Abstract
Glia are required for axon pathfinding along longitudinal trajectories, but it is unknown how this relates to the molecular paradigm of axon guidance across the midline. Most interneuron axons in bilateral organisms cross the midline only once. Preventing them from recrossing the midline requires the expression of Robo receptors on the axons. These sense the repulsive signal Slit, which is produced by the midline. The lateral positioning of longitudinal axons depends on the response to Slit by the combination of Robo receptors expressed by the axons, on selective fasciculation, and on longitudinal (lateral) glia. Here, we analyse how longitudinal glia influence reading of the ‘Robo code’ by axons. We show that whereas loss of robo1 alone only affects the most medial axons, loss of both glial cells missing (gcm) and robo1 causes a severe midline collapse of longitudinal axons, similar to that caused by the loss of multiple Robo receptors. Furthermore, whereas ectopic expression of robo2 is sufficient to displace the medial MP2 axons along a more lateral trajectory, this does not occur in gcm-robo1 double-mutant embryos, where axons either do not extend at all or they misroute exiting the CNS. Hence, lateral neuron-glia interactions steer the response of axons to the Robo code.
Keywords: Glia, axon guidance, robo, gcm, neuron-glia interactions
INTRODUCTION
The ‘decision’ of growth cones to cross the midline and never to recross it is a successful paradigm for the molecular dissection of growth-cone guidance (Bagri et al., 2002; Culverwell and Karlstrom, 2002; Dickson, 2002; Guthrie, 2001; Tear, 1999a; Tear, 1999b). Similar molecular mechanisms operate in other contexts in which either axons or cells navigate and migrate towards a target (Englund et al., 2002; Gilthorpe et al., 2002; Kinrade et al., 2001; Kramer et al., 2001; Wong et al., 2002). Coordination of left-right movement in all bilateral organisms requires that interneuron axons linking the motor systems with the brain cross the midline once only. The midline is the source of a repulsive diffusible signal that is encoded by slit. The Slit gradient is read by axons as they upregulate the expression of a combination of Robo receptors on crossing the midline (Ba-Charvet et al., 1999; Battye et al., 1999; Brose et al., 1999; Kidd et al., 1999; Kidd et al., 1998a; Kidd et al., 1998b; Rajagopalan et al., 2000; Simpson et al., 2000a; Simpson et al., 2000b). The expression by a neuron of a particular combination of robo1, robo2 and robo3 receptors determines the lateral position of its axon in response to different thresholds of the Slit gradient (Rajagopalan et al., 2000; Simpson et al., 2000a; Simpson et al., 2000b). Thus, axons that contain Robo1, Robo2 and Robo3 will project further away from the midline than those with Robo1 and Robo3, and, in turn, these will be further from the midline than axons with only Robo1.
Robo receptors are also thought to be involved in fasciculation (Rajagopalan et al., 2000; Simpson et al., 2000a; Simpson et al., 2000b). As an interneuron axon extends longitudinally, it ‘chooses’ which trajectory to follow, a process known as ‘selective fasciculation’ (Raper et al., 1984). Accordingly, an axon might follow a more lateral or medial trajectory, depending on selective fasciculation, which is dictated by the combination of Robo receptors expressed and other cell-adhesion molecules that are present in the axons, such as FasII (Rajagopalan et al., 2000; Simpson et al., 2000a; Simpson et al., 2000b). The intracellular domains of the different Robo receptors differ and Robo receptors can form homodimers (Hivert et al., 2002). Conceivably, Robos might also interact with other cell-surface molecules.
The ability of Robo receptors to determine axonal positioning has been demonstrated by ectopic expression of Robo2 and Robo3 in axons that project medially in normal embryos, which causes the lateral displacement of these axons (Rajagopalan et al., 2000; Simpson et al., 2000a). Normally, Robo2 is expressed over the lateral FasII fascicle, in the axons that project furthest away from the midline (Rajagopalan et al., 2000; Simpson et al., 2000a). The dMP2 and vMP2 axons at the end of embryogenesis normally project along the first, medial fascicle closest to the midline (Hidalgo and Brand, 1997). Ectopic expression of Robo2 in the MP2 axons displaces their trajectory laterally, to the central fascicle (Simpson et al., 2000a). However, ectopic expression of Robo2 does not displace MP2 axons sufficiently far from the midline to reach the domain of axons that express Robo2 endogenously. Although this might reflect either insufficient Robo2 levels, because increasing Robo2 concentration shifts the lateral displacement of other axons (Rajagopalan et al., 2000), or the need for these axons to also express robo3 in order to reach the most lateral domain, it might also indicate that other factors restrict the lateral positioning of axons.
Glia are necessary for axon guidance and axonal fasciculation (Bastiani et al., 1986; Hidalgo and Booth, 2000; Poeck et al., 2001; Rangarajan et al., 1999; Sepp and Auld, 2003; Sepp et al., 2001; Silver et al., 1982). Longitudinal glia (LG) overlie the longitudinal axons of the ventral nerve cord of the Drosophila embryo, whereas midline glia enwrap the commissural portion of the axons as they cross the midline. LG occupy positions where growth cones ‘make steering decisions’ during growth cone guidance (called choice points); they trigger fasciculation and defasciculation events; and they maintain neuronal survival and the integrity of the neuropile (Booth et al., 2000; Hidalgo and Booth, 2000). The major axonal fascicles along which the different robo-expressing axons extend are formed by interactions between the LG and the pioneer axons (Hidalgo and Booth, 2000). Multiple inputs, such as cell-survival interactions and cell contact between axons and glia confine axonal trajectories regardless of the presence of the Robo1 receptor on axons (Kinrade et al., 2001). In fact, when lateral neuron-glia interactions are disrupted, axons misroute across the midline despite the fact that they express robo1 and that the midline still produces Slit. This shows that interactions between neurons and glia have a crucial role in lateral positioning of axons that is independent of the Slit gradient. Here, we ask whether LG can influence the reading of the Robo code by axons.
Slit and Robo1 are involved in glial migration before axon guidance (Kinrade et al., 2001). LG migrate in two distinct phases (Hidalgo and Booth, 2000; Kinrade et al., 2001): in the first, neuron-independent, phase, the LG migrate medially from their site of origin at the edge of the neuroectoderm towards the midline, until they contact the cell bodies of the pioneer neurons (medial migration); in the second phase (during axon guidance), the LG migrate longitudinally, along the antero-posterior axis in close contact with extending pioneer growth cones (longitudinal migration). Longitudinal migration correlates with the movements of axons (Hidalgo and Booth, 2000) and if normal axonal patterns are disrupted LG will migrate to retain axonal contact (Kinrade et al., 2001). Medial migration takes place within domains of robo1-expressing cells. The glia stop migrating at a fixed distance from the midline as a consequence of Slit signalling, which coincides with the moment in which the LG contact the pioneer neuron cell bodies. In fact, LG severely collapse over the midline in slit mutants, and overexpression of slit in neurons can prevent the medial migration of LG (Kinrade et al., 2001). The expression of robo1 then declines in the LG, which do not express robo1 during longitudinal migration. Thus, during axon guidance, LG migration does not require Robo1. However, in many cases, Slits are required for cell migration (Englund et al., 2002; Gilthorpe et al., 2002; Kramer et al., 2001; Wong et al., 2002). Thus, it is possible that Robo2 and Robo3 influence glial migration during axon guidance.
Taking into account the Robo code and that neuron-glia interactions are required for axon guidance, fasciculation and lateral axonal positioning, there are two conceivable mechanisms organising longitudinal axonal patterns: (1) both axons and glia respond to the Slit gradient, LG produce Robo receptors and, thus, Slit is the overall organiser of the CNS neuropile. In this case, LG influence axon guidance but they might not influence the reading of the Robo code by axons. (2) Only axons respond to Slit and glial positioning follows other cues. In this later case, neuron-glia interactions constitute a separate input to which axons respond and LG might influence the output of the Robo code.
OBJECTIVE
Here, we ask whether LG influence the reading of the Robo code by axons. To approach this question we first asked whether LG might themselves respond directly to the Robo code. Hence, we tested whether the LG express robo2 and/or robo3 during axon guidance. Since the glia do not express robo2 and robo3 but they are involved in axon guidance, we next asked whether the absence of functional glial cells might alter the lateral displacements normally observed in conditions of loss and gain of function of Robo receptors. For this we looked at axonal patterns in gcm-robo1 double-mutant embryos and at the effects of expressing robo2 in medial neurons in these embryos.
