Abstract
Background and purpose:
The human, rat, and mouse P2X7 receptors have been previously characterized, and in this study we report the cloning and pharmacological properties of the guinea pig orthologue.
Experimental approach:
A cDNA encoding for the guinea pig P2X7 receptor was isolated from a guinea pig brain library. The receptor was expressed in U-2 OS cells using the BacMam viral expression system. A monoclonal antibody was used to confirm high levels of cell surface expression and the functional properties were determined in ethidium bromide accumulation studies.
Key results:
The predicted guinea pig protein is one amino acid shorter than the human and rat orthologues and over 70% identical to the rat and human receptors. In contrast to human and rat P2X7 receptors, 2′-&3′-O-(4benzoylbenzoyl)ATP (BzATP) was a partial agonist of the guinea pig P2X7 receptor when compared to ATP and acted as an antagonist in some assays. However, as at other species orthologues, BzATP was more potent than ATP. The guinea pig P2X7 receptor possessed higher affinity for 1-[N,O-bis(5-isoquinoline sulphonyl)-N-methyl-L-tyrosyl]-4-phenylpiperazine (KN62), suramin and Coomassie Brilliant Blue than human or rat P2X7 receptors suggesting that it is pharmacologically different to other rodent or human P2X7 receptors.
Conclusions and implications:
The guinea pig recombinant P2X7 receptor displays a number of unique properties that differentiate it from the human, rat and mouse orthologues and this structural and functional information should aid in our understanding of the interaction of agonists and antagonist with the P2X7 receptor.
Keywords: ion channel, BacMam, ATP, BzATP, U-2 OS
Introduction
The P2X7 receptor is a ligand-gated cation channel activated by extracellular ATP (for review see North, 2002; Burnstock and Knight, 2004; Liang and Schwiebert, 2005). The P2X7 receptor exhibits a number of unusual properties. Following brief activation by low concentrations of agonist, the receptor acts as a nonselective cation channel permeable to calcium, potassium and sodium. However, repeated or prolonged activation by higher agonist concentrations creates a much larger aqueous pore permeable to molecules up to 900 kDa and eventually leads to cell lysis. Recent studies suggest that the large pore may reflect coupling to a pannexin hemichannel (Pelegrin and Surprenant, 2006). The P2X7 receptors are expressed in immune cells and cells from immune origin (Surprenant et al., 1996), and it has been reported recently in neurons (Anderson and Nedergaard, 2006). The localization of P2X7 receptors on pro-inflammatory cells and its ability to modulate the release of the pro-inflammatory cytokine interleukin-1β (North, 2002) have suggested a role in inflammatory diseases. It has been reported that extracellular ATP acting through the P2X7 receptor may be an important modulator of the neuroinflammation in Alzheimer's disease (Rampe et al., 2004), and more recently it was demonstrated that P2X7 knockout mice show reduced inflammatory and neuropathic pain (Chessell et al., 2005).
The rat, human and mouse P2X7 receptors have been cloned and their pharmacological and functional properties determined (Surprenant et al., 1996; Rassendren et al., 1997; Chessell et al., 1998). These three species orthologues possess different pharmacological properties with the rat and human P2X7 receptors having higher affinity for the agonist 2′-&3′-O-(4benzoylbenzoyl) ATP (BzATP) than the mouse P2X7 receptor (Chessell et al., 1998). The species orthologues also show very marked differences with respect to antagonist potency particularly between human and rodent receptors. Thus, 1-[N, O-bis(5-isoquinolinesulphonyl)-N-methyl-L-tyrosyl]-4-phenylpiperazine (KN62) possesses much higher affinity for human than rat or mouse receptors, whereas Coomassie brilliant blue (CBB) possesses higher affinity for rat and mouse than human receptors (Humphreys et al., 1998; Hibell et al., 2001). More recently, novel P2X7 receptor antagonists such as AZ11645373 (Stokes et al., 2006) and even non-selective antagonists such as oxidized ATP, pyridoxal phosphate-6-azophenyl-2′, 4′-disulphonic acid (PPADS) and suramin (Hibell et al., 2001) have also been shown to possess higher affinity for human than for rodent receptors. These species differences in antagonist potency clearly complicate pre-clinical studies on P2X7 receptor antagonists, which are currently under investigation for various therapeutic indications (Baraldi et al., 2004). In this regard, KN62 was found to block a guinea-pig putative P2X7 receptor (Hu et al., 2001), which may indicate that the guinea-pig P2X7 receptor is pharmacologically similar to the human receptor. To study this further, we have cloned and pharmacologically characterized this species orthologue.
In the present study, we report the isolation of the cDNA encoding the guinea-pig P2X7 receptor from a brain library and its pharmacological characterization. For these studies, we used the BacMam (recombinant baculovirus in which the polyhedrin promoter has been replaced with a mammalian promoter) expression system (Kost et al., 2005) as an alternative to electroporation or stable cell line generation. This technology is safe, as the viruses are unable to replicate in mammalian cells, easy to use and amenable to use with a wide range of host cells. Furthermore, the BacMam technology has been used for the expression of recombinant proteins for cell-based functional assays, including G-protein-coupled receptors (Ames et al., 2004a), nuclear receptors (Boudjelal et al., 2005) and ion channels (Pfohl et al., 2002). In the present study, we evaluated this technology for functional expression of P2X7 receptors. We used U-2 OS (human osteosarcoma cell line) cells as they have a null background for P2Y receptors (Ames et al., 2004b) and do not express P2X7 receptors (Michel et al., 2007).
Pharmacological characterization of the guinea-pig P2X7 receptor showed that the receptor does have some pharmacological similarities to human P2X7 receptors but also that it has significant differences from human as well as rat and mouse P2X7 receptor orthologues.
Methods
Cloning of the guinea-pig P2X7 receptor
The 5′ and 3′ sequence ends of the guinea-pig P2X7 gene were determined via rapid amplification of cDNA ends PCR and cloning from guinea-pig brain and spleen SMART cDNA (Clontech, Mountain View, CA, USA). Primers were designed based on an alignment between the rat (NM_019256), mouse (NM_011027) and human (NM_002562) sequences and rapid amplification of cDNA ends PCR was performed to amplify these ends. Once the 5′ and 3′ sequence ends were confirmed between the two different tissue sources, primers were then designed to amplify the full-length cDNA open reading frame. The full-length open reading frame was obtained by PCR from guinea-pig brain SMART cDNA. This sequence was confirmed by comparison with cDNA clones obtained from multiple tissue sources. The final clone was flanked with a 5′ CACC for directional cloning into the pcDNA3.1_V5-His-TOPO vector. The GenBank accession number for the guineapig P2X7 receptor is EU275201.
Verification of sequence
To confirm the absence of amino acid at position 77 in the guinea-pig receptor compared with the human and rat orthologues (Figure 1), total RNA was isolated from guinea-pig brain using the RNeasy mini kit (Qiagen, Crawley, UK). cDNA synthesis and PCR were carried out using the Superscript One step reverse transcriptase-PCR with Platinum Taq kit (Invitrogen, Paisley, UK) and gene-specific primers (5′-CAAAGTCACCCGCATCCAGAGTAG-3′ and 5′-GTCCAGGTTGCAGTCCCAGTTGAT-3′) encompassing the amino acid of interest. A reaction containing no reverse transcriptase-PCR was used as a control. The 739 bp PCR product was agarose gel purified using Geneclean II (MP Biomedicals, Cambridge, Cambs, UK), cloned into pCR2.1 using the TOPO TA cloning kit (Invitrogen) and transformed into TOP10 cells. The resulting transformants were cultured overnight in Luria Bertani broth containing 100 μg ml−1 ampicillin and plasmid DNA was then isolated using the Miniprep spin kit (Qiagen). The insert sequence was then confirmed by DNA sequencing in both directions, confirming the amino acids between positions 17 and 262.
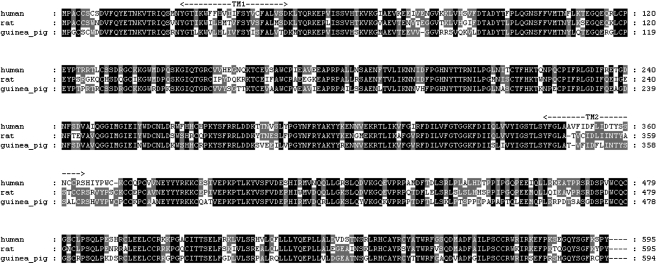
Figure 1.
