Abstract
Background and purpose:
We have investigated the ability of α1-adrenoceptor antagonists to affect the hyperthermia produced by methylenedioxy methamphetamine (MDMA) in conscious mice.
Experimental approach:
Mice were implanted with temperature probes under ether anaesthesia and allowed 2 weeks recovery. MDMA (20 mg kg−1) was administered subcutaneously 30 min after vehicle or test antagonist or combination of antagonists and effects on body temperature monitored.
Key results:
Following vehicle, MDMA produced a hyperthermia, reaching a maximum increase of 1.8 °C at 140 min. Prazosin (0.1 mg kg−1) revealed an early significant hypothermia to MDMA of −1.94 °C. The α1A-adrenoceptor antagonist RS 100329 (0.1 mg kg−1), or the α1D-adrenoceptor antagonist BMY 7378 (0.5 mg kg−1) given alone, did not reveal a hypothermia to MDMA, but the combination of the two antagonists revealed a significant hypothermia to MDMA. The putative α1B-adrenoceptor anatagonist cyclazosin (1 mg kg−1) also revealed a significant hypothermia to MDMA, but actions of cyclazosin at the other α1-adrenoceptor subtypes cannot be excluded.
Conclusions and implications:
More than one subtype of α1-adrenoceptor is involved in a component of the hyperthermic response to MDMA in mouse, probably both α1A- and α1D-adrenoceptors, and removal of this α1-adrenoceptor-mediated component reveals an initial hypothermia.
Keywords: MDMA, hyperthermia, α1-adrenoceptor, α1A-adrenoceptor, α1D-adrenoceptor
Introduction
Hyperthermia is a life-threatening acute consequence of methylenedioxy methamphetamine (MDMA) toxicity and is often seen when the drug is used at a rave, an environment where ambient temperature tends to be high and there is excessive physical exertion. In animal studies, it has also been shown that MDMA disrupts thermoregulation, causing either hypo- or hyperthermia depending on the ambient temperature (Malberg and Seiden, 1988). The mechanism(s) by which MDMA disrupts thermoregulation is still unclear, but both central and peripheral mechanisms have been implicated. As 5-hydroxytryptaminergic, noradrenergic and dopaminergic neurotransmitter systems have all been implicated in the mediation of hypothermia and hyperthermia, acute increases in these neurotransmitters induced by MDMA (White et al., 1996), or agonist actions of MDMA at receptors for these neurotransmitters (Lavelle et al., 1999; McDaid and Docherty 2001), may therefore influence the thermoregulatory system. Recently, we demonstrated that α2-adrenoceptors are involved in MDMA-induced hyperthermia and, in the absence of α2A-adrenoceptors, the monophasic hyperthermic response produced by MDMA became a biphasic response: hypothermia followed by hyperthermia (Bexis and Docherty, 2005). It is clear from our previous studies that α2-adrenoceptors alone do not mediate all of the MDMA-induced hyperthermia (Bexis and Docherty, 2005). In addition to α2-adrenoceptors, MDMA also has an affinity, although lower, for α1-adrenoceptors and β-adrenoceptors in the brain (Battaglia et al., 1988). Both α1- and β3-adrenoceptors have been shown to be involved in thermogenesis, and antagonism of α1- and β3-adrenoceptors reduces or abolishes MDMA-induced hyperthermia in the rat (Sprague et al., 2003, 2004).
It is established that there are three functional α1-adrenoceptor subtypes: α1A, α1B, α1D (see Guimaraes and Moura, 2001). The distribution of these α1-adrenoceptor subtypes varies between organs and tissues, as does their functional response. The main aim of this study was to investigate the role of α1-adrenoceptor subtypes in MDMA-mediated hyperthermia using the non-selective α1-adrenoceptor antagonist prazosin (α1A,1B,1D) and the selective α1-adrenoceptor subtype antagonists 5-methyl-3-(3-(4-(2-(2,2,2,-trifluoroethoxy)phenyl)-1-piperazinyl)propyl)-2,4-(1H)-pyrimidinedione (RS 100329) (α1A) (Williams et al., 1999), BMY 7378 (α1D) (Stone et al., 2001) and cyclazosin (putative α1B) (see Discussion) in wild-type (WT) mice.
Some of these results have been published in abstract form (Bexis and Docherty, 2006).
Methods
All studies conformed to the Declaration of Helsinki and were approved by the Department of Health and by the RCSI Research Ethics Committee. Male C-57 WT mice (22–35 g) were obtained from Harlan UK Limited (Shaw's Farm, Blackthorn, Bicester, UK).
Radiotelemetry
Under ether anaesthesia, animals were implanted with a radiotelemetric device enabling measurement of core body temperature (TAC50-PXT; Data Sciences International, St Paul, MN, USA). The implant was placed in the abdominal cavity and the abdomen was then closed. Animals were given temgesic (buprenorphine hydrochloride 0.05 mg kg−1, Schering-Plough, Welwyn, UK) subcutaneously (s.c.) postoperatively and allowed to recover for 14 days before experiments were performed.
Animals were housed individually, and home cages and bedding were used during temperature monitoring. On experimental days, a PhysiolTel-Receiver (model RPC-1) was placed under each animal cage, enabling recording of core body temperature. Data signals were acquired 90 min prior to and 300 min after vehicle (1 ml kg−1) or MDMA (20 mg kg−1) administration. All recordings were obtained at room temperature (23±0.2 °C).
