Abstract
Background and purpose:
Sensory neuropathy develops in the presence of cardiovascular risk factors (e.g. diabetes, dyslipidemia), but its pathological consequences in the heart are unclear. We have previously shown that systemic sensory chemodenervation by capsaicin leads to impaired myocardial relaxation and diminished cardiac nitric oxide (NO) content. Here we examined the mechanism of diminished NO formation and if it may lead to a reduction of peroxynitrite (ONOO−)-induced S-nitrosylation of sarcoendoplasmic reticulum Ca2+-ATPase (SERCA2a).
Experimental approach:
Male Wistar rats were treated with capsaicin for 3 days to induce sensory chemodenervation. Seven days later, myocardial function and biochemical parameters were measured.
Key results:
Capsaicin pretreatment significantly increased left ventricular end-diastolic pressure (LVEDP) decreased cardiac NO level, Ca2+-dependent NO synthase (NOS) activity, and NOS-3 mRNA. Myocardial superoxide content, xanthine oxidoreductase and NADPH oxidase activities did not change, although superoxide dismutase (SOD) activity increased. Myocardial and serum ONOO− concentration and S-nitrosylation of SERCA2a were significantly decreased.
Conclusions and implications:
Our results show that sensory chemodenervation decreases cardiac NO via decreased expression and activity of Ca2+-dependent NOS and increases SOD activity, thereby leading to decreased basal ONOO− formation and reduction of S-nitrosylation of SERCA2a, which causes impaired myocardial relaxation characterized by increased left ventricular end-diastolic pressure (LVEDP). This suggests that capsaicin sensitive sensory neurons regulate myocardial relaxation via maintaining basal ONOO− formation and SERCA S-nitrosylation.
Keywords: capsaicin, sensory nerves, myocardial function, free radicals, nitric oxide, peroxynitrite, S-nitrosylation, SERCA
Introduction
Sensory neuropathy develops in the presence of various risk factors for cardiovascular diseases, such as diabetes, dyslipidemia and obesity. However, it should be noted that other non-cardiovascular diseases are also associated with sensory neuropathy, such as trauma, HIV infection, pain disorders, and so on (Hughes et al., 2004; Polydefkis et al., 2004; Kassem et al., 2005; Abrams et al., 2007; Facer et al., 2007; Herman et al., 2007). Very little is known about the physiological and pathological role of sensory nerves in the regulation of cellular functions in the heart and other organs. The mechanism of the development of sensory neuropathy due to the risk factors for cardiovascular diseases is also unknown.
Capsaicin is a highly selective sensory neurotoxin that leads to a selective functional blockade and/or ablation of a morphologically well-defined population of primary sensory nerves (Jancso et al., 1968, 1977). Therefore, capsaicin has become one of the most important probes for investigations of sensory neural pathology and pharmacology (Szallasi et al., 2007). However, the exact role of capsaicin-sensitive sensory nerves and the capsaicin receptor (transient receptor potential vanilloid receptor 1) in cellular mechanisms under normal and pathological conditions are largely unknown especially in the heart (Wang and Wang, 2005; Holzer, 2006). Sensory nerve endings may act as potential sensor machinery for ischaemia, as ischaemia, hypoxia, lactate, K+ and low pH were shown to stimulate cardiac sensory nerves in association with a release of their transmitters (Franco-Cereceda, 1988). Sensory nerves may exert a strong influence on cardiac function, cardiac reflexes and adaptive responses due to their nitric oxide (NO) and vasoactive peptide content, such as calcitonin gene-related peptide and substance P (Franco-Cereceda, 1988; Ren and Ruda, 1995; Sosunov et al., 1995, 1996; Cinca and Rodriguez-Sinovas, 2000). In agreement with the hypotheses outlined above, through the use of capsaicin, we and others have previously shown that cardiac sensory nerves play a role in cardiac adaptation to ischaemic stress (Li et al., 1996; Ferdinandy et al., 1997; Wang and Wang, 2005; Zhong and Wang, 2007), in the regulation of cardiac NO–cGMP system (Csont et al., 2003) and in the mechanism of doxorubicin-induced heart failure (Katona et al., 2004). Furthermore, we have recently shown that cardiac sensory nerves strongly influence the gene expression pattern of rat hearts (Zvara et al., 2006). Therefore, it seems that capsaicin-sensitive cardiac nerves regulate a series of complex cellular events contributing to physiological and pathological myocardial function.
We have previously shown that systemic capsaicin pretreatment leads to impaired myocardial relaxation with a concomitant decrease of cardiac NO content, although the mechanism underlying these changes remained unclear (Ferdinandy et al., 1997; Csont et al., 2003). Decreased basal NO formation may lead to decreased peroxynitrite (ONOO−) formation (Ferdinandy and Schulz, 2003; Ferdinandy, 2006) and also decreased S-nitrosylation of sarcoendoplasmic reticulum Ca2+-ATPase (SERCA), the major player in myocardial relaxation. Nitrosylation of Cys349 has been shown to be responsible for the activation of the SERCA by ONOO− (Viner et al., 1999; Adachi et al., 2004). Consequently, it is plausible to speculate that sensory chemodenervation-induced decrease in NO formation may lead to impaired relaxation of the myocardium via decreased ONOO− formation and S-nitrosylation of SERCA.
Therefore, in the present study, we have systematically analysed myocardial functional parameters, cardiac NO, superoxide and ONOO− metabolism, as well as the degree of S-nitrosylation of SERCA in response to systemic sensory chemodenervation in rats.
Methods
Experimental protocols
All animal procedures conformed to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85-23, revised 1996).
Male Wistar rats weighing 300–350 g, housed in a room maintained at 12-h light–dark cycles and at a constant temperature of 22±2 °C, were used throughout the experiments. For selective chemodenervation of primary sensory nerves, rats were treated with solvent or capsaicin (1% w v−1; Fluka, Buchs, Switzerland, dissolved in physiological saline containing 6% v v−1 ethanol and 8% v v−1 Tween 80) s.c. in the sequence of 10, 30 and 50 mg kg−1 single daily doses in 1 ml kg−1 volume for 3 days as described (Ferdinandy et al., 1997).