METHODS
Flies
The following fly stocks were used: (1) gcmΔP1/CyOlacZ; (2) roboZ14/CyOlacZ; (3) robo28/Cyo wglacZ; (4) robo31/CyolacZ; (5) F263 (LG-lacZ reporter) (Jacobs et al., 1989); (6) 15J2 (MP2 GAL4); (7) UASrobo2; (8) UASGAPGFP. The following stocks were generated by conventional genetics: (1) gcmΔP1 roboZ14/CyOlacZ; (2) gcmDP1/CyO; UASGAPGFP; (3) gcmΔP1/CyO; 15J2; (4) gcmΔP1 roboZ14/CyOlacZ; 15J2; (5) gcmΔP1 roboZ14/CyOlacZ; UASGAPGFP; (6) gcmΔP1 roboZ14/CyOlacZ; UASrobo2-myc; (7) roboZ14/CyOlacZ; 15J2; (8) roboZ14/CyOlacZ; UASGAPGFP; (9) roboZ14/CyOlacZ; UASrobo2. Mutant embryos were identified by staining the reporter-lacZ marked balancers with anti-Bgal antibodies.
Immunohystochemistry
Antibody stainings were performed following a standard protocol (Patel, 1994). Antibodies were used in the following dilutions for fluorescence detection (for HRP detection, primary antibody concentrations were halved): rabbit anti-Bgal (Cappel), 1:2500; rabbit anti-Repo, 1:100; mouse anti-Repo, 1:10; mouse FasII, 1:2; mouse anti-Myc (9E10, Iowa Hybridoma Bank), 1:10; rabbit anti-GFP (AbCam), 1:250; mouse anti-Robo2 (HRP, Iowa), 1:500; mouse anti-Robo3 (HRP), 1:10; mouse 22c10, 1:5; mouse anti-Slit (HRP), 1:10. As secondary antibodies we used biotinylated antibodies at 1:300 (Jackson and Vector Labs) followed by streptavidin Alexa 488 and Alexa 546 at 1:250 or the Elite kit (Vector Labs), or directly conjugated to Alexa 488 and Alexa 546 at 1:250 (Molecular Probes). Anti-Myc was detected using the Tyramide Signal Amplification kit (PerkinElmer).
Microscopy and imaging
All fluorescence microscopy used a BioRad Radiance 2000 laser scanning confocal microscope. 3D imaging used Volocity (Improvision).
RESULTS
LG do not require the Robo code for longitudinal migration
To investigate whether LG respond to the Robo code, we looked at glial migration in the absence of signalling by Robo1, Robo2 and Robo3. We find that glial migration follows the positions of axons in all genetic backgrounds (Fig. 1). In robo1 mutants, the medial axonal fascicle misroutes across the midline, whereas the intermediate and lateral fascicles extend laterally: some LG migrate over the midline whereas some retain their lateral positions (Fig. 1B, 100% penetrance, n=5 embryos). In robo3 mutants, the medial and intermediate axonal fascicles fuse, some LG line up over the fused fascicles, whereas some remain laterally together with remaining lateral axons (Fig. 1C, 100% penetrance, n=9 embryos). In robo2 mutants, the axons are defasciculated and the LG are disorganised, but the phenotype is rather weak (Fig. 1D, 26%, penetrance, n=96 hemisegments) and the glia do not migrate abnormally closer to the midline. Because LG migration is affected in both robo1 and robo3 mutants, LG migration might depend on either the Robo code or on contact with axons.
Fig. 1.
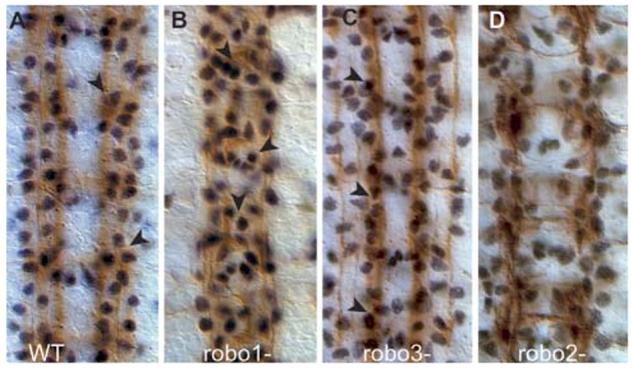
LG are misplaced in Robo-mutant embryos. Embryos stained with the glial nuclear anti-Repo (black) and neuronal FasII (brown) antibodies at stage 16. (A) In wild-type embryos glia (arrowheads) are deployed in discrete rows over the three FasII fascicles. (B) In robo1-mutant embryos, some glia migrate over the midline (arrowheads) and some remain over the lateral axons. (C) In robo3-mutant embryos, many glia are displaced medially and they overlie the fascicle formed by the fused medial and central fascicle (arrowheads). (D) In robo2 mutants the lateral FasII fascicle and glial patterns are only slightly disorganised. Anterior is to the top.
We already know that LG do not express robo1 at this stage (Kinrade et al., 2001), which indicates that longitudinal migration of LG requires contact with axons. To verify whether robo2 and robo3 are expressed in the LG during axon guidance, we looked at their distribution with anti-Robo2 and anti-Robo3 antibodies and visualised the LG with either the nuclear glial marker anti-Repo or anti-βgal in embryos bearing the LG-lacZ reporter (Fig. 2) (Jacobs et al., 1989). Robo2 is expressed at low levels from the end of stage 12. At this stage, the Robo2 signal that appears to surround LG cells is erratic (Fig. 2A,B) and more ventral than that of Repo in the LG (Fig. 2A,G). From stage 13/14 on, the Robo2 signal is more ventral (Fig. 2C) than the LG (Fig. 2D), and a lateral view shows that Robo2 signal is physically separated from the LG nuclei, which lie dorsally (Fig. 2H). At stage 16 and 17, the Robo2 signal appears to partially surround the LG from a frontal view (Fig. 2I), but from a transverse view it is clear that Repo+ LG lie dorsally over the Robo2 axons (Fig. 2J). These temporal profiles show that Robo2 is not expressed in the LG during axon guidance.
Fig. 2.
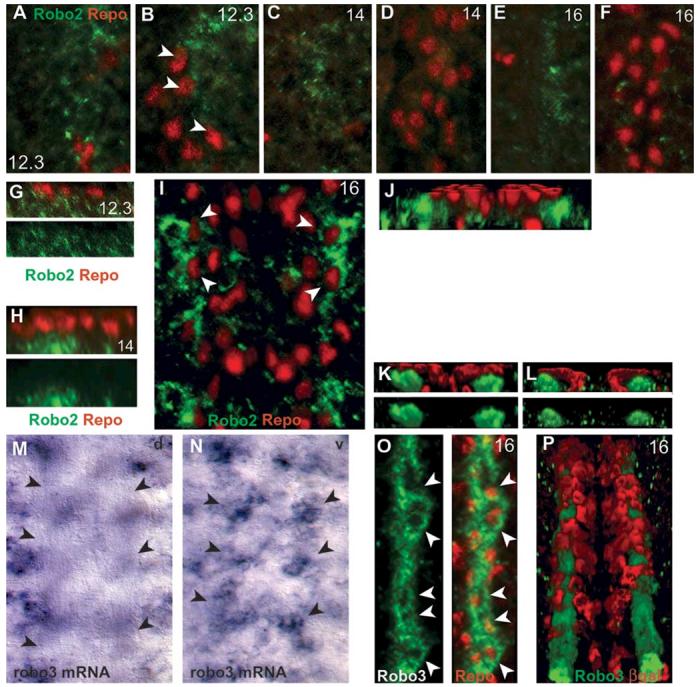
Robo2 and Robo3 are not expressed in LG. LG nuclei are visualised in wild-type embryos with the glial nuclear marker anti-Repo (red) (A-K,O), and LG cytoplasm with anti-βgal on the LG-lacZ reporter (red) (L,P). (A-J) Temporal profile of Robo2 distribution (green) in the ventral nerve cord. Ventral (A) and dorsal (B) longitudinal and (G) sagittal sections of the same stage-12.3 embryo. Robo2 is first detected at stage 12.3 lateral to the LG and mostly in a ventral plane under the LG. Some LG nuclei appear partly surrounded by Robo2 signal (arrowheads in B), but in a transverse view (G) the glial nuclei overlie Robo2 signal. At stage 14, from a frontal view Robo2 signal is ventral (C) and physically separate from the dorsal, Repo-positive LG nuclei (D,H). (G,H) Single channel images of anti-Robo2 are also shown (green). (E,F,I) At stage 16, Robo2 is lateral to the LG but it appears to surround some Repo-positive LG nuclei (arrowheads in I), however these are in separate planes: ventral Robo2 (E); dorsal Repo glia (F); and a whole-stack projection (I). (J) A tilted, 3D-reconstruction section of the same embryonic stack as in (I), showing that Robo2 lies ventrally and laterally to the LG. (M,N) Distribution of robo3 transcripts by in situ hybridisation with DIG probes. (M) There is no signal in the dorsal plane of the neuropile, axons are seen clear of signal by Nomarski optics (arrows). (N) All DIG signal is in a ventral plane (arrows) below the neuropile. (K,L,O,P) Distribution of anti-Robo3 (green) and either Repo or anti-βgal (red) in the LG at stage 16. (O,P) From a frontal view, Robo3 appears to surround the nuclei of LG (arrowheads, whole-stack projections). (O) Single channel image shown in green next to merged image. (K) 3D transverse view of the embryo in (O) reveals that the glial nuclei are dorsal to and separated from the Robo3 axons (bottom, single channel view of Robo3, green; top, merge). (P) LG are visualised with anti-βgal (red) using the LG-lacZ reporter overlie the Robo3 axons (tilted 3D reconstruction, whole stack). (L) 3D transverse view of the same embryo as in (P) revealing that the βgal glial signal overlies the Robo3 axonal bundles, but it is not coincident with them. All images were processed with Volocity (Improvision). Anterior is up. A-F,G,H show one hemisegment; O just over one hemisegment; the midline is to the left. I shows one segment; M,N, 4 segments; P, 5 segments; G,H, lateral sections; J,K,L, cross-section views. A-F are projections of sections restricted to or excluding glial nuclei; I,O,P are projections of the whole stack of sections through the neuropile.
