Abstract
Background and purpose:
Eupalmerin acetate (EPA) is a marine diterpene compound isolated from the gorgonian octocorals Eunicea succinea and Eunicea mammosa. The compound has been previously shown to modulate muscle-type and neuronal nicotinic acetylcholine receptors, which are inhibited in the presence of low micromolar concentrations of EPA. In this study, we examined the effect of EPA on another transmitter-gated ion channel, the GABAA receptor.
Experimental approach:
Whole-cell and single-channel recordings were made from HEK 293 cells transiently expressing rat wild-type and mutant α1β2γ2L GABAA receptors.
Key results:
Our findings demonstrate that, at micromolar concentrations, EPA potentiates the rat α1β2γ2L GABAA receptor. The analysis of single-channel currents recorded in the presence of EPA showed that the kinetic mode of action of EPA is similar to that of neuroactive steroids. Mutations to residues α1Q241 and α1N407/Y410, previously shown to affect receptor modulation by neurosteroids, also diminished potentiation by EPA. Exposure to a steroid antagonist, (3α,5α)-17-phenylandrost-16-en-3-ol, reduced potentiation by EPA. Additionally, exposure to EPA led to potentiation of GABAA receptors activated by very high concentrations (1–10 μM) of allopregnanolone. In tadpole behavioural assays, EPA caused loss of righting reflex and loss of swimming reflex.
Conclusions and implications:
We conclude that EPA either interacts with the putative neurosteroid binding site on the GABAA receptor or shares with neurosteroids the key transduction elements involved in channel potentiation by steroids. The results indicate that cembranoids represent a novel class of GABAA receptor modulators.
Keywords: GABAA receptor, channels, cembranoids, steroids
Introduction
Cembranoids are 14-membered ring containing diterpenes, found in marine invertebrates as well as in some plants and insects. Although the natural role of marine cembranoids is believed to be defensive against other marine species (Pawlik, 1993; Maia et al., 1999), many cembranoids have been shown to be biologically active in unrelated model systems with potential biomedical applications. Some cembrane derivatives have activity against the malaria-causing parasite Plasmodium falciparum (Gutierrez et al., 2005). Others can be neuroprotective (Ferchmin et al., 2005) or possess cytotoxic activity against tumour cell lines, thereby presenting as potentially useful anticancer agents (Reyes et al., 2004; Sanchez et al., 2006; Sawant et al., 2006; Iwamaru et al., 2007). But by far, the best-studied biological action of cembranoids is block of nicotinic transmission. It has been known since the 1980s that exposure to the cembranoid lophotoxin inhibits rat and frog skeletal muscle nicotinic receptors as well as embryonic type nicotinic receptors expressed in BC3H-1 cells (Atchison et al., 1984; Culver et al., 1984). Nicotinic receptors in autonomic ganglia of the chick and rat are similarly vulnerable to block by lophotoxin (Sorenson et al., 1987). The site of action for this cembranoid is the acetylcholine-binding site, where lophotoxin analogues label the tyrosine-190 residue in the α subunit (Abramson et al., 1989). More recent studies have revealed numerous other cembrane derivatives, which inhibit the function of both muscle- and neuronal-type nicotinic receptors, although in many cases, the site of action is considered to be distinct from the nicotinic agonist-binding site (Eterovic et al., 1993; Hann et al., 1998; Ferchmin et al., 2001; Ulrich et al., 2007).
The GABAA receptor is a member of the superfamily of transmitter-gated ion channels sharing many structural and functional features with the nicotinic receptor. The GABAA receptor mediates the major component of fast inhibitory transmission in the central nervous system, and potentiators of the GABAA receptor can act as anxiolytics, anticonvulsants or anaesthetics. In the present study, we investigated the modulation of the rat α1β2γ2L GABAA receptor by the marine cembranoid, eupalmerin acetate (EPA, Figure 1). Our data show that the drug acts as a potentiator of currents elicited by GABA or pentobarbital. Single-channel electrophysiology demonstrated that EPA reduces the mean closed-time duration and increases the mean open-time duration. Potentiation by EPA was reduced by coapplication of EPA with the steroid antagonist (3α,5α)-17-phenylandrost-16-en-3-ol (17-PA) or mutagenesis of residues previously shown to be involved in receptor modulation by neurosteroids. Interestingly, EPA also potentiated receptors activated by saturating concentrations of allopregnanolone, indicating that the binding sites for the two drugs are not overlapping.