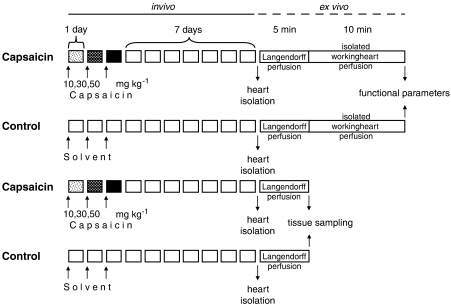
To exclude any nonspecific pharmacological effects of capsaicin, isolated heart experiments were commenced 7 days after the end of capsaicin treatment, when depletion of peptide-containing myocardial sensory nerves and elimination of capsaicin is complete (Ferdinandy et al., 1997; Csont et al., 2003). Hearts were excised after anaesthesia with diethylether and prepared for working heart perfused at 37 °C with Krebs-Henseleit bicarbonate buffer containing (in mM) 118 NaCl, 4.3 KCl, 2.4 CaCl2, 25 NaHCO3, 1.2 KH2PO4, 1.2 MgSO4 and 11.1 glucose and gassed with 95% O2–5% CO2 (Csonka et al., 1999; Ferdinandy et al., 2000). After a 5 min initial normoxic, normothermic Langendorff perfusion to normalize sinus rhythm, the perfusion was switched to working perfusion for 10 min to measure cardiac mechanical functional parameters, such as heart rate, coronary flow, aortic flow, cardiac output, left ventricular-developed pressure and its first derivatives (±dP dtmax−1), and left ventricular end-diastolic pressure (LVEDP) were monitored as described earlier (Csonka et al., 1999; Csont et al., 1999; Ferdinandy et al., 2000) (Figure 1). Ventricular tissue samples for biochemical measurements were taken in separate experiments after the initial 5 min perfusion to eliminate blood before tissue sampling (Figure 1).
Figure 1.
Experimental protocol. Male Wistar rats were treated with capsaicin s.c., giving 10, 30 and 50 mg kg−1 as single daily doses in 1 ml kg−1 volume for 3 days in the sequence shown. Solvent (physiological saline containing 6% v v−1 ethanol and 8% v v−1 Tween 80) was administered to control animals the same way. Seven days after the last injection (when depletion of sensory nerves and elimination of capsaicin are complete), hearts were excised and isolated working heart perfusion was carried out to measure cardiac functional parameters in a working heart mode after an initial 5 min Langendorff perfusion for stabilization of sinus rhythm. In separate experiments, after the initial Langendorff 5 min perfusion, left ventricles were frozen in liquid nitrogen and powdered to perform biochemical assays. In another set of separate experiments, 5 min before the isolation of the hearts, spin trapping of NO was performed by i.p. administration of 500 mg kg−1 diethyl-dithio-carbamate (DETC), 50 mg kg−1 FeSO4 and 200 mg kg−1 sodium citrate, and tissue sampling was performed after 1 min Langendorff perfusion to eliminate blood from the coronary circulation.
Measurement of cardiac NO metabolism
To measure cardiac NO content directly, in vivo spin trapping of NO was applied in separate experiments (Figure 1), followed by electron-spin resonance spectroscopy (ESR) analysis of left ventricular tissue samples as described (Csont et al., 1998; Onody et al., 2003). In brief, the spin trap diethyl-dithio-carbamate (500 mg kg−1), 50 mg kg−1 FeSO4 and 200 mg kg−1 sodium citrate were administered i.p. under diethyl ether anaesthesia. Five minutes after separate injections of diethyl-dithio-carbamate and FeSO4 plus citrate, hearts were isolated and perfused for 1 min to eliminate blood, and ventricular samples were placed into quartz ESR tubes and frozen in liquid nitrogen until assayed for ESR spectra of the NO–Fe2+(diethyl-dithio-carbamate)2 complex at a temperature of 160 K (Bruker ECS106, Rheinstetten, Germany).
To estimate endogenous enzymatic NO production, Ca2+-dependent and Ca2+-independent NOS activities in ventricular homogenates were measured by the conversion of [14C]L-arginine to [14C]L-citrulline as previously described (Ferdinandy et al., 2000). Powdered frozen ventricular tissue was placed in four volumes of ice-cold homogenization buffer (composition given by Schulz et al., 1992) and homogenized with an Ultra-Turrex disperser using three strokes of 20 s duration each. The homogenate was centrifuged (1000 g for 10 min) at 4 °C, and the supernatant was kept on ice for immediate assay of enzyme activities. Samples were incubated for 25 min at 37 °C in the presence or absence of EGTA (1 mM) or EGTA plus N-monomethyl-L-arginine (1 mM) to determine the level of Ca2+-dependent and Ca2+-independent NOS activities, respectively. NOS activities were expressed in pmol per min per mg protein.
Real-time quantitative reverse transcription-PCR was performed on a RotorGene 2000 instrument (Corbett Research, Sydney, Australia) with gene-specific primers and SybrGreen protocol to assess expression changes of three different NOS genes (NOS-1, NOS-2 and NOS-3) observed previously by microarrays. Total RNA (2 μg) from each sample was reverse transcribed in the presence of random primers in a total volume of 20 μl. After dilution with 20 μl of water, 1 μl of the diluted reaction mix was used as template in real-time quantitative reverse transcription-PCR. The 20 μl reaction volume contained 0.2 mM of dNTP, 1 × PCR reaction buffer (ABGene, Epsom, UK), 6 mM of each primer, 4 mM of MgCl2, 1 × SYBR Green I (Molecular Probes, Eugene, OR, USA) at final concentration and 0.5 U of thermostart Taq DNA polymerase (ABGene). The amplification was carried out with the following cycling parameters: 600 s heat start at 95 °C, 45 cycles of denaturation at 95 °C for 25 s, annealing at 60 °C for 25 s and fluorescence detection at 72 °C for 15 s. Relative expression ratios were normalized to β-actin. A non-template control sample was used for each PCR run to check the genomic DNA contaminations of cDNA template. Analysis of results was performed using the Pfaffl method (Pfaffl, 2001; Zvara et al., 2006). With the use of this calculation method, differences between the amplification efficiencies of reactions could be corrected.
