peptides in order of decreasing predicted binding affinity down to an algorithm score that predicts 75% of all binders (<2.617; an arbitrary cut-off point) (35). The full-length h-gp100 amino acid sequence (GenBank, accession no. M77348; 661 aa) was analyzed with this program, yielding 92 predicted candidate epitopes. The 24 peptides with the highest MHC binding affinities (Table I) were selected for further study, and two amino acids based on the native sequence were added to both the N terminus and C terminus. Twenty-one 13-residue candidate epitopes were then synthesized. Peptides ranked 5, 20, and 24 (start positions 614, 613, and 608, respectively) were omitted from analysis because their start positions were distal to the C terminus of the truncated recombinant h-gp100 protein used for immunization experiments (see above). The computer-assisted algorithm is currently available on the Surgery Branch, National Cancer Institute web site: http://surgery.nci.nih.gov.
Table I.
Candidate HLA-DRB1*040I-restricted peptide epitopes from h-gp100a
| Rank | AV Score |
Core Start Position |
Core xx(Sequence)xx |
IFN-γ (pg/ml) |
|---|---|---|---|---|
| 1 | 20 | 48 | RQ(LYPEWTEAQ)RL | 168 |
| 2 | 22 | 189 | TV(YHRRGSRSY)VP | 10 |
| 3 | 24 | 150 | SF(VYVWKTWGQ)YW | 14 |
| 4 | 32 | 276 | LV(VTHTYLEPG)PV | 10 |
| 5 | 35 | 616 | LI(YRRRLMKQD)FS | ND |
| 6 | 39 | 143 | GS(WSQKRSFVY)VW | 0 |
| 7 | 49 | 586 | LI(MPGQEAGLG)QV | 0 |
| 8 | 53 | 603 | GI(LLVLMAVVL)AS | 0 |
| 9 | 57 | 219 | VS(VSQLRALDG)GN | 0 |
| 10 | 59 | 557 | HQ(ILKGGSGTY)CL | 7 |
| 11 | 59 | 526 | AC(MEISSPGCQ)PP | 11 |
| 12 | 80 | 458 | PL(LDGTATLRL)VK | 0 |
| 13 | 89 | 498 | AE(ILQAVPSGE)GD | 0 |
| 14 | 114 | 121 | QP(VYPQETDDA)CI | 0 |
| 15 | 122 | 545 | PV(LPSPACQLV)LH | 0 |
| 16 | 122 | 242 | FA(LQLHDPSGY)LA | 0 |
| 17 | 150 | 499 | EI(LQAVPSGEG)DA | 0 |
| 18 | 154 | 88 | IA(LNFPGSQKV)LP | 6 |
| 19 | 162 | 423 | TE(WVETTAREL)PI | 6 |
| 20 | 170 | 615 | SL(IYRRRLMKQ)DF | ND |
| 21 | 179 | 431 | RE(LPIPEPEGP)DA | 32 |
| 22 | 185 | 553 | QL(VLHQILKGG)SG | 0 |
| 23 | 196 | 217 | FS(VSVSQLRAL)DG | 0 |
| 24 | 218 | 610 | AV(VLASLIYRR)RL | ND |
Peptides are listed in order of decreasing predicted binding affinities. The algorithm values (AV) determined by the program were multiplied by 1000 to generate an integer AV Score. For screening candidate epitopes, 13 amino acid peptides were generated by adding two amino acids to both the N and C termini of the 9-aa binding core. Peptides with predicted ranks of 5, 20, and 24 positions (h-gp100 start position 614, 613, and 608, respectively) were excluded from analysis (see Materials and Methods). Twenty-one of the top 24 candidate epitopes from h-gp100 were tested for ex vivo peptide reactivity using DR4-IE Tg mice previously vaccinated with recombinant h-gp100 protein. A total of 5 × 105 lymph node lymphocytes were cultured with individual candidate peptides added at 100 μg/ml. IFN-γ cytokine levels were measured by ELISA 24 h after the initial stimulation.
Generation of murine CD4+ T cell lines
DR4-IE Tg mice were immunized once with either 50 μg of the truncated, recombinant h-gp100 protein or with 100 μg of h-gp10046–58 peptide to initiate T cell lines A9895 and C1056, respectively. Both recombinant protein and peptide h-gp10046–58 were emulsified in CFA (final volume, 100 μL), divided equally, and administered s.c. into the rear footpads. On day 12, the animals were sacrificed and bilateral hindlimb popliteal and inguinal lymph nodes were harvested. Cells were cultured in 24-well plates at 5 × 106 cells per well with peptide h-gp10046–58 at 100 μg/ml. Twelve days after the first ex vivo stimulation, both lines were restimulated with peptide-pulsed, irradiated, syngeneic DR4-IE splenocytes. Splenocytes were pulsed with h-gp10046–58 at 600 μg/ml for 3 h at 37°C, washed, irradiated with 3000 rad, then added to each T cell culture at a 10:1 ratio (5 × 106 APCs per well). Culture medium (CM) containing IL-2 (Chiron, Emeryville, CA) at 7.5 CU/ml was added 2 days after the stimulation (day 14). Thereafter, lines were stimulated and maintained using the same methods every 13–15 days. Both lines were found to be >95% CD4+ by flow cytometry, following three in vitro stimulations (data not shown).
Generation of human CD41 T cell lines
Fresh PBMC from three HLA-DRB1*0401-positive patients (Te, Th, Wa) with documented h-gp100 expressing metastatic melanoma were used for ex vivo peptide sensitization (see above). For patient Te, PBMC were cultured at 1.5 × 105 cells per well in a flat-bottom 96-well plate in CM containing h-gp10044–59 at 50 μM and the mAb MAR4 (anti-CD29; IgG1; PharMingen) at 2.5 μg/ml (36). On day 9, cells were restimulated with autologous, peptide-pulsed, irradiated (3000 rad) PBMC. PBMC were pulsed with h-gp10044–59 at 100 μM for 3 h at 37°C, then washed and added at 1.5 × 105 cells per well in CM containing IL-2 at 50 CU/ml. On day 23, all wells from the original parental 96-well plate were tested for specific peptide reactivity using HLA-DRB1*0401-matched EBV-B cells pulsed with h-gp10044–59. The most reactive wells were selected and stimulated with autologous, irradiated, pulsed (as above) PBMC in 24-well flat-bottom plates at a 10:1 ratio (5 × 106 APCs per well). Two weeks after restimulation, each subline was screened for specific peptide and tumor reactivity. One line, Te-22, was selected for further analysis. For patients Th and Wa, fresh PBMC were initially depleted of CD8+ T cells with mAb-coated magnetic beads (Dynal, Lake Success, NY). Remaining cells were added to 96-well flat-bottom plates at 3 × 105 cells per well and cultured with GM-CSF 200 U/ml, IL-4 100 U/ml, and h-gp10044–59 at 50 μM. IL-2 at 25 CU/ml was added on day 4, and each microculture was restimulated on day 8 with pulsed (100 mM), irradiated (3000 rad), allogeneic DRB1*0401-matched EBV-B cells (106 per well). On day 22, individual wells were pooled as single lines and tested for peptide and tumor reactivity. All T cell lines were found to be >95% CD4+ by flow cytometry, following three in vitro stimulations (data not shown).