Drug treatments
Since treatment of the animals and recordings were performed in the laboratory and not in the animal facility, animals were allowed to acclimatize, in their home cages, to the surroundings in the laboratory for 2 days (5–6 h per day) before administration of any drugs. Animals were injected s.c. with the non-selective α1-adrenoceptor antagonist prazosin (0.1 mg kg−1), selective α1A-adrenoceptor antagonist RS 100329 (0.1 mg kg−1), selective α1D-adrenoceptor antagonist BMY 7378 (0.5 mg kg−1), RS 100329 (0.1 mg kg−1) plus BMY 7378 (0.5 mg kg−1) or the putative α1B-adrenoceptor antagonist cyclazosin (1.0 mg kg−1). Antagonists were administered 30 min prior to the injection of vehicle (1 ml kg−1) or MDMA (20 mg kg−1).
Statistics
Values are mean±s.e.mean from 5–9 experiments. Responses were compared between groups by repeated measures analysis of variance followed by the Bonferroni or Dunnett test. Statistical and graphical analysis was carried out using GraphPad Prism for Macintosh computers.
Drugs
(+/−)-3,4-methylenedioxy methamphetamine hydrochloride (MDMA) (Research Biochemicals, Natick, MA, USA and NIDA, Bethesda, MD, USA); BMY 7378 ((8-(2-(4-(2-methoxyphenyl)-1-piperazinyl)ethyl)-8-azaspiro(4.5) decane-7,9-dione); Tocris, Bristol, UK); cyclazosin HCl (Research Biochemicals International, Natick, MA, USA, now part of Sigma-Aldrich); prazosin HCl (Sigma, Dublin, Ireland); RS 100329 (5-methyl-3-(3-(4-(2-(2,2,2,-trifluoroethoxy)phenyl)-1-piperazinyl)propyl)-2,4-(1H)-pyrimidinedione; Tocris, Bristol, UK). All drugs were dissolved in distilled water.
Results
Resting core body temperature
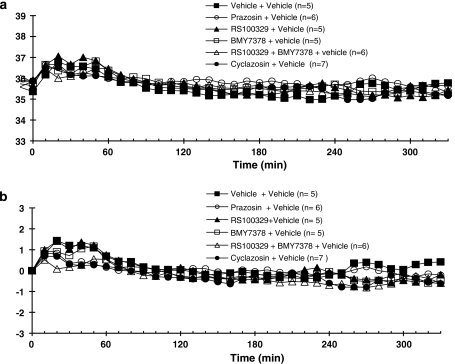
The resting body temperatures for all groups prior to drug treatment were not significantly different (see Figures 1a and 2a). However, body temperature transiently increased after the administration of vehicle (for antagonist) or antagonists (Figures 1 and 2). In all studies in which vehicle replaced MDMA, irrespective of which antagonist was used, there were no significant differences between groups in the response to vehicle, whether or not data were expressed as absolute temperature or change in temperature (Figures 1a and b).
Figure 1.
Core body temperature recordings in conscious WT mice given vehicle or antagonists 30 min prior to vehicle, at room temperature. All drugs or vehicles were administered s.c. Responses are expressed as (a) absolute body temperature and (b) change in body temperature. Vertical bars indicate the s.e.mean from 5–9 mice. Antagonist or vehicle was injected at time 0, and vehicle at 30 min.
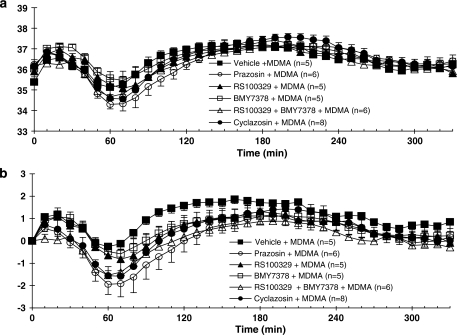
Figure 2.
Core body temperature recordings in conscious WT mice given vehicle, prazosin (0.1 mg kg−1), RS 100329 (0.1 mg kg−1), BMY 7378 (0.5 mg kg−1), RS 100329 (0.1 mg kg−1) plus BMY 7378 (0.5 mg kg−1) or cyclazosin (1 mg kg-1) 30 min prior to administration of MDMA (20 mg kg−1), at room temperature. All drugs were administered s.c. Responses are expressed as (a) absolute body temperature and (b) change in body temperature. Vertical bars indicate the s.e.mean from 5–9 mice. Antagonist or vehicle was injected at time 0, and MDMA at 30 min.
Effect of MDMA on core body temperature
Although baseline temperature did not significantly differ between groups prior to vehicle/antagonist and MDMA, baseline temperatures ranged between 35.4 and 36.2 °C, whereas maximum hypothermia was only about 2.0 °C (Figure 2a); therefore, effects of drugs on response to MDMA are best seen in terms of change in temperature from baseline (Figure 2b). Following vehicle injection, the administration of MDMA (20 mg kg−1) resulted in hyperthermia (Figure 2b). Significant hyperthermia was observed at 70 min post-MDMA with the maximal temperature occurring at 130 min, after which temperature gradually declined.