Measurement of cardiac superoxide metabolism
In situ detection of superoxide anion was performed by confocal laser scanning microscopy using a fluorescent dye, dihydroethidium (Sigma, Sigma-Aldrich Corp, St Louis, MO, USA) as described by Csont et al. (2007). Dihydroethidium is freely permeable to cell membranes and fluoresces red when oxidized by superoxide. Frozen native heart sections (30 μm) were placed on glass slides covered with PBS buffer (pH 7.4) and stored at +4 °C. Then slides were submerged in 1 μM dihydroethidium (Sigma) in PBS buffer (pH 7.4) and incubated at 37 °C for 30 min in a dark humidified container. Fluorescence in heart sections was detected by a confocal microscope (Olympus FV1000) using a 530 nm long pass filter for excitation. Images of the hearts that were treated with saline (negative control) were measured first. After the basal settings of the confocal microscope were adjusted, images of the hearts were collected digitally. Eight images were taken randomly of each slide and fluorescence intensity was analysed by ImageJ 129 program.
Activities of xanthine oxidoreductase (XOR; xanthine oxidase and xanthine dehydrogenase) and NADPH oxidase, major sources of superoxide in rat hearts, were determined from ventricular homogenates (protein concentration: 8–10 μg μl−1). XOR activity was determined by a fluorometric kinetic assay based on the conversion of pterine to isoxanthopterine in the presence (total XOR activity) and absence (xanthine oxidase activity) of the electron acceptor methylene blue, as described (Beckman et al., 1989). NADPH oxidase activity was measured by a chemiluminescence assay (Mohazzab et al., 1997; Csont et al., 2007) in Krebs-Henseleit solution containing 0.25 M HEPES (pH 7.4), 5 μM lucigenin and 30 μl of tissue homogenate in 1 ml of total volume using a Packard Bell liquid scintillation counter in out-of-coincidence mode. The increase in luminescence signal was monitored for 5 min after adding 100 μM NADPH. Ventricular homogenates were prepared as for the measurement of NOS activity.
Total activity of superoxide dismutase (SOD) was measured by a spectrophotometric assay using a Randox Laboratories kit. Approximately 100 mg ventricular tissue was homogenized in 10 × volumes of ice-cold phosphate buffer (0.01 M, pH 7.0). SOD activity in homogenates was determined by the inhibition of formazan dye formation due to superoxide generated by xanthine and xanthine oxidase.
Measurement of cardiac and systemic ONOO−
To estimate cardiac and systemic ONOO− formation, we measured free nitrotyrosine by ELISA (Cayman Chemical, Ann Arbor, MI, USA) in myocardial homogenates and in the serum as described (Ferdinandy et al., 2000; Csont et al., 2002). Briefly, samples were deproteinized by the addition of four volumes of ice-cold ethanol. After centrifugation, the supernatants were evaporated in nitrogen flow, dissolved in phosphate buffer and incubated overnight with anti-nitrotyrosine rabbit IgG and nitrotyrosine acetylcholinesterase tracer in precoated (mouse anti-rabbit IgG) microplates, followed by the development with Ellman's reagent.
S-nitrosylation of SERCA2a
To assess SERCA S-nitrosylation, sarcoplasmic reticulum was isolated as described previously (Nakanishi and Jarmakani, 1984; Komuro et al., 1989). All procedures were performed at 4 °C. The left ventricles (approximately 1.5 g) were minced with scissors and homogenized three times with a polytron tissue processor for 5 s in 4 × volume of the isolation solution containing 10 mM Na2HCO3 (pH 7.1 with 0.1 N HCl). The homogenate was centrifuged at 1000 g for 10 min. The supernatant was centrifuged at 14 000 g for 20 min three times to remove mitochondria. The supernatant from the third spin was centrifuged at 45 000 g for 30 min. The pellet was suspended in a solution containing 0.6 M KCl and 10 mM N-tris(hydroxymethyl) methyl-2-aminomethane-sulphonic acid (pH 7.1) using a glass–Teflon homogenizer. The suspension was centrifuged at 45 000 g for 30 min. The resulting pellet was suspended in solution containing 150 mM KCl and 1 mM N-tris(hydroxymethyl) methyl-2-aminomethane-sulphonic acid (pH 7.1) and used as sarcoplasmic reticulum fractions.
Isolated sarcoplasmic reticulum fractions were resuspended in 40 μl solution containing 150 mM KCl and 1 mM N-tris(hydroxymethyl) methyl-2-aminomethane-sulphonic acid (pH 7.1) to load 20 μg of total protein on 8% polyacrylamide gel. After electrophoresis and blotting, nitrocellulose membranes were incubated with mouse monoclonal anti-SERCA2a antibody or rabbit polyclonal SNO-Cys antibody (ab2861; Abcam plc., Cambridge, UK and NISC11-A; Alpha Diagnostics International Inc., San Antonio, TX, USA, respectively) for 1.5 h. Polyclonal rabbit anti-mouse IgG and polyclonal goat anti-rabbit IgG secondary antibodies (DakoCytomation Denmark A/S, Glostrup, Denmark) were used for incubation at room temperature for 1 h, as appropriate. Membranes were developed with an enhanced chemiluminescence kit (ECL Plus; GE Healthcare, Little Chalfont, Buckinghamshire, UK), exposed to X-ray film, scanned and density of SNO-Cys bands were measured and normalized to the density of SERCA2a bands, and SNO-Cys/SERCA2a ratio was expressed in arbitrary units.
Statistical analysis
Data were expressed as means±s.e.mean or s.d. and analysed with unpaired t-test. P⩽0.05 was accepted as statistically significant difference compared to control group.