Other cell lines
EBV-B cell lines 697 EBV-B, 1102 EBV-B, 1359 EBV-B (and 1359 EBV-B transfectants); melanoma lines 697 Mel, 888 Mel, 1102 Mel, 1300 Mel, 1498 Mel; breast cancer line MDA-231; and stable transfectant Myeloma #25 (expressing chimeric DR4-IE Tg MHC; a gift from K. Ito) were maintained in the appropriate CM, as previously described (murine (37) or human (38)). The human CD8+ T cell clone (K4/H5) reactive against h-gp100209–217 and human melanoma in the context of HLA-A*0201 (M. E. Dudley; unpublished observation) was maintained in CM with IL-2 (50 CU/ml). To generate 1359 EBV-B transfectants expressing h-gp100 or green fluorescence protein (GFP), the episomal vector pEAK-8 (Edgebiosystems, Gaithersburg, MD) containing the appropriate inserts was electroporated into B cells. Autologous human DCs were generated from fresh PBMC by culturing in CM with 500 ng/ml of both GM-CSF and IL-4 for 7 days.
Assessment of T cell responses
To assess ex vivo reactivity to either candidate epitopes or to the peptide h-gp10046–58, lymphocytes from lymph nodes were harvested from Tg mice 12 days after vaccination (as described above) and plated at 5 × 105 cells per well in 96-well U-bottom plates. Protein or peptides were added to each microculture well at 100 μg/ml, then cultured for 24 h. To assess the reactivity of the CD4+ murine T cell lines A9895 and C1056, DR4-IE splenocytes or EBV-B cells were incubated for 12–18 h with peptide, protein, or with freeze-thaw lysates of tumor cells (105 cell equivalents per well). APCs were then cocultured (105 cells per well) with T cells (105 cells per well) in U-bottom 96-well plates for 24 h. To assess recognition of whole tumor cells, individual human or murine CD4+ T cell lines were cultured in U-bottom 96-well plates at 105 cells per well in the presence of irradiated tumor cells (12,000 rad) at 105 cells per well for 24 h. All melanomas, except 1102 Mel (constitutive expression of MHC class II), were pretreated with IFN-γ 500 U/ml for 48 h to up-regulate MHC class II expression. Culture supernatants were assayed for IFN-γ, GM-CSF, IL-4, IL-5, and TNF-α using commercially available ELISA kits (Endogen, Woburn, MA). In addition, commercially available ELISA kits (Endogen) were used to assess chemokines macrophage inflammatory protein (MIP)-1α, MIP-1β, and RANTES. ELISAs for chemokines MIP-3α and MIP-3β were prepared by coating 96-well plates at 2 μg/ml with primary Abs (PeproTech, Rocky Hill, NJ) and were detected with biotinylated secondary Abs at 2 μg/ml (R&D Systems, Minneapolis, MN). In blocking experiments, mAbs were used to inhibit specific Ag recognition by T cells. Abs included L243 (against HLA-DR; IgG2a; PharMingen), W6/32 (against HLA-A, B, C; IgG2a; American Type Culture Collection, Manassas, VA), and H129.19 (against murine CD4; IgG2a; PharMingen). For cytolysis experiments, target cells were pulsed with 200 μCi of 51Cr for 60 min, washed, and distributed onto U-bottom 96-well plates at 104 cells per well with effector T cells added at varying E:T ratios. Percent lysis was calculated using the standard formula: [(experimental 51Cr release − spontaneous release)/(total release − spontaneous release)] × 100. All experiments were performed between two and four times with similar results using different but comparable targets, thus demonstrating the generalizability of the experimental findings. For example, a variety of DR4+ or DR4− and gp100+ or gp100− targets were used to address the specificity of T cell responses.
Results
Initial screening of candidate epitopes for h-gp100 forecasted by a computer-assisted, DRB1*0401 prediction algorithm
To test the predictive capacity of the computer-assisted algorithm described above (35), we selected protein sequences from preproinsulin and OspA. HLA-DRB1*0401-restricted autoimmune epitopes for both proteins have been described and identified by separate methods (39, 40). The algorithm predicted the correct epitope in the number 3 and number 1 ranked positions, respectively (data not shown), thus demonstrating the potential for accurately forecasting candidate HLA-DRB1*0401 epitopes. In each case, the program forecasted >90 candidate epitopes.
To identify a shared HLA-DRB1*0401-restricted epitope for h-gp100, we screened 21 of the top 24 candidate epitopes for h-gp100 (Table I) for ex vivo peptide reactivity using DR4-IE Tg mice previously vaccinated with recombinant h-gp100 protein. IFN-γ, GM-CSF, and IL-4 cytokine levels were measured by ELISA 24 h after the initial stimulation with candidate peptide epitopes. Only one peptide, h-gp10046–58 (ranked 1 of 92), elicited significant amounts of IFN-γ secretion (168 pg/ml; Table I) and GM-CSF (10 pg/ml; data not shown), while IL-4 production was not detected with any of the peptides, except that induced with a control anti-CD3 mAb (data not shown). Importantly, a longer version of this peptide, h-gp10044–59, was previously eluted from MHC II-peptide complexes from DR4+ melanoma cells (41, 42). This peptide was included in subsequent experiments and compared with peptides h-gp10046–58 and murine-gp10046–58 (see Table II).
Table II.
Candidate epitope gp10046–58a
| Candidate Epitopes | Sequence |
|---|---|
| m-gp10046–58 | RQLQPEWTEVQGS |
| h-gp10046–58 | RQLYPEWTEAQRL |
| h-gp10044–59 | WNRQLYPEWTEAQRLD |
Human and murine variants of the candidate epitope h-gp10046–58 are displayed in alignment.
Murine CD4+ T cell lines recognize human melanoma
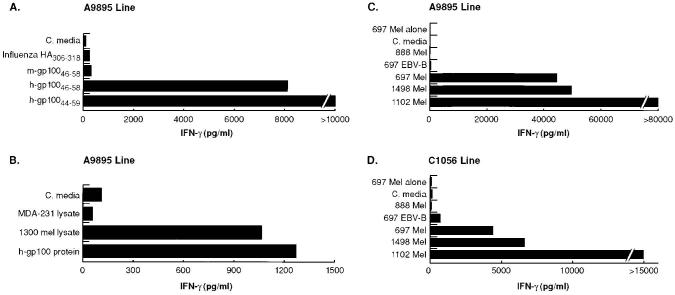
To characterize the specific reactivity of murine CD4+ T cells to peptides (h-gp10044–59 and h-gp10046–58), melanoma lysate processed by APCs, and intact melanoma cells, two distinct lymphoid lines (A9895 and C1056, as described above) were generated. Using DR4-IE Tg splenocytes as APCs, specific reactivity was detected to peptides h-gp10044–59 and h-gp10046–58, as well as to the recombinant h-gp100 protein and a lysate of a h-gp100 expressing human melanoma (1300 Mel) (Fig, 1, A and B). As seen previously, no recognition was detected to the murine variant of gp10046–58, the control peptide HA306–318, or the irrelevant tumor lysate (breast carcinoma; MDA-231). Comparable reactivity to peptides h-gp10044–59 and h-gp10046–58 was seen when using a human myeloma line expressing the DR4-IE chimeric MHC as a source of Ag presentation (data not shown). In both sets of experiments, recognition was markedly stronger using the longer peptide h-gp10044–59, indicating that amino acids outside the nine-residue core were important in enhancing T cell recognition. To assess the recognition of endogenously processed h-gp10044–59, A9895 and C1056 CD4+ T cell lines were cultured for 24 h with various human tumor targets (Fig. 1, C and D). HLA-DRB1*0401-matched tumors expressing h-gp100 (697 Mel, 1498 Mel, 1102 Mel) were recognized by both T cell lines. Control tumors 888 Mel and 697 EBV-B, mismatched for the appropriate restriction element and Ag expression, respectively, were not recognized by either murine CD4+ T cell line.