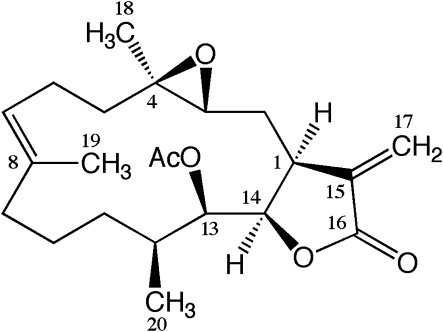
Figure 1.
Structure of eupalmerin acetate (EPA). The compound was isolated from the Caribbean gorgonian octocorals E. succinea and E. mammosa, where it is believed to have a defensive role against other marine organisms.
Methods
The experiments were conducted on HEK 293 cells transiently expressing rat α1β2γ2L GABAA receptors as described previously (Akk et al., 2001, 2004; Li et al., 2006). The subunit cDNAs were subcloned into the pcDNA3 expression vector (Invitrogen, Carlsbad, CA, USA) and transiently transfected into HEK cells using a calcium phosphate precipitation-based transfection technique (Akk, 2002). The α1 subunit is epitope (FLAG) tagged in the N-terminal end of the subunit (Ueno et al., 1996; Einhauer and Jungbauer, 2001). The presence of the FLAG tag is without effect on channel kinetics (Ueno et al., 1996).
The electrophysiological experiments were carried out using whole-cell voltage clamp and cell-attached single-channel patch clamp methods. The bath solution contained (in mM) 140 NaCl, 5 KCl, 1 MgCl2, 2 CaCl2, 10 glucose and 10 HEPES at pH 7.4. In whole-cell recordings, the pipette solution contained (in mM) 140 CsCl, 4 NaCl, 4 MgCl2, 0.5 CaCl2, 5 EGTA and 10 HEPES at pH 7.4. In single-channel recordings, the pipette solution contained (in mM) 120 NaCl, 5 KCl, 10 MgCl2, 0.1 CaCl2, 20 tetraethylammonium, 5 4-aminopyridine, 10 glucose and 10 HEPES at pH 7.4.
The agonist and modulators (GABA, pentobarbital, steroids and EPA) were added to the pipette solution in single-channel recordings, or applied through the bath using an SF-77B fast perfusion stepper system (Warner Instruments, Hamden, CT, USA) in whole-cell experiments. The stock solutions of steroids and EPA were initially made in dimethyl sulphoxide (DMSO), and diluted immediately before the experiment. The maximal DMSO concentration in diluted solutions was 0.1%. Channel activation by GABA was not affected by the presence of up to 0.3% DMSO (Li et al., 2007a). All experiments were carried out at room temperature.
The recording and analysis of whole-cell currents have been described previously (Li et al., 2006). The cells were clamped at −60 mV. Typically, the cells were exposed to the drugs for 4 s with 30 s washouts separating successive applications. In some experiments, longer drug applications were employed. The current traces were low-pass filtered at 2 kHz and digitized at 10 kHz. The analysis of whole-cell currents was carried out using the pClamp 9.0 software package and was aimed at determining the peak amplitude.