Results
Effects of sensory chemodenervation by capsaicin on myocardial function
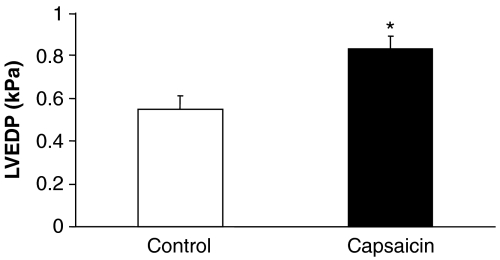
We measured parameters of myocardial haemodynamics to assess the effect of capsaicin-induced chemodenervation on basal cardiac function in isolated heart preparations. LVEDP, the most sensitive marker for myocardial relaxation, was significantly increased when hearts were isolated after systemic capsaicin treatment, whereas other functional parameters were not affected as compared to solvent-treated controls (Figure 2 and Table 1).
Figure 2.
Effect of sensory chemodenervation by systemic capsaicin pretreatment on left ventricular end-diastolic pressure (LVEDP) in isolated rat hearts. Values are means±s.e.mean (n=6 in each group; *P<0.01 vs control).
Table 1.
Effect of systemic capsaicin pretreatment on cardiac functional parameters in isolated rat hearts
| HR (bpm) | CF (ml min−1) | AF (ml min−1) | LVDP (kPa) | +dP dtmax−1 (kPa s−1) | −dP dtmax−1 (kPa s−1) | |
|---|---|---|---|---|---|---|
| Control | 278±6 | 22.0±1.0 | 44.3±2.9 | 18.2±0.6 | 904±63 | 472±24 |
| Capsaicin | 270±11 | 22.7±0.7 | 46.7±1.1 | 18.7±0.4 | 903±58 | 458±32 |
Abbreviations: AF, aortic flow; CF, coronary flow; HR, heart rate; LVDP, left ventricular developed pressure.
Values are means±s.e.mean (n=6 in each group).
Effects of sensory chemodenervation on cardiac NO
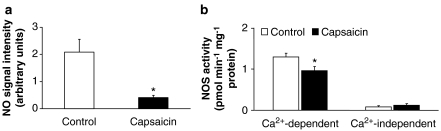
In the solvent-treated group, basal cardiac NO content was detected by ESR spectroscopy. In the capsaicin-treated group, the specific signal for NO was markedly reduced to a level near the detection limit (Figure 3a).
Figure 3.
(a) NO content of left ventricular tissue samples in solvent and capsaicin-pretreated groups as measured by electron spin resonance spectroscopy (values are means±s.e.mean; n=6 in each group, *P<0.01 vs control, ESR parameters: X band, 100 kHz modulation frequency, 160 K, 10 mW microwave power, 2.85 G modulation amplitude, 340 G sweep width and 3350 G central field). (b) Myocardial activities of NOS. Values are means±s.e.mean (n=6 in each group, *P<0.01 vs control).
To further explore cardiac NO synthesis, activities of NOS were measured. Ca2+-dependent activity significantly decreased in the capsaicin-pretreated group, whereas Ca2+-independent activity did not change (Figure 3b).
We also examined the gene expression pattern of the three different isoforms of NOS in the heart by real-time quantitative PCR. The mRNA level of endothelial NOS (NOS-3) was significantly downregulated by capsaicin treatment; however, the expression of neuronal NOS (NOS-1) or inducible NOS (NOS-2) was not affected (Table 2).
Table 2.
Effect of chemodenervation by systemic capsaicin pretreatment on gene expression pattern of NOS isoforms measured by real-time QPCR in rat hearts
| Gene product | Access no. | Real-time PCR | Forward primer | Reverse primer |
|---|---|---|---|---|
| Endothelial NOS (NOS-3) | AF085195 | −1.96 (0.19)* | TGGCAACAGCGACAATTTGA | CACCCGAAGACCAGAACCAT |
| Inducible NOS (NOS-2) | NM012611 | −0.27 (0.12) | CGGCTGCCCGGAAAA | TCGTCGGCCAGCTCTTTCT |
| Neuronal NOS (NOS-1) | U67309 | −0.68 (0.10) | CAAACCGAGGCAATCTTCGT | CCCGGCCAGCGTAGCT |
Abbreviation: QPCR, quantitative PCR.
Values are mean fold changes±s.d.
*P<0.05 significant difference as compared to control.
Effects of sensory chemodenervation on cardiac superoxide
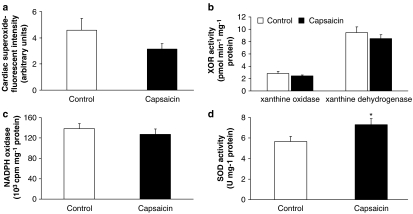
To further test the mechanism of reduced cardiac NO due to systemic capsaicin pretreatment, we systematically analysed myocardial superoxide synthesis and ONOO− formation. Myocardial superoxide, as assessed by dihydroethidium staining, was approximately 30% lower in the capsaicin-treated group, but this difference was not statistically significant (Figure 4a). We also measured the activity of XOR and NADPH oxidase enzyme activities, the major enzymatic sources of superoxide in rat hearts. There was no significant difference in activities of xanthine oxidase, xanthine dehydrogenase (Figure 4b) and NADPH oxidase (Figure 4c).
Figure 4.
Effect of sensory chemodenervation by systemic capsaicin pretreatment on cardiac superoxide (a), cardiac activities of xanthine oxidoreductase (XOR, b), NADPH oxidase (c) and superoxide dismutase (SOD, d). Values are mean±s.e.mean (n=11 in each group; *P<0.05 vs control).
We also tested the total activity of SOD in the heart, the major enzyme responsible for endogenous detoxification of superoxide, and found that SOD activity was significantly increased in the capsaicin-pretreated group (Figure 4d).