Pairwise alignment of primary amino-acid sequences of human, rat and guinea-pig P2X7 proteins. The two predicted transmembrane-spanning regions are annotated as TM1 and TM2 with dashes and arrows. Completely conserved residues are coloured black, whereas positions that differ between all three sequences are coloured white. Amino-acid positions that display an intermediate level of conservation are shaded grey.
Construction of pFastBac-Mam-1 expression plasmids and BacMam expression viruses
Guinea-pig P2X7 cDNA was subcloned as a 1860 bp Hind3/Not1 fragment obtained from the plasmid pcDNA3.1/guinea pig P2X7 into the Hind3 and Not1 sites of pFastBac-Mam-1. The BacMam baculovirus transfer vector pFastBac-Mam-1 has been described previously (Condreay et al., 1999). The resulting plasmid, pFBMam-1/guinea-pig P2X7, was then used to generate the BacMam baculovirus BacMam-guinea-pig P2X7. The rat, mouse and human P2X7 receptor sequences (Surprenant et al., 1996; Rassendren et al., 1997; Chessell et al., 1998) were excised from the plasmid pCDNA3 and then ligated into the pFastBac-Mam-1 vector using conventional techniques (Condreay et al., 1999). In these studies, the mouse sequence described by Young et al. (2007) was utilized. This differs from the mouse P2X7 receptor described by Chessell et al. (1998) at three amino acids, specifically, leucine instead of phenylalanine at amino acid 11, threonine instead of alanine at position 221 and threonine instead of methionine at position 283. Identical results were obtained using the mouse P2X7 receptor described by Chessell et al. (1998) (data not shown). All BacMam baculoviruses were generated from their transfer plasmids in Sf9 insect cells using standard methods described previously (Clay et al., 2003).
Cell culture and viral transductions
Human osteosarcoma U-2 OS cells (obtained from ATCC, see Ames et al., 2004b) were maintained in adherent culture conditions in the presence of Dulbecco's modified Eagle's medium:nutrient mixture F-12 supplemented with Glutamax (DMEM:F12+Glutamax, Invitrogen) and 10% foetal bovine serum (PAA laboratories GmBH, Pasching, Austria) at 37 °C, 5% CO2. One day prior to assay, cells were harvested from the culture flasks using 0.05% trypsin/EDTA (Invitrogen), pelleted and resuspended at a concentration of ∼750 × 103 cells ml−1 in culture media in the presence of varying concentrations of the test BacMam viruses (1.25–20% BacMam virus in culture media v/v). Unless stated, human, rat and guinea-pig P2X7 BacMam viruses were used at 10% (v/v), whereas the mouse P2X7 receptor was used at 2.5% (v/v). For ethidium bromide and quantitative antibody binding fluorometry studies, cells (70–80 000) were plated into individual wells of poly-L-lysine pretreated 96-well plates (Costar, High Wycombe, UK) and the plates were incubated at 37 °C, 5% CO2 overnight. Alternatively, for immunocytochemistry (ICC) studies, cells were plated in 22 mm diameter poly-D-lysine-treated BioCoat coverslips (BD Biosciences, Oxford, UK). For the experiments reported here, titres of the different viruses were as follows: 2 × 108 plaque-forming units (PFU) ml−1 for the human P2X7-BacMam, 2.2 × 108 PFU ml−1 for the rat P2X7-BacMam, 1.8 × 108 PFU ml−1 for the guinea-pig P2X7-BacMam and 1.22 × 109 PFU ml−1 for the mouse P2X7-BacMam.
ICC and quantitative antibody binding fluorimetry
ICC was performed as described elsewhere (Fonfria et al., 2002), using a monoclonal antibody specific for human P2X7 receptor (P2X7mAb) (Buell et al., 1998) diluted 1:500, and an Alexa-488-conjugated goat anti-mouse secondary antibody (Invitrogen), diluted 1:200. At the end of the procedure, cells were washed in phosphate-buffered saline (Invitrogen) and mounted onto glass slides using ProLong Gold antifade mounting media (Invitrogen). Fluorescent images were captured using a Leica DMR microscope (Leica Microsystems GmbH, Germany).
Quantitative antibody binding fluorimetry was performed using the same primary and secondary antibodies as above. The assay buffer comprised 2% BSA (Sigma-Aldrich, Poole, UK) in phosphate-buffered saline (Invitrogen). Briefly, adherent cells in 96-well plates were washed twice with 350 μl of assay buffer, and exposed to a mixture of increasing concentrations of primary antibody (0.1–3 μg ml−1) and 10 μg ml−1 of secondary antibody in assay buffer. Plates were covered with aluminium foil and shaken at 10 r.p.m. min−1 in an orbital shaker (Stuart Scientific, Staffordshire, UK) for 3 h at room temperature. After the incubation period, cells were washed three times with assay buffer and 100 μl of fresh assay buffer was added to the cells. P2X7 antibody binding was determined by measuring fluorescence (excitation wavelength of 485 nm and emission wavelength of 530 nm) from below the plate with a FlexStation (Molecular Devices, Wokingham, UK).
Ethidium bromide accumulation assays
For the ethidium bromide accumulation studies, the assay buffer comprised (in mM): HEPES 10, N-methyl-D-glucamine 5, KCl 5.6, D-glucose 10, CaCl2 0.5 (pH 7.4) and was supplemented with either 140 mM NaCl (NaCl buffer) or 280 mM sucrose (sucrose buffer). Studies were performed as described previously (Michel et al., 2006a). Cells grown in poly-L-lysine pretreated 96-well plates (see above) were washed with 350 μl of assay buffer and incubated for 30 min at room temperature (19–21 °C) in the presence or absence of the P2X7 receptor antagonists before addition of agonist (ATP or BzATP, Sigma-Aldrich) and ethidium bromide (100 μM final assay concentration, supplied as 10 mM stock by Pierce, Cramlington, UK). Incubations were continued for the length of time indicated and were rapidly terminated by addition of 25 μl of 1.3 M sucrose assay buffer containing 5 mM of the P2X7 receptor antagonist, Reactive black 5. Cellular accumulation of ethidium in the cell monolayer was determined by measuring fluorescence (excitation wavelength of 530 nm and emission wavelength of 620 nm) from below the plate with a FlexStation (Molecular Devices). For studies using BzATP as an antagonist, a 15 min pre-incubation period was used. The antagonists CBB, KN62, PPADS and decavanadate were obtained from Sigma-Aldrich. Decavanadate solutions were prepared as described previously (Michel et al., 2006b).
Data analysis
Data are mean±s.e.mean of three to four independent experiments. Curve fitting and statistical analysis were performed using GraphPad Prism 3 (GraphPad Software Inc., San Diego, CA, USA). For quantitative immunocytochemical studies, nonspecific binding of the P2X7mAb to non-transduced U-2 OS cells was measured at each mAb concentration and subtracted from the binding signal measured in the U-2 OS cells transduced with the various P2X7 receptors using the BacMam virus expression system in order to measure specific binding of the P2X7mAb. For studies on HEK293 cells stably expressing human or rat P2X7 receptors, nonspecific binding was measured in wild-type HEK293 cells and subtracted from total binding to determine specific binding of the P2X7mAb. For statistical tests, one-way ANOVA followed by Dunnett's or Tukey's post hoc test was used.
Results
Cloning of the guinea-pig P2X7 receptor
The sequence of the guinea-pig P2X7 receptor was identical in cDNA clones generated from two different cDNA tissue sources, guinea-pig brain and guinea-pig spleen SMART cDNA. The predicted final protein sequence is 594 amino acids in length, one amino acid less than the human and rat orthologues, which are both 595 amino acids in length (Figure 1). The difference was due to the absence of the glutamic acid at position 77, which is present in the human, rat and mouse orthologues. The absence of the residue was confirmed by PCR amplification of this region of the receptor from mRNA obtained from guinea-pig brain. This residue is present across all P2X receptors (North, 2002). However, for the P2X1-P2X6 receptors, several residues between amino acids 72 and 86 (P2X7 nomenclature) in exon 2 are missing or show a high degree of divergence (North, 2002).