Robo3 is expressed first at stage 15, and expression is maintained in the same pattern to the end of embryogenesis. By in situ hybridisation, we find that robo3 transcripts are not located in the dorsal plane of the neuropile (Fig. 2M) but in a ventral plane (Fig. 2N). Thus, Robo3 transcripts are not in the plane along which LG are located, implying that robo3 is not expressed in the LG. Fluorescent imaging of Robo3 together with the glial markers reveals apparent colocalization in the LG when the whole stack is projected onto one single image. However, this is deceiving. From a frontal view, the domains of Robo3 include the LG, and they surround nuclei that express Repo (Fig. 2O) but, again, the axonal staining of Robo3 is ventral and physically separated from the overlying Repo signal in LG (Fig. 2K). When LG are visualised with the lacZ reporter and anti-βgal antibodies, the cytoplasmic projections of the LG are seen to enwrap, but not mix with, the axonal bundles that express Robo3 (Fig. 2P), which can also be seen in transverse sections (Fig. 2L). Thus, Robo3 is not expressed in the LG.
To further test that glia do not express robo2 and robo3, we checked whether they are expressed in embryos that lack functional glia in glial cells missing (gcm) mutants. We found that at stage 13 robo2 is expressed in clusters in the same antero-posterior location as the LG (Fig. 3A). In gcm mutants (Fig. 3D), Robo2 signal is still present in all hemisegments, confirming that at stage 12 robo2 expression is neuronal. Nevertheless, there is a slight decrease in Robo2 signal in these embryos, which could be caused by loss of neurons through apoptosis in the absence of glia (Booth et al., 2000). Later, at stage 16, robo2 is still expressed in clusters in gcm embryos (Fig. 3B,E). However it is absent from the axons, which is consistent with the fact that axons cannot extend in the absence of glia, and with an increase in neuronal death (Booth et al., 2000; Hidalgo and Booth, 2000). Robo3 expression starts later, at stage 15 and the pattern does not change for the remainder of embryogenesis (Fig. 3C). Robo3 is still present in clusters in gcm-mutant embryos (Fig. 3F). However, as with robo2, signal is lost along the axonal tracts. Interestingly, we do not see overexpression of either robo2 or robo3 in gcm-mutant embryos, in which glia are presumed to adopt a neuronal fate. These data confirm that expression of robo2 and robo3 is restricted to neurons.
Fig. 3.
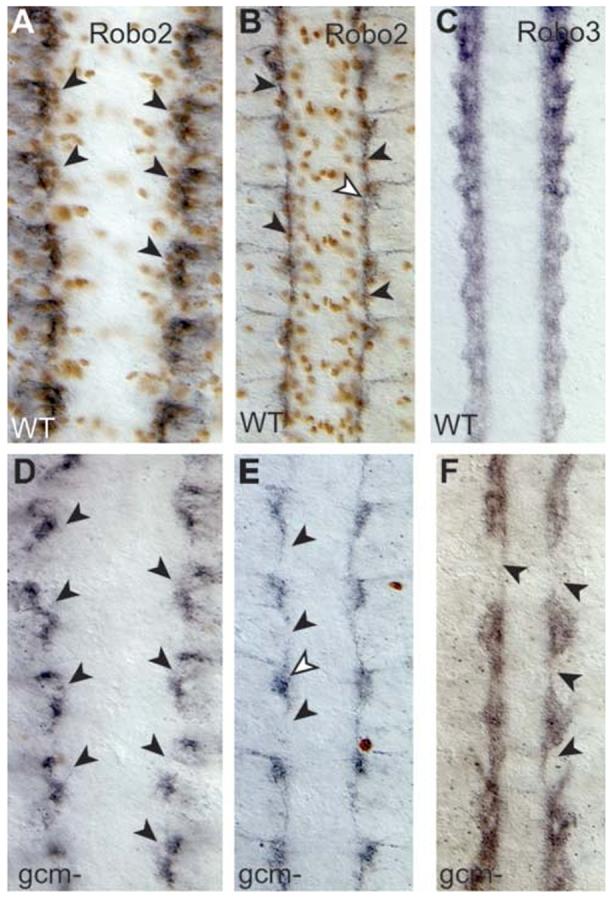
Robo2 and Robo3 in the absence of glia. Anti-Robo2 (black) and anti-Repo (brown) in wild-type embryos (A,B) and gcm mutant (D,E) embryos. Anti-Robo3 in wild-type (C) and gcm mutant (F) embryos. At stage 13 in wild-type embryos, Robo2 clusters lie under the LG (A). Robo2 is present but disarranged in gcm mutants (D). At stage 16, Robo2 longitudinal axons are frequently missing in gcm mutants (E, arrowheads), but Robo2 signal (white arrowhead) is present at the base of the commissures. (F) Robo3 is still present in gcm mutants, but the longitudinal projection of axons is severely compromised (arrowheads). Anterior is up.
Because robo2 and robo3 are not expressed in the LG, longitudinal migration of LG does not rely on the integration of the Robo code cell-autonomously in the glia. Glia are always displaced following axons in the robo1, robo2 and robo3-mutant embryos, which indicates that glial migration depends on interactions with axons and on other molecular mechanisms.
Loss of glia induces midline collapse in loss-of-function robo1 mutants
We wondered whether, reciprocally, glial-cell function might explain the different effects of robo1 mutations on axonal trajectories. In robo1 mutants, only the medial FasII fascicle misroutes over the midline; the outermost fascicles extend normally, parallel to the midline (Kidd et al., 1998a) (Fig. 4B). Double-mutant robo2-robo1 embryos have a dramatic midline collapse of the outer fascicles as well, supporting the notion that multiple Robo receptors keep axons away from the midline (Simpson et al., 2000a; Simpson et al., 2000b). We wondered if LG might also help the lateral fascicles retain their positions in robo1 mutants.
Fig. 4.