FIGURE 1.
Murine CD4+ T cells lines recognize intact human melanoma. Line A9895 was generated by vaccinating DR4-IE Tg mice with recombinant h-gp100 protein, followed by serial restimulation with peptide h-gp10046–58 in vitro. Line C1056 was generated by vaccinating DR4-IE Tg mice with peptide h-gp10046–58, followed by serial restimulation with h-gp10046–58 in vitro. A, A9895 line recognizes peptides h-gp10046–58 h-gp10044–59 (100 μM peptides) pulsed onto DR4-IE splenocytes. There was no evidence of reactivity against control peptides HA306–318 and murine-gp10046–58. B, A9895 line recognizes recombinant h-gp100 protein (10 μg) and human melanoma lysate (1300 Mel; known to express h-gp100) pulsed onto DR4-IE splenocytes. There was no evidence of reactivity against the control breast carcinoma lysate MDA-231. T cell lines A9895 (C) and C1056 (D) react with intact DR4+ human melanomas 697 Mel, 1498 Mel, and 1102 Mel. In both cases, control tumors 888 Mel and 697 EBV-B, mismatched for the appropriate restriction element and Ag expression, respectively, were not recognized by either murine CD4+ T cell line. For all experiments, T cells and targets were cocultured for 24 h and supernatants were measured for IFN-γ secretion by ELISA. All experiments were repeated at least three times with similar results using different but comparable targets, thus demonstrating the generalizability of the experimental findings.
Additional characteristics of the murine CD4+ T cell line A9895 were also studied. Line A9895 was found to secrete IFN-γ, IL-2, GM-CSF, TNF-α, but not IL-5, consistent with a Th1 cytokine profile, and, in addition, produced significant amounts of the chemokine RANTES (Fig. 2A). To verify that recognition of melanoma by line A9895 was CD4 directed and HLA-DR restricted, mAbs directed against murine CD4, HLA-DR, and human class I were used to inhibit T cell recognition (Fig. 2B). A human CD8+ T cell clone (K4/H5), previously shown to recognize h-gp100209–217 in the context of HLA-A*0201 (see Materials and Methods), was used to test for nonspecific or deleterious effects of either mAb on target cell interaction. Monoclonal Abs L243 (anti-HLA-DR) and H129.19 (anti-murine CD4) inhibited recognition of 697 Mel and 1102 Mel by line A9895. In the control group, recognition by the CD8+ T cell clone K4/H5 of 697 Mel and 1102 Mel (both HLAA*0201) was inhibited by mAb W6/32 (anti-class I) and unaffected by the mAb directed against murine CD4. A9895 CD4+ T cell line was also tested for cytolytic activity against two h-gp100-expressing melanoma lines (Fig. 2C). Specific lysis of 1102 Mel (DR4+ melanoma) at 61% was observed at an E:T ratio of 30:1, while no evidence of lysis was observed to 888 Mel (DR4− melanoma). To confirm that lysis of 1102 Mel was indeed DR restricted, the mAb L243 (anti-DR) was shown to specifically block cytolysis by line A9895 (data not shown).
FIGURE 2.
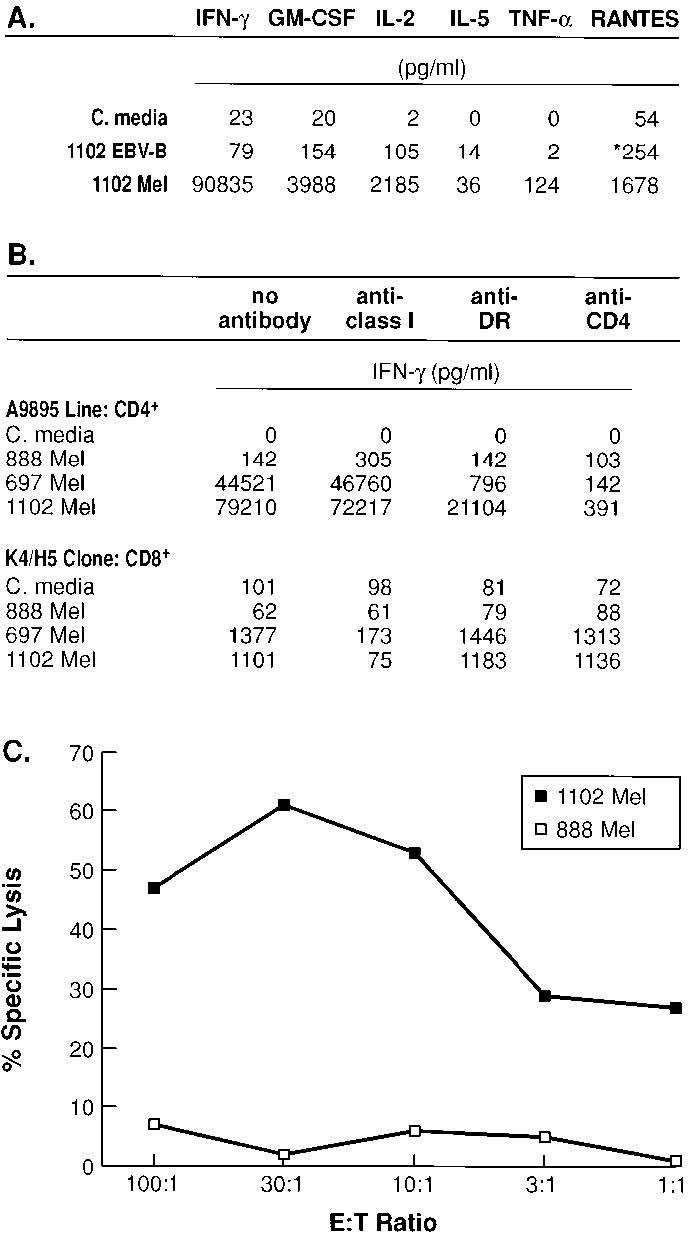
Characterization of CD4+ T cell line A9895. A, CD4+ T cell line A9895 exhibits a Th1-like cytokine profile. Secretion of cytokines IFN-γ, GM-CSF, IL-2, and TNF-α, but not IL-5, is seen against target melanoma line (1102 Mel). There was no reactivity seen against the control target (1102 EBV-B). The line also produces significant amounts of RANTES against melanoma target (1102 Mel), but not against control target (*, 888 Mel). Background reactivity for each cytokine and chemokine (baseline tumor production at 24 h) were subtracted from individual total values, respectively. B, Reactivity of line A9895 is MHC class II restricted. T cells were coincubated with targets in the presence of anti-HLA-A, -B, -C, anti-DR, and anti-murine CD4 mAbs. As a control for mAb blocking, the h-gp100209–217-specific, HLA-A*0201-restricted, human CD8+ T cell clone (K4/H5) was cocultured with identical targets. Recognition of 697 Mel and 1102 Mel by line A9895 was blocked by mAbs anti-DR and anti-CD4, but unaffected by mAb anti-HLA-A, -B, -C. In the control group, mAb anti-HLA-A, -B, -C blocked recognition of 697 Mel and 1102 Mel (both express HLA-A*0201), but was unaffected by mAbs anti-DR and anti-CD4. C, Line A9895 specifically lyses DR4+ human melanoma (1102 Mel), but not control melanoma (888 Mel), in a 15-h 51Cr release assay. For A and B, T cells and targets were cocultured for 24 h and supernatants were measured for individual cytokine and chemokine secretion by ELISA. All experiments were repeated twice with similar results using different but comparable targets, thus demonstrating the generalizability of the experimental findings.