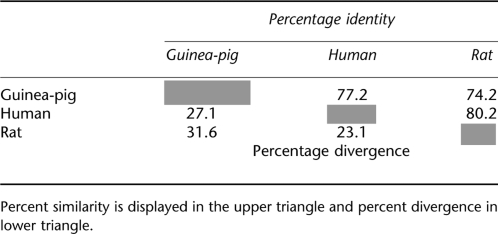
The homology of the generated guinea-pig P2X7 receptor clone is 77% to the human, and 74% to the rat P2X7 receptors (Table 1). Figure 1 shows the alignments of the guinea-pig clone compared to previously reported human and rat P2X7 receptors (Surprenant et al., 1996; Rassendren et al., 1997). The absence of glutamine 77 in the guinea-pig receptor complicates comparison across species, so all comparisons have been made based on the sequence alignment in Figure 1 and using the human numbering. The guinea-pig P2X7 receptor had the same 10 conserved cysteine residues present in the other P2X receptors at positions 119, 129, 135, 152, 162, 168, 216, 226, 260 and 269 (North, 2002). In addition, the guinea-pig receptor contained the putative N-glycosylation sites (Asparagine-Xaa-Serine/Threonine) at asparagine residues 187, 202, 213 and 241 present in rat, mouse and human receptors. The rat has two extra putative N-glycosylation sites at asparagine 74 and 284, this later site also being present in human and mouse receptors, but these residues were not present in the guinea-pig receptor (Figure 1 and Young et al., 2007).
Table 1.
Pair distances using ClustalW method (settings=slow/accurate, Gonnet)
Several single nucleotide polymorphisms of the human and mouse P2X7 receptor have been identified. Histidine 155 to tyrosine is a reported gain of function polymorphism in human receptors (Cabrini et al., 2005), and in guinea-pig receptors, the corresponding residue was tyrosine as is in rat and mouse (Figure 1 and Young et al., 2007). Human polymorphisms arginine 307 to glutamine (Gu et al., 2004), threonine 357 to serine (Shemon et al., 2006), glutamate 496 to alanine (Gu et al., 2001), isoleucine 568 to asparagine (Wiley et al., 2003) and arginine 574 to histidine (Fernando et al., 2005) are reported to affect function of human P2X7 receptors either by altering the trafficking to the plasma membrane or producing defective pore formation. In guinea-pig, rat and mouse, the corresponding residues are arginine at position 307, threonine at position 357, glutamine at position 496, isoleucine at position 568 and arginine at position 574 (human numbering, Figure 1 and Young et al., 2007).
The mouse P2X7 receptor exhibits loss-of-function mutations in positions serine 342 to phenylalanine (Denlinger et al., 2003) and proline 451 to leucine (Adriouch et al., 2002). These two residues were serine 342 and proline 451 in guinea-pig P2X7 receptor and were conserved among the guinea-pig, rat and human orthologues (Figure 1 and Young et al., 2007).
Finally, the mouse and rat receptors possess differential sensitivity to ATP and BzATP and it is thought that the lysine at residue 127 in rat (alanine in mouse) and asparagine at residue 284 in rat (aspartate in mouse) are responsible for this difference (Young et al., 2007). In the guinea-pig receptor, the residues are threonine and glutamine, respectively, whereas the human orthologue has threonine and asparagine (Figure 1). Note that if the missing amino acid at position 77 in the guinea-pig P2X7 receptor affects the relative location of the other residues then the corresponding amino acid at position 127 would be arginine.
ICC of P2X7 receptors expressed in U-2 OS cells
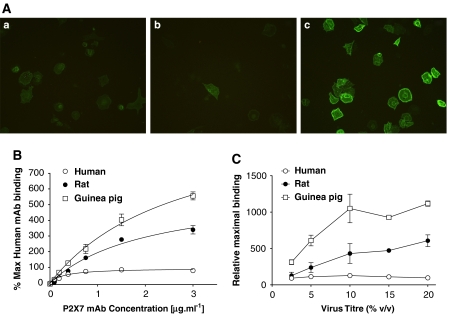
Expression of P2X7 receptor orthologues was studied in U-2 OS cells transduced with 10% of each individual BacMam virus (v/v) using ICC. The primary P2X7mAb, which recognizes the human P2X7 receptor (Buell et al., 1998) was used, with a secondary antibody conjugated to the fluorophore Alexa-488. Images in Figure 2 upper panels show a positive cell surface staining for human, rat and guinea-pig P2X7 orthologues, suggesting that this P2X7mAb also detects rat and guinea-pig P2X7 receptors. Non-transduced U-2 OS cells showed negative staining (data not shown). All micrographs were captured under the same conditions in order to enable comparisons of the relative levels of labelling between the species orthologues.
Figure 2.
Characterization of P2X7 receptor expression on BacMam-transduced U-2 OS cells. (A) Immunofluorescence images for U-2 OS cells transduced with the human (a), rat (b) and guinea-pig (c) recombinant P2X7 receptors. Expression was detected using a primary anti-P2X7 antibody (Buell et al., 1998) and an Alexa-488-conjugated secondary antibody. Images shown are representative of three micrographs taken from three independent experiments. (B) Specific binding of the P2X7mAb to U-2 OS cells transduced with the human, rat or guinea-pig recombinant P2X7 receptors. (C) Effect of virus titre on the maximal level of mAb binding to U-2 OS cells transduced with the human, rat or guinea-pig recombinant P2X7 receptors. Data are shown as mean±s.e.mean of three independent experiments run in duplicate.
The staining in cells transduced with the guinea-pig P2X7 receptor was greater than observed in cells transduced with either the rat or human P2X7 receptor. To investigate the reason for this, we used a quantitative method to analyse antibody binding to the different orthologues (Figure 2, lower panels). Binding of the antibody to each of the species orthologues increased in a concentration-dependent manner and showed signs of saturation at the highest concentration of 3 μg ml−1 tested (Figure 2B). For the guinea-pig and rat orthologues, expression increased with increasing levels of virus used for the transduction (Figure 2C) with maximal expression at approximately 10% virus (one-way ANOVA P>0.05 when comparing the mAb binding with titres of 10, 15 or 20%). For the human orthologue, even 2.5% virus produced nearly maximal levels of binding (Figure 2C). In cells transduced with 15 and 20% BacMam virus, the cell density on the plates after the extensive wash procedures used to measure the mAb bound appeared less than in cells transduced with the lower virus titres. The loss of cells did not appear to differ greatly between cells transduced with the different species orthologues. In cells transduced with the highest virus titre of 20%, the pKD values (g ml−1) for the antibody were 5.53±0.08, 5.68±0.07 and 6.51±0.09, respectively, for guinea-pig, rat and human P2X7 receptors (n=3 independent experiments). Similar pKD values were obtained at the other virus titres (data not shown). In cells transduced with 20% of the human, rat and guinea-pig receptor BacMam viruses, the maximal fluorescence was approximately 2-, 4.7- and 6.8-fold, respectively, of the background binding in non-transduced cells. When the data were expressed relative to the human P2X7 receptor, the estimated maximal levels of binding obtained from the curve fits to the data for the rat and guinea-pig receptor were 6.07±0.8- and 11.2±0.04-fold, respectively. Taken together, these findings suggest that the higher staining observed in the immunocytochemical studies reflects a higher level of expression of the guinea-pig receptor compared to the human and rat orthologues.
To further validate the methodology, stably transfected recombinant human and rat orthologues in HEK cells, previously shown to possess functional P2X7 receptors (Surprenant et al., 1996; Rassendren et al., 1997), were also tested using this technique. A 17-fold and 4-fold increase in mAb binding over wild-type HEK293 cells was seen in cells expressing human and rat P2X7 receptors, respectively. The pKD of the antibody was 6.43±0.07 for human and 5.78±0.02 for rat P2X7 receptors (n=3 independent experiments), which is in close agreement with the respective pKD values determined using the BacMam-transduced U-2 OS cells.