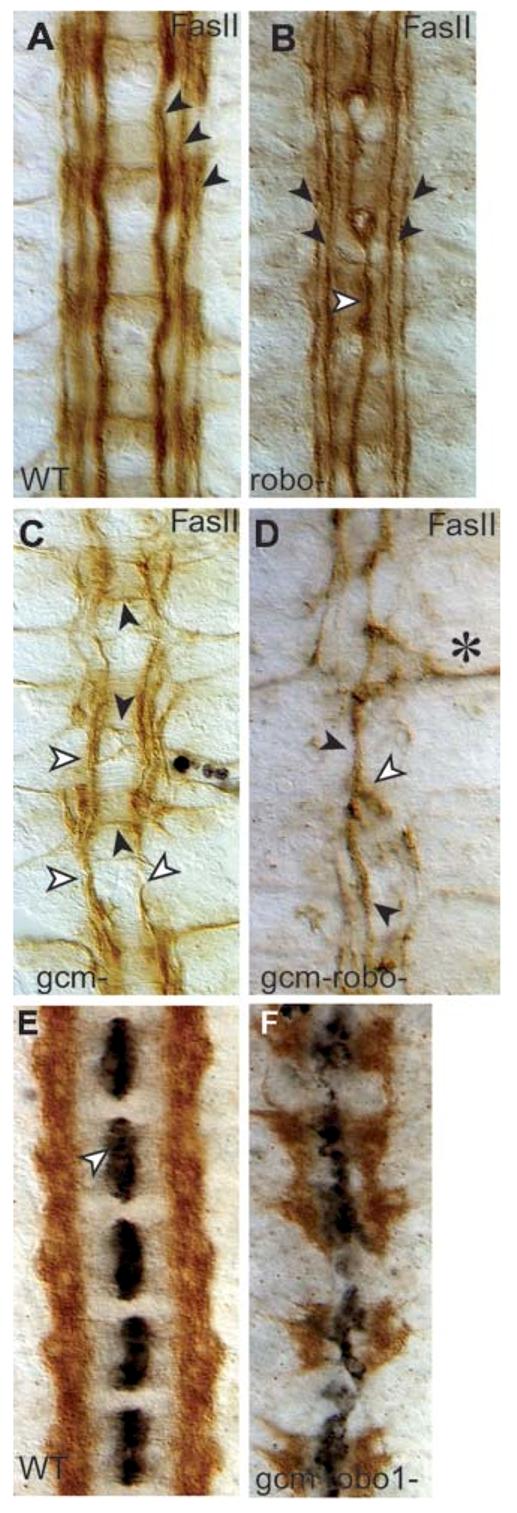
Midline collapse of longitudinal axons in gcm-robo1 double-mutant embryos. Axons are visualised with anti-FasII antibodies (brown) at stage 16 (A-D). (A) Wild-type embryo. (B) robo1 mutant. Note that the medial fascicle runs along the midline (white arrowhead) whereas the outer two fascicles are well formed and run parallel to the midline (black arrowheads). (C) In gcm mutants, axons sporadically misroute across the midline (black arrowheads) and the lateral fascicles can be fused into one that runs parallel to the midline along the central trajectory (white arrowheads). This embryo has also been stained with anti-Repo (in black, there are a few glia present). (D) In gcm-robo1 double-mutant embryos, axons can fuse (white arrowhead) into one single fascicle running along the midline (black arrowhead) or exit the CNS (asterisk) and grow towards the muscle, but no axons run along the lateral trajectories. (E,F) Axons are stained with anti-Robo3 (brown) and midline glia with anti-Slit (black) at stage 16. (E) Robo3 axons never cross the midline in wild-type embryos, and anti-Slit stains the midline glia in each segment. (F) gcm-robo1 double mutant. Slit (black) is still produced by the midline glia, although the midline glia are disorganised. No longitudinal axons remain, instead axons cross the midline despite expressing robo3. Anterior is up.
We looked at the FasII fascicles in robo1 mutants that also lack normal glial function, that is, gcm-robo1 double-mutant embryos (Fig. 4). To our surprise, in gcm-robo1 double-mutant embryos all fascicles can collapse along the midline (Fig. 4D), with full collapse observed in 12.5% of cases (n=144 segments) and collapse from one side of the embryo in 22.6% (n=288 hemisegments). This phenotype is never observed in either mutant alone. In gcm-robo1 double mutants, 31% of axons also misroute towards the muscle (n=288 hemisegments) (Fig. 4D, asterisk), a phenotype observed with less frequency in gcm mutants. Thus, in a gcm-robo1 double-mutant background, the absence of glia causes a disruption to the lateral fascicles that is comparable to the removal of multiple Robo receptors.
Axons might project along the midline in gcm-robo1 double mutants if the either midline glia are absent, despite the fact that gcm is not expressed in the midline glia, or if no repulsive Slit signal is produced. Thus, we verified whether Slit is produced in the double mutants. We find that, in gcm-robo1 double-mutant embryos, Slit signal is produced (Fig. 4E,F) and the mildine glia are present but disorganised. These results show that midline collapse in gcm-robo1 double mutants is not caused by lack of repulsion from the midline.
Axons might collapse over the midline because of extensive loss of Robo2 and Robo3 neurons, caused by neuronal apoptosis induced by lack of glia (Booth et al., 2000). To test whether Robo3 neurons, which are amongst the most abundant, are still present in gcm-robo1 double-mutants embryos, we stained embryos with anti-Robo3 antibodies (Fig. 4F). We find abundant distribution of Robo3 axons. Interestingly, most of these axons now cross the midline aberrantly (Fig. 4F). This means that midline collapse in gcm-robo1 double-mutant embryos is not only caused by extensive neuronal apoptosis.
Longitudinal axons project along the midline in gcm-robo1 mutant embryos even though they express Robo3. This is consistent with previous findings that show that interfering with lateral neuron-glia interactions causes midline crossing despite robo1 expression and repulsion from the midline (Kinrade et al., 2001). Thus, in the absence of glia, axons misroute to find alternative trajectories, regardless of midline-derived repulsion and the expression of Robo receptors by the axons. Because Robo3 labels only follower axons, LG are necessary to keep follower axons away from the midline independent of the Robo code.
Different requirements for robo1 and glia among pioneer axons
To determine whether these dramatic axonal defects are specific to follower neurons or due to primary defects in axon guidance that can be traced back to abnormal function of pioneer neurons, we looked at the extension of pioneer axons in gcm-robo1 double mutants. In wild-type the descending dMP2/MP1 pioneer fascicle meets the ascending vMP2/pCC pioneer fascicle to form a characteristic chain-like structure (Fig. 5A) (Hidalgo and Brand, 1997). In gcm mutants the vMP2/pCC fascicle extends normally, but the dMP2/MP1 fascicle extends outwards straight towards the muscle (88.2%, n=144 hemisegments) (Fig. 5B) (see also Hidalgo and Booth, 2000). In robo1 mutants the converse is found and only the pCC/vMP2 fascicle is affected, collapsing over the midline (100%, n=191) (Fig. 5C). In gcm-robo1 double-mutant embryos both fascicles are affected, as the vMP2/pCC fascicle collapses completely over the midline (100%, n=174) and the dMP2/MP1 fascicle either does not extend or misroutes towards the muscle (89%, n=174) (Fig. 4D). Slightly later, at stage 14, these phenotypes become even clearer. Wild-type embryos have two distinct fascicles, the outer dMP2/MP1 and the inner vPM2/pCC fascicles (Fig. 5F). In gcm mutants only the vMP2/pCC fascicle is present (68.5%, n=92) (Fig. 5G), whereas in robo1 mutants only the dMP2/MP1 fascicle extends normally (27.7%, n=94) (Fig. 5H). In gcm-robo1 double-mutants, there is a synergistic effect on both fascicles (Fig. 5I): the vMP2/pCC fascicle is completely collapsed over the midline (96%, n=156), and the dMP2/MP1 fascicle is missing (97%, n=156), meaning that it might have either misrouted out of the CNS or collapsed over the midline. These results demonstrate that loss of glia in a robo1-mutant background alters the formation of axonal trajectories from the beginning of pioneer-growth-cone extension. Thus, absence of functional glia in a robo1 loss-of-function background synergistically affects axon guidance of both pioneer and follower neurons.
Fig. 5.
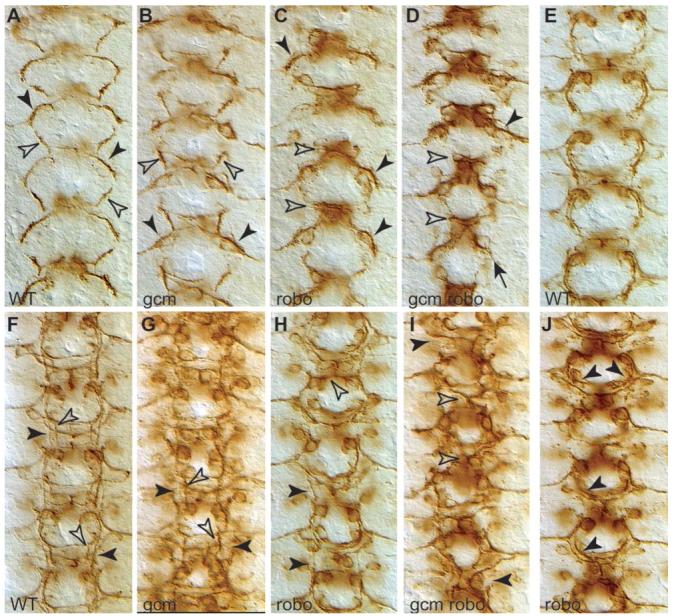
Axonal defects occur from the earlier phases of pioneer axon extension. Pioneer axons visualised with 22c10 antibodies in wild-type (A,E,F), gcm-mutant embryo (B,G), robo1 mutant (C,H,J) and gcm-robo1 double mutant (D,I) at stages 12.1 (A,B,C,D); 13 (E,J) and 14 (F,G,H,I). (A) In wild-type embryos, the descending dMP2/MP1 axons (black arrowhead) meet the ascending vMP2/pCC axons (white arrowhead) at the end of stage 12. (B) In gcm mutants, the dMP2/MP1 fascicle misroutes to the muscle, so its descending trajectory is missing (black arrowheads), but the vMP2/pCC fascicle is unaffected (white arrowheads). (C) In robo mutants, the pCC/vMP2 fascicle does not grow but is trapped in the midline (white arrowheads), whereas the dMP2/MP1 fascicle begins its normal descending trajectory. This is also seen slightly later, at stage 13, (J) where the dMP2/MP1 fascicle extends normally all the way down (arrowheads) and only then curves towards the midline as it fails to encounter the ascending vMP2/pCC fascicle, which is missing (compare to E, wild-type). (D) In gcm-robo1 double-mutant embryos, the pCC/vMP2 fascicle is completely collapsed on the midline (white arrowheads) and the dMP2/vMP2 fascicle is either missing or misrouted to the muscle (black arrowheads). (F) At stage 14, the two fascicles, dMP2/MP1 (black arrowheads) and pCC/vMP2 (white arrowheads) are clearly distinguishable. (G) In gcm mutants, only the pCC/vMP2 fascicle is present (white arrowheads) and the dMP2/MP1 fascicle is missing (black arrowheads). (H) In robo1 mutants, the pCC/vMP2 fascicle collapses on the midline (white arrowhead) but the dMP2/MP1 fascicle (black arrowhead) is normal. (I) In gcm-robo1 double mutants, the pCC/vMP2 fascicle collapses on the midline, but the dMP2/MP1 fascicle is not discernible. Anterior is up.