The optimal peptide for T cell recognition is h-gp10044–59
To compare T cell recognition of the algorithm-predicted h-gp10046–58 to the naturally processed h-gp10044–59, 1102 EBV-B cells were pulsed with decreasing concentrations of each peptide (Fig. 3A). The naturally processed 16 amino acid peptide, h-gp10044–59, was capable of inducing significant recognition (>1000 pg/ml of IFN-γ) at concentrations of 0.3 μM or higher. The 13 amino acid peptide h-gp10046–58 required concentrations of 3–10 μM to induce significant reactivity.
FIGURE 3.
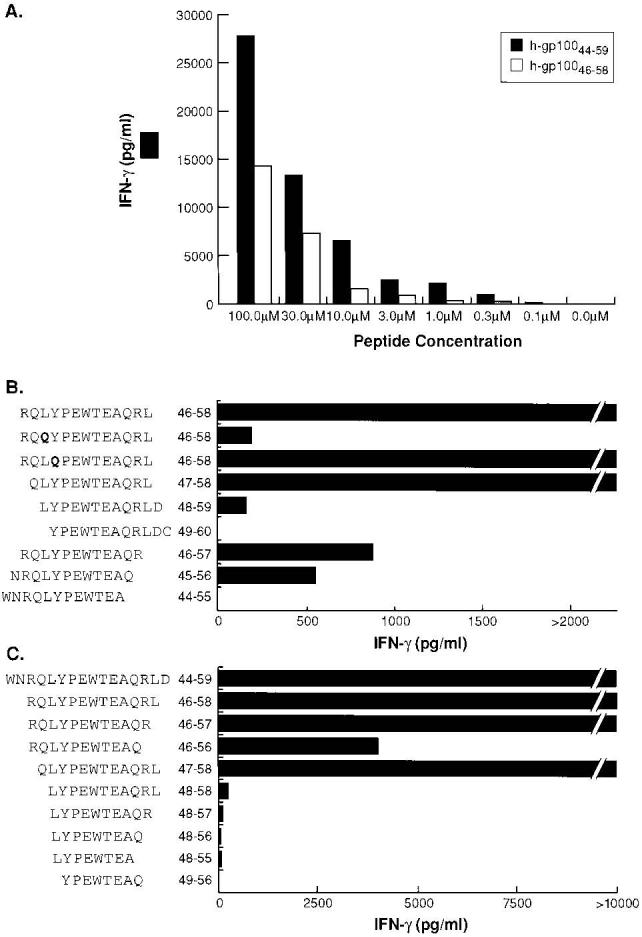
Characterization of the HLA-DRB1*0401 immunodominant epitope for h-gp100. A, The naturally processed 16-aa epitope h-gp10044–59 provides stronger stimulation for the CD4+ T cell line A9895 than the nested variant h-gp10046–58 used in the initial screening of candidate epitopes. Peptide-pulsed 1102 EBV-B cells were prepared at titrating peptide concentrations. B and C, Determinations of the P1 and P9 anchor positions for the DRB1*0401-restricted h-gp100 epitope. 1102 EBV-B cells were pulsed with indicated peptides at 10 μM (B) and 100 μM (C). T cells and targets were cocultured for 24 h and supernatants were measured for IFN-γ secretion by ELISA. All experiments were repeated three times with similar results using different but comparable targets, thus demonstrating the generalizability of the experimental findings.
To determine the P1-P9 anchor positions of h-gp10044–59, a series of truncated and modified peptides of h-gp10046–58 were tested for T cell recognition (Fig. 3B). Optimal P1 binding peptides, characterized by large nonpolar residues, have been based on the x-ray crystal structure of DRB1*0401 complexed with human collagen II1168–1180 (43), as well as sequence motifs based on phage display and synthetic peptide libraries (44-46). A nonconservative substitution involving glutamine (Q) for leucine (L) in position 48 substantially reduced T cell recognition, while the substitution Y49Q had no effect, suggesting L48 as the P1 anchor. Sequential truncations of h-gp10046–58 demonstrated a dramatic step-off in T cell recognition with the loss of L48 (P1) and abrogation of T cell recognition with the removal of Q56 (P9 position). The core nine-residue sequence (LYPEWTEAQ) based on this analysis represents the precise sequence predicted by the computer-assisted DRB1*0401 algorithm (Table I). The importance of amino acids outside the core nine-residue sequence were also determined by altering T cell recognition by making sequential truncations of h-gp10044–59 (Fig. 3C). The removal of glutamine (Q) in position 47 and arginine (R) in position 57 markedly reduced T cell recognition from peptides h-gp10046–56 and h-gp10048–58, respectively.
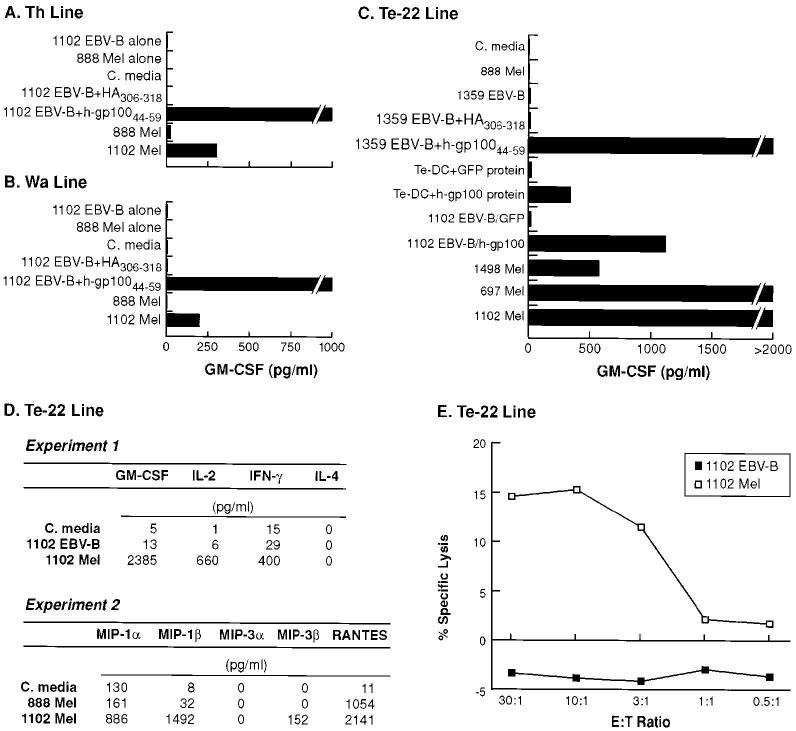
Human lymphocytes sensitized ex vivo to h-gp10044–59 recognize melanoma
The peptides h-gp10044–59 and h-gp10046–58 differ from their murine counterpart at several positions, particularly those centered over putative TCR contact points (P2 and P8 positions; see Table II), and are thus foreign Ags in mice. To test if this self-Ag could be of equivalent immunogenicity in humans, fresh PBMC from three patients with confirmed h-gp100-expressing metastatic melanoma were sensitized ex vivo to h-gp10044–59 (as described above). Bulk CD4+ T cell lines from patients Th, Wa, and line 22 from patient Te (Te-22) were screened for peptide and tumor reactivity. In Fig. 4, A and B, lines from patients Th and Wa are shown to react against DR4+ EBV-B cells (1102 EBV-B) pulsed with peptide h-gp10044–59 and DR4+ intact melanoma line (1102 Mel) expressing h-gp100 endogenously. There was no reactivity detected against control targets 888 Mel (DR4−) and 1102 EBV-B pulsed with the irrelevant peptide (HA306–318). In Fig. 4C, CD4+ T cell line Te-22 is shown to react with DR4+ EBV-B cells pulsed with peptide h-gp10044–59, recombinant h-gp100 protein pulsed onto autologous DCs, DR4+ EBV-B cells transfected with h-gp100, and DR4+ intact melanoma lines expressing h-gp100 endogenously. As before, there was no reactivity against the control peptide (HA306–318), autologous DCs pulsed with the irrelevant recombinant GFP protein, EBV-B cells transfected with GFP, or against the DR4− melanoma line (888 Mel). Importantly, these experiments demonstrate that the epitope h-gp10044–59 can be processed through both exogenous and endogenous pathways and deliberately imitate the design of the first experiment in which the recombinant protein was used to begin the search for CD4+ T cell reactivity with candidate epitopes.