Agonist-induced ethidium bromide accumulation in recombinant P2X7 U-2 OS cells
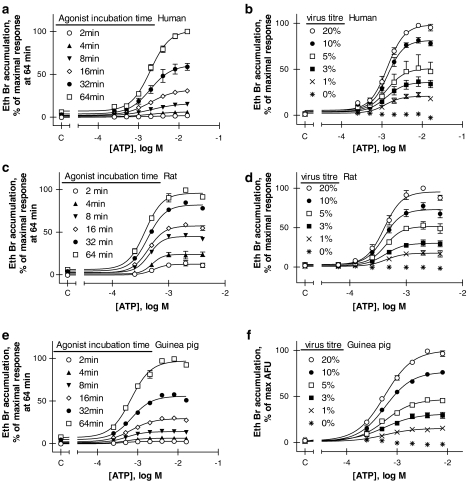
The functional properties of the guinea-pig P2X7 receptor were evaluated using ethidium bromide accumulation assays. Initial experiments on functional expression were performed at a fixed transduction rate of 10% BacMam virus (v/v), as this condition showed optimal cell surface expression of the human, rat and guinea-pig P2X7 orthologues (Figure 2). Figure 3 shows concentration-dependent ethidium bromide accumulation following ATP exposure for 2–64 min for the human receptor (panel A), rat receptor (panel C) and guinea-pig receptor (panel E). ATP potency was not affected by agonist exposure time (one-way ANOVA, P>0.05), whereas the maximal effect increased over time and after 64 min agonist exposure resulted in a ∼20-fold increase in fluorescence for each orthologue. The maximal rate of ethidium accumulation at the highest concentrations of ATP studied could be described by a single exponential (data not shown) and was characterized by a Kobs of 0.012, 0.058 and 0.01 l min−1 for cells expressing human, rat and guinea-pig P2X7 receptors, respectively. The maximum ethidium accumulation measured after 64 min in the presence of the highest concentration of ATP was 215 338±22 068, 216 467±20 934 and 150 505±9862 relative fluorescence units in cells expressing human, rat and guinea-pig P2X7 receptors, respectively, and these values were not significantly different from each other (P>0.05, one-way ANOVA followed by Tukeys's post hoc test). To account for the differences in kinetics at the species orthologues, the predicted maximal ethidium accumulation for each orthologue was also calculated from the curve fits of the rate data. The values were 392 106±6376, 217 865±23 807 and 299 985±53 812 relative fluorescence units in cells expressing human, rat and guinea-pig P2X7 receptors, respectively. The predicted maximal accumulation in cells expressing the rat P2X7 receptor was slightly, but significantly, lower than in the cells expressing human or guinea-pig P2X7 receptors (P<0.05, one-way ANOVA followed by Tukeys's post hoc test).
Figure 3.
ATP-stimulated ethidium (Eth Br) bromide accumulation in U-2 OS cells transiently transduced with P2X7 orthologues. (a, c, e) U-2 OS cells were transduced with BacMam virus at 10% (v/v) and ethidium bromide accumulation following ATP exposure (2–64 min) was measured in cells transduced with human (a), rat (c) or guinea-pig (e) recombinant P2X7 receptors. (b, d, f) U-2 OS cells were transduced with various titres of BacMam virus (1.25–20%, v/v) and ethidium bromide accumulation following ATP exposure was measured in cells transduced with human (b), rat (d) or guinea-pig (f) recombinant P2X7 receptors. ATP exposure was 16 min for human and guinea-pig orthologues, and 8 min for the rat orthologue. NaCl buffer was used in all studies. Control values obtained in the absence of ATP are shown on the abscissa (denoted as c). Data are mean±s.e.mean of three experiments run in duplicate.
Agonist exposure times for subsequent studies were chosen, so that uptake was still on the linear phase of the time–response relationship. This was 16 min for human and guinea-pig receptors, and 8 min for the rat receptor. Exposure of U-2 OS cells to increasing amounts of human, rat or guinea-pig P2X7 receptor BacMam virus (1.25–20% v/v) resulted in a titre-dependent increase in maximal fluorescence for all three species orthologues (Figure 3b, d and f, for human, rat and guinea-pig, respectively). The effect of varying virus concentration on maximal rates of ethidium accumulation was different from that seen when measuring expression using the mAb (Figure 2C). Whereas maximal levels of expression were observed at 2.5–10% virus (Figure 2C), there was no clearly defined maximal response in the ethidium accumulation studies even at 20% virus. The pEC50 for ATP did not vary with virus titre at the human, rat and guinea-pig receptors, respectively, suggesting that potency was not affected by the level of expression. For all subsequent experiments on human, rat and guinea-pig receptors functional responses were measured using 10% BacMam virus.
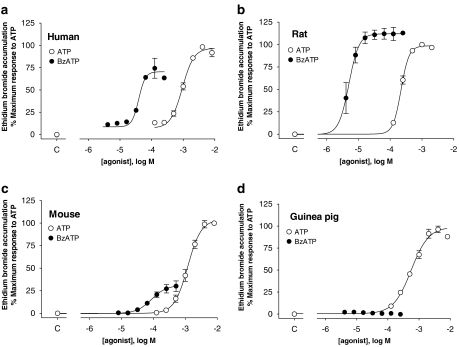
One of the features of P2X7 receptors is the difference in sensitivity to BzATP and ATP among the species orthologues (Young et al., 2007). Figure 4 shows a direct comparison of ATP and BzATP potency at the human, rat, mouse and guinea-pig P2X7 receptors in NaCl buffer. BzATP and ATP were agonists of rat, human and mouse receptors, although there were differences in the relative potencies and intrinsic activities between the orthologues (Figure 4 and Table 2). Most notably, BzATP and ATP produced comparable effects at rat P2X7 receptors, but BzATP was a partial agonist when compared with ATP at human and mouse P2X7 receptors and produced no effect at the guinea-pig P2X7 receptor. Furthermore, BzATP was an antagonist of ATP-mediated responses in NaCl buffer (Figures 5a and b), although it was not a competitive antagonist as it reduced the maximum response to ATP without greatly affecting ATP potency.
Figure 4.
Comparison of BzATP- and ATP-stimulated ethidium bromide (Eth Br) accumulation in U-2 OS cells transiently transduced with P2X7 orthologues. Ethidium bromide accumulation following agonist exposure was measured in cells transduced with human (a), rat (b), mouse (c) or guinea-pig (d) recombinant P2X7 receptors. Agonist exposure was 16, 8, 8 and 32 min, respectively, for cells transduced with the human, rat, mouse or guinea-pig P2X7 receptors. NaCl buffer was used in all studies. Control values obtained in the absence of ATP or BzATP are shown on the abscissa (denoted as c). Data are mean±s.e.mean of three experiments run in duplicate. BzATP, 2′-&3′-O-(4benzoylbenzoyl) ATP.
Table 2.
Relative potencies and intrinsic activities of ATP and BzATP at the rat, human, mouse and guinea-pig P2X7 orthologues
| P2X7 orthologue | pEC50 BzATP | pEC50 ATP | Intrinsic activity BzATPa |
|---|---|---|---|
| Rat | 5.30±0.08 | 3.66±0.02 | 112±6% |
| Human | 4.43±0.05* | 3.06±0.04* | 74±11%* |
| Mouse | 4.06±0.03*,† | 2.92±0.05* | 30±6%*,† |
| Guinea-pig | — | 3.22±0.10*,‡ | 2±0.3%*,†,‡ |
Studies were performed in NaCl buffer.
Compared to full agonist ATP (100%). Data are mean±s.e.mean, n=3 experiments. One-way ANOVA followed by Tukey's post hoc test revealed statistically significant differences vs rat (*P<0.05), human (†P<0.05) or mouse P2X7 (‡P<0.05).
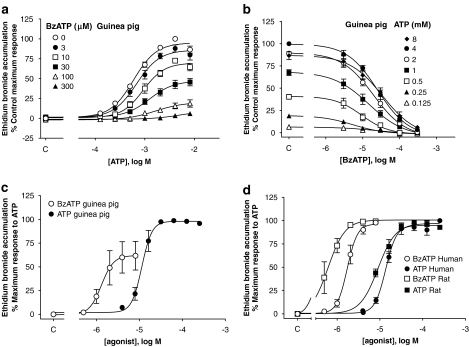
Figure 5.
BzATP- and ATP-stimulated ethidium bromide (Eth Br) accumulation in U-2 OS cells transiently transduced with P2X7 orthologues. (a, b) Cells were pretreated with the indicated concentrations of BzATP 15 min before the addition of ATP and ethidium. Ethidium bromide accumulation was measured after a further 32 min incubation in the presence of ATP and BzATP. These studies were performed in NaCl buffer: (a) effect of BzATP on ATP concentration–effect curve and (b) inhibition curves for BzATP at each ATP concentration employed in (a). (c, d) Concentration–effect curves for BzATP and ATP determined in sucrose buffer: (c) guinea-pig and (d) human and rat receptors. Agonist exposure was 2 min for human and rat and 8 min for the guinea-pig orthologue. Control values obtained in the absence of ATP or BzATP are shown on the abscissa (denoted as c). Data are mean±s.e.mean of three experiments run in duplicate. BzATP, 2′-&3′-O-(4benzoylbenzoyl) ATP.
