Abstract
Background and purpose:
The cGMP-dependent protein kinase (PKG) is a key enzyme for nitrovasodilator-induced vasodilation. The present study was to determine its role in nitrate tolerance.
Experimental approach:
isolated porcine coronary arteries were incubated for 24 h with nitroglycerin (NTG) and their relaxant responses were determined. PKG activity was assayed by measuring the incorporation of 32P into BPDEtide. PKG protein was determined by Western blotting and PKG mRNA by real-time PCR.
Key results:
A 24 h incubation with NTG attenuated relaxation of coronary arteries to NTG, which was associated with decreased PKG activity. The nitrate tolerance induced with NTG at 10−7 M was affected by a scavenger of reactive oxygen species and the tolerance induced with NTG at 10−6 and 10−5 M showed cross-tolerance to DETA NONOate and 8-Br-cGMP (a cell permeable cGMP analogue). PKG protein and mRNA were down-regulated by a 24 h incubation with NTG at 10−5 M but not at 10−7 M. Acute exposure to exogenous superoxide inhibited PKG activity stimulated by NTG at 10−7 M but not at 10−5 M. Superoxide had no effect on PKG activity stimulated with exogenous cGMP.
Conclusions and implications:
Nitrate tolerance induced by NTG at low concentrations may result from an increased production of reactive oxygen species acting on sites upstream of PKG. The tolerance induced by NTG at higher concentrations may be in part due to suppression of PKG expression resulting from sustained activation of the enzyme. These distinct mechanisms of nitrate tolerance may be of clinical significance.
Keywords: nitric oxide, 8-Br-cGMP, Rp-8-Br-PET-cGMPS
Introduction
Nitroglycerine (NTG) is a widely used vasodilator in the treatment of angina pectoris and acute heart failure. It causes vasodilation after being converted inside the cell to nitric oxide (NO) or an NO-related intermediate, which elevates cyclic GMP (cGMP) following the activation of soluble guanylyl cyclase (Ignarro et al., 1981). The effectiveness of NTG is often plagued by the development of tolerance. This phenomenon was recognized a century ago (Stewart, 1888) and the underlying mechanisms are not yet fully understood (Parker, 2004; Csont and Ferdinandy, 2005; Münzel et al., 2005; Chen and Stamler, 2006). There is compelling evidence that an increased production of reactive oxygen species (ROS) might inhibit the biotransformation of NTG by thiol oxidation of mitochondrial aldehyde dehydrogenase (Münzel et al., 1995; Difabio et al., 2003; Sydow et al., 2004; Chen et al., 2005; Kollau et al., 2005). In addition, many other mechanisms seem to be involved, including decreased endothelial uptake of L-arginine (Gold et al., 1989; Abou-Mohamed et al., 2000), endothelial NO synthase uncoupling (Münzel et al., 2000), desensitization of soluble guanylyl cyclase (Molina et al., 1987; Romanin and Kukovetz, 1989) and upregulation of phosphodiesterases (Pagani et al., 1993; Kim et al., 2001; MacPherson et al., 2006). The mechanisms underlying nitrate tolerance may also differ depending on species and study models used (Parker, 2004; Münzel et al., 2005).
Cyclic GMP-dependent protein kinase (PKG) is a key mediator for relaxation of smooth muscle induced by cGMP-elevating agents such as NTG and NO (Hofmann et al., 2000). In human arteries and veins, nitrate tolerance is associated with decreased PKG activity (Schulz et al., 2002). In the arteries of rats and rabbits, nitrate tolerance induced by low-dose NTG is associated with decreased PKG activity, while the tolerance induced by high-dose NTG is associated with decreased PKG protein level and activity (Mülsch et al., 2001). Nitrate tolerance is also associated with an increased production of superoxide (Mülsch et al., 2001; Schulz et al., 2002). In the study by Mülsch et al. (2001), treatment with vitamin C improved tolerance, reduced vascular superoxide production and increased PKG activity. Hence, the decreased activity of PKG may result in part from an increased production of ROS.
In transgenic mice overexpressing endothelial NO synthase, basal cGMP levels of aortas are higher but PKG protein expression and activity are decreased (Ohashi et al., 1998; Yamashita et al., 2000). The decrease in PKG in these transgenic mice is probably not due to enhanced production of ROS, as increased ROS production would have bound to NO and resulted in decreased cGMP levels. Rather, it may be due to desensitization of PKG following continuous exposure to high basal levels of NO (Gao et al., 2004). This speculation has led us to hypothesize that following prolonged exposure to NTG, PKG may be downregulated by a negative feedback mechanism independent of ROS, by the action of a sustained high level of cGMP resulting from continuous activation of soluble guanylyl cyclase. The present study was undertaken to determine whether such a mechanism is involved in nitrate tolerance. Our results suggest that the decreased PKG activity associated with nitrate tolerance of porcine coronary arteries may result from two distinct mechanisms. The tolerance caused by NTG at low concentrations is mainly due to increased production of ROS, which act on sites upstream of PKG, whereas that caused by NTG at higher concentrations may be at least in part due to a direct suppression of PKG by a cGMP-mediated negative feedback mechanism, independent of ROS.
Methods
Porcine coronary arteries preparations
Fresh porcine hearts were collected from a local slaughterhouse. The porcine coronary left circumflex and left anterior descending arteries were carefully dissected and cut into rings (length 5 mm) in ice-cold modified Krebs–Ringer bicarbonate buffer (composition (in mM): NaCl, 118.3; KCl, 4.7; CaCl2, 2.5; MgSO4, 1.2; KH2PO4, 1.2; NaHCO3, 25.0; glucose, 11.1).
Organ chamber study
Rings of coronary arteries were incubated for 24 h in serum-free Dulbecco's modified Eagle's medium (Gibco, Paisley, Scotland, UK; supplemented with 2 mM glutamate, 100 U ml−1 penicillin, 100 μg ml−1 streptomycin, 0.25 μg ml−1 amphotericin B; 37±0.5 °C, 20% O2–5% CO2, pH 7.4) in the presence of solvent, NTG, or DETA NONOate [2,2′-(hydroxynitrosohydrazono) bis(ethanamine)] (a stable NO donor; Mooradian et al., 1995). In some experiments, 1H-(1,2,4)oxadiazolo(4,3-a)quinoxalin-1-one (ODQ) (3 × 10−5 M, an inhibitor of soluble guanylyl cyclase; Gerlach et al., 1993), N-acetyl-L-cysteine (NAC (10−2 M), a scavenger of ROS that acts a glutathione precursor; Nowicki et al., 2001; Park and Lee, 2006) or ebselen (10−4 M, a scavenger of ROS that acts by mimicking the antioxidants glutathione peroxidase, thioredoxin reductase and thioredoxin peroxidase; Zhao and Holmgren, 2002) was present.
To eliminate the possible involvement of endogenous prostanoids and endothelium-derived NO, indomethacin (10−5 M) and nitro-L-arginine (10−4 M) were included in the medium and present throughout the experiments (Gao et al., 2003).
Following incubation, vessel rings were repeatedly rinsed and suspended in organ chambers filled with 10 ml of modified Krebs-Ringer bicarbonate solution maintained at 37±0.5 °C and aerated with 95% O2-5% CO2 (pH 7.4). Two stirrups passed through the lumen suspended each ring. One stirrup was anchored to the bottom of the organ chamber and the other one connected to a strain gauge, and the isometric force was measured with a ML785 PowerLab/8sp recording and Analysis System (ADInstruments Pty Ltd, Castle Hill, Sydney, Australia) (Wang et al., 2006).