Effect of the non-selective α1-adrenoceptor antagonist prazosin on MDMA-induced hyperthermia
Prazosin (0.1 mg kg−1), followed by a vehicle injection, did not produce any change in core body temperature when compared to the vehicle group (Figure 1).
Following pretreatment of mice with prazosin (0.1 mg kg−1), MDMA administration produced a significant decrease in core temperature, which was then followed by an increase in core temperature, reaching temperatures similar to those obtained in mice treated with MDMA alone (Figure 2b).
Effect of selective α1A- and α1D-adrenoceptor antagonists on MDMA-induced hyperthermia
The selective α1A-adrenoceptor antagonist RS 100329 (0.1 mg kg−1) or α1D-adrenoceptor antagonist BMY 7378 (0.5 mg kg−1) when given alone did not produce any change in core body temperature in relation to the vehicle group (Figure 1). Following pretreatment of mice with RS 100329 (0.1 mg kg−1) or BMY 7378 (0.5 mg kg−1), MDMA produced a slight decrease in core body temperature that was not significantly different from temperatures obtained in mice treated with MDMA (post-vehicle) followed by a hyperthermic response (Figure 2b).
Co-administration of RS 100329 (0.1 mg kg−1) and BMY 7378 (0.5 mg kg−1) in vehicle experiments did not significantly change core body temperature compared to the vehicle group (Figure 1). Pretreatment of mice with RS 100329 (0.1 mg kg−1) and BMY 7378 (0.5 mg kg−1) in combination altered the monophasic hyperthermic response induced by MDMA to a biphasic response: a hypothermic response followed by a hyperthermic response (Figure 2b). The decrease in core temperature was significantly different from the effects of MDMA following vehicle and similar to the effect of MDMA post-prazosin (see above).
Effect of putative α1B-adrenoceptor antagonist on MDMA-induced hyperthermia
The putative α1B-adrenoceptor antagonist cyclazosin (1 mg kg−1) in vehicle experiments did not significantly alter the body temperature when compared to vehicle group (Figure 1). The pretreatment of mice with cyclazosin significantly altered the effect of MDMA on core body temperature (Figure 2b). In the presence of cyclazosin, MDMA caused an initial decrease in core temperature that was significantly different from core temperatures in mice treated with MDMA post-vehicle. After reaching a minimum temperature 40 min after MDMA administration, core temperature began to rise, reaching temperatures similar to those observed in mice treated with MDMA post-vehicle (Figure 2b).
The onset of hypothermia in all of the treatment groups occurred 10 min after the injection of MDMA and a minimum core temperature was reached 40 min after drug administration. The maximum core temperature was reached between 120 and 160 min after the injection of MDMA, followed by a gradual decrease in core temperature towards baseline levels (Figures 2a and b). MDMA produced a significant hyperthermia (P<0.05) in all groups of experiment, irrespective of the antagonist employed, as compared to baseline absolute temperature (Figure 2b). Although all antagonist treatments tended to reduce the maximum hyperthermia to MDMA, only the combination of RS 100329 and BMY 7378 significantly reduced this (P<0.05; comparison of changes in body temperature from baseline) (see Figure 2b).
Discussion
Hyperthermia is one of the most detrimental acute toxic effects of MDMA ingestion in humans. The mechanism(s) by which MDMA may disrupt thermoregulation still remain elusive, hindering therapeutic intervention. It is well established that the sympathetic nervous system is involved in thermoregulation, both centrally and peripherally, in terms of both heat production and transfer. Changes in sympathetic outflow to cutaneous blood vessels will alter blood flow and thereby heat transfer, and the sympathetic innervation regulates brown adipose tissue metabolism, which is important for heat production (Morrison, 2004). Non-shivering thermogenesis may occur in skeletal muscle, and, in part, metabolic alterations occur as a result of changes to blood flow involving sympathetic nerves (Ye and Colquhoun, 1998). The hyperthermic response induced by MDMA has been suggested to be a result of both a reduction in the ability of the animal to dissipate heat and increased metabolism (Gordon et al., 1991; Pedersen and Blessing, 2001; Blessing et al., 2006). Indirect actions of MDMA to release noradrenaline (White et al., 1996) and direct agonist actions of MDMA at adrenergic receptors (Lavelle et al., 1999; McDaid and Docherty 2001) may be involved in the hyperthermic response.
Before discussing the results obtained, it is appropriate to consider the antagonist concentrations chosen. Prazosin (0.1 mg kg−1, 0.24 μmol kg−1) was chosen as a dose that revealed a significant hypothermia to MDMA, while producing only threshold effects at α2-adrenoceptors (see below). BMY 7378 (0.5 mg kg−1, 1.1 μmol kg−1) was chosen to block α1D-adrenoceptors (pKi of 8.78, -log M; Ki of 0.016 μM), with only threshold effects at α1A-adrenoceptors (6.32, 0.48 μM) and limited effects at α1B-adrenoceptors (6.74, 0.18 μM) (see Honner and Docherty, 1999). RS 100329 (0.1 mg kg−1, 0.214 μmol kg−1) was chosen to block α1A-adrenoceptors (pKi of 9.6, 0.25 nM), with only limited effects at α1D-adrenoceptors (7.9, 0.013 μM) and α1B-adrenoceptors (pKi of 7.50, 0.032 μM) (Williams et al., 1999). Cyclazosin (1 mg kg−1, 2.1 μmol kg−1) was chosen to block α1B-adrenoceptors, given the uncertainty of its potency at these receptors (pA2/pKa values; 8.85, 1.4 nM, Marucci et al., 2005; 7.96, 0.011 μM, Stam et al., 1998).