To explore if the lack of effect of BzATP was due to low efficacy in this assay, additional studies were undertaken in sucrose buffer, as in this buffer, agonist potency and the ability of P2X7 agonists to stimulate ethidium accumulation is more easily demonstrated (Michel et al., 1999, 2000). In sucrose buffer, ATP potency was increased and BzATP produced an agonist effect, although it was still a partial agonist (Figure 5c). Similarly, the maximal effect of BzATP, relative to ATP, at the human receptor was increased, and BzATP was a full agonist in sucrose buffer (Figure 5d).
P2X7 antagonist effects at the guinea-pig P2X7 receptor
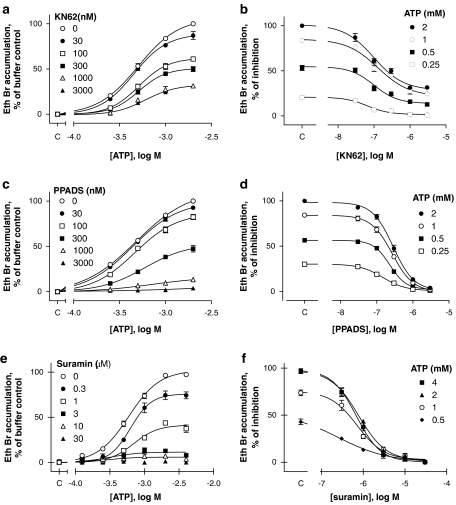
Antagonist potency was determined in ethidium bromide accumulation assays performed on U-2 OS cells transduced with 10% guinea-pig P2X7 receptor BacMam virus. Figure 6 shows data for antagonists that are more potent at the human receptor than at the rat or mouse orthologue (Anderson and Nedergaard, 2006). KN62 has a pIC50 value of 6.5 at human P2X7 receptors and <5 at rat receptors (Hibell et al., 2001); in this study, it was a potent antagonist (pIC50=6.88±0.09, n=4) of 1 mM ATP-stimulated ethidium bromide accumulation in cells expressing guinea-pig P2X7 receptors (Figures 6a and b). One-way ANOVA revealed no statistically significant differences in the antagonist potency with respect to the doses of ATP used (P>0.05). PPADS has a pIC50 value of 6.7 and 5.9, respectively, at human and rat P2X7 receptors (Hibell et al., 2001) and here it also inhibited the guinea-pig receptor (Figures 6c and d), with relatively high potency (pIC50=6.54–6.82, n=4). One-way ANOVA followed by Dunnett's post hoc test revealed a small but statistically significant (P<0.001) difference in its potency against 250 μM ATP (pIC50=6.82±0.02, n=4) compared to its potency against 2 mM ATP (pIC50=6.54±0.04, n=4). Suramin possesses a pIC50 of 4.9 and 4.8 at rat and human receptors, respectively (Hibell et al., 2001), but in this study was a much more potent antagonist (pIC50=6.11±0.02, n=3, against 1 mM ATP) at the guinea-pig receptor (Figures 6e and f). One-way ANOVA revealed no statistically significant differences in antagonist potency with respect to the doses of ATP used (P>0.05). In this study, PPADS and suramin were non-competitive antagonists of the guinea-pig P2X7 receptor, as they insurmountably and completely reduced the maximal response to ATP. KN62 was also a non-competitive antagonist but, unlike the other antagonists, the reduction in maximal response to ATP was saturable, the inhibition of ATP responses produced by 1 and 3 μM KN62 being identical (Figures 6a and b).
Figure 6.
Antagonist effects on ATP-induced ethidium bromide (Eth Br) accumulation in U-2 OS cells transiently transduced with the guinea-pig P2X7 receptor. Effects of KN62 are shown in (a, b), PPADS in (c, d) and suramin in (e, f). (a, c, e) The effects of the antagonists on the concentration–effect curve to ATP. In (b, d, f), the data from (a, c, e) are re-plotted to present antagonist inhibition curves at each of the indicated ATP concentrations. NaCl buffer was used in all studies. Control values obtained in the absence of the antagonists or ATP are shown on the abscissa (denoted as c). Results shown are mean±s.e.mean of 3–4 independent experiments run in duplicate.
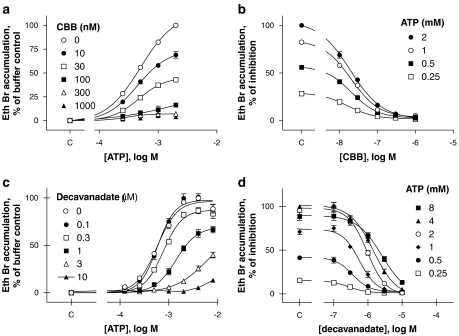
Figure 7 shows the effects of antagonists of the P2X7 receptor, which are selective for the rat, compared to the human, orthologue, namely CBB (Hibell et al., 2001) or equipotent at both, namely decavanadate (Michel et al., 2006b). CBB was a potent non-competitive antagonist of the guinea-pig P2X7 receptor (pIC50=7.63±0.02, n=3, against 1 mM ATP), and one-way ANOVA revealed no statistically significant differences in the antagonist potency with respect to the doses of ATP used (P>0.05). Decavanadate potency varied quite markedly with agonist concentration (Figures 7c and d) with its potency against 250 μM ATP (pIC50=6.53±0.11, n=4) being significantly higher than against 8 mM ATP (pIC50=5.61±0.02, n=4; P<0.001, one-way ANOVA followed by Dunnett's post hoc test). Although decavanadate did not produce a clearly competitive antagonist effect, it was the only antagonist to significantly reduce ATP potency (one-way ANOVA followed by Dunnett's post hoc test). Thus, the control ATP potency (pEC50) at the guinea-pig receptor was 3.24±0.02 (n=3), whereas in the presence of 1 μM decavanadate it was 2.85±0.06 (n=3, P<0.001) and in the presence of 3 μM decavanadate it was 2.40±0.03 (n=3, P<0.001).
Figure 7.
Antagonist effects on ATP-induced ethidium bromide (Eth Br) accumulation in U-2 OS cells transiently transduced with the guinea-pig P2X7 receptor. Effects of CBB are shown in (a, b), and effects of decavanadate in (c, d). (a, c) The effects of the antagonists on the concentration–effect curve to ATP. In (b, d), the data from (a, c) are re-plotted to present antagonist inhibition curves at each of the indicated ATP concentrations. NaCl buffer was used in all studies. Control values obtained in the absence of the antagonists or ATP are shown on the abscissa (denoted as c). Results shown are mean±s.e.mean of 3–4 independent experiments run in duplicate. CBB, Coomassie brilliant blue.
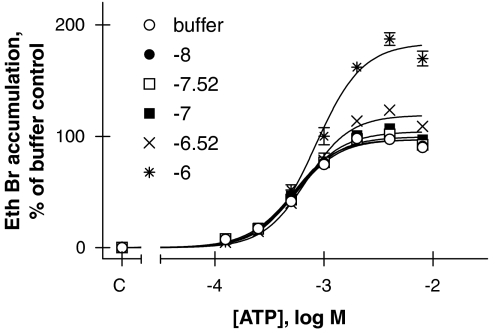
Finally, the P2X7mAb used to label cell surface P2X7 receptors (Figure 3), which is an antagonist of the human receptor (Buell et al., 1998), was also tested as antagonist of the guinea-pig orthologue. Figure 8 shows that this mAb increased rather than inhibited ATP-induced ethidium bromide accumulation in guinea-pig. Thus, maximal ethidium accumulation was increased to 119±2.6% (n=3, P<0.001) at 300 ng ml−1 and to 184±2.8% (n=3, P<0.001) at 1 μg ml−1 when compared to maximal ethidium accumulation in the absence of the antibody (97±1.6%, n=3). At a concentration of 1 μg ml−1, the antibody slightly decreased ATP potency (one-way ANOVA followed by Dunnett's post hoc test, P<0.001) from a pEC50 of 3.27±0.03 (n=3) in the absence of the antibody to 3.08±0.04 (n=3).
Figure 8.
Effect of a range of concentrations of mAb against the P2X7 receptor (Figure 3) on ATP-induced ethidium bromide (Eth Br) accumulation in U-2 OS cells transiently transduced with the guinea-pig P2X7 orthologue. The different concentrations of antibody incubated with the cells are shown in the figure as log10(g antibody protein per ml); thus −6.52=300ng ml−1. NaCl buffer was used and the control values obtained in the absence of ATP are shown on the abscissa (denoted as c). Results shown are mean±s.e.mean of three independent experiments run in duplicate. mAb, monoclonal antibody.