The recording and analysis of single-channel currents were carried out as described in detail previously (Akk et al., 2001, 2004). All currents were obtained at 50 μM GABA, a concentration that corresponds to ∼EC40 in the open probability concentration–effect curve (Steinbach and Akk, 2001). The pipette potential was held at +60 to +80 mV, which translates to an approximately −120 to −100 mV potential difference across the patch membrane. The currents were recorded using an Axopatch 200B amplifier, low-pass filtered at 10 kHz and acquired with a Digidata 1320 series interface at 50 kHz using pClamp software (Molecular Devices, Union City, CA, USA). The key feature of the analysis of single-channel currents is that the analysis was limited to clusters, that is, episodes of intense activity originating from the activation of a single ion channel or fragments of clusters containing no overlapping currents. The digitized currents were low-pass filtered at 2–3 kHz and idealized using the segmented-k-means algorithm (Qin et al., 1996). The open and closed times were estimated from the idealized currents using a maximum likelihood method, which incorporates a correction for missed events (QuB Suite; http://www.qub.buffalo.edu).
Molecular modelling of EPA was performed with the MacroModel programme (Mohamadi et al., 1990). The structure was built by hand and then submitted to a Monte Carlo Multiple Minimum conformational search using 2500 steps, keeping those conformations within 50 kJ mol−1 of the global minimum. Each conformer was then minimized using the MMFF force field to a gradient of 0.01. This search resulted in a total of 136 unique conformations. A similar process was followed for allopregnanolone, but due to the rigidity of the steroid backbone only 12 unique conformations were found. The conformations found for both EPA and allopregnanolone were then exported as a multimolecule file for use in similarity alignment using the Rapid Overlay of Chemical Structures (ROCS; OpenEye Scientific Software, Santa Fe, NM, USA) programme. The EPA conformations were then superimposed against the allopregnanolone conformations using a combination of the best shape match and chemical features; this produced a total of 31 possible matches. The best scoring alignments are shown in Figure 6.
In Xenopus laevis tadpole behavioural assays, EPA was added to beakers, each containing 100 ml of oxygenated 1 × Tadpole Ringer's solution. The Tadpole Ringer's solution contained 5.8 mM NaCl, 67 μM KCl, 34 μM Ca(NO3)2, 83 MgSO4, 419 μM Tris-HCl and 80 μM Tris-Base. The final concentration of DMSO was at or below 0.1% in these trials, and a beaker containing 0.1% DMSO served as a control. Ten tadpoles were distributed into each beaker, after which they were allowed to equilibrate in the Tadpole Ringer's solution for 3 h. At the end of the equilibration period, the loss of righting reflex (LRR) and the loss of swimming reflex (LSR) were measured. To measure LRR, a hooked glass rod was used to get underneath a tadpole's tail to flip the tadpole over. LRR was defined as the inability of a tadpole to right itself after 5 s on its back, for three consecutive trials. If at any time, the tadpole was able to right itself within the 5 s period during any of the three trials, then that specific tadpole did not have LRR. To measure LSR, a tadpole was gently swirled around the beaker for 5 s, while its tail was observed for signs of swimming. The swimming reflex is not a large twitch at the base of the tail, near the body, but is a rapid fluttering of the tail, which is best seen at the tip of the tail. The lack of rapid fluttering was defined as LSR. The tests were conducted in the presence of 1, 3, 10, 20 and 30 μM EPA. A total of 20 tadpoles, in two replicates of 10 tadpoles each, were used in the presence of 3, 10 and 20 μM EPA, while a single experiment with 10 tadpoles was conducted in the presence of 1 and 30 μM EPA.
Data analysis
Statistical analysis was carried out by use of Student's t-test and a P-value less than 0.05 was assumed to denote a significant difference.
Compounds
Eupalmerin acetate was purified and characterized as described previously (Rodríguez et al., 2001). Allopregnanolone and other chemicals were purchased from Sigma Chemical Co. (St Louis, MO, USA). 17-PA was a gift from Drs DF Covey and K Krishnan (Washington University School of Medicine).