Effects of sensory chemodenervation on cardiac ONOO− and S-nitrosylation of SERCA
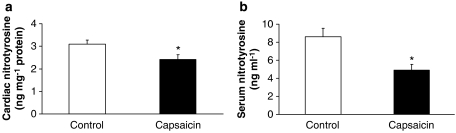
We then studied whether decreased NO synthesis and increased SOD activity due to chemodenervation changed basal cardiac ONOO− formation in cardiac and extracardiac tissues. Therefore, the concentration of free nitrotyrosine (as a marker for systemic ONOO− formation) was measured in myocardium and serum. Both serum free nitrotyrosine and myocardial free nitrotyrosine were markedly decreased in capsaicin-pretreated groups when compared to controls (Figures 5a and b).
Figure 5.
Myocardial- (a) and serum- (b) free nitrotyrosine measured by ELISA in the serum and in the cardiac homogenates as markers of ONOO− generation. Values are mean±s.e.mean (n=11 in each group; *P<0.05 vs control).
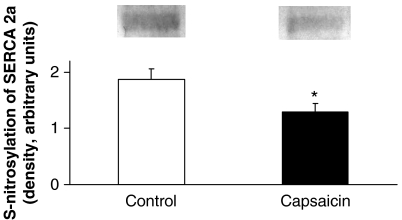
To assess if decreased ONOO− may decrease the degree of S-nitrosylation of cardiac SERCA (SERCA2a), we measured S-nitrosylation in cardiac sarcoplasmic reticulum preparations isolated from both groups by western blotting. We found that sensory chemodenervation significantly decreased S-nitrosylation of SERCA2a in the capsaicin-pretreated group when compared to solvent-treated controls (Figure 6).
Figure 6.
S-nitrosylation of SERCA2a as demonstrated by representative western blots and mean values are shown in the bar graph. Values are mean±s.e.mean (n=9 in each group; *P<0.05 vs control).
Discussion and conclusions
We have found in the present study that systemic sensory chemodenervation decreases cardiac NO availability via decreased expression of Ca2+-dependent NOS and increases SOD activity thereby leading to decreased basal ONOO− formation and a reduction of S-nitrosylation of SERCA2a, which causes impaired myocardial relaxation characterized by increased LVEDP. This is the first demonstration that capsaicin-sensitive sensory neurons regulate myocardial relaxation via maintaining basal ONOO− formation and SERCA S-nitrosylation.
Focusing mostly on the well-known adrenergic and cholinergic effector innervation of the heart, cardiovascular researchers generally neglect the rich sensory innervation of the myocardium and the coronary vascular system, which may have considerable influence on cardiac physiology and pathology due to its content of NO, calcitonin gene-related peptide, substance P and other neurokinins (Franco-Cereceda, 1988; Sosunov et al., 1995, 1996; Szallasi and Blumberg, 1999). For example, calcitonin gene-related peptide affects atrial contractility and relaxation (Huang et al., 1999), and induces a prolongation of the action potential (Franco-Cereceda et al., 1988). Furthermore, it has become well established that sensory neuropathy develops in the presence of major cardiovascular risk factors, such as diabetes (Polydefkis et al., 2004; Facer et al., 2007), dyslipidemia (Hughes et al., 2004; Kassem et al., 2005) and obesity (Herman et al., 2007). Nevertheless, still very little is known about the physiological and pathological role of sensory nerves in the regulation of the cardiovascular system. Since the original breakthrough observation by Jancso et al. (1977), in the late 1970s, capsaicin has become one of the most important probes for investigation of sensory neural pathology, as selective sensory chemodenervation by capsaicin and its analogues enabled the development of animal models for sensory neuropathy (Jancso et al., 1968; Khan et al., 2002; Szolcsanyi, 2004).
We have previously shown that sensory chemodenervation leads to impaired myocardial relaxation with a concomitant decrease in cardiac NO content; however, its mechanism remained unclear (Ferdinandy et al., 1997; Csont et al., 2003). In the present study, we confirmed that capsaicin-induced sensory denervation led to impaired cardiac relaxation characterized by elevation of LVEDP and to decreased cardiac NO availability. Here, we further examined the mechanism of reduced cardiac NO content and showed that it is due to decreased expression of endothelial NOS and therefore decreased enzymatic activity of Ca2+-dependent NOS.
To further examine if reduced basal NO content may lead to decreased basal ONOO− formation, we systematically measured superoxide formation, as ONOO− is formed from the rapid nonenzymatic reaction of NO and superoxide (Ferdinandy and Schulz, 2001, 2003). Activities of two major enzymatic sources of superoxide in the heart, XOR and NADPH oxidase, were not changed. However, cardiac activity of SOD, the major enzyme for superoxide removal, was significantly increased by systemic sensory chemodenervation. Myocardial superoxide content as assessed by dihydroethidium staining was approximately 30% lower in the capsaicin-treated group, although this was not statistically significant. Nevertheless, these findings indicate that at least there is a tendency of decreased basal superoxide availability in the myocardium when sensory neural function was ablated by capsaicin. To examine if diminished NO and superoxide availability in the myocardial tissue may lead to decreased formation of ONOO−, we measured markers of ONOO−-induced nitration, that is, levels of cardiac nitrotyrosine. Myocardial free nitrotyrosine was significantly decreased after capsaicin pretreatment. This was further supported by decreased nitrotyrosine level in the serum, a marker for systemic ONOO− formation. These results clearly show that after systemic sensory chemodenervation, there is a decrease in basal cardiac and systemic ONOO− formation.