Discussion and conclusions
In this study, we have isolated a cDNA encoding the guinea-pig P2X7 receptor from guinea-pig brain and functionally characterized this orthologue. This orthologue has some structural differences to the human and rodent receptors and distinctive pharmacological properties with both similarities and differences to the three previously characterized mammalian P2X7 receptor orthologues.
The homology between the guinea-pig P2X7 and the human, rat and mouse P2X7 receptors was approximately 70%. The receptor was one amino acid shorter than the other species orthologues missing a glutamine at position 77 (human nomenclature). The guinea-pig receptor possessed the same conserved cysteines as present in all other P2X receptors, including the same conserved cysteine residue pairs, thought to be responsible for the tertiary structure of the receptor at positions 119–168, 129–152, 135–162, 216–226 and 260–269 (human nomenclature) (North, 2002).
P2X7 receptors have up to six potential N-linked glycosylation sites on asparagine residues at positions 74, 187, 202, 213, 241 and 284. The guinea-pig receptor only possessed four potential N-linked glycosylation sites on asparagine residues corresponding to positions 187, 202, 213 and 241 (human nomenclature). The absence of asparagine at position 284 is of interest, as a recent study has implicated this region as being important to species differences in ATP and BzATP potency (Young et al., 2007). Despite having fewer potential N-linked glycosylation sites, the guinea-pig P2X7 receptor expressed at high levels and was functional (see below). Several loss-of-function single nucleotide polymorphisms have been described in the literature for P2X7 (Gu et al., 2001; Adriouch et al., 2002; Denlinger et al., 2003; Wiley et al., 2003; Gu et al., 2004; Fernando et al., 2005; Shemon et al., 2006), but the guinea-pig P2X7 receptor did not express any of these, which is consistent with it being functional.
We found that the guinea-pig P2X7 receptor could be labelled using the P2X7mAb previously used to label human P2X7 receptors (Buell et al., 1998), although the P2X7mAb possessed slightly lower affinity for guinea-pig than for human P2X7 receptors. Immunocytochemical studies using this P2X7mAb revealed even higher levels of cell surface receptor expression for guinea-pig P2X7 receptors than for human or rat P2X7 receptors. This may be due to a greater efficiency of transduction for the guinea-pig BacMam virus compared with the human or rat constructs, or represent a more efficient expression of the guinea-pig P2X7 receptor, possibly due to the nature of the amino-acid residues in its intracellular C-terminus. Certainly, studies on P2X7 receptors have identified multiple intracellular residues that affect cell surface expression of the P2X7 receptor (Denlinger et al., 2003; Wiley et al., 2003; Fernando et al., 2005). Where positive labelling was obtained, it could be seen in the immunocytochemical micrographs that different cells had different degrees of staining, indicative of a non-homogenous expression of the transduced orthologues among the U-2 OS cell population. More quantitative saturation binding studies with the P2X7mAb confirmed the high levels of cell surface expression of the guinea-pig P2X7 receptor and showed that receptor expression could be increased with increasing titres of virus with evidence of maximal expression being achieved at the highest virus titres used. These data demonstrate that the BacMam viral expression system is suitable for producing a graded expression of P2X7 receptors, although there are still cell-to-cell variations and gradation of expression is only apparent when dealing with populations of cells.
The response to ATP at the human, rat and guinea-pig orthologues in ethidium accumulation studies also increased with increasing virus titre and ATP pEC50 remained constant regardless of virus titre or agonist exposure time. This observation suggests that agonist potency was independent of receptor density at the expression levels examined in this study. There were some discrepancies between ethidium accumulation and expression studies, as expression appeared to saturate at the higher virus titres tested, whereas receptor function did not clearly saturate. This may be due to binding of the mAb being underestimated in immunocytochemical studies when using the higher virus titres. Certainly, there was a loss of cells from the 96-well plates in the immunocytochemical studies, but not the ethidium accumulation studies, when using the higher virus titres. This may reflect toxic effects of either the virus or high levels of P2X7 receptor expression on cell adhesion leading to cell loss, which was only evident in the immunocytochemical studies due to the greater number of cell washing steps in those studies. Nevertheless, both techniques confirmed that a graded expression of the receptor is possible with the BacMam virus expression system.
The immunocytochemical studies suggested that rat and guinea-pig P2X7 receptors were expressed at 6- to 11-fold higher levels than the human P2X7 receptor. However, there was at most a twofold difference in maximal ethidium accumulation between the species orthologues. This mismatch may not be unexpected, as cellular ethidium accumulation is likely to be limited by total cellular DNA or RNA levels, which should be similar in all cases. Furthermore, recent studies have suggested that cellular entry of dyes such as ethidium occurs independently of the P2X7 receptor (Jiang et al., 2005) and may occur via the pannexin hemichannel (Pelegrin and Surprenant, 2006). In such a situation, the maximal ethidium accumulation may reflect coupling of the P2X7 receptor to endogenous pannexin channels, which should be present at similar levels in all cases. The rate of ethidium accumulation was faster in cells transduced with the rat than with the human or guinea-pig orthologues. This may reflect differences in the intracellular C-termini of the species orthologues and their ability to couple to pathways responsible for cellular entry of ethidium.
There were differences in agonist effect at the guinea-pig P2X7 receptor compared with the other species orthologues. Thus, in studies on the rat, human and mouse P2X7 receptors, ATP is a partial agonist compared to BzATP (Wiley et al., 1998; Young et al., 2007). However, in NaCl buffer, BzATP had no agonist activity at the guinea-pig P2X7 receptor and instead was able to block responses to ATP. Interestingly, BzATP was also a partial agonist at the human and mouse P2X7 receptors when studied in NaCl buffer, although this contrasts with previous studies (Wiley et al., 1998; Young et al., 2007). The reasons for the discrepancies in intrinsic activity between assay formats are not known, but these studies suggest that BzATP intrinsic activity differs at the species orthologues and is higher at rat and human P2X7 receptors than at mouse or guinea-pig P2X7 receptors. We have previously observed that P2X7 receptor-simulated ethidium accumulation is more readily demonstrated in sucrose buffer than in NaCl buffer (Michel et al., 1999) and in sucrose buffer BzATP was able to evoke a response, although its intrinsic activity was still less than ATP.
The reasons for the lower intrinsic activity of BzATP at the guinea-pig receptor are not known but Surprenant and co-workers (Young et al., 2007) reported that amino-acid residues at positions 127 and 284 in rat and mouse P2X7 orthologues affect agonist potency and also affected the intrinsic activity of BzATP. However, any comparison of the residues present at these positions with those in the guinea-pig receptor is complicated by the missing amino acid at position 77 in the guinea-pig P2X7 receptor. Consequently, the guinea-pig amino acid corresponding to position 127 in the other orthologues may be arginine or threonine depending upon how the missing amino acid affects receptor structure. At position 127, arginine would differ from that present in all other orthologues, whereas threonine is present in the human receptor. Similarly, at position 284, the corresponding guinea-pig residues could be either glutamate 283 or glutamate 284, different from those in the other species orthologues. As amino acids at positions 130, 134 and 136 were also implicated in BzATP potency (Young et al., 2007), it seems likely that multiple residues affect agonist potency and efficacy. Consequently, determining the reasons for the low intrinsic activity of BzATP at guinea-pig P2X7 receptors will be difficult.
Several antagonists that discriminate between the rat, mouse and human orthologues (Anderson and Nedergaard, 2006) were examined as antagonists of the guinea-pig P2X7 receptor. KN62 and PPADS, which display some selectivity for the human receptor (Hibell et al., 2001), were also potent antagonists of the guinea-pig receptor. However, the effect of KN62 was clearly not competitive, as it produced only a small and apparently saturating shift in the ATP concentration–effect curve and produced incomplete inhibition of responses to ATP, which complicated analysis of its effects. We have observed similar behaviour with KN62 at human P2X7 receptors (Michel et al., 2000) and believe this is due to it being an allosteric inhibitor of the P2X7 receptor (Michel et al., 2007). Nevertheless, KN62 was a potent inhibitor of guinea-pig P2X7 receptors with a pIC50 of 6.8 that was similar to that obtained at the human P2X7 receptor (pIC50 of 6.5; Hibell et al., 2001). In the case of PPADS, the pIC50 value of 6.5 was also similar to its pIC50 of 6.7 at the human receptor (Hibell et al., 2001). Note that as antagonist affinities differ considerably between techniques, and even with buffer composition (Hibell et al., 2001), we have compared potencies only to those obtained with the same technique and in NaCl buffer.