Results
Eupalmerin acetate potentiates macroscopic responses elicited by GABA or pentobarbital
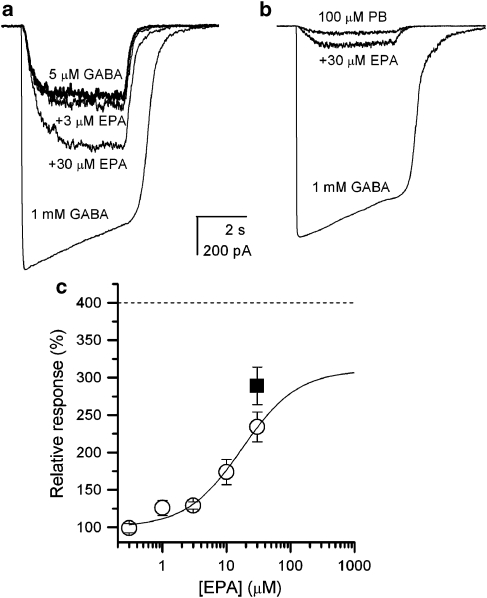
Coapplication of EPA with GABA or pentobarbital results in potentiation of current response. Sample recordings demonstrating whole-cell responses to the application of 5 μM GABA (∼EC25) or 100 μM pentobarbital, in the absence and presence of EPA, are shown in Figure 2. The response to 5 μM GABA was potentiated by EPA in a dose-dependent manner. The peak current was increased to 234±20% (mean±s.e.mean; n=5 cells) in the presence of 30 μM EPA, while curve fitting yielded the maximal potentiation of 310% and an EC50 of 17.4 μM (Figure 2c). The application of 30 μM EPA alone, in the absence of GABA or pentobarbital, did not elicit currents (data not shown).
Figure 2.
Cembranoid eupalmerin acetate (EPA) potentiates macroscopic currents elicited by GABA or pentobarbital. (a) Whole-cell currents from an HEK cell expressing α1β2γ2L receptors. The cell was exposed to 5 μM (∼EC25), 1 mM GABA (a saturating concentration) or 5 μM GABA plus 3 or 30 μM EPA. (b) Whole-cell currents elicited by 100 μM pentobarbital (PB) in the absence and presence of 30 μM EPA. For reference, the response of the same cell to 1 mM GABA is shown. (c) The potentiation concentration–effect relationship for currents activated by 5 μM GABA. Open circles show mean±s.e.mean from five cells. The curve was fitted to the following equation: response=100%+(maximal response−100%) ([EPA]/([EPA]+EC50)) yielding a maximal response of 310±40% and an EC50 of 17.4±6.9 μM. Because of limited aqueous solubility, higher EPA concentrations were not tested. Curve fitting was done using the NFIT programme (Medical Branch at Galveston, the University of Texas). The solid square represents the effect of 30 μM EPA on currents elicited by 100 μM pentobarbital (mean±s.e.mean from five cells). The dashed line shows the extent of the maximal possible potentiation of currents elicited by an EC25 concentration of GABA, assuming that the modulator affects channel kinetics but not single-channel conductance.
We note that, due to poor water solubility, the maximal EPA concentrations used in the present study were limited to 30–40 μM, and the lack of data points at higher drug concentrations leaves room for error in the dose–response curve fitting (see Figure 2c). Therefore, our estimate for EC50 of EPA actions on whole-cell currents should be treated with some caution. Nevertheless, the maximal potentiation estimated from the fit (310%) is comparable to the theoretically possible extent of potentiation, assuming that EPA only modifies the kinetic properties of the GABAA receptor without affecting single-channel conductance.
Potentiation by EPA was diminished when higher concentrations of GABA were used to activate the receptor. In the presence of 1 mM GABA (a saturating concentration), the addition of 1, 3, 10 or 30 μM EPA yielded a peak response of 100±2, 99±3, 97±3 and 96±3% of control (n=3 cells), respectively.
Currents elicited by a submaximal concentration of pentobarbital were also potentiated by EPA. In the presence 100 μM pentobarbital, the addition of 30 μM EPA enhanced the peak response to 289±25% of control (n=5 cells).