In this study, we have demonstrated that specific blockade of α1-adrenoceptors with prazosin altered the hyperthermic response to MDMA in mice. In the presence of prazosin, the monophasic hyperthermic response produced by MDMA in WT mice became a biphasic response, with an initial hypothermic response followed by a hyperthermic response. Our results are consistent with other studies demonstrating the involvement of α1-adrenoceptors in the increase in core temperature seen after treatment with MDMA (Sprague et al, 2003). α1-Adrenoceptors have been shown to be present on thermoregulatory pathways within the CNS, which regulate sympathetic outflow to cutaneous vessels and brown adipose tissue (Boulant 2000; Mallick et al., 2002). In the periphery, α1-adrenoceptors are found on cutaneous blood vessels regulating blood flow by vasoconstriction. They are also found on brown adipocytes where their activation potentiates β-adrenoceptor-mediated thermogenesis (Zhao et al., 1997). MDMA-induced hyperthermia has been shown to involve both cutaneous vasoconstriction and brown adipose tissue thermogenesis due to central sympathetic activation (Pedersen and Blessing, 2001; Blessing et al., 2006). Therefore, it can be suggested that α1-adrenoceptor blockade attenuates both central and peripheral sympathetic outflow to brown adipose tissue and cutaneous blood vessels, causing a decrease in heat production and an increase in heat loss leading to a decrease in body temperature. In addition to a decrease in sympathetic outflow, direct peripheral antagonism of α1-adrenoceptors on the vasculature and brown adipocytes could also contribute to the hypothermic response seen in animals pretreated with prazosin prior to the administration of MDMA.
As prazosin alone did not significantly affect temperature and as prazosin revealed a hypothermia to MDMA, it seems that prazosin targets α1-adrenoceptors in vasculature and/or adipocytes that are tonically activated only in the presence of MDMA, or that block of α1-adrenoceptors reveals a hypothermic response to MDMA involving other receptors.
Even when prazosin and other antagonists or combinations revealed a hypothermia to MDMA, a later hyperthermia developed. Although pharmacokinetic factors cannot be ruled out, it seems more likely that this can be explained by actions of MDMA on other receptor systems or another component of temperature regulation. It can be noted from Figure 2 that the recovery time from hypothermia to hyperthermia is similar for all drugs. Other authors have reported that prazosin can prevent but not reverse the hyperthermia to MDMA in mice (Fantegrossi et al., 2004), suggesting that the later hyperthermia following prazosin in our studies has little to do with pharmacokinetics and more to do with a later hyperthermic component to the response to MDMA.
Prazosin is an antagonist selective for α1-adrenoceptors but displays high affinity for all the three subtypes: α1A-, α1B-, α1D-adrenoceptors (pKi>9) (Docherty, 1998; Guimaraes and Moura, 2001). All the three subtypes are found both centrally and peripherally, but their distribution and function varies between tissues. In the CNS, localization and the precise functional roles of the specific subtypes remain uncertain (Tanoue et al, 2002; Hague et al, 2003). In the vascular tree, all the three subtypes have been shown to be expressed and there is diversity in the α1-adrenoceptor subtype mediating vascular contraction (Tanoue et al, 2002). Studies have shown that one or two of the α1-adrenoceptor subtypes usually predominate in the contraction of a given blood vessel. Functional studies using isolated blood vessels suggest that α1A- and α1D-adrenoceptors primarily control vasoconstriction with a minor contribution from α1B-adrenoceptors (Docherty, 1998; Guimaraes and Moura, 2001; Hague et al., 2003). Studies have also demonstrated that both α1A- and α1D-adrenoceptor mRNA expressions are high in brown adipose tissue, with α1B-adrenoceptor expression being very low or not expressed at all (Granneman et al., 1997; Kikuchi-Utsumi et al, 1997; Chernogubova et al., 2005), although α1D-adrenoceptor-binding sites were not detected (levels <2% of α1A binding) (Granneman et al., 1997). However, in rat vas deferens, α1D-adrenoceptors are difficult to detect by ligand-binding studies, except after chemical sympathectomy, because they are usually localized in junctional areas to mediate actions of nerve-released neurotransmitter (Cleary et al., 2004).