At the beginning of the experiment, each vessel ring was stretched to its optimal resting tension. This was achieved by stepwise stretching until the active contraction of the vessel ring to 100 mM KCl reached a plateau. The optimal resting tension of porcine coronary arteries was ∼2.5 g. After the vessels were brought to their optimal resting tension, 1 h of equilibration was allowed. Effects of NTG, DETA NONOate and 8-bromo-guanosine 3′5′-cyclic monophosphate (8-Br-cGMP) (a cell membrane-permeable analogue of cGMP; Meyer and Miller, 1974) were determined in vessels constricted with (9,11)-dideoxy-(11α,9α)-epoxymethanoprostaglandin F2α (U46619). The concentration–response curves to these dilators were constructed in a cumulative fashion.
PKG activity assay
Isolated porcine coronary arteries were incubated in serum-free Dulbecco's modified Eagle's medium for 24 h with NTG or DETA NONOate in the presence and absence of ODQ (3 × 10−5 M), an inhibitor of soluble guanylyl cyclase (Ignarro et al., 1981). They were then homogenized on ice in a buffer containing 50 mM Tris HCl (pH 7.4), 10 mM EDTA, 2 mM dithiothreotol, 1 mM isobutylmethylxanthine, 100 μM nitro-L-arginine and 10 μM indomethacin. The homogenate was sonicated and centrifuged at 14 000 g for 10 min at 4 °C. Supernatants were assayed for PKG activity by measuring the incorporation of 32P from γ-32P-labelled ATP into a specific PKG substrate BPDEtide (Biomol Research Laboratories, Plymouth Meeting, PA, USA). Aliquots (20 μl) of supernatant were added to a mixture (total volume 50 μl) containing 50 mM Tris HCl (pH 7.4), 20 mM MgCl2, 0.1 mM isobutylmethylxanthine, 10 μM indomethacin, 100 μM nitro-L-arginine, 150 μM BPDEtide, 1 μM PKI (a synthetic protein kinase A (PKA) inhibitor; Peninsula Laboratories, Belmont, CA, USA), and 0.2 mM γ-32P-labelled ATP (specific activity 3000 Ci mM−1). The mixture was incubated at 30 °C for 10 min in the presence or absence of 3 μM exogenous cGMP. The reaction was terminated by spotting 40-μl aliquots of mixture onto phosphocellulose papers (2 × 2 cm; P81 Whatman) and placing them in ice-cold 75 mM phosphoric acid. The filter papers were washed, dried and counted in a liquid scintillation counter. Assays were performed in triplicate with appropriate controls. PKG activity is expressed as picomoles of 32P incorporated into the PKG substrate per min per mg protein. Experiments confirmed the linearity of PKG activity at the protein concentration used (data not shown).
In some experiments, the effect of exogenous superoxide on PKG activity of isolated coronary arteries and of vessel homogenates was determined using xanthine oxidase (XO) plus pterine as a generator of superoxide (Atlante et al., 2000). In studies with isolated vessels, coronary arteries were incubated with NTG at 10−7 and 10−5 M for 10 min in the presence of XO (10 mU ml−1) plus pterine (10−4 M) or solvent. The vessels were then snap frozen, homogenized and assayed for the activity of PKG as described above. In studies with vessel homogenates, PKG activity was stimulated with exogenous cGMP in the presence XO (10 mU ml−1) plus pterine (10−4 M) or solvent.
Western blot analysis of PKG protein
Tissue lysates were prepared from isolated porcine coronary arteries incubated for 24 h, as described earlier, in the presence of solvent, NTG (10−7 M), NTG (10−5 M) or DETA NONOate (10−5 M). In some experiments, ODQ (3 × 10−5 M) or β-Phenyl-1,N2-etheno-8-bromoguanosine-3′,5′-cyclic monophosphorothioate, Rp isomer (Rp-8-Br-PET-cGMPS, 3 × 10−5 M) was included in the incubation medium.
The lysates, each containing 30 μg of protein, were subjected to SDS-polyacrylamide gel electrophoresis and electro-transferred to polyvinylidene difluoride. Nonspecific binding of antibody was blocked by washing with tris-buffered saline (TBS) buffer containing 10% milk for 1 h. The blot was then subjected to two brief washes with TBS plus 0.5% Tween-20, incubated with the PKG antibody (an affinity-purified polyclonal antibody detecting a 75-kDa protein, corresponding to the apparent molecular mass of PKG type Iα and Iβ on SDS-polyacrylamide gel electrophoresis immunoblot, of human, mouse and Xenopus origins (Stressgen, Victoria, Canada); 1:5000 dilution) for 1 h and the secondary antibody (1:5000 dilution) for 40 min. It was developed using the chemiluminescent detection method (Amersham ECL, Amersham Pharmacia Biotech, Buckinghamshire, UK). The PKG protein present in blots was quantified by densitometry using a Gel Doc 2000 densitometre (Bio-Rad, Hercules, CA, USA) and normalized to scanning signals of actin (Calbiochem, La Jolla, CA, USA).
Real-time polymerase chain reaction for PKG Iα and PKG Iβ mRNAs
Total RNA was extracted from coronary arteries using TRIZOL reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer's protocol. The blood vessels were preincubated for 24 h, as described earlier, in the presence of solvent, NTG, DETA NONOate or 8-Br-cGMP (10−4 M). In some experiments, Rp-8-Br-PET-cGMPS was present in the incubation medium.
Complementary DNA was generated from 5 μg of total RNA using Promega reverse transcription reagent. Polymerase chain reaction (PCR) was carried out using primers for PKG Iα (sense: 5′-cTGGAGGAAGACTTTGCCAAGATTC-3′ and antisense: 5′-TCGGATTTGGTGAACTTCCGGAATG-3′ (GenBank accession number X160886 at base pair 16–40 and 269–245, respectively)) and PKG Iβ (sense: 5′-CCCTGCGGGATTTACAGTATG-3′ and antisense: 5′-GGTCACATGGCTGAGATCCTG-3′ (GenBank accession number X54289 at base pair 171–191 and 448–428, respectively)). As an internal control, the porcine β-actin cDNA was amplified using the following primers: sense, 5′-TCCCTGGAGAAGAGCTACGAG-3′ and antisense, 5′-GGGCGATGATCTTGATCTTCA-3′ (GenBank accession number SSU07786 at base pair 337–357 and 608–588, respectively).
Real-time PCR was performed using the DNA Engine Opticon (MJ Research, Reno, NV, USA). Briefly, a mixture of the following reaction components was prepared to the indicated final –concentration: 0.5 μM sense primer (AuGCT, Beijng, China), 0.5 μM antisense primer (AuGCT), 1 × PCR buffer (Promega, Madison, WI, USA), 1.5 mM MgCl2 (Promega), 200 μM dNTP (KeHaoDa Biotechnology, Beijing, China), 1 × SYBR Green I (Bio-Vision, XiaMen, China) and 50 000 U l−1 Taq DNA polymerase (Promega). Then, 18 μl of the mixture was filled into one of Low tube strips (Bio-Rad) and 2 μl of cDNA (produced as described above) was added as the PCR template. Low tube strips (Bio-Rad) were closed by Flat cap strips (Bio-Rad) and placed into the DNA Engine Opticon. The following experimental protocol was used: denaturation (94 °C for 5 min) followed by an amplification program repeated for 40 cycles (94 °C for 30 s, gradient 55–57 °C for 45 s and then 72 °C for 60 s) using a single fluorescence measurement. Also, a melting-curve program (60–95 °C with a heating rate of 0.1 °C s−1 with continuous fluorescence measurement) was run and finally a cooling step to 4 °C. The specificity of each PCR product was verified by the melting-curve analysis and gel electrophoresis.