Our results also show that robo1 is not required in the early stages of dMP2/MP1 fascicle extension. In fact, we find that whereas in robo mutants the vMP2/pCC fascicle is trapped in the midline, the dMP2/MP1 fascicle begins to extend normally with its characteristic curved trajectory (Fig. 5C). Slightly later in wild-type embryos, the dMP2/MP1 fascicle can be seen as it separates from the vMP2/pCC fascicle (Fig. 5E). In robo1 mutants, the dMP2/MP1 fascicle extends its normal trajectory, and curves medially slightly more than in wild-type only at the most posterior end (Fig. 5J), possibly because of the lack of the ascending vMP2/pCC fascicle that the dMP2/MP1 axons normally encounter. Later still, at stage 14, the vMP2/pCC fascicle is always collapsed over the midline in robo1 mutants, but the dMP2/MP1 fascicle can form normally (Fig. 5H). This indicates that the pioneer neurons dMP2 and MP1 do not require robo1 during growth-cone guidance.
The finding that robo1 is not required in the pioneer dMP2/ MP1 fascicle contrasts with previous reports suggesting that robo1 is required by all pioneer axons, despite the fact that robo1 has not been shown to be expressed in the MP1 and dMP2 neurons (Kidd et al., 1998a; Rajagopalan et al., 2000; Simpson et al., 2000b).
Lateral displacement of axons upon ectopic expression of robo2 requires glia
The ectopic expression of robo2 in neurons that normally project over the medial fascicle is sufficient to displace their trajectory laterally to the central fascicle (Fig. 6E and Fig. 7B; Rajagopalan et al., 2000; Simpson et al., 2000a). This is one of the key experiments that demonstrates the nature of the Robo code. We wondered whether the response of axons to gain-of-function in the Robo code might require normal glial environment and function.
Fig. 6.
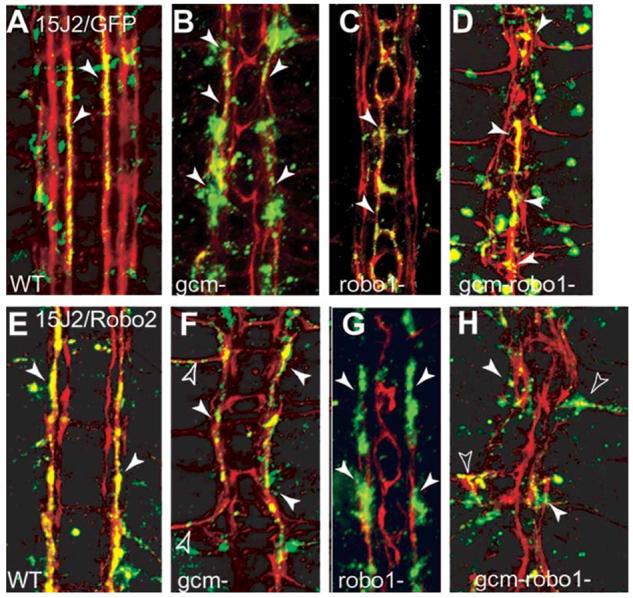
Ectopic expression of robo2 does not displace the MP2 axons along lateral longitudinal tracts in the absence of glia. FasII axons are visualised in red at stage 16. The MP2 axons are visualised with anti-GFP (green) by the expression of 15J2/UASGAPGFP in multiple genotypes (A-D). Colocalisation of GFP and FasII is seen in yellow. Ectopic expression of robo2 with 15J2/UASrobo2-myc, visualised with anti-Myc (green) antibodies in embryos of different genotypes (E-H). Colocalisation of Myc and FasII is seen in yellow. (A) Wild-type. The MP2 axons run parallel to the midline along the medial FasII fascicle at this stage (arrowheads, 15J2/UASGAPGFP). (E) When expression of robo2 is driven ectopically in the MP2 axons, their trajectories are displaced to the central fascicle (arrowheads, 15J2/UASRobo2myc). (B) In gcm-mutant embryos, the MP2 axons still run along the medial fascicle (arrowheads, gcmΔP1/gcmΔP1; 15J2/UASGAPGFP). (F) When robo2 is expressed ectopically in the MP2 neurons in gcm mutants (gcmΔP1/gcmΔP1; 15J2/UASrobo2myc), the MP2 axons stay within the fused, single fascicle (arrowheads). This gcm-mutant embryo is phenotypically more severe than in B, where the fasicles are defasciculated but not fused. (C) In robo1 mutants, the MP2 axons run on the medial fascicle, collapsed along the midline (arrowheads, robo1/robo1; 15J2/UASGAPGFP). (G) Expression of robo2 in the MP2 axons in robo1 mutants (robo1/robo1; 15J2/UASrobo2myc) displaces the MP2 axons laterally onto the central fascicle (arrowheads). (D) gcm-robo1 double mutant with midline collapse of the longitudinal fascicles, in which the MP2 axons run along the midline (arrowheads, gcmΔP1 robo/gcmΔP1 robo; 15J2/UASGAPGFP). (H) gcm-robo1 double mutant embryo with midline collapse of the longitudinal fascicles, expressing robo2 in the MP2 axons (gcmΔP1 robo1/gcmΔP1 robo1; 15J2/UASrobo2myc): the MP2 axons leave the CNS and extend towards the periphery (empty arrowheads) or they do not grow at all (white arrowheads). There are no MP2 axons projecting longitudinally in these embryos. Anterior is up.
Fig. 7.
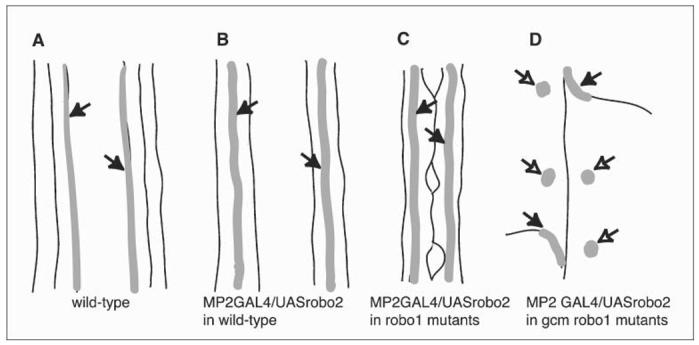
Schematic of the behaviour of MP2 longitudinal axons in different genetic backgrounds. In wild-type embryos, MP2 axons extend along the medial fascicle (A, arrows) and are displaced laterally on expression of robo2 (B, arrows). Lateral displacement still takes place in robo1 mutant embryos (C, arrows). However, if embryos are double mutant for robo1 and gcm and, therefore, lack functional glia, MP2 axons either do not grow (D, empty arrows) or they misroute out of the CNS towards the muscle (D, arrows).
To test whether Robo2 can displace axons laterally along a longitudinal trajectory in embryos that lack glia and in which fascicles misroute over the midline, we targeted the expression of Robo2 in gcm-robo1 double-mutant embryos (Fig. 6). In controls, ectopic expression of robo2 can displace axons laterally and longitudinally in robo1-mutant embryos (Fig. 6G and Fig. 7C), indicating that robo1 is not required for the displacement induced by robo2 expression. In gcm mutants, the three FasII fascicles might be defasciculated but are distinctly present, and MP2 axons visualised with GFP project normally over the medial fascicle (78.5%, n=28 hemisegments) (Fig. 6B). Ectopic expression of robo2 in gcm-mutant embryos might cause: (1) lateral displacement in embryos that have a mild phenotype (the expressivity of the gcm phenotype is variable); (2) in embryos with a more severe phenotype, the axons that misexpress robo2 project over a single, fused, FasII fascicle (35%, n=20 hemisegments) (Fig. 6F); and (3) axons misroute to the muscle exiting the CNS (10%, n=20 hemisegments) (Fig. 6F). Thus, axons that express robo2 ectopically are not necessarily displaced over a lateral longitudinal trajectory in the absence of functional glia.