The main purpose of our study was to investigate the relative participation of the three α1-adrenoceptor subtypes in the hyperthermic response induced by MDMA. We compared the effects of the α1A-adrenoceptor selective antagonist RS 100329, the α1D-adrenoceptor selective antagonist BMY 7378 and the putative α1B-adrenoceptor antagonist cyclazosin on MDMA-induced hyperthermia. Individually, RS 100329 and BMY 7378 did not significantly alter the hyperthermic response produced by MDMA. However, when RS 100329 and BMY 7378 were administered simultaneously, a biphasic response to MDMA was obtained: hypothermia followed by hyperthermia. The hypothermic response was significantly different from the hyperthermic response seen in mice treated with MDMA alone. From these results, it is suggested that both α1A- and α1D-adrenoceptors are involved, and blockade of only one subtype was not sufficient to alter the thermoregulatory effect of MDMA. As BMY 7378, at a dose of 0.25 mg kg−1, s.c., has been shown to reduce central 5-HT release (Hjorth et al, 1995), it cannot be ruled out that the reversal of the hyperthermic response also involves 5-HT1A autoreceptor agonism but admittedly only in the presence of α1A-adrenoceptor blockade. Given the similar actions of prazosin, it is more likely that BMY 7378 is acting as an α1D-adrenoceptor antagonist when effective in combination with RS 100329.
Admittedly, cyclazosin also revealed a biphasic response to MDMA. Discrepancies exist in the literature regarding the selectivity of cyclazosin. Studies have demonstrated that cyclazosin either is highly selective for α1B-adrenoceptors (see Marucci et al, 2005) or shows relatively equal affinity for all three α1-adrenoceptor subtypes (Stam et al, 1998). The hypothermic response seen with cyclazosin or with the co-administration of RS 100329 and BMY 7378 was similar to the hypothermic response seen with prazosin alone. Perhaps the crucial fact to suggest that α1B-adrenoceptors are not important in these responses is that RS 100329 has limited potency, but it is more potent than BMY 7378, at α1B-adrenoceptors (compare Williams et al., 1999 with values quoted in Honner and Docherty, 1999). Furthermore, RS 100329 has similar affinities for α1B- and α1D-adrenoceptors (Williams et al., 1999); therefore, any action at α1B-adrenoceptors should be matched by action at α1D-adrenoceptors. Hence, it is difficult to see how the combination of RS 100329 and BMY 7378 would have markedly more effect than RS 100329 alone at α1B-adrenoceptors.
In a previous study, we demonstrated that α2A-adrenoceptors were involved in the hyperthermic response produced by MDMA and in their absence a biphasic response was obtained (Bexis and Docherty, 2005). Briefly, α2-adrenoceptors are found presynaptically as inhibitory receptors regulating the release of noradrenaline (autoreceptors) and other neurotransmitters, such as dopamine and 5-HT (heteroreceptors) in the central and peripheral nervous systems (Philipp et al., 2002; Brede et al., 2004), as well as being present postsynaptically in a number of cell types including smooth muscle. Since monoaminergic systems are interconnected and can influence each other, it was thereby suggested that, under the condition of increased extracellular levels of the three monoamines produced by MDMA, concomitant activation of neuronal α2A-adrenoceptors could result in a component of the hyperthermic response. In addition, α2A-adrenoceptors have been shown to contribute to systemic vasoconstriction (Gavin and Docherty, 1996; Duka et al, 2000) and thus may contribute to cutaneous vasoconstriction. However, α2A-adrenoceptors have been demonstrated to counteract β-adrenoceptor-mediated lipolysis (Stich et al., 2003); therefore, it is perhaps surprising that hypothermia results in the α2A-adrenoceptor knock-out mouse.
As tests in α2A-adrenoceptor knock-out mice or α2A-adrenoceptor antagonism in WT mice also reveals a hypotensive component of the response to MDMA, we have to consider whether actions of prazosin or BMY 7378 in combination with RS 100329 could be explained by α2A-adrenoceptor blockade. We have a number of reasons to suggest that the actions of these agents are mainly at α1-adrenoceptors. Firstly, prazosin (0.1 mg kg−1, 0.24 μmol kg−1) would have threshold effects only at α2A-adrenoceptors (pKi of 6.24, 0.58 μM) and minimal effects at α2B-adrenoceptors (7.12, 0.08 μM) and α2C-adrenoceptors (6.92, 0.12 μM) (Ho et al., 1998). Secondly, BMY 7378 (0.5 mg kg−1, 1.1 μmol kg−1) would have threshold effects at α2C-adrenoceptors (pKi of 6.54, 0.29 μM), but no effect would be expected at α2A-adrenoceptors (pKi of 5.48, 3.3 μM) or α2B-adrenoceptors (pKi of 5.23, 5.9 μM) (Cleary et al., 2005). Incidentally, BRL 44408 (1 mg kg−1, 3.02 μmol kg−1) would have major effects at α2A-adrenoceptors (7.77, 0.017 μM) but threshold effects at α2B-adrenoceptors (6.11, 0.78 μM) and α2C-adrenoceptors (6.28, 0.52 μM) (Ho et al., 1998). Thirdly, RS 100329 (0.1 mg kg−1, 0.214 μmol kg−1) would have minimal effects at α2A-adrenoceptors (prejunctional actions above 0.1 μM in functional studies; Cleary et al., 2003). Overall, it is difficult to see how α2-adrenoceptor antagonism could explain actions of prazosin or BMY 7378/RS 100329 in this study. Indeed, BRL 44408 in the dose used is more likely to have a component of its actions at α1A-adrenoceptors (pKi of 6.59, 0.26 μM; Cleary et al., 2003).