Data analyses
Data are shown as means±s.e.mean. When mean values of two groups were compared, Student's t-test for unpaired observations was used. Student's t-test for paired observations was used when the mean values of the same group before and after stimulation were compared. Comparison of mean values of more than two groups was performed with one-way analysis of variance test with Student–Newman–Keuls test for post hoc testing of multiple comparisons. Statistical significance was accepted when the P-value (two tailed) was less than 0.05. In all experiments, n represents the number of animals.
Drugs
The following drugs were used (unless otherwise specified, all were obtained from Sigma, St Louis, MO, USA): 8-bromo-adenosine 3′,5′-cyclic monophosphate, 8-Br-cGMP, DETA NONOate, ebselen, indomethacin, nitro-L-arginine, N-acetyl-L-cysteine, NTG (Beijing Yimin Pharmaceutical Co. Ltd, Beijing, China), ODQ, pterine, Rp-8-Br-PET-cGMPS (Biolog Life Science Institute, Bremen, Germany), U46619 ((9,11)-dideoxy-(11α,9α)-epoxymethanoprostaglandin F2α) and XO.
1H-(1,2,4)oxadiazolo(4,3-a)quinoxalin-1-one and U46619 were dissolved in dimethyl sulphoxide (final concentration <0.2%). Preliminary experiments showed that dimethyl sulphoxide at the concentration used had no effect on contraction to U46619 and relaxation induced by the NO donor and cGMP analogues. The other drugs were prepared using distilled water.
Results
Organ chamber studies
Rings of porcine coronary arteries were incubated for 24 h with NTG or DETA NONOate (a stable NO donor), and then repeatedly rinsed and constricted with U46619 from 10−7 to 3 × 10−7 M to a similar tension level before testing their relaxant responses. The increase in tension of different vessel groups evoked by U46619 was not significantly different (ranged from 7.44±1.30 to 8.64±1.33 g, n=6–10 for each group, P>0.05).
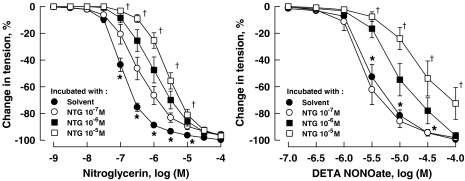
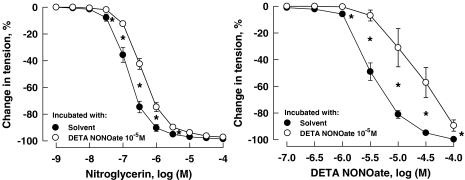
A 24 h incubation of coronary arteries with NTG significantly attenuated its subsequent response to the nitrovasodilator. Incubation of the vessels with NTG at higher concentrations (10−6 and 10−5 M) but not the low concentration (10−7 M) also reduced the response of coronary arteries to DETA NONOate (Figure 1; Table 1). Incubation of the vessels for 24 h with DETA NONOate (10−5 M) reduced the response to both the NO donor and NTG (Figure 2; Table 1). The attenuation of the relaxation response of coronary arteries induced by 24 h incubation with NTG at the high concentration of 10−5 M was reversed by 26.2±3.1%, 24 h after washout of NTG (data not shown, n=7–10, P<0.05).
Figure 1.
Relaxations of porcine coronary arteries to NTG (left panel) and DETA NONOate (right panel) following 24h incubation with NTG. Vessels were constricted with U46619 (10−7 to 3 × 10−7 M) before testing their relaxant responses. Data are shown as means±s.e.mean; n=7–10 for each group. *Significantly different from vessels incubated with NTG from 10−7 to 10−5 M (left panel) or with NTG at 10−6 and 10−5 M (right panel) (P<0.05); †significantly different from vessels incubated with NTG at 10−7 and 10−6 M (P<0.05). DETA NONOate, [2,2′-(hydroxynitrosohydrazono) bis(ethanamine)]; NTG, nitroglycerine; U46619, (9,11)-dideoxy-(11α,9α)-epoxymethanoprostaglandin F2α.
Table 1.
Effect of 24 h incubation with NTG and DETA NONOate (DETA NO) on response of porcine coronary arteries
|
Relaxation to NTG |
Relaxation to DETA NO |
|||
|---|---|---|---|---|
| EC50, −log (M) | Max relaxation, % | EC50, −log (M) | Max relaxation, % | |
| 24 h incubation with: | ||||
| Solvent | 6.82±0.09 | 99.74±0.20 | 5.48±0.08 | 99.60±0.32 |
| NTG, 10−7 M | 6.32±0.11a | 96.80±1.65 | 5.57±0.10 | 98.22±1.12 |
| NTG, 10−6 M | 6.01±0.17a | 96.38±1.13 | 4.99±0.14a | 96.64±1.89 |
| NTG, 10−5 M | 5.58±0.10a,b | 95.47±2.48 | 4.37±0.33a,b | 72.54±11.86a |
| 24 h incubation with: | ||||
| Solvent | 6.82±0.04 | 99.09±0.07 | 5.45±0.06 | 99.73±0.07 |
| DETA NO, 10−5 M | 6.39±0.04a | 98.28±0.07 | 4.67±0.17a | 89.41±0.07a |
Abbreviations: DETA NONOate, [2,2′-(hydroxynitrosohydrazono) bis(ethanamine)]; NTG, nitroglycerine.
Relaxation of porcine coronary arteries to NTG and DETA NO were determined in vessels constricted with U46619 (10−7 to 3 × 10−7 M) to a similar the tension level. Data are expressed as means±s.e.mean, n=6–10 for each group.
Significantly different from vessels incubated with solvent (P<0.05).
Significantly different from vessels incubated with NTG at 10−7 and 10−6 M (P<0.05).
Figure 2.
Relaxations of porcine coronary arteries to NTG (left panel) and DETA NONOate (right panel) following 24 h incubation with DETA NONOate at 10−5 M. Vessels were constricted with U46619 (10−7 to 3 × 10−7 M) before testing their relaxant responses. Data are shown as means±s.e.mean; n=6 for each group. *Significant difference between vessels incubated with solvent and those with DETA NONOate at 10−5 M (P<0.05). DETA NONOate, [2,2′-(hydroxynitrosohydrazono) bis(ethanamine)]; NTG, nitroglycerine; U46619, (9,11)-dideoxy-(11α,9α)-epoxymethanoprostaglandin F2α.
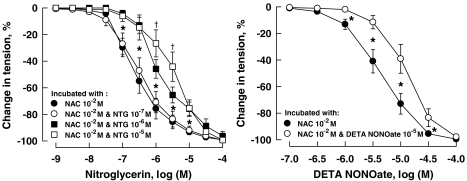
The attenuated response of coronary arteries to NTG caused by a 24 h incubation with the nitrovasodilator at low concentration (10−7 M) was prevented by the presence in the incubation medium of NAC (10−2 M), a scavenger of ROS, which acts as a glutathione precursor. The effect of incubation with NTG at higher concentrations (10−6 or 10−5 M) or with DETA NONOate (10−5 M) was not affected by NAC (Figure 3; Table 2). The effect of incubation with the higher concentration of NTG was also not affected by ebselen, a scavenger of ROS that mimics the activities of the antioxidant enzymes, glutathione peroxidase, thioredoxin reductase and thioredoxin peroxidase. The EC50 of relaxation to NTG was −5.58±0.10 (log M) and −5.84±0.17 (log M) for vessels incubated for 24 h with NTG (10−5 M) alone and those incubated with ebselen (10−4 M) plus NTG (10−5 M), respectively (n=9–10 for each group, P>0.05). There was also no significant difference in the EC50 of relaxation to NTG between vessels incubated with solvent and those with ebselen (10−4 M; −6.82±0.09 (log M) and −7.11±0.17 (log M), respectively; n=9–10 for each group, P>0.05).
Figure 3.