The effect of glial loss is exacerbated in severe gcm-robo1 double-mutant embryos in which the longitudinal fascicles either collapse along the midline or misroute severely towards the periphery and exit the CNS (Fig. 6D and Fig. 7D). When the MP2 axons in these double mutants are visualised with GFP, they are seen to project all along the midline (63.3%, n=30 segments) (Fig. 6D) or misroute out of the CNS (13.3%, n=60 hemisegments). However, when MP2 axons in these embryos misexpress robo2, they do not extend longitudinally. Instead, they either do not grow (36.6%, n=166 hemisegments) and remain stunted at the base of the commissures, or they leave the CNS and misroute towards the muscle (30.7%, n=166 hemisegments) (Fig. 6H). Therefore, in the absence of glia as well as robo1, ectopic Robo2 cannot define a more lateral position for axons along a longitudinal pathway. Instead, the fact that longitudinal axons either do not grow or exit the CNS, means that axons respond to ectopic robo2 by being repelled along other pre-existing major fascicles and that in the absence of glia there are not sufficient fasciculation cues for axons to extend longitudinally.
CONCLUSIONS
Longitudinal migration of LG does not require the Robo code cell-autonomously. Thus, a Slit gradient is not sufficient to define neuropile structure.
Longitudinal axons collapse along the midline in gcm-robo1 double mutants, which mimics loss-of-function of multiple Robo receptors.
In gcm-robo1 double-mutant embryos, axons that ectopically express robo2 cannot extend longitudinally and they either do not grow or exit the CNS.
These findings mean that axons require LG to read the Robo code.
DISCUSSION
Glia are required for the reading of the Robo code by axons
We demonstrate here that interfering with neuron-glia interactions alters how axons respond to the Robo code. Thus, normal neuron-glia interactions are necessary for the correct functioning of the axonal Robo code.
We have shown that LG do not migrate normally during axon guidance in embryos mutant in the different robo genes. Instead, glial positions follow axonal pattern changes in these mutants. We have shown that neither robo2 nor robo3 are expressed in the LG during their longitudinal migration. Previously, we have shown that robo1 is expressed in LG during their medial migration before axon guidance, but not later during longitudinal migration (Kinrade et al., 2001). Furthermore, both robo2 and robo3 are still expressed in the absence of glia in gcm-mutant embryos, meaning that loss of glia does not cause loss of the robo code. Thus, these findings indicate that the lateral position of LG relative to the midline does not depend directly on Slit and the Robo code, but that it depends on interactions with axons and on other signalling mechanisms. This means that the Slit gradient is not sufficient to define all aspects of neuropile structure. Because glia are necessary for axon guidance, but longitudinal migration of glia is not controlled by Slit, axons receive multiple, Slit-dependent and Slit-independent inputs that define their lateral trajectories.
We have shown that lateral neuron-glia interactions influence how axons read the Robo code. We have shown that removing functional glia with gcm mutations in robo1 loss-of-function mutants causes a severe, midline-collapse phenotype, comparable to the removal of multiple Robo receptors. This means that glia are necessary for the reading of the Robo code by axons. It also means that, in addition to Robo-code signalling on axons, LG are necessary to keep axons away from the midline. We have also shown that in gcm-robo embryos with severe, midline-collapse phenotypes, the ectopic expression of robo2 does not redirect axons to a more lateral, but still longitudinal pathway. Instead, MP2 axons either do not grow at all or they leave the CNS and project towards the muscle. Either way, they do not project longitudinally. Thus, glia are also required for axonal displacement caused by gain-of-function of Robo receptors. This demonstrates that neuron-glia interactions along the longitudinal pathways are necessary for the function of the axonal Robo code, and to define and maintain the longitudinal trajectories of axons. Both follower and pioneer neurons require inputs from both the Robo code and glia to define their trajectories, thus, these two inputs are necessary for the establishment of longitudinal trajectories from the beginning and throughout axon guidance.
Different requirements for the Robo code among pioneer neurons
Different pioneer axons have different requirements for glia and the Robo code. Loss of glia in gcm mutants prevents the dMP2/MP1 fascicle from descending longitudinally. Instead, loss of robo causes a very dramatic collapse on the midline of the pCC/vMP2 fascicle, whereas the dMP2 growth cone extends normally. The fact that robo1 mutations do not affect early dMP2/MP1 pioneer growth-cone guidance might help explain why the intermediate (MP1) FasII fascicle is not affected in robo mutants. In fact, the intermediate fasII fascicle originates from neurons that never required robo1, and where robo1 has not been shown to be expressed.
Later, when dMP2 fasciculates with pCC along the medial fascicle, it does require robo function. The fact that ectopic expression of robo2 in dMP2 and vMP2 neurons causes displacement only to the intermediate fascicle and not more laterally, could be because the medial and intermediate fascicles become fasciculated together earlier (Hidalgo and Brand, 1997) and the ectopic expression of Robo2 prevents their defasciculation (Simpson et al., 2000a). This is consistent with a primary role of Robo2 in fasciculation.
The differential requirement for robo1 in the pioneer neurons also helps to define the different shape of their trajectories: the pCC axon extends very straight and parallel to the midline, and it clearly requires robo1 whereas the dMP2 axons take a turn outwards before turning back inwards (Hidalgo and Brand, 1997): such a complex trajectory might not be achieved with a constant activation of Robo signalling, because this would ensure a constant distance from the midline, hence a parallel trajectory.
Possible mechanisms involving glia and fasciculation
Our results have shown that the Robo code is insufficient to determine the longitudinal trajectories of axons, and that axons also sense a second input based on local interactions with glia. In the context of neuron-glia interactions, the Slit gradient could still be the driving force organising axonal patterns if the Robo code directly determines glial migration as well as axonal positioning. However, we have shown that LG do not express Robo receptors and that their migration depends on local and other cues. Thus, our data support the notion that axons receive two instructions that define their longitudinal trajectories: one in the form of signalling from Robo receptors, and one from interactions with LG. Furthermore, being an interactive system, glia can influence the outcome of the axonal Robo code.
The molecular mechanism by which glia steer the response of axons to midline signalling is unknown. We have shown that axons can cross the midline in the absence of glia and neuron-glia interactions despite the expression of multiple Robo receptors (Kinrade et al., 2001; and this work) and that axons of interneurons that do not normally cross the midline either do not extend at all or misroute to the muscle when forced to overexpress robo2 in gcm-robo double mutants. Perhaps Robo receptors are involved directly in fasciculation/adhesion between axons and glia. Although Robo receptors have been shown to function as homophilic binding molecules (Hivert et al., 2002), they might also interact with other proteins on the glial cell membrane, causing different responses in the axon in the absence and presence of glia. Another possibility is that glia change growth-cone behaviour by influencing the small GTPases (Kuhn et al., 1999) that function downstream of Robo (Patel and Van Vactor, 2002) so that, in the absence of glia, normal signalling by Robo is abrogated. Perhaps Robo receptors interact with tyrosine kinases (Wong et al., 2003) in the growth cone that respond to interactions with glia and, in the absence of glia, the input to the Robo code is lost. Last, glia are likely to influence the molecular environment of extending axons. For example, loss of glia might change the relative concentration of heparan sulfate proteoglycans and other extracellular components that influence signalling by Robo (Johnson et al., 2004; Steigemann et al., 2004) and, presumably, other pathways involved in neuron-glia interactions. Together, it appears that the roles played by the Robos are dependent on context. If the cellular environment changes then the Robos cannot display the normal repulsive signalling function as a read-out of distance from the midline.
Whatever the mechanism, we conclude that axons require fasciculation cues present in both glia and axons to trace their trajectories. Thus, loss of fasciculation cues in gcm-robo double mutants prevents the longitudinal extension of robo2-expressing axons independently of midline repulsion. Given that axons do extend longitudinally in either gcm or robo single mutants, the loss of both glia and axonal fasciculation cues in gcm-robo1 double mutants exacerbates the disorientation of the axons. In gcm-robo1 double mutants, interneuron longitudinal axons can extend over the motoraxons that exit the CNS, which indicates that there they encounter fasciculation cues that they can adhere to. This is consistent with Robo2 playing a key role in fasciculation. It has been observed in other contexts that the roles of the different Robo receptors vary greatly (Godenschewege et al., 2002; Zlatic et al., 2003) and that both Robo receptors and Slits have relevant roles in fasciculation and axonal growth (Hivert et al., 2002; Hutson and Chien, 2002; Shu et al., 2003). Our results support the notion that Robo2 and, perhaps, Robo3 are involved in fasciculation, and that fasciculation might also involve direct interactions with membrane proteins on the glial surfaces.