Our results further confirm the involvement of both α2A- and α1-adrenoceptors in MDMA-induced hyperthermia. Other studies have demonstrated the involvement of β3-adrenoceptors in MDMA-induced hyperthermia, at least in rats (Sprague et al, 2004, 2005). However, antagonism of α-adrenoceptors does not prevent the later component of the hyperthermia produced by MDMA, suggesting that other neurotransmitters and/or hormones are involved. Changes in the level of thyroid hormone influence the thermogenic response of brown adipose tissue (Silva, 2003), and thyroid hormone is involved in MDMA-induced hyperthermia (Sprague et al., 2003). Centrally released 5-HT and dopamine have been demonstrated to increase thermoregulatory cutaneous and brown adipose tissue sympathetic outflow (Ootsuka et al, 2004; Ootsuka and Blessing, 2006). It has also been demonstrated that activation of the uncoupling protein-3, a skeletal thermogenic protein, is involved in MDMA-induced hyperthermia (Mills et al, 2003).
In conclusion, more than one subtype of α1-adrenoceptor is involved in a component of the hyperthermic response to MDMA in mouse, probably both α1A- and α1D-adrenoceptors, and removal of this α1-adrenoceptor-mediated component reveals an initial hypothermia.
Acknowledgments
Supported by the Health Research Board (Ireland). MDMA was generously supplied under the NIDA Drug Supply Program.
Abbreviations
- BMY 7378
(8-(2-(4-(2-methoxyphenyl)-1-piperazinyl)ethyl)-8-azaspiro(4.5) decane-7,9-dione
- MDMA
methylenedioxy methamphetamine
- RS 100329
5-methyl-3-(3-(4-(2-(2,2,2,-trifluoroethoxy)phenyl)-1-piperazinyl)propyl)-2,4-(1H)-pyrimidinedione
- WT
wild type
Conflict of interest
The authors state no conflict of interest.
References
- Battaglia G, Brooks BP, Kulsakdinun C, De Souza EB. Pharmacologic profile of MDMA (3,4-methylenedioxymethamphetamine) at various brain recognition sites. Eur J Pharmacol. 1988;149:159–163. doi: 10.1016/0014-2999(88)90056-8. [DOI] [PubMed] [Google Scholar]
- Bexis S, Docherty JR. Role of alpha2A-adrenoceptors in the effects of MDMA on body temperature in the mouse. Br J Pharmacol. 2005;146:1–6. doi: 10.1038/sj.bjp.0706320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bexis S, Docherty JR.Involvement of adrenoceptors in the temperature effects of MDMA in the mouse 2006. Proceedings of the British Pharmacological Society at
- Blessing WW, Zilm A, Ootsuka Y. Clozapine reverses increased brown adipose tissue thermogenesis induced by 3,4-methylenedioxymethamphatamine and by cold exposure in conscious rats. Neuroscience. 2006;141:2067–2073. doi: 10.1016/j.neuroscience.2006.05.050. [DOI] [PubMed] [Google Scholar]
- Boulant JA. Role of the preoptic-anterior hypothalamus in thermoregulation and fever. Clin Infect Dis. 2000;31:S157–S161. doi: 10.1086/317521. [DOI] [PubMed] [Google Scholar]
- Brede M, Philipp M, Knaus A, Mothig V, Hein L. Alpha2-adrenergic receptor subtypes—novel functions uncovered in gene-targeted mouse models. Biol Cell. 2004;96:343–348. doi: 10.1016/j.biolcel.2004.03.006. [DOI] [PubMed] [Google Scholar]
- Chernogubova E, Hutchinson DS, Nedergaard J, Bengtsson T. α1- and β1-adrenoceptor signaling fully compensates for β3-adrenoceptor deficiency in brown adipocyte norepinephrine-stimulated glucose uptake. Endocrinology. 2005;146:2271–2284. doi: 10.1210/en.2004-1104. [DOI] [PubMed] [Google Scholar]
- Cleary L, Slattery J, Bexis S, Docherty JR. Sympathectomy reveals alpha1A- and alpha1D-adrenoceptor components to contractions to noradrenaline in rat vas deferens. Br J Pharmacol. 2004;143:745–752. doi: 10.1038/sj.bjp.0705987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleary L, Murad K, Bexis S, Docherty JR. The alpha1D-adrenoceptor antagonist BMY 7378 is also an alpha2C-adrenoceptor antagonist. Auton Autacoid Pharmacol. 2005;25:135–141. doi: 10.1111/j.1474-8673.2005.00342.x. [DOI] [PubMed] [Google Scholar]
- Cleary L, Vandeputte C, Docherty JR. Investigation of postjunctional alpha1- and alpha2-adrenoceptor subtypes in vas deferens from wild-type and alpha(2A/D)-adrenoceptor knockout mice. Br J Pharmacol. 2003;138:1069–1076. doi: 10.1038/sj.bjp.0705137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Docherty JR. Subtypes of functional alpha1- and alpha2-adrenoceptors. Eur J Pharmacol. 1998;361:1–15. doi: 10.1016/s0014-2999(98)00682-7. [DOI] [PubMed] [Google Scholar]