Relaxations of porcine coronary arteries to NTG (left panel) and DETA NONOate (right panel) following 24 h incubation with the respective nitrovasodilators plus NAC (10−2 M). Vessels were constricted with U46619 (10−7 to 3 × 10−7 M) before testing their relaxant responses. Data are shown as means±s.e.mean; n=6–8 for each group. *Significant difference between vessels incubated with NAC and those with NTG at 10−6 M plus NAC (left panel) or between vessels incubated with NAC and DETA NONOate at 10−5 M plus NAC (right panel) (P<0.05); †significantly different from vessels incubated with NTG at 10−6 M plus NAC (left panel) (P<0.05). DETA NONOate, [2,2′-(hydroxynitrosohydrazono) bis(ethanamine)]; NTG, nitroglycerine; NAC, N-acetyl-L-cysteine; U46619, (9,11)-dideoxy-(11α,9α)-epoxymethanoprostaglandin F2α.
Table 2.
Effect of 24 h incubation with NTG and DETA NONOate (DETA NO) plus NAC on relaxation of porcine coronary arteries
|
Relaxation to NTG |
||
|---|---|---|
| EC50, −log (M) | Max relaxation, % | |
| 24 h incubation with | ||
| NAC alone | 6.63±0.19 | 99.15±0.72 |
| NAC and NTG, 10−7 M | 6.64±0.20 | 99.34±0.30 |
| NAC and NTG, 10−6 M | 5.90±0.15a | 96.27±2.49 |
| NAC and NTG, 10−5 M | 5.56±0.22a,b | 99.74±0.20 |
|
Relaxation to DETA NO |
||
| |
EC50, −log (M) |
Max relaxation, % |
| 24 h incubation with | ||
| NAC alone | 5.43±0.07 | 99.54±0.42 |
| DETA NO, 10−5 M | 4.67±0.17a | 97.84±1.16 |
Abbreviations: DETA NONOate, [2,2′-(hydroxynitrosohydrazono) bis(ethanamine)]; NAC, N-acetyl-L-cysteine; NTG, nitroglycerine.
Relaxation of porcine coronary arteries to NTG and DETA NO were determined in vessels constricted with U46619 (10−7 to 3 × 10−7 M) to a similar the tension level. Data are expressed as means±s.e.mean, n=6–8 for each group.
Significantly different from vessels incubated with NAC alone (P<0.05).
Significantly different from vessels incubated with NTG at 10−6 M plus NAC (P<0.05).
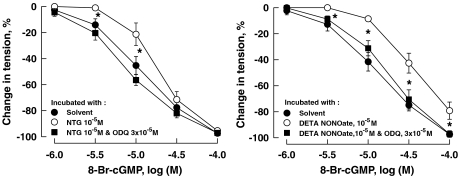
A 24 h incubation of the coronary arteries with NTG at 10−5 M or with DETA NONOate at 10−5 M also significantly attenuated the relaxation induced by 8-Br-cGMP, a cell membrane-permeable analogue of cGMP. This attenuation was prevented by the presence in the incubation medium of ODQ (3 × 10−5 M), a specific inhibitor of soluble guanylyl cyclase (Figure 4; Table 3).
Figure 4.
Relaxations of porcine coronary arteries to 8-Br-cGMP following 24 h incubation with NTG (left panel) or DETA NONOate (right panel). Vessels were constricted with U46619 (10−7 to 3 × 10−7 M) before testing their relaxant responses. Data are shown as means±s.e.mean; n=6–9 for each group. *Significant difference between vessels incubated with solvent and those with NTG at 10−5 M (left panel) or between vessels incubated with solvent and those with DETA NONOate at 10−5 M (right panel) (P<0.05). 8-Br-cGMP, 8-bromo-guanosine 3′5′-cyclic monophosphate; DETA NONOate, [2,2′-(hydroxynitrosohydrazono) bis(ethanamine)]; NTG, nitroglycerine.
Table 3.
Effect of 24 h incubation with NTG and DETA NONOate (DETA NO) on response of porcine coronary arteries to 8-Br-cGMP
|
Relaxation to 8-Br-cGMP |
||
|---|---|---|
| EC50, −log (M) | Max relaxation, % | |
| 24 h incubation with: | ||
| Solvent | 4.98±0.07 | 96.97±1.02 |
| NTG, 10−5 M | 4.72±0.05a | 95.38±1.33 |
| NTG, 10−5 M and ODQ, 10−4 M | 5.09±0.06 | 97.13±1.77 |
| 24 h incubation with: | ||
| Solvent | 4.90±0.07 | 97.33±0.91 |
| DETA NO, 10−5 M | 4.40±0.08a | 79.18±4.20a |
| DETA NO, 10−5 M and ODQ, 10−4 M | 4.75±0.09 | 97.14±2.56 |
Abbreviations: 8-Br-cGMP, 8-bromo-guanosine 3′5′-cyclic monophosphate; DETA NONOate, [2,2′-(hydroxynitrosohydrazono) bis(ethanamine)]; NTG, nitroglycerine; ODQ, 1H-(1,2,4)oxadiazolo(4,3-a)quinoxalin-1-one.
Relaxation of porcine coronary arteries to NTG and DETA NO were determined in vessels constricted with U46619 (10−7 to 3 × 10−7 M) to a similar the tension level. Data are expressed as means±s.e.mean, n=6–9 for each group.
Significantly different from vessels incubated with solvent (P<0.05).
The experiments described above were performed in coronary arteries in the presence of the inhibitors of NO synthase and cyclooxygenase, nitro-L-arginine and indomethacin, respectively. These inhibitors had no significant effects on the tolerance of porcine coronary arteries induced by NTG (10−5 M). There was also no significant difference in the tolerance to NTG between vessels with and without endothelium (Table 4).
Table 4.
Effects of endothelium removal and indomethacin plus nitro-L-arginine on nitrate tolerance of porcine coronary arteries induced by 24 h incubation with NTG
|
Relaxation to NTG |
||
|---|---|---|
| EC50, −log (M) | Max relaxation, % | |
| With endothelium, incubated with: | ||
| Solvent | 6.79±0.27 | 99.84±0.14 |
| NTG, 10−5 M | 5.64±0.23a | 96.65±1.56 |
| Without endothelium, incubated with: | ||
| Solvent | 6.71±0.14 | 100.00±0.00 |
| NTG, 10−5 M | 5.76±0.10a | 99.27±0.57 |
| With endothelium treated with indomethacin plus nitro-L-arginine, incubated with: | ||
| Solvent | 6.82±0.09 | 99.74±0.20 |
| NTG, 10−5 M | 5.58±0.10a | 95.47±2.48 |
Abbreviation: NTG, nitroglycerine.
Relaxation of porcine coronary arteries to NTG were determined in vessels constricted with U46619 (10−7 to 3 × 10−7 M) to a similar the tension level. Indomethacin, 10−5 M; nitro-L-arginine, 10−4 M. Data are expressed as means±s.e.mean, n=5–7 for each group.
Significantly different from the respective solvent group (P<0.05).
PKG activity
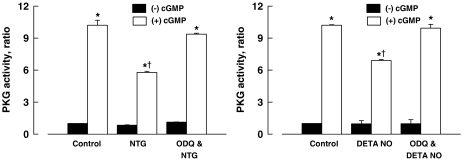
The basal activity of PKG of the coronary arteries incubated for 24 h with NTG (10−5 M) or DETA NONOate (10−5 M) was not significantly different from that of the control. The activity of PKG stimulated with cGMP (3 × 10−6 M) of coronary arteries incubated for 24 h with NTG (10−5 M) or DETA NONOate (10−5 M) was significantly less than those of the control vessels. The effects of NTG and DETA NONOate were prevented by ODQ (3 × 10−5 M) (Figure 5).
Figure 5.