Given that glia are necessary for axon guidance and extension of axons in the correct direction (Hidalgo and Booth, 2000), it is paradoxical that the reciprocal interactions between axons and glia during extension and migration acquire directionality. One mechanism that confers some directionality is the non-autonomous maintenance of cell survival, which places an instructive positional pressure on cells. In fact, the survival of some LG depends on neurons. The descending fascicle of the dMP2 pioneer neuron extends the longest distance during axon guidance, and it does so completely surrounded and anticipated by glial cells (Hidalgo and Booth, 2000). Neuregulin Vein produced by the dMP2 neurons maintains the survival of a subset of these glia (Hidalgo et al., 2001). Follower-neuron survival also depends on glial cells, but pioneer-neuron survival does not (Booth et al., 2000). Because the pioneer neurons are the first neurons to navigate and trace out a scaffolding of the major axonal fascicles, their independence from glia for survival provides directionality during axon guidance (Booth et al., 2000).
It is conceivable that in gcm-robo1 double mutants the collapse of axons over the midline might correlate with an increase in neuronal apoptosis because there is extensive apoptosis in gcm-mutant embryos. We have shown previously that interfering with cell survival disrupts the lateral positions of axons. However, the induction of apoptosis cannot be the main cause of midline collapse in the absence of robo1 and gcm. First, we observe axon-guidance defects in the pioneer axons, which do not undergo apoptosis in gcm-robo1 double mutants. Second, complete lack of cell death in H99 embryos that also lack glia (gcm-H99 double mutants) also induces severe midline crossing by axons. This means that neuronal apoptosis might be a correlation but not a cause of the gcm-robo1 double-mutant phenotype. Instead, these findings support the notion that neuron-glia interactions maintain the survival of both cell types and restrict their positions.
Glia during vertebrate axon guidance
In vertebrates, neuron-glia interactions might play an analogous role in the confinement of axonal trajectories and influencing the final outcome of other guidance cues. For example, the longitudinal axons of the spinal cord in vertebrates are also associated with a type of glial cells, oligodendrocytes, whose cell bodies are found all along the length of the spinal cord. As with ablation of glia in Drosophila, elimination of oligodendrocytes causes disruptions to axonal patterns, such as defasciculation and misrouting, which indicate that myelinating cells create a ‘channel’ along which axons extend (Schwab and Schnell, 1991). Similarly, motor axons also use mechanical cues, in the form of non-neuronal ‘tubes’, to find their trajectories during regeneration, implying that axons follow cues of diverse nature during pathfinding (Nguyen et al., 2002). Regeneration experiments have also shown that re-growth of broken axonal tracts requires that oligodendrocytes are aligned in the direction of axonal growth, and it has been shown that transplanting glial cells to the sites of axonal injury can restore axonal trajectories and neuronal function (Ramon-Cueto et al., 2000). These and other findings indicate that glia are also required for the formation of axonal tracts in vertebrates and that axons navigate by sensing multiple inputs from their environment. Slits and Robos are conserved in vertebrates. Understanding the complexity of cellular and molecular events that regulate Slit-Robo mechanisms and neuron-glia interactions during axon guidance is necessary for the therapeutic implementation of CNS repair.
To conclude, we have shown that in the absence of glia and, most dramatically, in a gcm-robo1 double-mutant background, axons lose their ability to extend longitudinally in conditions of either loss- or gain-of-function of the Robo code. Our results show that neuron-glia interactions are necessary for the correct functioning of the axonal Robo code.
ACKNOWLEDGEMENTS
We thank members of our group for helpful discussions. We are grateful to Akira Chiba, Barry Dickson, Corey Goodman, Iowa Hybridoma Bank, Roger Jacobs, Matthias Landgraf, Susana Romani, Guy Tear and Andrew Travers for antibodies and flies. E.F.V.K. is supported by a BBSRC studentship; A.H.’s work is funded by a Wellcome Trust CDF, MRC CEG and EMBO YIP.
REFERENCES
- Ba-Charvet KTN, Brose K, Marillat V, Kidd T, Goodman CS, Tessier-Lavigne M, Sotelo C, Chedotal A. Slit2-mediated chemorepulsion and collapse of developing forebrain axons. Neuron. 1999;22:463–473. doi: 10.1016/s0896-6273(00)80702-3. [DOI] [PubMed] [Google Scholar]
- Bagri A, Martin O, Plump AS, Mak J, Pleasure SJ, Rubenstein JLR, Tessier-Lavigne M. Slit proteins prevent midline crossing and determine the dorsoventral position of major axonal pathways in the mammalian forebrain. Neuron. 2002;33:233–248. doi: 10.1016/s0896-6273(02)00561-5. [DOI] [PubMed] [Google Scholar]
- Bastiani MJ, du Lac S, Goodman CS. Guidance of neuronal growth cones in the grasshopper embryo. I. Recognition of a scpecific pathway by the pCC neuron. Journal of Neuroscience. 1986;6:3518–3531. doi: 10.1523/JNEUROSCI.06-12-03518.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battye R, Stevens A, Jacobs R. Axon repulsion from the midline requires Slit function. Development. 1999;126:2475–2481. doi: 10.1242/dev.126.11.2475. [DOI] [PubMed] [Google Scholar]
- Booth G, Kinrade E, Hidalgo A. Glia maintain follower neuron survival during Drosophila CNS development. Development. 2000;127:237–244. doi: 10.1242/dev.127.2.237. [DOI] [PubMed] [Google Scholar]
- Brose K, Bland KS, Wang KH, Arnott D, Henzel W, Goodman CS, Tessier-Lavigne M, Kidd T. Slit proteins bind Robo receptors and have an evolutionary conserved role in repulsive axon guidance. Cell. 1999;96:795–806. doi: 10.1016/s0092-8674(00)80590-5. [DOI] [PubMed] [Google Scholar]
- Culverwell J, Karlstrom RO. Making the connection: retinal axon guidance in the zebrafish. Seminars in Cell and Developmental Biology. 2002;13:497–506. doi: 10.1016/s1084952102001039. [DOI] [PubMed] [Google Scholar]
- Dickson BJ. Molecular mechanisms of axon guidance. Science. 2002;298:1959–1964. doi: 10.1126/science.1072165. [DOI] [PubMed] [Google Scholar]
- Englund C, Steneberg P, Falileeva L, Xylourgidis N, Samakovlis C. Attractive and repulsive functions of Slit are mediated by different receptors in the Drosophila trachea. Development. 2002;129:4941–4951. doi: 10.1242/dev.129.21.4941. [DOI] [PubMed] [Google Scholar]
- Gilthorpe JD, Papantoniou E-K, Chedotal A, Lumsden A, Wingate RJT. The migration of cerebellar rhombic lip derivatives. Development. 2002;129:4719–4728. doi: 10.1242/dev.129.20.4719. [DOI] [PubMed] [Google Scholar]
- Godenschewege TA, Simpson JH, Shan J, Bashaw GJ, Goodman CS, Murphey RK. Ectopic expression in the giant fiber system of Drosophila reveals distinct roles for Roundabout (Robo), Robo2 and Robo3 in dendritic guidance and synaptic connectivity. Journal of Neuroscience. 2002;22:3117–3129. doi: 10.1523/JNEUROSCI.22-08-03117.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guthrie S. Axon guidance: Robos make the rules. Current Biology. 2001;11:R300–R303. doi: 10.1016/s0960-9822(01)00169-5. [DOI] [PubMed] [Google Scholar]
- Hidalgo A, Booth G. Glia dictate trajectories of pioneer neurons in the Drosophila embryonic CNS. Development. 2000;127:393–402. doi: 10.1242/dev.127.2.393. [DOI] [PubMed] [Google Scholar]
- Hidalgo A, Brand AH. Targeted neuronal ablation: the role of pioneer neurons in guidance and fasciculation in the CNS of Drosophila. Development. 1997;124:3253–3262. doi: 10.1242/dev.124.17.3253. [DOI] [PubMed] [Google Scholar]