FIGURE 4.
Three different human CD4+ T cell lines from three separate patients recognize human melanoma. A and B, Lines Th and Wa recognize h-gp10044–59 pulsed on DR4+ EBV-B cells and DR4+ intact human melanoma cells expressing h-gp100 endogenously. There was no reactivity detected against control targets 888 Mel (DR4−) and 1102 EBV-B pulsed with the irrelevant peptide (HA306–318). C, Line Te-22 recognizes h-gp10044–59, h-gp100 protein, h-gp100 EBV-B transfectants, and DR4+ intact human melanoma cells. There was no evidence of cytokine release against the irrelevant peptide (HA306–318), irrelevant protein (GFP protein), control transfectant (GFP), and control tumor targets (888 Mel and 1102 EBV-B). Peptides (100 μM) and protein (10 μg/ml) were pulsed onto APCs overnight, and effectors were added at 1:1 ratio (105/well). D, Experiment 1, Line Te-22 displays a Th1 cytokine profile with secretion of IFN-γ, GM-CSF, and IL-2, but not IL-4 against 1102 Mel. D, Experiment 2, Line Te-22 secretes chemokines MIP-1α, MIP-1β, MIP-3β, and RANTES, but not MIP-3α against 1102 Mel. In both experiments, there was minimal cytokine and chemokine release against control tumor targets 1102 EBV-B and 888 Mel, respectively. Background reactivity for each cytokine and chemokine (baseline tumor production at 24 h) were subtracted from individual total values, respectively. E, Line Te-22 specifically lyses 1102 Mel in a 12-h 51Cr release assay. There was no evidence of lysis against control tumor 1102 EBV-B. All experiments were repeated at least twice with similar results using different but comparable targets, thus demonstrating the generalizability of the experimental findings.
To understand the reactivity of line Te-22 in greater detail, its cytolytic activity and cytokine and chemokine profiles were studied. The line displayed a Th1-like cytokine profile, secreting IFN-γ, GM-CSF, IL-2 but not IL-4 (Fig. 4D, experiment 1) and producing significant amounts of chemokines MIP-1α, MIP-1β, MIP-3β, and RANTES (Fig. 4D, experiment 2) when stimulated with the specific Ag (1102 Mel). This pattern of cytokine and chemokine secretion parallel that of the murine CD4+ T cell line A9895 (see Fig. 2A). In a 12-h chromium release assay, lytic activity was observed at titrating E:T ratios with 15.3% specific lysis seen at an E:T of 10:1 (Fig. 4E).
Discussion
We describe in this study the identification and characterization of a shared MHC class II-restricted epitope for h-gp100. Our experiments begin with the immunization of DR4-IE Tg mice with recombinant h-gp100 protein, followed by the screening of candidate epitopes derived from a computer-assisted prediction algorithm for HLA-DRB1*0401. MHC class II Tg mice have been used to identify several candidate autoantigen epitopes (40, 47-49), and DR4-IE Tg mice have been used to identify an HLA-DRB1*0401-restricted autoimmune epitope from LFA-1 in treatment-resistant Lyme disease (47). Other investigators have used A2/Kb Tg mice to identify HLA-A*0201-restricted epitopes for p53 (50, 51). However, this work represents the first example of Tg mice used to identify an MHC class II-restricted epitope capable of inducing tumor-reactive CD4+ T cells.
Although our initial screening of candidate peptides demonstrated reactivity to h-gp10046–58, we elected to study in subsequent experiments the naturally processed epitope h-gp10044–59. This peptide was previously shown by reversed-phase HPLC to be on the surface of DRB1*0401-matched human melanoma after eluting MHC-peptide complexes (41, 42). When compared with h-gp10046–58, the naturally processed, endogenously presented ligand induced nearly 50% greater reactivity from the murine CD4+ T cell line A9895 at a peptide concentration of 100 μM when pulsed onto DRB1*0401-matched EBV-B cells. These results are consistent with the specific binding affinity of peptide h-gp10044–59 to isolated HLA-DRB1*0401 molecules, previously measured at IC50= 7 μM using an in vitro binding and competition assay (41). The ability to detect by reversed-phase HPLC endogenously processed peptides from eluted MHC class II-peptide complexes represents a technically challenging, but potentially useful, method for identifying other immunodominant Ags.
CD4+ T cells generated from DR4-IE Tg mice were found to react against specific peptides and unexpectedly against HLA-DRB1*0401-matched human melanomas. Residues 110 and 139 within the β2 domain of the murine MHC class II molecule have been shown to be critical for optimal interactions with the murine CD4 molecule (52). Despite differences within the β2 domain of human MHC class II, murine CD4+ T cell lines described above were capable of specific xeno-interactions with human melanoma targets. This finding suggested a high-avidity interaction between murine T cell and human tumor, limiting the contribution of species-specific CD4 molecules required for T cell recognition. The ability to test the reactivity of murine CD4+ T cell lines against human melanoma may permit for the potential identification of endogenously presented MHC class II-restricted epitopes derived from other melanoma differentiation Ags (tyrosinase, TRP-1, TRP-2, MART-1, OA1, and P. polypeptide). We are currently investigating these Ags within the context of DR4-IE Tg mice.
Computer-assisted algorithms based on the peptide binding motifs for individual MHC class I alleles have been used to predict tumor-associated epitopes (53, 54). Such an approach is more difficult for MHC class II molecules because they have an “open” peptide-binding grove. The majority of MHC class II molecules bind peptides between 10 and 20 residues in length, with sizes between 13 and 16 amino acids being the most frequently observed (55, 56). Thus, binding epitopes have no definitive amino or carboxyl termini, making prediction of the primary anchor positions more difficult. In the absence of allele-specific epitope forecasts, gene truncations or overlapping peptides covering the full length of the protein were required for the identification of MHC class II-restricted epitopes. Computer-assisted algorithms, such as the one described here, can be used to forecast the most avid binding candidate epitopes for proteins of any length. Indeed, the DRB1*0401 algorithm described by Southwood et al. (35) accurately predicted immunodominant epitopes from pre-proinsulin and OspA in the number 3 and number 1 ranked positions, respectively. In both cases, overlapping peptide libraries were used in the original studies to identify the epitopes described (39, 40).