PKG activity of porcine coronary arteries after 24 h incubation with NTG (10−5 M; left panel) or DETA NONOate (DETA NO, 10−5 M; right panel). (−) cGMP, without cGMP; (+) cGMP, cGMP at 3 × 10−6 M. Data are shown as means±s.e.; n=6 for each group. *Significantly different from those without cGMP (P<0.05); †significantly different from the control group (P<0.05). cGMP, cyclic GMP; DETA NONOate, [2,2′-(hydroxynitrosohydrazono) bis(ethanamine)]; NTG, nitroglycerine; PKG, cGMP-dependent protein kinase.
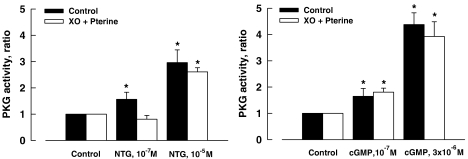
To determine the effect of ROS on the activity of PKG, isolated coronary arteries and the homogenate preparations of the vessels were stimulated with NTG and cGMP, respectively, in the presence and absence of exogenous superoxide (generated by addition of XO (10 mU ml−1) plus pterine (10−4 M)). Addition of exogenous superoxide inhibited the activity of PKG that was stimulated by NTG at a low concentration (10−7 M) but not that stimulated by NTG at a higher concentration (10−5 M). The comparable increases in PKG activity stimulated with cGMP were not affected by the presence of exogenous superoxide (Figure 6).
Figure 6.
PKG activity of porcine coronary arteries activated with NTG (control, 10−7 or 10−5 M; left panel) or cGMP (control, 10−7 or 3 × 10−7 M; right panel). XO (10 mU ml−1) and pterine (10−4 M). Data are shown as means±s.e.; n=6–10 for each group. *Significantly different from those the control group (P<0.05). cGMP, cyclic GMP; NTG, nitroglycerine; PKG, cGMP-dependent protein kinase; XO, xanthine oxidase.
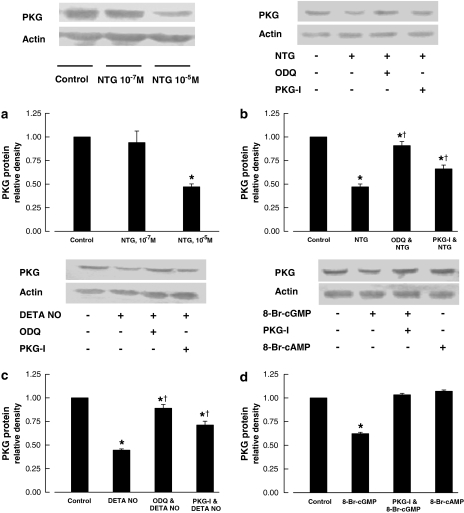
PKG protein
A reduced PKG protein level of coronary arteries was observed after 24 h incubation with NTG at 10−5 M, DETA NONOate at 10−5 M, but not with NTG at 10−7 M. The effect was prevented by ODQ (3 × 10−5 M) and Rp-8-Br-PET-cGMPS (3 × 10−5 M), inhibitors of soluble guanylyl cyclase and PKG, respectively (Figure 7). The inhibition of PKG protein expression was also observed after 24 h incubation with 8-Br-cGMP (10−4 M) but not with 8-bromo-adenosine 3′5′-cyclic monophosphate (10−4 M; a cell membrane-permeable analogue of cAMP). The effect of 8-Br-cGMP was inhibited by the inclusion of Rp-8-Br-PET-cGMPS (3 × 10−5 M) in the incubation medium (Figure 7).
Figure 7.
PKG protein of porcine coronary arteries following 24 h incubation with NTG (a, b), DETA NO (c) or 8-Br-cGMP or 8-bromo-adenosine 3′5′-cyclic monophosphate (d). The upper panels are western blots of PKG. The lower panels are the densitometric scanning of PKG protein normalized to actin. NTG, 10−5 M unless otherwise stated; DETA NO,10−5 M; 8-Br-cGMP or 8- 8-bromo-adenosine 3′5′-cyclic monophosphate, 10−5 M; ODQ, 3 × 10−5 M; PKG-I, (Rp-8-Br-PET-cGMPS), 3 × 10−5 M. Data shown as means±s.e.mean; n=4–6 for each group. *Significantly different from the control group; †significantly different from vessels incubated with NTG (b) or incubated with DETA NO (c) (P<0.05). 8-Br-cGMP, 8-bromo-guanosine 3′5′-cyclic monophosphate; DETA NONOate, [2,2′-(hydroxynitrosohydrazono) bis(ethanamine)]; NTG, nitroglycerine; ODQ, 1H-(1,2,4)oxadiazolo(4,3-a)quinoxalin-1-one; PKG, cGMP-dependent protein kinase.
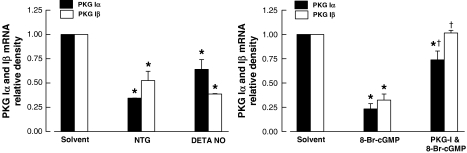
PKG Iα and Iβ mRNAs
Coronary arteries incubated for 24 h with NTG (10−5 M), DETA NONOate (10−5 M) or 8-Br-cGMP (10−4 M) showed a significant decrease in mRNA levels of PKG Iα and PKG Iβ in comparison with the control. The effect induced by 8-Br-cGMP (10−4 M) was blocked by the inclusion of Rp-8-Br-PET-cGMPS (3 × 10−5 M) in the incubation medium (Figure 8).
Figure 8.
Real-time PCR of mRNA fragments of PKG Iα and Iβ of porcine coronary arteries incubated for 24 h with NTG (10−5 M) or DETA NONOate (DETA NO, 10−5 M (left panel) and with 8-Br-cGMP (10−4 M) (right panel). The densitometric scanning of mRNA fragments of PKG is normalized to β-actin. PKG-I (Rp-8-Br-PET-cGMPS), 3 × 10−5 M. Data shown as means±s.e.mean; n=4–8 for each group. *Significantly different from vessels incubated with solvent (P<0.05); †significantly different from vessels not treated with PKG-I (P<0.05). 8-Br-cGMP, 8-bromo-guanosine 3′5′-cyclic monophosphate; DETA NONOate, [2,2′-(hydroxynitrosohydrazono) bis(ethanamine)]; NTG, nitroglycerin; ODQ, 1H-(1,2,4)oxadiazolo(4,3-a)quinoxalin-1-one; PCR, polymerase chain reaction; PKG, cGMP-dependent protein kinase.
Discussion
In the present study, attenuation of relaxation of coronary arteries to NTG caused by a 24 h incubation with this nitrovasodilator at a low concentration (10−7 M) was prevented by NAC, a scavenger of ROS that acts as a glutathione precursor (Nowicki et al., 2001; Park and Lee, 2006). This suggests that the tolerance might result from an increased production of ROS. This finding is consistent with other published data indicating that ROS generated during continuous exposure to NTG might inhibit mitochondrial aldehyde dehydrogenase, a key enzyme for the biotransformation of NTG and other high-potent nitrates. This mechanism may play a central role in the development of nitrate tolerance when nitrates are used in low doses (Münzel et al., 1995; Difabio et al., 2003; Sydow et al., 2004; Chen et al., 2005; Kollau et al., 2005). The studies by Schulz et al. (2002) and Mülsch et al. (2001) showed that nitrate tolerance is associated with an increased production of superoxide and decreased PKG activity. In the study by Mülsch and co-workers, treatment with vitamin C improved tolerance, reduced vascular superoxide production and increased PKG activity. These results suggest that the decreased activity of PKG may result in part from an increased production of ROS.