- Duka I, Gavras I, Johns C, Handy DE, Gavras H. Role of the postsynaptic alpha2-adrenergic receptor subtypes in catecholamine-induced vasoconstriction. Gen Pharmacol. 2000;34:101–106. doi: 10.1016/s0306-3623(00)00051-3. [DOI] [PubMed] [Google Scholar]
- Fantegrossi WE, Kiessel CL, Leach PT, Van Martin C, Karabenick RL, Chen X, et al. Nantenine: an antagonist of the behavioral and physiological effects of MDMA in mice. Psychopharmacology. 2004;173:270–277. doi: 10.1007/s00213-003-1741-2. [DOI] [PubMed] [Google Scholar]
- Gavin KT, Docherty JR. Investigation of the subtype of alpha2-adrenoceptor mediating pressor responses in the pithed rat. Eur J Pharmacol. 1996;318:81–87. doi: 10.1016/s0014-2999(96)00780-7. [DOI] [PubMed] [Google Scholar]
- Gordon CJ, Watkinson WP, O'Callaghan JP, Miller DB. Effects of 3,4-methylenedioxymethamphatamine on autonomic thermoregulatory responses of the rat. Pharmacol Biochem Behav. 1991;38:339–344. doi: 10.1016/0091-3057(91)90288-d. [DOI] [PubMed] [Google Scholar]
- Granneman JG, Zhai Y, Lahners KN. Selective up-regulation of α1A-adrenergic receptor protein and mRNA in brown adipose tissue by neural and β3-adrenergic stimulation. Mol Pharmacol. 1997;51:644–650. doi: 10.1124/mol.51.4.644. [DOI] [PubMed] [Google Scholar]
- Guimaraes S, Moura D. Vascular adrenoceptors: an update. Pharmacol Rev. 2001;53:319–356. [PubMed] [Google Scholar]
- Hague C, Chen Z, Uberti M, Minneman KP. α1-Adrenergic receptor subtypes: non-identical triplets with different dancing partners. Life Sci. 2003;74:411–418. doi: 10.1016/j.lfs.2003.07.008. [DOI] [PubMed] [Google Scholar]
- Hjorth S, Bengtsson HJ, Milano S, Lundberg JF, Sharp T. Studies on the role of 5HT1A autoreceptors and α1-adrenoceptors in the inhibition of 5HT release- I. BMY7378 and prazosin. Neuropharmacology. 1995;34:615–620. doi: 10.1016/0028-3908(95)00038-8. [DOI] [PubMed] [Google Scholar]
- Ho SL, Honner V, Docherty JR. Investigation of the subtypes of alpha2-adrenoceptor mediating prejunctional inhibition in rat atrium and cerebral cortex. Naunyn Schmiedebergs Arch Pharmacol. 1998;357:634–639. doi: 10.1007/pl00005218. [DOI] [PubMed] [Google Scholar]
- Honner V, Docherty JR. Investigation of the subtypes of alpha1-adrenoceptor mediating contractions of rat vas deferens. Br J Pharmacol. 1999;128:1323–1331. doi: 10.1038/sj.bjp.0702913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuchi-Utsumi K, Kikuchi-Utsumi M, Cannon B, Nedergaard J. Differential regulation of the expression of α1-adrenergic receptor subtype genes in brown adipose tissue. Biochem J. 1997;322:417–424. doi: 10.1042/bj3220417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavelle A, Honner V, Docherty JR. Investigation of the prejunctional α2-adrenoceptor mediated actions of MDMA in rat atrium and vas deferens. Br J Pharmacol. 1999;128:975–980. doi: 10.1038/sj.bjp.0702875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malberg JE, Seiden LS. Small changes in ambient temperature cause large changes in 3,4-methylenedioxymethamphetamine (MDMA)-induced serotonin neurotoxicity and core body temperature in the rat. J Neurosci. 1988;18:5086–5094. doi: 10.1523/JNEUROSCI.18-13-05086.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallick BN, Jha SK, Islam F. Presence of α1-adrenoceptors on thermosensitive neurons in the medial preoptic-anterior hypothalamic area in rats. Neuropharmacology. 2002;42:697–705. doi: 10.1016/s0028-3908(02)00016-3. [DOI] [PubMed] [Google Scholar]
- Marucci G, Angeli P, Buccioni M, Gulini U, Melchiorre C, Sagratini G, et al. Cyclazosin, a selective α1B-adrenoceptor antagonist: functional evaluation in rat and rabbit tissues. Eur J Pharmacology. 2005;522:100–107. doi: 10.1016/j.ejphar.2005.08.044. [DOI] [PubMed] [Google Scholar]
- McDaid J, Docherty JR. Vascular actions of MDMA involve α1 and α2-adrenoceptors in the anaesthetized rat. Br J Pharmacol. 2001;133:429–437. doi: 10.1038/sj.bjp.0704094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills EM, Banks ML, Sprague JE, Toren F. Uncoupling the agony from the ecstasy. Nature. 2003;426:403–404. doi: 10.1038/426403a. [DOI] [PubMed] [Google Scholar]
- Morrison SF. Central pathway controlling brown adipose tissue thermogenesis. News Physiol Sci. 2004;19:67–74. doi: 10.1152/nips.01502.2003. [DOI] [PubMed] [Google Scholar]