We sought to link class I- and II-restricted epitopes capable of binding individual MHC alleles with maximal prevalence in patients with metastatic melanoma (HLA-A*0201 and DRB1*0401). The coadministration of immunodominant, MHC class I- and II-restricted epitopes derived from the same Ag could potentially increase the immunogenicity and therapeutic efficacy of CTL through the activation or “conditioning” of a common, intermediary APC (17, 19, 20). Following immunization with the full-length Ag, or specific class I and II epitopes, APCs within draining lymph nodes or the spleen present Ag to cognate CD4+ T cells principally through the interaction of CD40 and its ligand. Activated APCs secrete IL-12, up-regulate costimulatory molecules CD54, CD80, and CD86, and cross-present Ag through MHC class I to cognate CD8+ T cells either within the same draining lymph node basin or in remote locations. The secretion of chemokines such as RANTES, MIP-1α, MIP-1β, and MIP-3β (see Fig. 4D) from activated CD4+ T cells could potentiate the immune response further still through the attraction, amplification, and differentiation of NK cells, monocytes, T cells (CD4+ and CD8+), and DCs (57-59).
Although we were able to detect significant reactivity to peptide h-gp10046–58 during the initial screening of candidate HLA-DRB1*0401 epitopes, more than one potential epitope is likely to exist (see Table I). The detection of cytokine production by ELISA within 24 h after ex vivo stimulation may be ineffective at detecting low-level T cell precursor frequency, a problem potentially circumvented with the addition of the enzyme-linked immunospot techniques, [3H]thymidine proliferation assays, and quantitative RT/PCR assays. However, a more conservative and probably more reliable method would involve serial restimulation with all candidate epitopes. T cell cultures that proliferate can then be tested for specific peptide reactivity as well as the recognition of endogenously processed epitopes.
This simple strategy emphasizes the value of Tg mice and allele-specific epitope forecasts used to identify and characterize candidate MHC class I- and class II-restricted epitopes. In retrospect, we would have arrived at similar results had we initiated our search for T cell reactivity using ex vivo-sensitized human lymphocytes similar to that of investigators who have identified MHC class II-restricted epitopes for MAGE-3 (23, 24). By using DR4-IE Tg mice, we circumvented the need to sensitize human lymphocytes to a large number of peptides, allowed for the naturally processed, immunodominant epitope to be presented in vivo, and established the basis for a workable tumor treatment model. The data from this study has led to the implementation of a clinical trial at the Surgery Branch, National Cancer Institute. Following the coimmunization with immunodominant class I- and class II-restricted epitopes from h-gp100, we plan to evaluate both clinical response as well as the role of Ag-specific T cell help in antitumor immunity.
Acknowledgements
We thank Martha Blalock for assistance with graphics, Donald White for assistance with computer programming, and Dr. Kouichi Ito for providing helpful discussion and criticism.
Footnotes
Abbreviations used in this paper: DC, dendritic cell; h, human; TIL, tumor-infiltrating lymphocytes; CM, culture medium; GFP, green fluorescence protein; Tg, transgenic; MIP, macrophage inflammatory protein.
References
- 1.Boon T, Coulie PG, Van den Eynde B. Tumor antigens recognized by T cells. Immunol. Today. 1997;18:267. doi: 10.1016/s0167-5699(97)80020-5. [DOI] [PubMed] [Google Scholar]
- 2.Restifo NP, Rosenberg SA. Developing recombinant and synthetic vaccines for the treatment of melanoma. Curr. Opin. Oncol. 1999;11:50. doi: 10.1097/00001622-199901000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rosenberg SA, Yang JC, Schwartzentruber DJ, Hwu P, Marincola FM, Topalian SL, Restifo NP, Dudley ME, Schwarz SL, Spiess PJ, et al. Immunologic and therapeutic evaluation of a synthetic peptide vaccine for the treatment of patients with metastatic melanoma. Nat. Med. 1998;4:321. doi: 10.1038/nm0398-321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marchand M, van Baren N, Weynants P, Brichard V, Dreno B, Tessier MH, Rankin E, Parmiani G, Arienti F, Humblet Y, et al. Tumor regressions observed in patients with metastatic melanoma treated with an antigenic peptide encoded by gene MAGE-3 and presented by HLA- A1. Int. J. Cancer. 1999;80:219. doi: 10.1002/(sici)1097-0215(19990118)80:2<219::aid-ijc10>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 5.Nestle FO, Alijagic S, Gilliet M, Sun Y, Grabbe S, Dummer R, Burg G, Schadendorf D. Vaccination of melanoma patients with peptide- or tumor lysate-pulsed dendritic cells. Nat. Med. 1998;4:328. doi: 10.1038/nm0398-328. [DOI] [PubMed] [Google Scholar]
- 6.Pardoll DM, Topalian SL. The role of CD4+ T cell responses in antitumor immunity. Curr. Opin. Immunol. 1998;10:588. doi: 10.1016/s0952-7915(98)80228-8. [DOI] [PubMed] [Google Scholar]
- 7.Parry SL, Hall FC, Olson J, Kamradt T, Sonderstrup G. Auto-reactivity versus autoaggression: a different perspective on human autoantigens. Curr. Opin. Immunol. 1998;10:663. doi: 10.1016/s0952-7915(98)80086-1. [DOI] [PubMed] [Google Scholar]
- 8.Mata M, Paterson Y. Th1 T cell responses to HIV-1 Gag protein delivered by a Listeria monocytogenes vaccine are similar to those induced by endogenous listerial antigens. J. Immunol. 1999;163:1449. [PubMed] [Google Scholar]
- 9.Zajac AJ, Blattman JN, Murali-Krishna K, Sourdive DJ, Suresh M, Altman JD, Ahmed R. Viral immune evasion due to persistence of activated T cells without effector function. J. Exp. Med. 1998;188:2205. doi: 10.1084/jem.188.12.2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goedegebuure PS, Eberlein TJ. The role of CD4+ tumor-infiltrating lymphocytes in human solid tumors. Immunol. Res. 1995;14:119. doi: 10.1007/BF02918172. [DOI] [PubMed] [Google Scholar]
- 11.Maccalli C, Mortarini R, Parmiani G, Anichini A. Multiple sub-sets of CD4+ and CD8+ cytotoxic T-cell clones directed to autologous human melanoma identified by cytokine profiles. Int. J. Cancer. 1994;57:56. doi: 10.1002/ijc.2910570111. [DOI] [PubMed] [Google Scholar]
- 12.Sahin U, Tureci O, Pfreundschuh M. Serological identification of human tumor antigens. Curr. Opin. Immunol. 1997;9:709. doi: 10.1016/s0952-7915(97)80053-2. [DOI] [PubMed] [Google Scholar]