The P2X7mAb is an antagonist of the human P2X7 receptor (Buell et al., 1998) but, surprisingly, it potentiated rather than inhibited ATP-induced responses at the guinea-pig P2X7 receptor. This potentiation may represent positive cooperativity between the antibody and ATP binding sites. Previously, we have shown that the P2X7mAb produces non-competitive blockade of response in functional studies (Buell et al., 1998), and in radioligand binding studies it partially inhibited radioligand binding to the human P2X7 receptor, suggesting that it may be an allosteric regulator of the P2X7 receptor (Michel et al., 2007). Presumably, structural differences in the human and guinea-pig receptor result in positive rather than negative cooperativity at the guinea-pig receptor.
CBB is selective for the rat over human P2X7 receptors and its pIC50 of 7.5 at guinea-pig P2X7 receptors was even higher than that observed at rat receptors (pIC50=6.8; Hibell et al., 2001). Decavanadate is a competitive antagonist equipotent at the rat and human receptors (Michel et al., 2006b). This compound possessed similar potency at guinea-pig receptors (pIC50=6.5–5.6) as at human or rat P2X7 receptors (pIC50=6.2–6.5), although it did not display such a clearly defined competitive interaction against ATP responses as it did of BzATP-mediated responses at human and rat P2X7 receptors (Michel et al., 2006b).
Suramin is a relatively non-selective antagonist of human, rat and mouse P2X7 receptors but possessed 10- to 20-fold higher affinity at the guinea-pig P2X7 receptor. Interestingly, the guinea-pig P2X7 receptor has one less amino acid than the other species orthologues and this deletion is in a region of the receptor (glutamine 77, human sequence) that can affect suramin potency at P2X receptors. Thus, the equivalent residue in the P2X4 receptor (amino acid 78) has been shown to affect sensitivity to suramin (Garcia-Guzman et al., 1997), as replacing glutamine in the rat P2X4 receptor with lysine increased suramin potency. Clearly, the differences in amino-acid composition between P2X4 and P2X7 receptors make such comparisons complex, but it seems plausible that the absence of the glutamine at position 77 in the guinea-pig P2X7 receptor could contribute to the higher potency of suramin at the P2X7 receptor.
Several of the compounds tested as antagonists in this study produced an insurmountable antagonist effect. The reasons for this behaviour are not known but may reflect a limitation of the methodology with respect to agonist and antagonist equilibration. Certainly, BzATP would not be expected to be a non-competitive antagonist, as it presumably binds at the ATP binding site. In the case of PPADS, the non-competitive effect is very likely a reflection of its slow dissociation kinetics, as other studies have shown that it binds at the ATP binding site (Michel et al., 2006b, 2007).
In conclusion, here we report the cloning of the guinea-pig P2X7 orthologue. The pharmacological characterization of this new orthologue revealed similar sensitivity to ATP as at the human, rat and mouse receptors but a greatly reduced efficacy of BzATP and a different sensitivity to P2X7 receptor antagonists than observed at the human, rat and mouse orthologues. The receptor displays a number of unique properties that differentiate it from the human, rat and mouse orthologues and the structural information may aid in our understanding of the interaction of agonists and antagonist with the P2X7 receptor.
Abbreviations
- BacMam
recombinant baculovirus in which the polyhedrin promoter has been replaced with a mammalian promoter
- BzATP
2′-&3′-O-(4benzoylbenzoyl) ATP
- CBB
Coomassie brilliant blue
- KN62
1-[N, O-bis(5-isoquinolinesulphonyl)-N-methyl-L-tyrosyl]-4-phenylpiperazine
- mAb
monoclonal antibody
- PFU
plaque-forming units
- PPADS
pyridoxal phosphate-6-azophenyl-2′, 4′-disulphonic acid
- U-2 OS
human osteosarcoma cell line
Conflict of interest
The authors are employed by GlaxoSmithKline R&D Ltd.
References
- Adriouch S, Dox C, Welge V, Seman M, Koch-Nolte F, Haag F. Cutting edge: a natural P451L mutation in the cytoplasmic domain impairs the function of the mouse P2X7 receptor. J Immunol. 2002;169:4108–4112. doi: 10.4049/jimmunol.169.8.4108. [DOI] [PubMed] [Google Scholar]
- Ames R, Fornwald J, Nuthulaganti P, Trill J, Foley J, Buckley P, et al. BacMam recombinant baculoviruses in G protein-coupled receptor drug discovery. Receptors Channels. 2004a;10:99–107. doi: 10.1080/10606820490514969. [DOI] [PubMed] [Google Scholar]
- Ames R, Nuthulaganti P, Fornwald J, Shabon U, van der Keyl H, Elshourbagy N. Heterologous expression of G protein-coupled receptors in U-2 OS osteosarcoma cells. Receptors Channels. 2004b;10:117–124. doi: 10.1080/10606820490515012. [DOI] [PubMed] [Google Scholar]
- Anderson CM, Nedergaard M. Emerging challenges of assigning P2X7 receptor function and immunoreactivity in neurons. Trends Neurosci. 2006;29:257–262. doi: 10.1016/j.tins.2006.03.003. [DOI] [PubMed] [Google Scholar]
- Baraldi PG, Di Virgilio F, Romagnoli R. Agonists and antagonists acting at P2X7 receptor. Curr Top Med Chem. 2004;4:1707–1717. doi: 10.2174/1568026043387223. [DOI] [PubMed] [Google Scholar]
- Boudjelal M, Mason SJ, Katso RM, Fleming JM, Parham JH, Condreay JP, et al. The application of BacMam technology in nuclear receptor drug discovery. Biotechnol Annu Rev. 2005;11:101–125. doi: 10.1016/S1387-2656(05)11003-5. [DOI] [PubMed] [Google Scholar]
- Buell G, Chessell IP, Michel AD, Collo G, Salazzo M, Herren S, et al. Blockade of human P2X7 receptor function with a monoclonal antibody. Blood. 1998;92:3521–3528. [PubMed] [Google Scholar]
- Burnstock G, Knight GE. Cellular distribution and functions of P2 receptor subtypes in different systems. Int Rev Cytol. 2004;240:31–304. doi: 10.1016/S0074-7696(04)40002-3. [DOI] [PubMed] [Google Scholar]
- Cabrini G, Falzoni S, Forchap SL, Pellegatti P, Balboni A, Agostini P, et al. A His-155 to Tyr polymorphism confers gain-of-function to the human P2X7 receptor of human leukemic lymphocytes. J Immunol. 2005;175:82–89. doi: 10.4049/jimmunol.175.1.82. [DOI] [PubMed] [Google Scholar]
- Chessell IP, Hatcher JP, Bountra C, Michel AD, Hughes JP, Green P, et al. Disruption of the P2X7 purinoceptor gene abolishes chronic inflammatory and neuropathic pain. Pain. 2005;114:386–396. doi: 10.1016/j.pain.2005.01.002. [DOI] [PubMed] [Google Scholar]
- Chessell IP, Simon J, Hibell AD, Michel AD, Barnard EA, Humphrey PP. Cloning and functional characterisation of the mouse P2X7 receptor. FEBS Lett. 1998;439:26–30. doi: 10.1016/s0014-5793(98)01332-5. [DOI] [PubMed] [Google Scholar]
- Clay WC, Condreay JP, Moore LB, Weaver SL, Watson MA, Kost TA, et al. Recombinant baculoviruses used to study estrogen receptor function in human osteosarcoma cells. Assay Drug Dev Technol. 2003;1:801–810. doi: 10.1089/154065803772613435. [DOI] [PubMed] [Google Scholar]
- Condreay JP, Witherspoon SM, Clay WC, Kost TA. Transient and stable gene expression in mammalian cells transduced with a recombinant baculovirus vector. Proc Natl Acad Sci USA. 1999;96:127–132. doi: 10.1073/pnas.96.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denlinger LC, Sommer JA, Parker K, Gudipaty L, Fisette PL, Watters JW, et al. Mutation of a dibasic amino acid motif within the C terminus of the P2X7 nucleotide receptor results in trafficking defects and impaired function. J Immunol. 2003;171:1304–1311. doi: 10.4049/jimmunol.171.3.1304. [DOI] [PubMed] [Google Scholar]