- Ootsuka Y, Blessing WW. Thermogenesis in brown adipose tissue: increase by 5HT2A receptor activation and decrease by 5HT1A receptor activation in conscious rats. Neurosci Lett. 2006;395:170–174. doi: 10.1016/j.neulet.2005.10.062. [DOI] [PubMed] [Google Scholar]
- Ootsuka Y, Nalivaiko E, Blessing WW. Spinal 5HT2A receptors regulate cutaneous sympathetic vasomotor outflow in rabbits and rats; relevance for cutaneous vasoconstriction elicited by MDMA (3,4-methylenedioxymethamphetamine, ‘Ecstasy') and its reversal by clozapine. Brain Res. 2004;1014:34–44. doi: 10.1016/j.brainres.2004.03.058. [DOI] [PubMed] [Google Scholar]
- Pedersen NP, Blessing WW. Cutaneous vasoconstriction contributes to hyperthermia induced by 3,4-methylenedioxymethamphetamine (Ecstasy) in conscious rabbits. J Neurosci. 2001;21:8648–8654. doi: 10.1523/JNEUROSCI.21-21-08648.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philipp M, Brede M, Hein L. Physiological significance of α2-adrenergic receptor subtype diversity: one receptor is not enough. Am J Physiol. 2002;283:287–295. doi: 10.1152/ajpregu.00123.2002. [DOI] [PubMed] [Google Scholar]
- Silva JE. The thermogenic effect of thyroid hormone and its clinical implications. Ann Intern Med. 2003;139:205–213. [PubMed] [Google Scholar]
- Sprague JE, Banks ML, Cook VJ, Mills EM. Hypothalamic–pituitary–thyroid axis and sympathetic nervous system involvement in hyperthermia induced by 3,4-methylenedioxymethamphatamine (Ecstasy) J Pharmacol Exp Ther. 2003;305:159–166. doi: 10.1124/jpet.102.044982. [DOI] [PubMed] [Google Scholar]
- Sprague JE, Brutcher RE, Mills EM, Caden D, Rusyniak DE. Attenuation of 3,4-methylenedioxymethamphatamine (MDMA, Ecstasy)-induced rhabdomyolysis with α1- plus β3-adrenoceptor antagonists. Br J Pharmacol. 2004;142:667–670. doi: 10.1038/sj.bjp.0705823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprague JE, Moza P, Caden D, Rusyniak DE, Homes C, Goldstein DS, et al. Carvedilol reverses hyperthermia and attenuates rhabdomyolysis induced by 3,4-methylenedioxymethamphatamine (MDMA, Ecstasy) in an animal model. Crit Care Med. 2005;33:1311–1316. doi: 10.1097/01.ccm.0000165969.29002.70. [DOI] [PubMed] [Google Scholar]
- Stam WB, Van Ser Graaf PH, Saxena PR. Functional characterization of the pharmacological profile of the putative α1B-adrenoceptor antagonist (+)-cyclazosin. Eur J Pharmacol. 1998;361:79–83. doi: 10.1016/s0014-2999(98)00735-3. [DOI] [PubMed] [Google Scholar]
- Stich V, Pelikanova T, Wohl P, Sengenes C, Zakaroff-Girard A, Lafontan M, et al. Activation of a2-adrenergic receptors blunts epinephrine-induced lipolysis in subcutaneous adipose tissue during a hyperinsulinemic euglycemic clamp in men. Am J Physiol Endicronol Metab. 2003;285:E599–E607. doi: 10.1152/ajpendo.00502.2002. [DOI] [PubMed] [Google Scholar]
- Stone EA, Lin Y, Itteera A, Quartermain D. Pharmacological evidence for the role of central alpha1B-adrenoceptors in the motor activity and spontaneous movement of mice. Neuropharmacology. 2001;40:254–261. doi: 10.1016/s0028-3908(00)00151-9. [DOI] [PubMed] [Google Scholar]
- Tanoue A, Koshimizu T, Tsujimoto G. Transgenic studies of α1-adrenergic receptor subtype function. Life Sci. 2002;71:2207–2215. doi: 10.1016/s0024-3205(02)02012-x. [DOI] [PubMed] [Google Scholar]
- White SR, Obradovic T, Imel KM, Wheaton MJ. The effects of methylenedioxymethamphetamine (MDMA, ‘ecstasy') on monoaminergic neurotransmission in the central nervous system. Prog Neurobiol. 1996;49:455–479. doi: 10.1016/0301-0082(96)00027-5. [DOI] [PubMed] [Google Scholar]
- Williams TJ, Blue DR, Daniels DV, Davis B, Elworthy T, Gever JR, et al. In vitro α1-adrenoceptor pharmacology of Ro70-0004 and RS-100329, novel α1A-adrenoceptor selective antagonists. Br J Pharmacology. 1999;127:252–258. doi: 10.1038/sj.bjp.0702541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye J-M, Colquhoun EQ. Altered muscle metabolism associated with vasoconstriction in spontaneously hypertensive rats. Am J Physiol Endocrinol Metab. 1998;275:1007–1015. doi: 10.1152/ajpendo.1998.275.6.E1007. [DOI] [PubMed] [Google Scholar]
- Zhao J, Cannon B, Nedergaard J. α1-adrenergic stimulation potentiates the thermogenic action of β3-adrenoceptor-generated c-AMP in brown fat cells. J Biol Chem. 1997;275:32847–32856. doi: 10.1074/jbc.272.52.32847. [DOI] [PubMed] [Google Scholar]