- 12a.Surman DR, Dudley ME, Overwijk WW, Restifo NP. Cutting Edge: CD4+ T cell control of CD8+ T cell reactivity to an immunogenic model tumor antigen. J Immunol. 2000;164:562. doi: 10.4049/jimmunol.164.2.562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ossendorp F, Mengede E, Camps M, Filius R, Melief CJ. Specific T helper cell requirement for optimal induction of cytotoxic T lymphocytes against major histocompatibility complex class II negative tumors. J. Exp. Med. 1998;187:693. doi: 10.1084/jem.187.5.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Matloubian M, Concepcion RJ, Ahmed R. CD4+ T cells are required to sustain CD8+ cytotoxic T-cell responses during chronic viral infection. J. Virol. 1994;68:8056. doi: 10.1128/jvi.68.12.8056-8063.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.von Herrath MG, Yokoyama M, Dockter J, Oldstone MB, Whitton JL. CD4-deficient mice have reduced levels of memory cytotoxic T lymphocytes after immunization and show diminished resistance to subsequent virus challenge. J. Virol. 1996;70:1072. doi: 10.1128/jvi.70.2.1072-1079.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cardin RD, Brooks JW, Sarawar SR, Doherty PC. Progressive loss of CD8+ T cell-mediated control of a γ-herpesvirus in the absence of CD4+ T cells. J. Exp. Med. 1996;184:863. doi: 10.1084/jem.184.3.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bennett SR, Carbone FR, Karamalis F, Flavell RA, Miller JF, Heath WR. Help for cytotoxic-T-cell responses is mediated by CD40 signalling. Nature. 1998;393:478. doi: 10.1038/30996. [DOI] [PubMed] [Google Scholar]
- 18.Bennett SR, Carbone FR, Karamalis F, Miller JF, Heath WR. Induction of a CD8+ cytotoxic T lymphocyte response by cross-priming requires cognate CD4+ T cell help. J. Exp. Med. 1997;186:65. doi: 10.1084/jem.186.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ridge JP, Di Rosa F, Matzinger P. A conditioned dendritic cell can be a temporal bridge between a CD4+ T- helper and a T-killer cell. Nature. 1998;393:474. doi: 10.1038/30989. [DOI] [PubMed] [Google Scholar]
- 20.Schoenberger SP, Toes RE, van der Voort EI, Offringa R, Melief CJ. T-cell help for cytotoxic T lymphocytes is mediated by CD40-CD40L interactions. Nature. 1998;393:480. doi: 10.1038/31002. [DOI] [PubMed] [Google Scholar]
- 21.Pieper R, Christian RE, Gonzales MI, Nishimura MI, Gupta G, Settlage RE, Shabanowitz J, Rosenberg SA, Hunt DF, Topalian SL. Biochemical identification of a mutated human melanoma antigen recognized by CD4+ T cells. J. Exp. Med. 1999;189:757. doi: 10.1084/jem.189.5.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang RF, Wang X, Atwood AC, Topalian SL, Rosenberg SA. Cloning genes encoding MHC class II-restricted antigens: mutated CDC27 as a tumor antigen. Science. 1999;284:1351. doi: 10.1126/science.284.5418.1351. [DOI] [PubMed] [Google Scholar]
- 23.Chaux P, Vantomme V, Stroobant V, Thielemans K, Corthals J, Luiten R, Eggermont AM, Boon T, van der Bruggen P. Identification of MAGE-3 epitopes presented by HLA-DR molecules to CD4+ T lymphocytes. J. Exp. Med. 1999;189:767. doi: 10.1084/jem.189.5.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Manici S, Sturniolo T, Imro MA, Hammer J, Sinigaglia F, Noppen C, Spagnoli G, Mazzi B, Bellone M, Dellabona P, Protti MP. Melanoma cells present a MAGE-3 epitope to CD4+ cytotoxic T cells in association with histocompatibility leukocyte antigen DR11. J. Exp. Med. 1999;189:871. doi: 10.1084/jem.189.5.871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Topalian SL, Gonzales MI, Parkhurst M, Li YF, Southwood S, Sette A, Rosenberg SA, Robbins PF. Melanoma-specific CD4+ T cells recognize nonmutated HLA-DR-restricted tyrosinase epitopes. J. Exp. Med. 1996;183:1965. doi: 10.1084/jem.183.5.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang RF, Wang X, Rosenberg SA. Identification of a novel major histocompatibility complex class II-restricted tumor antigen resulting from a chromosomal rearrangement recognized by CD4+ T cells. J. Exp. Med. 1999;189:1659. doi: 10.1084/jem.189.10.1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cormier JN, Hijazi YM, Abati A, Fetsch P, Bettinotti M, Steinberg SM, Rosenberg SA, Marincola FM. Heterogeneous expression of melanoma-associated antigens and HLA-A2 in metastatic melanoma in vivo. Int. J. Cancer. 1998;75:517. doi: 10.1002/(sici)1097-0215(19980209)75:4<517::aid-ijc5>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 28.Kawakami Y, Eliyahu S, Delgado CH, Robbins PF, Sakaguchi K, Appella E, Yannelli JR, Adema GJ, Miki T, Rosenberg SA. Identification of a human melanoma antigen recognized by tumor-infiltrating lymphocytes associated with in vivo tumor rejection. Proc. Natl. Acad. Sci. USA. 1994;91:6458. doi: 10.1073/pnas.91.14.6458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kawakami Y, Eliyahu S, Jennings C, Sakaguchi K, Kang X, Southwood S, Robbins PF, Sette A, Appella E, Rosenberg SA. Recognition of multiple epitopes in the human melanoma antigen gp100 by tumor-infiltrating T lymphocytes associated with in vivo tumor regression. J. Immunol. 1995;154:3961. [PubMed] [Google Scholar]
- 30.Parkhurst MR, Salgaller ML, Southwood S, Robbins PF, Sette A, Rosenberg SA, Kawakami Y. Improved induction of melanoma-reactive CTL with peptides from the melanoma antigen gp100 modified at HLA-A*0201-binding residues. J. Immunol. 1996;157:2539. [PubMed] [Google Scholar]
- 31.Marincola FM, Shamamian P, Rivoltini L, Salgaller M, Cormier J, Restifo NP, Simonis TB, Venzon D, White DE, Parkinson DR. HLA associations in the antitumor response against malignant melanoma. J. Immunother. Emphasis. Tumor Immunol. 1995;18:242. doi: 10.1097/00002371-199511000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ito K, Bian HJ, Molina M, Han J, Magram J, Saar E, Belunis C, Bolin DR, Arceo R, Campbell R, et al. HLA-DR4-IE chimeric class II transgenic, murine class II-deficient mice are susceptible to experimental allergic encephalomyelitis. J. Exp. Med. 1996;183:2635. doi: 10.1084/jem.183.6.2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wakeland E, Morel L, Achey K, Yui M, Longmate J. Speed congenics: a classic technique in the fast lane (relatively speaking) Immunol. Today. 1997;18:472. doi: 10.1016/s0167-5699(97)01126-2. [DOI] [PubMed] [Google Scholar]
- 34.Topalian SL, Rivoltini L, Mancini M, Ng J, Hartzman RJ, Rosenberg SA. Melanoma-specific CD4+ T lymphocytes recognize human melanoma antigens processed and presented by Epstein-Barr virus-transformed B cells. Int. J. Cancer. 1994;58:69. doi: 10.1002/ijc.2910580113. [DOI] [PubMed] [Google Scholar]