- Hidalgo A, Kinrade EFV, Georgiou M. The Drosophila Neuregulin Vein maintains glial survival during axon guidance in the CNS. Developmental Cell. 2001;1:679–690. doi: 10.1016/s1534-5807(01)00074-0. [DOI] [PubMed] [Google Scholar]
- Hivert B, Liu Z, Chuang C-Y, Doherty P, Sundaresan V. Robo1 and Robo2 are homophilic binding molecules that promote axonal growth. Molecular and Cellular Neuroscience. 2002;21:534–545. doi: 10.1006/mcne.2002.1193. [DOI] [PubMed] [Google Scholar]
- Hutson LD, Chien C-B. Pathfinding and error correction by retinal axons: the role of astray/robo2. Neuron. 2002;33:205–217. doi: 10.1016/s0896-6273(01)00579-7. [DOI] [PubMed] [Google Scholar]
- Jacobs JR, Hiromi Y, Patel NH, Goodman CS. Lineage, migrations and morphogenesis of longitudinal glia in the Drosophila CNS as revealed by a molecular lineage marker. Neuron. 1989;2:1621–1635. doi: 10.1016/0896-6273(89)90051-2. [DOI] [PubMed] [Google Scholar]
- Johnson KG, Ghose A, Epstein E, Lincecum J, O’Connor MB, Van Vactor DL. Axonal heparan sulfate proteoglycans regulate the distribution and efficiency of the repellent Slit during midline axon guidance. Current Biology. 2004;14:499–504. doi: 10.1016/j.cub.2004.02.005. [DOI] [PubMed] [Google Scholar]
- Kidd T, Bland KS, Goodman CS. Slit is the midline repellent for the Robo receptor in Drosophila. Cell. 1999;96:785–794. doi: 10.1016/s0092-8674(00)80589-9. [DOI] [PubMed] [Google Scholar]
- Kidd T, Brose K, Mitchell KJ, Fetter RD, Tessier-Lavigne M, Goodman CS, Tear G. Roundabout controls axon crossing of the CNS midline and defines a novel subfamily of evolutionary conserved guidance receptors. Cell. 1998a;92:205–215. doi: 10.1016/s0092-8674(00)80915-0. [DOI] [PubMed] [Google Scholar]
- Kidd T, Russell C, Goodman CS, Tear G. Dosage-sensitive and complementary functions of roundabout and commisureless control axon crossing of the CNS midline. Neuron. 1998b;20:25–33. doi: 10.1016/s0896-6273(00)80431-6. [DOI] [PubMed] [Google Scholar]
- Kinrade EFV, Brates T, Tear G, Hidalgo A. Roundabout signalling, cell contact and trophic support confine longitudinal glia and axons in the drosophila CNS. Development. 2001;128:207–216. doi: 10.1242/dev.128.2.207. [DOI] [PubMed] [Google Scholar]
- Kramer SG, Kidd T, Simpson JH, Goodman CS. Switching repulsion to attraction: changing responses to Slit during transition in mesoderm migration. Science. 2001;292:737–739. doi: 10.1126/science.1058766. [DOI] [PubMed] [Google Scholar]
- Kuhn TB, Brown MD, Wilcox CL, Raper JA, Bamburg JR. Myelin and Collapsin-1 induce motor neuron growth cone collapse through different pathways: inhibition of collapse by opposing mutants of rac1. Journal of Neuroscience. 1999;19:1965–1975. doi: 10.1523/JNEUROSCI.19-06-01965.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen QT, Sanes JR, Lichtman JW. Pre-existing pathways promote precise projection patterns. Nature Neuroscience. 2002;5:861–867. doi: 10.1038/nn905. [DOI] [PubMed] [Google Scholar]
- Patel BN, Van Vactor DL. Axon guidance: the cytoplasmic tail. Current Opinion in Cell Biology. 2002;14:221–229. doi: 10.1016/s0955-0674(02)00308-3. [DOI] [PubMed] [Google Scholar]
- Patel NH. Imaging neuronal subsets and other cell types in whole-mount Drosophila embryos and larvae using antibody probes. In: Goldstein LSB, Fyrberg EA, editors. Drosophila melanogaster: Practical Uses in Cell and Molecular Biology. Vol. 44. Academic Press; 1994. pp. 446–485. [DOI] [PubMed] [Google Scholar]
- Poeck B, Fischer S, Gunning D, Zipursky SL, Salecker I. Glial cells mediate target layer selection of retinal axons in the developing visual system of Drosophila. Neuron. 2001;29:99–113. doi: 10.1016/s0896-6273(01)00183-0. [DOI] [PubMed] [Google Scholar]
- Rajagopalan S, Vivancos V, Nicolas E, Dickson BJ. Selecting a longitudinal pathway: Robo receptors specify the lateral position of axons in the Drosophila CNS. Cell. 2000;103:1033–1045. doi: 10.1016/s0092-8674(00)00207-5. [DOI] [PubMed] [Google Scholar]
- Ramon-Cueto A, Cordero M, Santos-Benito F, Avila J. Functional recovery of paraplegic rats and motor axon regeneration in their spinal cords by olfactory ensheathing glia. Neuron. 2000;25:425–435. doi: 10.1016/s0896-6273(00)80905-8. [DOI] [PubMed] [Google Scholar]
- Rangarajan R, Gong R, Gaul U. Migration and function of glia in the developing Drosophila eye. Development. 1999;126:3285–3292. doi: 10.1242/dev.126.15.3285. [DOI] [PubMed] [Google Scholar]
- Raper J, Bastiani MJ, Goodman CS. Pathfinding by neuronal growth cones in grasshopper embryos. IV. The effects of ablating the A and P axons upon the behaviour of the G growth cone. Journal of Neuroscience. 1984;4:2329–2345. doi: 10.1523/JNEUROSCI.04-09-02329.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwab ME, Schnell L. Channelling of developing rat corticospinal tract axons by myelin-associated neurite growth inhibitors. Journal of Neuroscience. 1991;11:709–721. doi: 10.1523/JNEUROSCI.11-03-00709.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sepp KJ, Auld VJ. Reciprocal interactions between neurons and glia are required for Drosophila peripheral nervous system development. Journal of Neuroscience. 2003;10:8221–8230. doi: 10.1523/JNEUROSCI.23-23-08221.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sepp KJ, Schulte J, Auld VJ. Peripheral glia direct axon guidance across the CNS/PNS transition zone. Developmental Biology. 2001;238:47–63. doi: 10.1006/dbio.2001.0411. [DOI] [PubMed] [Google Scholar]
- Shu T, Sundaresan V, McCarthy MM, Richards LJ. Slit2 guides both precrossing and postcrossing callosal axons at the midline in vivo. Journal of Neuroscience. 2003;23:8176–8184. doi: 10.1523/JNEUROSCI.23-22-08176.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver J, Lorenz SE, Wahlstein D, Coughlin J. Axonal guidance during development of the great cerebral commissures: descriptive and experimental studies in vivo on the role of the preformed glial pathways. Journal of Comparative Neurology. 1982;210:10–29. doi: 10.1002/cne.902100103. [DOI] [PubMed] [Google Scholar]
- Simpson JH, Bland KS, Fetter RD, Goodman CS. Short-range and long-range guidance by Slit and its Robo receptors: a combinatorial code of Robo receptors control lateral position. Cell. 2000a;103:1019–1032. doi: 10.1016/s0092-8674(00)00206-3. [DOI] [PubMed] [Google Scholar]
- Simpson JH, Kidd T, Bland KS, Goodman CS. Short-range and long-range guidance by Slit and its Robo receptors: Robo and Robo2 play distinct roles in midline guidance. Neuron. 2000b;28:753–766. doi: 10.1016/s0896-6273(00)00151-3. [DOI] [PubMed] [Google Scholar]
- Steigemann P, Molitor A, Fellert S, Jäckle H, Vorbrüggen G. Heparan Sulfate Proteoglycan Sybdecan promotes axonal and myotube guidance by Slit/Robo signalling. Current Biology. 2004;14:225–230. doi: 10.1016/j.cub.2004.01.006. [DOI] [PubMed] [Google Scholar]
- Tear G. Axon guidance at the central nervous system midline. Cellular and Molecular Life Sciences. 1999a;55:1365–1376. doi: 10.1007/s000180050377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tear G. Neuronal guidance: a genetic perspective. Trends in Genetics. 1999b;15:113–117. doi: 10.1016/s0168-9525(98)01686-2. [DOI] [PubMed] [Google Scholar]
- Wong EV, Kerner JA, Jay DG. Convergent and divergent signaling mechanisms of growth cone collapse by EphrinA5 and Slit2. Journal of Neurobiology. 2003;59:66–81. doi: 10.1002/neu.10342. [DOI] [PubMed] [Google Scholar]
- Wong K, Tae Park H, Wu JY, Rao Y. Slit proteins: molecular guidance cues for cells ranging from neurons to leucocytes. Current Opinion in Genetics and Development. 2002;12:583–591. doi: 10.1016/s0959-437x(02)00343-x. [DOI] [PubMed] [Google Scholar]
- Zlatic M, Landgraf M, Bate M. Genetic specification of axonal arbors: atonal regulates robo3 to position terminal branches in the Drosophila nervous system. Neuron. 2003;37:41–51. doi: 10.1016/s0896-6273(02)01131-5. [DOI] [PubMed] [Google Scholar]