Although earlier ONOO− was thought to be a purely toxic reactive nitrogen species (Ferdinandy and Schulz, 2001, 2003), recently it has been shown that basal ONOO− production plays an important role as a regulator of several cellular mechanisms via protein nitrosylation of some enzymes (Ferdinandy, 2006; Pacher et al., 2007). Nitrosylation of Cys349 has been shown to be responsible for the activation of the SERCA by ONOO− thereby maintaining the normal physiological function of SERCA in myocardial relaxation (Viner et al., 1999; Adachi et al., 2004). Here, we have found that decreased basal ONOO− formation due to sensory chemodenervation resulted in a significant decrease in S-nitrosylation of SERCA2a and impaired relaxation of the heart characterized by increased LVEDP, the most sensitive haemodynamic marker for impairment of myocardial relaxation. Nevertheless, other molecular targets of ONOO− could be also involved in regulation of myocardial relaxation (Viappiani and Schulz, 2006). Recently, a total of 48 putative proteins containing nitrotyrosine were identified in whole heart homogenates by a proteomic approach (Kanski et al., 2005). Through S-nitrosylation, ONOO− can activate matrix metalloproteinases (MMP-9, MMP-2) and the resultant proteolysis of novel intracellular targets, including troponin I and myosin light chain 1, might also take part in the regulation of myocardial contractile function (Chow et al., 2007).
There are some limitations of the study. First of all, owing to its very short half-life at physiological pH, endogenous formation of ONOO− cannot be directly detected in biological systems. Therefore, to estimate basal ONOO− formation, we measured the most widely accepted marker of ONOO−, free nitrotyrosine, probably underestimating local ONOO− formation (Ferdinandy, 2006). Furthermore, our present study does not show a direct link between S-nitrosylation of SERCA2a and relaxation, as there is no validated method to replace physiological levels of ONOO− in the intact heart (Ferdinandy, 2006). Finally, we have measured all biochemical and cardiac functional parameters 7 days after the last administration of capsaicin, when depletion of peptide-containing myocardial sensory nerves and elimination of capsaicin were complete (Ferdinandy et al., 1997; Csont et al., 2003). However, a nonspecific systemic effect of capsaicin other than depletion of sensory nerves cannot be excluded (Szallasi and Blumberg, 1999).
We conclude that our present study provides the first evidence that sensory nerves play a significant role in the regulation of myocardial relaxation via maintaining basal cardiac NO, superoxide and ONOO− formation and thereby the physiological level of SERCA S-nitrosylation. The possible clinical relevance of our results are substantial, as it may emphasize the need for protection of sensory nerves in the presence of various risk factors for cardiovascular diseases, such as diabetes, dyslipidemia and obesity, associated with the development of sensory neuropathy.
Acknowledgments
This work was supported by grants from the Hungarian Ministry of Health (ETT 597/2006, ETT 515/2003), the Hungarian Scientific Research Fund (OTKA F 046810, T046417) and the National Office for Research and Technology (RET OMFB-00067/2005, Asboth-2005, Jedlik NKFP-A1-2006-0029). TC holds a János Bolyai fellowship.
Abbreviations
- AF
aortic flow
- CF
coronary flow
- ESR
electron-spin resonance spectroscopy
- LVEDP
left ventricular end-diastolic pressure
- ONOO−
peroxynitrite
- SERCA
sarcoendoplasmic reticulum Ca2+-ATPase
- SNO-Cys
S-nitroso-cysteine
- SOD
superoxide dismutase
- XOR
xanthine oxidoreductase
Conflict of interest
The authors state no conflict of interest.
References
- Abrams DI, Jay CA, Shade SB, Vizoso H, Reda H, Press S, et al. Cannabis in painful HIV-associated sensory neuropathy: a randomized placebo-controlled trial. Neurology. 2007;68:515–521. doi: 10.1212/01.wnl.0000253187.66183.9c. [DOI] [PubMed] [Google Scholar]
- Adachi T, Weisbrod RM, Pimentel DR, Ying J, Sharov VS, Schoneich C, et al. S-glutathiolation by peroxynitrite activates SERCA during arterial relaxation by nitric oxide. Nat Med. 2004;10:1200–1207. doi: 10.1038/nm1119. [DOI] [PubMed] [Google Scholar]
- Beckman JS, Parks DA, Pearson JD, Marshall PA, Freeman BA. A sensitive fluorometric assay for measuring xanthine dehydrogenase and oxidase in tissues. Free Radic Biol Med. 1989;6:607–615. doi: 10.1016/0891-5849(89)90068-3. [DOI] [PubMed] [Google Scholar]
- Chow AK, Cena J, Schulz R. Acute actions and novel targets of matrix metalloproteinases in the heart and vasculature. Br J Pharmacol. 2007;152:189–205. doi: 10.1038/sj.bjp.0707344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cinca J, Rodriguez-Sinovas A. Cardiovascular reflex responses induced by epicardial chemoreceptor stimulation. Cardiovasc Res. 2000;45:163–171. doi: 10.1016/s0008-6363(99)00319-3. [DOI] [PubMed] [Google Scholar]
- Csonka C, Szilvassy Z, Fulop F, Pali T, Blasig IE, Tosaki A, et al. Classic preconditioning decreases the harmful accumulation of nitric oxide during ischemia and reperfusion in rat hearts. Circulation. 1999;100:2260–2266. doi: 10.1161/01.cir.100.22.2260. [DOI] [PubMed] [Google Scholar]