In the present study we found that the decreased PKG activity associated with nitrate tolerance of porcine coronary arteries induced by NTG at a low concentration (10−7 M) was prevented by a ROS scavenger, but not that induced by NTG at a higher concentration (10−5 M). Acute treatment of the vessels with exogenous superoxide fully inhibited the activity of PKG stimulated by NTG at 10−7 M but not at 10−5 M. Superoxide had no effect on PKG activity stimulated by cGMP. These results suggest that the inhibition of PKG activity caused by NTG at low concentrations after 24 h exposure may be due to the action of ROS generated in the vessels on sites upstream of PKG.
In contrast with the NTG tolerance of coronary arteries developed to the nitrate at low concentrations, the tolerance obtained following exposure to higher concentrations (10−5 M) was not sensitive to either NAC or ebselen (a scavenger of ROS that mimics the activities of the antioxidant glutathione peroxidase, thioredoxin reductase and thioredoxin peroxidase; Müller et al., 1984; Zhao and Holmgren, 2002). The tolerance caused by higher concentrations of NTG was associated with a cross-tolerance to DETA NONOate, a stable NO donor (Mooradian et al., 1995). Moreover, a tolerance to NTG could also be induced with the NO donor. This suggests that the tolerance induced by NTG at high concentrations and that induced by NO share a common mechanism. It is well recognized that vasodilation induced by NTG and NO is mainly mediated by cGMP resulting from the activation of soluble guanylyl cyclase (Ignarro et al., 1981). In the present study, a 24 h incubation either with NTG at the higher concentrations or with the NO donor attenuated relaxation of the vessels to 8-Br-cGMP. This effect was prevented by the presence of ODQ, a specific inhibitor of soluble guanylyl cyclase (Gerlach et al., 1993). These results indicate that a sustained elevation in cGMP may be involved in the development of the tolerance.
Smooth muscle relaxation caused by cGMP is predominantly mediated by PKG (Francis and Corbin, 1994; Lincoln et al., 2001; Hofmann et al., 2006). In our study, a 24 h incubation of coronary arteries with NTG at 10−5 M or the NO donor caused not only a significant decrease in PKG activity but also a decrease in PKG protein expression. These effects were blocked by inhibition of soluble guanylyl cyclase with ODQ. Furthermore, a decrease in PKG protein level was also observed after a 24 h incubation with 8-Br-cGMP. These results suggest that the decrease in PKG protein and activity may be caused by the continuous exposure to high levels of cGMP during continuous exposure to the nitrovasodilators.
In transgenic mice overexpressing endothelial NO synthase, plasma nitrite and nitrate levels (a measure of endogenous NO production) as well as basal cGMP levels of the aortas are significantly higher as compared with that of wild type mice. The aortas of transgenic mice show a marked reduction in relaxation to sodium nitroprusside and 8-Br-cGMP (van Deel et al., 2007). Furthermore, PKG protein expression and activity of the aortas of transgenic mice are significantly decreased in comparison to that of wild type mice (Ohashi et al., 1998; Yamashita et al., 2000). Attenuation of PKG-mediated relaxation and of PKG protein expression and activity has also been observed by us in ovine pulmonary veins after continuous exposure to NO (Gao et al., 2004). This previous study in ovine pulmonary vessels supports our speculation that a sustained elevation of cGMP level may be the cause of the attenuated PKG-mediated relaxation and the decreased PKG protein expression and activity reported in this study.
The present study shows that the decrease in expression of PKG protein caused by prolonged exposure to NTG, DETA NONOate and 8-Br-cGMP was prevented by Rp-8-Br-PET-cGMPS, a specific inhibitor of PKG (Butt et al., 1995). It appears that the downregulation of PKG expression was caused by a negative feedback mechanism resulting from sustained activation of the enzyme itself. PKG is present as two types in mammalian cells, type I and II. The type I PKG has two isoforms, PKG Iα and Iβ. In smooth muscle cells, both PKG Iα and Iβ isoforms are highly expressed. Studies so far indicate that both isoforms are involved in mediating vasodilation induced by cGMP, although Iα seems to be more important (Francis and Corbin, 1994; Lincoln et al., 2001; Hofmann et al., 2006). We found that the mRNA levels of both PKG Iα and Iβ were downregulated following a 24 h incubation with NTG (10−5 M), DETA NONOate (10−5 M) or 8-Br-cGMP (10−4 M). Also the effect induced by 8-Br-cGMP (10−4 M) was blocked by the inclusion of Rp-8-Br-PET-cGMPS in the incubation medium. Hence, suppression of total PKG protein expression may represent reduced transcription of both PKG isoforms.
In rat and bovine aortic smooth muscle cells, expression of PKG Iα is attenuated by inhibition of the transcription factor Sp1 following prolonged exposure to higher concentrations of nitrovasodilators, theophylline, cGMP and cAMP. The transcription factor Sp1 may be inhibited directly by NO or indirectly via cAMP-dependent protein kinase (PKA) (Soff et al., 1997; Sellak et al., 2002; Browner et al., 2004). It is postulated that PKA-mediated suppression of PKG expression may be operative under pathological conditions when the expression of iNOS is induced by inflammatory cytokines, which leads to increased and prolonged NO production (Anderson et al., 2000). It is noted that in these studies, PKA activity is activated by nitrovasodilators and that cAMP analogues are more potent than cGMP analogues in suppressing PKG expression. Moreover, this phenomenon is inhibited by inhibitors of PKA but not affected by inhibitors of PKG (Soff et al., 1997; Sellak et al., 2002). In the present study, PKG protein and mRNA levels of coronary arteries were not affected by the cAMP analogue but were markedly suppressed by the cGMP analogue at similar concentrations. The effects of the cGMP analogue were significantly blocked by the PKG inhibitor. We also found that NTG and DETA NONOate at concentrations used in our study had no effect on the activity of PKA (Qin et al., 2007). Therefore, it is likely that the PKG-dependent suppression of PKG itself observed in the present study represents a negative regulation mechanism of the cGMP–PKG signalling. Such a mechanism may occur when the intracellular level of cGMP is continuously elevated but its level is not high enough to cross-activate PKA. When cGMP is elevated to a level high enough to cross-activate PKA, PKA-dependent suppression of PKG may occur. This situation occurs more likely under inflammatory conditions when there is a robust production of NO following induction of iNOS (Anderson et al., 2000).
In summary, the present study suggests that two distinct mechanisms appear to be involved in the development of tolerance of porcine coronary arteries to nitrovasodilators. The tolerance induced by NTG at low concentrations may result from an increased ROS production that acts at sites upstream of PKG. The tolerance induced by NTG at higher concentrations is probably mediated by a PKG-mediated negative feedback mechanism, which results in decreased PKG protein and mRNA expression, and decreased PKG activity. Clinically, human plasma concentrations of NTG, when given intravenously, range from 1.8 nM to 0.53 μM (Hashimoto and Kobayashi, 2003). The concentration of 1 μM NTG was used in the present study to trigger PKG-dependent PKG downregulation, a concentration that is close to the upper range of clinically relevant concentrations. Therefore, it is within the realm of possibility that in certain clinical situations, when high doses of NTG are being used, a decrease in PKG expression might contribute to the development of nitrate tolerance. Such a speculation is supported by the findings that although acetylcholine-induced production of NO from aortas of transgenic mice overexpressing endothelial NO synthase is only twofold greater than that of wild-type mice, PKG protein expression and activity as well as nitrovasodilator-induced relaxation of the vessels are markedly attenuated (Yamashita et al., 2000).
Acknowledgments
This study was supported in part by the National Natural Science Foundation of China Grant no. 30470629, no. 30770789, the Major National Basic Research Program of PR China (no. 2006CB503802) and the National Institute of Health Grants HL-059435 and HL-075187, USA.