- 35.Southwood S, Sidney J, Kondo A, del Guercio MF, Appella E, Hoffman S, Kubo RT, Chesnut RW, Grey HM, Sette A. Several common HLA-DR types share largely overlapping peptide binding repertoires. J. Immunol. 1998;160:3363. [PubMed] [Google Scholar]
- 36.Tanaka Y, Ogawa M, Nishimura Y, Matsushita S. Efficient induction of human CD4+ T cell lines reactive with a self-K- ras-derived peptide in vitro, using a mAb to CD29. Hum. Immunol. 1998;59:343. doi: 10.1016/s0198-8859(98)00031-7. [DOI] [PubMed] [Google Scholar]
- 37.Overwijk WW, Tsung A, Irvine KR, Parkhurst MR, Goletz TJ, Tsung K, Carroll MW, Liu C, Moss B, Rosenberg SA, Restifo NP. gp100/pmel 17 is a murine tumor rejection antigen: induction of “self”-reactive, tumoricidal T cells using high-affinity, altered peptide ligand. J. Exp. Med. 1998;188:277. doi: 10.1084/jem.188.2.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Topalian SL, Rivoltini L, Mancini M, Markus NR, Robbins PF, Kawakami Y, Rosenberg SA. Human CD4+ T cells specifically recognize a shared melanoma-associated antigen encoded by the tyrosinase gene. Proc. Natl. Acad. Sci. USA. 1994;91:9461. doi: 10.1073/pnas.91.20.9461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Congia M, Patel S, Cope AP, De Virgiliis S, Sonderstrup G. T cell epitopes of insulin defined in HLA-DR4 transgenic mice are derived from pre-proinsulin and proinsulin. Proc. Natl. Acad. Sci. USA. 1998;95:3833. doi: 10.1073/pnas.95.7.3833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gross DM, Forsthuber T, Tary-Lehmann M, Etling C, Ito K, Nagy ZA, Field JA, Steere AC, Huber BT. Identification of LFA-1 as a candidate autoantigen in treatment-resistant Lyme arthritis. Science. 1998;281:703. doi: 10.1126/science.281.5377.703. [DOI] [PubMed] [Google Scholar]
- 41.Halder T, Pawelec G, Kirkin AF, Zeuthen J, Meyer HE, Kun L, Kalbacher H. Isolation of novel HLA-DR restricted potential tumor-associated antigens from the melanoma cell line FM3. Cancer Res. 1997;57:3238. [PubMed] [Google Scholar]
- 42.Li K, Adibzadeh M, Halder T, Kalbacher H, Heinzel S, Muller C, Zeuthen J, Pawelec G. Tumour-specific MHC-class-II-restricted responses after in vitro sensitization to synthetic peptides corresponding to gp100 and Annexin II eluted from melanoma cells. Cancer Immunol. Immunother. 1998;47:32. doi: 10.1007/s002620050501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dessen A, Lawrence CM, Cupo S, Zaller DM, Wiley DC. X-ray crystal structure of HLA-DR4 (DRA*0101, DRB1*0401) complexed with a peptide from human collagen II. Immunity. 1997;7:473. doi: 10.1016/s1074-7613(00)80369-6. [DOI] [PubMed] [Google Scholar]
- 44.Hammer J, Bono E, Gallazzi F, Belunis C, Nagy Z, Sinigaglia F. Precise prediction of major histocompatibility complex class II-peptide interaction based on peptide side chain scanning. J. Exp. Med. 1994;180:2353. doi: 10.1084/jem.180.6.2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hammer J, Belunis C, Bolin D, Papadopoulos J, Walsky R, Higelin J, Danho W, Sinigaglia F, Nagy ZA. High-affinity binding of short peptides to major histocompatibility complex class II molecules by anchor combinations. Proc. Natl. Acad. Sci. USA. 1994;91:4456. doi: 10.1073/pnas.91.10.4456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hammer J, Valsasnini P, Tolba K, Bolin D, Higelin J, Takacs B, Sinigaglia F. Promiscuous and allele-specific anchors in HLA-DR-binding peptides. Cell. 1993;74:197. doi: 10.1016/0092-8674(93)90306-b. [DOI] [PubMed] [Google Scholar]
- 47.Sonderstrup G, McDevitt H. Identification of autoantigen epitopes in MHC class II transgenic mice. Immunol. Rev. 1998;164:129. doi: 10.1111/j.1600-065x.1998.tb01215.x. [DOI] [PubMed] [Google Scholar]
- 48.Andersson EC, Hansen BE, Jacobsen H, Madsen LS, Andersen CB, Engberg J, Rothbard JB, McDevitt GS, Malmstrom V, Holmdahl R, et al. Definition of MHC and T cell receptor contacts in the HLA-DR4-restricted immunodominant epitope in type II collagen and characterization of collagen-induced arthritis in HLA-DR4 and human CD4 transgenic mice. Proc. Natl. Acad. Sci. USA. 1998;95:7574. doi: 10.1073/pnas.95.13.7574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rosloniec EF, Brand DD, Myers LK, Esaki Y, Whittington KB, Zaller DM, Woods A, Stuart JM, Kang AH. Induction of autoimmune arthritis in HLA-DR4 (DRB1*0401) transgenic mice by immunization with human and bovine type II collagen. J. Immunol. 1998;160:2573. [PubMed] [Google Scholar]
- 50.Theobald M, Biggs J, Hernandez J, Lustgarten J, Labadie C, Sherman LA. Tolerance to p53 by A2.1-restricted cytotoxic T lymphocytes. J. Exp. Med. 1997;185:833. doi: 10.1084/jem.185.5.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Theobald M, Biggs J, Dittmer D, Levine AJ, Sherman LA. Targeting p53 as a general tumor antigen. Proc. Natl. Acad. Sci. USA. 1995;92:11993. doi: 10.1073/pnas.92.26.11993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pan S, Trejo T, Hansen J, Smart M, David CS. HLA-DR4 (DRB1*0401) transgenic mice expressing an altered CD4-binding site: specificity and magnitude of DR4-restricted T cell response. J. Immunol. 1998;161:2925. [PubMed] [Google Scholar]
- 53.Vonderheide RH, Hahn WC, Schultze JL, Nadler LM. The telomerase catalytic subunit is a widely expressed tumor-associated antigen recognized by cytotoxic T lymphocytes. Immunity. 1999;10:673. doi: 10.1016/s1074-7613(00)80066-7. [DOI] [PubMed] [Google Scholar]
- 54.Parkhurst MR, Fitzgerald EB, Southwood S, Sette A, Rosenberg SA, Kawakami Y. Identification of a shared HLA-A*0201-restricted T-cell epitope from the melanoma antigen tyrosinase-related protein 2 (TRP2) Cancer Res. 1998;58:4895. [PubMed] [Google Scholar]
- 55.Rudensky AY, Preston-Hurlburt P, Hong SC, Barlow A, Janeway CA., Jr. Sequence analysis of peptides bound to MHC class II molecules. Nature. 1991;353:622. [Google Scholar]
- 56.Chicz RM, Urban RG, Lane WS, Gorga JC, Stern LJ, Vignali DA, Strominger JL. Predominant naturally processed peptides bound to HLA-DR1 are derived from MHC-related molecules and are heterogeneous in size. Nature. 1992;358:764. doi: 10.1038/358764a0. [DOI] [PubMed] [Google Scholar]
- 57.Zlotnik A, Morales J, Hedrick JA. Recent advances in chemokines and chemokine receptors. Crit. Rev. Immunol. 1999;19:1. [PubMed] [Google Scholar]
- 58.Dieu MC, Vanbervliet B, Vicari A, Bridon JM, Oldham E, Ait-Yahia S, Briere F, Zlotnik A, Lebecque S, Caux C. Selective recruitment of immature and mature dendritic cells by distinct chemokines expressed in different anatomic sites. J. Exp. Med. 1998;188:373. doi: 10.1084/jem.188.2.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rollins BJ. Chemokines. Blood. 1997;90:909. [PubMed] [Google Scholar]