- Fernando SL, Saunders BM, Sluyter R, Skarratt KK, Wiley JS, Britton WJ. Gene dosage determines the negative effects of polymorphic alleles of the P2X7 receptor on adenosine triphosphate-mediated killing of mycobacteria by human macrophages. J Infect Dis. 2005;192:149–155. doi: 10.1086/430622. [DOI] [PubMed] [Google Scholar]
- Fonfria E, Dare E, Benelli M, Sunol C, Ceccatelli S. Translocation of apoptosis-inducing factor in cerebellar granule cells exposed to neurotoxic agents inducing oxidative stress. Eur J Neurosci. 2002;16:2013–2016. doi: 10.1046/j.1460-9568.2002.02269.x. [DOI] [PubMed] [Google Scholar]
- Garcia-Guzman M, Soto F, Gomez-Hernandez JM, Lund PE, Stuhmer W. Characterization of recombinant human P2X4 receptor reveals pharmacological differences to the rat homologue. Mol Pharmacol. 1997;51:109–118. doi: 10.1124/mol.51.1.109. [DOI] [PubMed] [Google Scholar]
- Gu BJ, Sluyter R, Skarratt KK, Shemon AN, Dao-Ung LP, Fuller SJ, et al. An Arg307 to Gln polymorphism within the ATP-binding site causes loss of function of the human P2X7 receptor. J Biol Chem. 2004;279:31287–31295. doi: 10.1074/jbc.M313902200. [DOI] [PubMed] [Google Scholar]
- Gu BJ, Zhang W, Worthington RA, Sluyter R, Dao-Ung P, Petrou S, et al. A Glu-496 to Ala polymorphism leads to loss of function of the human P2X7 receptor. J Biol Chem. 2001;276:11135–11142. doi: 10.1074/jbc.M010353200. [DOI] [PubMed] [Google Scholar]
- Hibell AD, Thompson KM, Xing M, Humphrey PP, Michel AD. Complexities of measuring antagonist potency at P2X7 receptor orthologs. J Pharmacol Exp Ther. 2001;296:947–957. [PubMed] [Google Scholar]
- Hu HZ, Gao N, Lin Z, Gao C, Liu S, Ren J, et al. P2X7 receptors in the enteric nervous system of guinea-pig small intestine. J Comp Neurol. 2001;440:299–310. doi: 10.1002/cne.1387. [DOI] [PubMed] [Google Scholar]
- Humphreys BD, Virginio C, Surprenant A, Rice J, Dubyak GR. Isoquinolines as antagonists of the P2X7 nucleotide receptor: high selectivity for the human versus rat receptor homologues. Mol Pharmacol. 1998;54:22–32. doi: 10.1124/mol.54.1.22. [DOI] [PubMed] [Google Scholar]
- Jiang L-H, Rassendren F, Mackenzie A, Zhang Y-H, Surprenant A, North RA. N-methyl-D-glucamine and propidium dyes utilize different permeation pathways at rat P2X7 receptors. Am J Physiol—Cell Physiol. 2005;289:C1295–C1302. doi: 10.1152/ajpcell.00253.2005. [DOI] [PubMed] [Google Scholar]
- Kost TA, Condreay JP, Jarvis DL. Baculovirus as versatile vectors for protein expression in insect and mammalian cells. Nat Biotechnol. 2005;23:567–575. doi: 10.1038/nbt1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang L, Schwiebert EM. Large pore formation uniquely associated with P2X7 purinergic receptor channels. Focus on ‘Are second messengers crucial for opening the pore associated with P2X7 receptor?'. Am J Physiol Cell Physiol. 2005;288:C240–C242. doi: 10.1152/ajpcell.00532.2004. [DOI] [PubMed] [Google Scholar]
- Michel AD, Chambers LJ, Clay WC, Condreay JP, Walter DS, Chessell IP. Direct labelling of the human P2X7 receptor and identification of positive and negative cooperativity of binding. Br J Pharmacol. 2007;151:103–114. doi: 10.1038/sj.bjp.0707196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel AD, Chessell IP, Humphrey PP. Ionic effects on human recombinant P2X7 receptor function. Naunyn Schmiedebergs Arch Pharmacol. 1999;359:102–109. doi: 10.1007/pl00005328. [DOI] [PubMed] [Google Scholar]
- Michel AD, Kaur R, Chessell IP, Humphrey PP. Antagonist effects on human P2X7 receptor-mediated cellular accumulation of YO-PRO-1. Br J Pharmacol. 2000;130:513–520. doi: 10.1038/sj.bjp.0703368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel AD, Thompson KM, Simon J, Boyfield I, Fonfria E, Humphrey PP. Species and response dependent differences in the effects of MAPK inhibitors on P2X7 receptor function. Br J Pharmacol. 2006a;149:948–957. doi: 10.1038/sj.bjp.0706938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel AD, Xing M, Thompson KM, Jones CA, Humphrey PP. Decavanadate, a P2X receptor antagonist, and its use to study ligand interactions with P2X7 receptors. Eur J Pharmacol. 2006b;534:19–29. doi: 10.1016/j.ejphar.2006.01.009. [DOI] [PubMed] [Google Scholar]
- North RA. Molecular physiology of P2X receptors. Physiol Rev. 2002;82:1013–1067. doi: 10.1152/physrev.00015.2002. [DOI] [PubMed] [Google Scholar]
- Pelegrin P, Surprenant A. Pannexin-1 mediates large pore formation and interleukin-1beta release by the ATP-gated P2X7 receptor. EMBO J. 2006;25:5071–5082. doi: 10.1038/sj.emboj.7601378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfohl JL, Worley JF, III, Condreay JP, An G, Apolito CJ, Kost TA, et al. Titration of KATP channel expression in mammalian cells utilizing recombinant baculovirus transduction. Receptors Channels. 2002;8:99–111. [PubMed] [Google Scholar]
- Rampe D, Wang L, Ringheim GE. P2X7 receptor modulation of beta-amyloid- and LPS-induced cytokine secretion from human macrophages and microglia. J Neuroimmunol. 2004;147:56–61. doi: 10.1016/j.jneuroim.2003.10.014. [DOI] [PubMed] [Google Scholar]
- Rassendren F, Buell GN, Virginio C, Collo G, North RA, Surprenant A. The permeabilizing ATP receptor, P2X7. Cloning and expression of a human cDNA. J Biol Chem. 1997;272:5482–5486. doi: 10.1074/jbc.272.9.5482. [DOI] [PubMed] [Google Scholar]
- Shemon AN, Sluyter R, Fernando SL, Clarke AL, Dao-Ung LP, Skarratt KK, et al. A Thr357 to Ser polymorphism in homozygous and compound heterozygous subjects causes absent or reduced P2X7 function and impairs ATP-induced mycobacterial killing by macrophages. J Biol Chem. 2006;281:2079–2086. doi: 10.1074/jbc.M507816200. [DOI] [PubMed] [Google Scholar]
- Stokes L, Jiang LH, Alcaraz L, Bent J, Bowers K, Fagura M, et al. Characterization of a selective and potent antagonist of human P2X7 receptors, AZ11645373. Br J Pharmacol. 2006;149:880–887. doi: 10.1038/sj.bjp.0706933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surprenant A, Rassendren F, Kawashima E, North RA, Buell G. The cytolytic P2Z receptor for extracellular ATP identified as a P2X receptor (P2X7) Science. 1996;272:735–738. doi: 10.1126/science.272.5262.735. [DOI] [PubMed] [Google Scholar]
- Wiley JS, Dao-Ung LP, Li C, Shemon AN, Gu BJ, Smart ML, et al. An Ile-568 to Asn polymorphism prevents normal trafficking and function of the human P2X7 receptor. J Biol Chem. 2003;278:17108–17113. doi: 10.1074/jbc.M212759200. [DOI] [PubMed] [Google Scholar]
- Wiley JS, Gargett CE, Zhang W, Snook MB, Jamieson GP. Partial agonists and antagonists reveal a second permeability state of human lymphocyte P2Z/P2X7 channel. Am J Physiol. 1998;275:C1224–C1231. doi: 10.1152/ajpcell.1998.275.5.C1224. [DOI] [PubMed] [Google Scholar]
- Young MT, Pelegrin P, Surprenant A. Amino acid residues in the P2X7 receptor that mediate differential sensitivity to ATP and BzATP. Mol Pharmacol. 2007;71:92–100. doi: 10.1124/mol.106.030163. [DOI] [PubMed] [Google Scholar]