Abbreviations
- 8-Br-cGMP
8-bromo-guanosine 3′5′-cyclic monophosphate
- NAC
N-acetyl-L-cysteine
- NTG
nitroglycerine
- ODQ
1H-(1,2,4)oxadiazolo(4,3-a)quinoxalin-1-one
- PKG
cGMP-dependent protein kinase
- Rp-8-Br-PET-cGMPS
β-Phenyl-1,N2-etheno-8-bromoguanosine-3′,5′-cyclic monophosphorothioate, Rp isomer
- U46619
(9,11)-dideoxy-(11α,9α)-epoxymethanoprostaglandin F2α
- XO
xanthine oxidase
Conflict of interest
The authors state no conflict of interest.
References
- Abou-Mohamed G, Kaesemeyer WH, Caldwell RB, Caldwell RW. Role of L-arginine in the vascular actions and development of tolerance to nitroglycerin. Br J Pharmacol. 2000;130:211–218. doi: 10.1038/sj.bjp.0703293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson PG, Boerth NJ, Liu M, McNama DB, Cornwell TL, Lincoln TM. Cyclic CMP-dependent protein kinase expression in coronary arterial smooth muscle in response to balloon catheter injury. Arterioscler Thromb Vasc Biol. 2000;20:2192–2197. doi: 10.1161/01.atv.20.10.2192. [DOI] [PubMed] [Google Scholar]
- Atlante A, Valenti D, Gagliardi S, Passarella S. A sensitive method to assay the xanthine oxidase activity in primary cultures of cerebellar granule cells. Brain Res Brain Res Protoc. 2000;6:1–5. doi: 10.1016/s1385-299x(00)00030-1. [DOI] [PubMed] [Google Scholar]
- Browner NC, Sellak H, Lincoln TM. Downregulation of cGMP-dependent protein kinase expression by inflammatory cytokines in vascular smooth muscle cells. Am J Physiol Cell Physiol. 2004;287:C88–C96. doi: 10.1152/ajpcell.00039.2004. [DOI] [PubMed] [Google Scholar]
- Butt E, Pohler D, Genieser HG, Huggins JP, Bucher B. Inhibition of cyclic GMP-dependent protein kinase-mediated effects by (Rp)-8-bromo-PET-cyclic GMPS. Br J Pharmacol. 1995;116:3110–3116. doi: 10.1111/j.1476-5381.1995.tb15112.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Foster MW, Zhang J, Mao L, Rockman HA, Kawamoto T, et al. An essential role for mitochondrial aldehyde dehydrogenase in nitroglycerin bioactivation. Proc Natl Acad Sci USA. 2005;102:12159–12164. doi: 10.1073/pnas.0503723102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Stamler JS. Bioactivation of nitroglycerin by the mitochondrial aldehyde dehydrogenase. Trends Cardiovasc Med. 2006;16:259–265. doi: 10.1016/j.tcm.2006.05.001. [DOI] [PubMed] [Google Scholar]
- Csont T, Ferdinandy P. Cardioprotective effects of glyceryl trinitrate: beyond vascular nitrate tolerance. Pharmacol Ther. 2005;105:57–68. doi: 10.1016/j.pharmthera.2004.10.001. [DOI] [PubMed] [Google Scholar]
- Difabio J, Ji Y, Vasiliou V, Thatcher GR, Bennett BM. Role of mitochondrial aldehyde dehydrogenase in nitrate tolerance. Mol Pharmacol. 2003;64:1109–1116. doi: 10.1124/mol.64.5.1109. [DOI] [PubMed] [Google Scholar]
- Francis SH, Corbin JD. Structure and function of cyclic nucleotide-dependent protein kinases. Annu Rev Physiol. 1994;56:237–272. doi: 10.1146/annurev.ph.56.030194.001321. [DOI] [PubMed] [Google Scholar]
- Gao Y, Dhanakoti S, Trevino EM, Sander FC, Portugal AM, Raj JU. Effect of oxygen on cyclic GMP-dependent protein kinase-mediated relaxation in ovine fetal pulmonary arteries and veins. Am J Physiol. 2003;285:L611–L618. doi: 10.1152/ajplung.00411.2002. [DOI] [PubMed] [Google Scholar]
- Gao Y, Dhanakoti S, Trevino EM, Wang X, Sander FC, Portugal AD, et al. Role of cGMP-dependent protein kinase in development of tolerance to nitric oxide in pulmonary veins of newborns lambs. Am J Physiol Lung Cell Physiol. 2004;286:L786–L792. doi: 10.1152/ajplung.00314.2003. [DOI] [PubMed] [Google Scholar]
- Gerlach H, Pappert D, Lewandowski K, Rossaint R, Falke KJ. Long-term inhalation with evaluated low doses of nitric oxide for selective improvement of oxygenation in patients with adult respiratory distress syndrome. Intensive Care Med. 1993;19:443–449. doi: 10.1007/BF01711084. [DOI] [PubMed] [Google Scholar]
- Gold ME, Bush PA, Ignarro LJ. Depletion of arterial L-arginine causes reversible tolerance to endothelium-dependent relaxation. Biochem Biophys Res Commun. 1989;164:714–721. doi: 10.1016/0006-291x(89)91518-0. [DOI] [PubMed] [Google Scholar]
- Hashimoto S, Kobayashi A. Clinical pharmacokinetics and pharmacodynamics of glyceryl trinitrate and its metabolites. Clin Pharmacokinet. 2003;42:205–221. doi: 10.2165/00003088-200342030-00001. [DOI] [PubMed] [Google Scholar]
- Hofmann F, Ammendola A, Schlossmann J. Rising behind NO: cGMP-dependent protein kinases. J Cell Sci. 2000;113:1671–1676. doi: 10.1242/jcs.113.10.1671. [DOI] [PubMed] [Google Scholar]
- Hofmann F, Feil R, Kleppisch T, Schlossmann J. Function of cGMP-dependent protein kinases as revealed by gene deletion. Physiol Rev. 2006;86:1–23. doi: 10.1152/physrev.00015.2005. [DOI] [PubMed] [Google Scholar]
- Ignarro LJ, Lippton H, Edwards JC, Baricos WH, Hyman AL, Kadowitz PJ, et al. Mechanism of vascular smooth muscle relaxation by organic nitrates, nitrites, nitroprusside and nitric oxide: evidence for the involvement of S-nitrosothiols as active intermediates. J Pharmacol Exp Ther. 1981;218:739–749. [PubMed] [Google Scholar]
- Kim D, Rybalkin SD, Pi XC, Wang Y, Zhang C, Münzel T, et al. Upregulation of phosphodiesterase 1A1 expression is associated with the development of nitrate tolerance. Circulation. 2001;104:2338–2343. doi: 10.1161/hc4401.098432. [DOI] [PubMed] [Google Scholar]
- Kollau A, Hofer A, Russwurm M, Koesling D, Keung WM, Schmidt K, et al. Contribution of aldehyde dehydrogenase to mitochondrial bioactivation of nitroglycerin: evidence for activation of purified soluble guanylate cyclase through direct formation of nitric oxide. Biochem J. 2005;385:769–777. doi: 10.1042/BJ20041354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lincoln TM, Dey N, Sellak H. cGMP-dependent protein kinase signaling mechanisms in smooth muscle: from the regulation of tone to gene expression. J Appl Physiol. 2001;91:1421–1430. doi: 10.1152/jappl.2001.91.3.1421. [DOI] [PubMed] [Google Scholar]
- MacPherson JD, Gillespie TD, Dunkerley HA, Maurice DH, Bennett BM. Inhibition of phosphodiesterase 5 selectively reverses nitrate tolerance in the venous circulation. J Pharmacol Exp The. 2006;317:188–195. doi: 10.1124/jpet.105.094763. [DOI] [PubMed] [Google Scholar]