- Csont T, Bereczki E, Bencsik P, Fodor G, Görbe A, Zvara Á, et al. Hypercholesterolemia increases myocardial oxidative and nitrosative stress thereby leading to cardiac dysfunction in apoB-100 transgenic mice. Cardiovasc Res. 2007;76:100–109. doi: 10.1016/j.cardiores.2007.06.006. [DOI] [PubMed] [Google Scholar]
- Csont T, Csonka C, Kovacs P, Jancso G, Ferdinandy P. Capsaicin-sensitive sensory neurons regulate myocardial nitric oxide and cGMP signaling. Eur J Pharmacol. 2003;476:107–113. doi: 10.1016/s0014-2999(03)02117-4. [DOI] [PubMed] [Google Scholar]
- Csont T, Csonka C, Onody A, Gorbe A, Dux L, Schulz R, et al. Nitrate tolerance does not increase production of peroxynitrite in the heart. Am J Physiol Heart Circ Physiol. 2002;283:H69–H76. doi: 10.1152/ajpheart.00817.2001. [DOI] [PubMed] [Google Scholar]
- Csont T, Pali T, Szilvassy Z, Ferdinandy P. Lack of correlation between myocardial nitric oxide and cyclic guanosine monophosphate content in both nitrate-tolerant and -nontolerant rats. Biochem Pharmacol. 1998;56:1139–1144. doi: 10.1016/s0006-2952(98)00167-1. [DOI] [PubMed] [Google Scholar]
- Csont T, Szilvassy Z, Fulop F, Nedeianu S, Pali T, Tosaki A, et al. Direct myocardial anti-ischaemic effect of GTN in both nitrate-tolerant and nontolerant rats: a cyclic GMP-independent activation of KATP. Br J Pharmacol. 1999;128:1427–1434. doi: 10.1038/sj.bjp.0702929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Facer P, Casula MA, Smith GD, Benham CD, Chessell IP, Bountra C, et al. Differential expression of the capsaicin receptor TRPV1 and related novel receptors TRPV3, TRPV4 and TRPM8 in normal human tissues and changes in traumatic and diabetic neuropathy. BMC Neurol. 2007;7:11. doi: 10.1186/1471-2377-7-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferdinandy P. Peroxynitrite: just an oxidative/nitrosative stressor or a physiological regulator as well. Br J Pharmacol. 2006;148:1–3. doi: 10.1038/sj.bjp.0706693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferdinandy P, Schulz R. Peroxynitrite: toxic or protective in the heart. Circ Res. 2001;88:E12–E13. doi: 10.1161/01.res.88.2.e12. [DOI] [PubMed] [Google Scholar]
- Ferdinandy P, Csont T, Csonka C, Torok M, Dux M, Nemeth J, et al. Capsaicin-sensitive local sensory innervation is involved in pacing-induced preconditioning in rat hearts: role of nitric oxide and CGRP. Naunyn Schmiedebergs Arch Pharmacol. 1997;356:356–363. doi: 10.1007/pl00005062. [DOI] [PubMed] [Google Scholar]
- Ferdinandy P, Danial H, Ambrus I, Rothery RA, Schulz R. Peroxynitrite is a major contributor to cytokine-induced myocardial contractile failure. Circ Res. 2000;87:241–247. doi: 10.1161/01.res.87.3.241. [DOI] [PubMed] [Google Scholar]
- Ferdinandy P, Schulz R. Nitric oxide, superoxide, and peroxynitrite in myocardial ischaemia–reperfusion injury and preconditioning. Br J Pharmacol. 2003;138:532–543. doi: 10.1038/sj.bjp.0705080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco-Cereceda A. Calcitonin gene-related peptide and tachykinins in relation to local sensory control of cardiac contractility and coronary vascular tone. Acta Physiol Scand Suppl. 1988;569:1–63. [PubMed] [Google Scholar]
- Franco-Cereceda A, Lundberg JM, Saria A, Schreibmayer W, Tritthart HA. Calcitonin gene-related peptide: release by capsaicin and prolongation of the action potential in the guinea-pig heart. Acta Physiol Scand. 1988;132:181–190. doi: 10.1111/j.1748-1716.1988.tb08316.x. [DOI] [PubMed] [Google Scholar]
- Herman RM, Brower JB, Stoddard DG, Casano AR, Targovnik JH, Herman JH, et al. Prevalence of somatic small fiber neuropathy in obesity. Int J Obes (Lond) 2007;31:226–235. doi: 10.1038/sj.ijo.0803418. [DOI] [PubMed] [Google Scholar]
- Holzer P. Efferent-like roles of afferent neurons in the gut: blood flow regulation and tissue protection. Auton Neurosci. 2006;125:70–75. doi: 10.1016/j.autneu.2006.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang MH, Knight PR, III, Izzo JL., Jr Ca2+-induced Ca2+ release involved in positive inotropic effect mediated by CGRP in ventricular myocytes. Am J Physiol. 1999;276:R259–R264. doi: 10.1152/ajpregu.1999.276.1.R259. [DOI] [PubMed] [Google Scholar]
- Hughes RA, Umapathi T, Gray IA, Gregson NA, Noori M, Pannala AS, et al. A controlled investigation of the cause of chronic idiopathic axonal polyneuropathy. Brain. 2004;127:1723–1730. doi: 10.1093/brain/awh192. [DOI] [PubMed] [Google Scholar]
- Jancso G, Kiraly E, Jancso-Gabor A. Pharmacologically induced selective degeneration of chemosensitive primary sensory neurones. Nature. 1977;270:741–743. doi: 10.1038/270741a0. [DOI] [PubMed] [Google Scholar]
- Jancso N, Jancso-Gabor A, Szolcsanyi J. The role of sensory nerve endings in neurogenic inflammation induced in human skin and in the eye and paw of the rat. Br J Pharmacol Chemother. 1968;33:32–41. doi: 10.1111/j.1476-5381.1968.tb00471.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanski J, Behring A, Pelling J, Schoneich C. Proteomic identification of 3-nitrotyrosine-containing rat cardiac proteins: effects of biological aging. Am J Physiol Heart Circ Physiol. 2005;288:H371–H381. doi: 10.1152/ajpheart.01030.2003. [DOI] [PubMed] [Google Scholar]
- Kassem HS, Azar ST, Zantout MS, Sawaya RA. Hypertriglyceridemia and peripheral neuropathy in neurologically asymptomatic patients. Neuro Endocrinol Lett. 2005;26:775–779. [PubMed] [Google Scholar]
- Katona M, Boros K, Santha P, Ferdinandy P, Dux M, Jancso G. Selective sensory denervation by capsaicin aggravates adriamycin-induced cardiomyopathy in rats. Naunyn Schmiedebergs Arch Pharmacol. 2004;370:436–443. doi: 10.1007/s00210-004-0985-7. [DOI] [PubMed] [Google Scholar]
- Khan GM, Chen SR, Pan HL. Role of primary afferent nerves in allodynia caused by diabetic neuropathy in rats. Neuroscience. 2002;114:291–299. doi: 10.1016/s0306-4522(02)00372-x. [DOI] [PubMed] [Google Scholar]
- Komuro I, Kurabayashi M, Shibazaki Y, Takaku F, Yazaki Y. Molecular cloning and characterization of a Ca2+ + Mg2+-dependent adenosine triphosphatase from rat cardiac sarcoplasmic reticulum. Regulation of its expression by pressure overload and developmental stage. J Clin Invest. 1989;83:1102–1108. doi: 10.1172/JCI113989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li YJ, Xiao ZS, Peng CF, Deng HW. Calcitonin gene-related peptide-induced preconditioning protects against ischemia–reperfusion injury in isolated rat hearts. Eur J Pharmacol. 1996;311:163–167. doi: 10.1016/0014-2999(96)00426-8. [DOI] [PubMed] [Google Scholar]
- Mohazzab H, Kaminski PM, Wolin MS. Lactate and PO2 modulate superoxide anion production in bovine cardiac myocytes: potential role of NADH oxidase. Circulation. 1997;96:614–620. doi: 10.1161/01.cir.96.2.614. [DOI] [PubMed] [Google Scholar]
- Nakanishi T, Jarmakani JM. Developmental changes in myocardial mechanical function and subcellular organelles. Am J Physiol. 1984;246:H615–H625. doi: 10.1152/ajpheart.1984.246.4.H615. [DOI] [PubMed] [Google Scholar]
- Onody A, Csonka C, Giricz Z, Ferdinandy P. Hyperlipidemia induced by a cholesterol-rich diet leads to enhanced peroxynitrite formation in rat hearts. Cardiovasc Res. 2003;58:663–670. doi: 10.1016/s0008-6363(03)00330-4. [DOI] [PubMed] [Google Scholar]
- Pacher P, Beckman JS, Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiol Rev. 2007;87:315–424. doi: 10.1152/physrev.00029.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polydefkis M, Hauer P, Sheth S, Sirdofsky M, Griffin JW, McArthur JC. The time course of epidermal nerve fibre regeneration: studies in normal controls and in people with diabetes, with and without neuropathy. Brain. 2004;127:1606–1615. doi: 10.1093/brain/awh175. [DOI] [PubMed] [Google Scholar]
- Ren K, Ruda MA. Nitric oxide synthase-containing neurons in sensory ganglia of the rat are susceptible to capsaicin-induced cytotoxicity. Neuroscience. 1995;65:505–511. doi: 10.1016/0306-4522(94)00510-c. [DOI] [PubMed] [Google Scholar]
- Schulz R, Nava E, Moncada S. Induction and potential biological relevance of a Ca(2+)-independent nitric oxide synthase in the myocardium. Br J Pharmacol. 1992;105:575–580. doi: 10.1111/j.1476-5381.1992.tb09021.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sosunov AA, Hassall CJ, Loesch A, Turmaine M, Burnstock G. Ultrastructural investigation of nitric oxide synthase-immunoreactive nerves associated with coronary blood vessels of rat and guinea-pig. Cell Tissue Res. 1995;280:575–582. doi: 10.1007/BF00318361. [DOI] [PubMed] [Google Scholar]
- Sosunov AA, Hassall CJ, Loesch A, Turmaine M, Burnstock G. Nitric oxide synthase-containing neurones and nerve fibres within cardiac ganglia of rat and guinea-pig: an electron-microscopic immunocytochemical study. Cell Tissue Res. 1996;284:19–28. doi: 10.1007/s004410050563. [DOI] [PubMed] [Google Scholar]
- Szallasi A, Blumberg PM. Vanilloid (capsaicin) receptors and mechanisms. Pharmacol Rev. 1999;51:159–212. [PubMed] [Google Scholar]
- Szallasi A, Cortright DN, Blum CA, Eid SR. The vanilloid receptor TRPV1: 10 years from channel cloning to antagonist proof-of-concept. Nat Rev Drug Dis. 2007;6:357–372. doi: 10.1038/nrd2280. [DOI] [PubMed] [Google Scholar]
- Szolcsanyi J. Forty years in capsaicin research for sensory pharmacology and physiology. Neuropeptides. 2004;38:377–384. doi: 10.1016/j.npep.2004.07.005. [DOI] [PubMed] [Google Scholar]
- Viappiani S, Schulz R. Detection of specific nitrotyrosine-modified proteins as a marker of oxidative stress in cardiovascular disease. Am J Physiol Heart Circ Physiol. 2006;290:H2167–H2168. doi: 10.1152/ajpheart.00128.2006. [DOI] [PubMed] [Google Scholar]
- Viner RI, Williams TD, Schoneich C. Peroxynitrite modification of protein thiols: oxidation, nitrosylation, and S-glutathiolation of functionally important cysteine residue(s) in the sarcoplasmic reticulum Ca-ATPase. Biochemistry. 1999;38:12408–12415. doi: 10.1021/bi9909445. [DOI] [PubMed] [Google Scholar]
- Wang L, Wang DH. TRPV1 gene knockout impairs postischemic recovery in isolated perfused heart in mice. Circulation. 2005;112:3617–3623. doi: 10.1161/CIRCULATIONAHA.105.556274. [DOI] [PubMed] [Google Scholar]
- Zhong B, Wang DH. TRPV1 gene knockout impairs preconditioning protection against myocardial injury in isolated perfused hearts in mice. Am J Physiol Heart Circ Physiol. 2007;293:H1791–H1798. doi: 10.1152/ajpheart.00169.2007. [DOI] [PubMed] [Google Scholar]
- Zvara A, Bencsik P, Fodor G, Csont T, Hackler L, Jr, Dux M, et al. Capsaicin-sensitive sensory neurons regulate myocardial function and gene expression pattern of rat hearts: a DNA microarray study. FASEB J. 2006;20:160–162. doi: 10.1096/fj.05-4060fje. [DOI] [PubMed] [Google Scholar]