- Meyer RBJ, Miller JP. Analogs of cyclic AMP and cyclic GMP: general methods of synthesis and the relationship of structure to enzymic activity. Life Sci. 1974;14:1019–1040. doi: 10.1016/0024-3205(74)90228-8. [DOI] [PubMed] [Google Scholar]
- Molina CR, Andersen JW, Rapoport RM, Waldman S, Murad F. Effect of in vivo nitroglycerin therapy on endothelium-dependent and independent vascular relaxation and cyclic GMP accumulation in rat aorta. J Cardiovasc Pharmacol. 1987;10:371–378. doi: 10.1097/00005344-198710000-00001. [DOI] [PubMed] [Google Scholar]
- Mooradian DL, Hutsell TC, Keefer LK. Nitric oxide (NO) donor molecules: effect of NO release rate on vascular smooth muscle cell proliferation in vitro. J Cardiovasc Pharmacol. 1995;25:674–678. [PubMed] [Google Scholar]
- Müller A, Cadenas E, Graf P, Sies H. A novel biologically active seleno-organic compound—I. Glutathione peroxidase-like activity in vitro and antioxidant capacity of PZ 51 (ebselen) Biochem Pharmacol. 1984;33:3235–3239. doi: 10.1016/0006-2952(84)90083-2. [DOI] [PubMed] [Google Scholar]
- Mülsch A, Oelze M, Klöss S, Mollnau H, Töpfer A, Smolenski A, et al. Effects of in vivo nitroglycerin treatment on activity and expression of the guanylyl cyclase and cGMP-dependent protein kinase and their downstream target vasodilator-stimulated phosphoprotein in Aorta. Circulation. 2001;103:2188–2194. doi: 10.1161/01.cir.103.17.2188. [DOI] [PubMed] [Google Scholar]
- Münzel T, Daiber A, Mülsch A. Explaining the phenomenon of nitrate tolerance. Circ Res. 2005;97:618–628. doi: 10.1161/01.RES.0000184694.03262.6d. [DOI] [PubMed] [Google Scholar]
- Münzel T, Li H, Mollnau H, Hink U, Matheis E, Hartmann M, et al. Effects of long-term nitroglycerin treatment on endothelial nitric oxide synthase (NOS III) gene expression, NOS III-mediated superoxide production, and vascular NO bioavailability. Circ Res. 2000;86:E7–E12. doi: 10.1161/01.res.86.1.e7. [DOI] [PubMed] [Google Scholar]
- Münzel T, Sayegh H, Freeman BA, Tarey MM, Harrison DG. Evidence for enhanced vascular superoxide anion production in nitrate tolerance. A novel mechanism underlying nitrate tolerance and cross tolerance. J Clin Invest. 1995;95:187–194. doi: 10.1172/JCI117637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowicki PT, Flavahan S, Hassanain H, Mitra S, Holland S, Goldschmidt-Clermont PJ, et al. Redox signaling of the arteriolar myogenic response. Circ Res. 2001;89:114–116. doi: 10.1161/hh1401.094367. [DOI] [PubMed] [Google Scholar]
- Ohashi Y, Kawashima S, Hirata K, Yamashita T, Ishida T, Inoue N, et al. Hypotension and reduced nitric oxide-elicited vasorelaxation in transgenic mice overexpressing endothelial nitric oxide synthase. J Clin Invest. 1998;102:2061–2071. doi: 10.1172/JCI4394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagani ED, VanAller GS, O'Connor B, Silver PJ. Reversal of nitroglycerin tolerance in vitro by the cGMP–phosphodiesterase inhibitor zaprinast. Eur J Pharmacol. 1993;243:141–147. doi: 10.1016/0014-2999(93)90373-p. [DOI] [PubMed] [Google Scholar]
- Park SJ, Lee YC. Antioxidants as novel agents for asthma. Mini Rev Med Chem. 2006;6:235–240. doi: 10.2174/138955706775475948. [DOI] [PubMed] [Google Scholar]
- Parker JD. Nitrate tolerance, oxidative stress, and mitochondrial function: another worrisome chapter on the effects of organic nitrates. J Clin Invest. 2004;113:352–354. doi: 10.1172/JCI21003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin X, Zheng X, Qi H, Dou D, Raj JU, Gao Y. cGMP-dependent protein kinase in regulation of basal tone and in nitroglycerin- and nitric-oxide-induced relaxation in porcine coronary artery. Pflügers Arch–Eur J Physiol. 2007;454:913–923. doi: 10.1007/s00424-007-0249-8. [DOI] [PubMed] [Google Scholar]
- Romanin C, Kukovetz WR. Tolerance to nitroglycerin is caused by reduced guanylate cyclase activation. J Mol Cell Cardiol. 1989;21:41–48. doi: 10.1016/0022-2828(89)91491-0. [DOI] [PubMed] [Google Scholar]
- Schulz E, Tsilimingas N, Rinze R, Reiter B, Wendt M, Oelze M, et al. Functional and biochemical analysis of endothelial (dys)function and NO/cGMP signaling in human blood vessels with and without nitroglycerin pretreatment. Circulation. 2002;105:1170–1175. doi: 10.1161/hc1002.105186. [DOI] [PubMed] [Google Scholar]
- Sellak H, Yang X, Cao X, Cornwell T, Soff GA, Lincoln T. Sp1 transcription factor as a molecular target for nitric oxide- and cyclic nucleotide-mediated suppression of cGMP-dependent protein kinase-Ialpha expression in vascular smooth muscle cells. Circ Res. 2002;90:405–412. doi: 10.1161/hh0402.105898. [DOI] [PubMed] [Google Scholar]
- Soff GA, Cornwell TL, Cundiff DL, Gately S, Lincoln TM. Smooth muscle cell expression of type I cyclic GMP-dependent protein kinase is suppressed by continuous exposure to nitrovasodilators, theophylline, cyclic GMP, and cyclic AMP. J Clin Invest. 1997;100:2580–2587. doi: 10.1172/JCI119801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart DD. Remarkable tolerance to nitroglycerine. Polyclinic. 1888;6:43. [Google Scholar]
- Sydow K, Daiber A, Oelze M, Chen Z, August M, Wednt M, et al. Central role of mitochondrial aldehyde dehydrogenase and reactive oxygen species in nitroglycerin tolerance and cross-tolerance. J Clin Invest. 2004;113:482–489. doi: 10.1172/JCI19267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Deel ED, Merkus D, van Haperen RJ, de Waard MC, de Crom R, Duncker DJ. Vasomotor control in mice overexpressing human endothelial nitric oxide synthase. Am J Physiol Heart Circ Physiol. 2007;293:1144–1153. doi: 10.1152/ajpheart.00773.2006. [DOI] [PubMed] [Google Scholar]
- Wang X, Tong M, Chinta S, Raj JU, Gao Y. Hypoxia-induced reactive oxygen species downregulate ETB receptor-mediated contraction of rat pulmonary arteries. Am J Physiol. 2006;290:L570–L578. doi: 10.1152/ajplung.00262.2005. [DOI] [PubMed] [Google Scholar]
- Yamashita T, Kawashima S, Ohashi Y, Ozaki M, Rikitake Y, Inoue N, et al. Mechanisms of reduced nitric oxide/cGMP-mediated vasorelaxation in transgenic mice overexpressing endothelial nitric oxide synthase. Hypertension. 2000;36:97–102. doi: 10.1161/01.hyp.36.1.97. [DOI] [PubMed] [Google Scholar]
- Zhao R, Holmgren A. A novel antioxidant mechanism of ebselen involving ebselen diselenide, a substrate of mammalian thioredoxin and thioredoxin reductase. J Biol Chem. 2002;277:39456–39462. doi: 10.1074/jbc.M206452200. [DOI] [PubMed] [Google Scholar]