Single-channel analysis of currents recorded in the presence of EPA
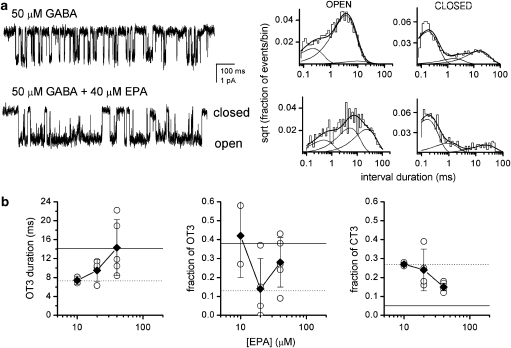
For a more detailed insight into the mechanisms of GABAA receptor modulation by EPA, we conducted single-channel patch clamp recordings in which we examined the effect of EPA on currents elicited by 50 μM GABA. Sample single-channel clusters obtained under control conditions (GABA only) and in the presence of 40 μM EPA are shown in Figure 3a. The currents recorded in the presence of EPA had a higher intracluster open probability resulting from changes in open- and closed-time distributions. The nature of the specific changes was similar to that observed in the presence of neuroactive steroids. The mean duration of the longest-lived open-time component (OT3) roughly doubled, from 7.3 to 14.3 ms, and the relative frequency of OT3 increased from 13 to 28% in the presence of 40 μM EPA. Neither effect, however, reached statistical significance, undoubtedly because of the relatively prominent patch-to-patch variability when EPA was coapplied with GABA. The application of EPA also resulted in a statistically significant reduction in the relative frequency of the longest-lived intracluster closed-time component (CT3). The CT3 closed-time component was reduced from 27 to 15% in the presence of 40 μM EPA. In addition to these changes, the duration of OT1 was increased in the presence of EPA. The results of the kinetic analysis are summarized in Table 1.
Figure 3.
The effect of eupalmerin acetate (EPA) on single-channel currents from HEK cells expressing α1β2γ2L receptors. (a) Sample clusters elicited by 50 μM GABA in the absence (top trace) and presence of 40 μM EPA (bottom trace). Open- and closed-time histograms from the respective patches are given next to the current traces. For 50 μM GABA, the open times were 0.21 ms (24%), 3.4 ms (72%) and 9.3 ms (4%), and the closed times were 0.16 ms (64%), 1.6 ms (11%) and 15.2 ms (25%). For GABA+40 μM EPA, the open times were 0.45 ms (21%), 4.8 ms (41%) and 22.2 ms (38%), and the closed times were 0.15 ms (66%), 0.8 ms (18%) and 21.3 ms (15%). A summary of averaged data for all patches under these conditions is given in Table 1. (b) Concentration–effect relationships for the duration of OT3, fraction of OT3 and fraction of CT3. The open circles give values from individual patches, solid diamonds show mean values±s.d. from all patches. The dotted line gives the mean value for the parameters in the presence of 50 μM GABA in the absence of modulators. The solid horizontal line gives the mean value for the parameters in the presence of 50 μM GABA and 1 μM allopregnanolone (Li et al., 2007a).
Table 1.
The summary of single-channel kinetic analysis of currents from the α1β2γ2L GABAA receptor exposed to GABA and EPA
| Pipette | OT1 (ms) | Fraction of OT1 | OT2 (ms) | Fraction of OT2 | OT3 (ms) | Fraction of OT3 |
|---|---|---|---|---|---|---|
| 50 μM GABA | 0.28±0.05 | 0.22±0.02 | 3.0±0.7 | 0.65±0.06 | 7.3±3.2 | 0.13±0.07 |
| 50 μM GABA+40 μM EPA | 0.41±0.05* | 0.25±0.07 | 4.0±0.8 | 0.47±0.17 | 14.3±5.9 | 0.28±0.13 |
|
Pipette |
CT1 (ms) |
Fraction of CT1 |
CT2 (ms) |
Fraction of CT2 |
CT3 (ms) |
Fraction of CT3 |
| 50 μM GABA | 0.15±0.01 | 0.60±0.10 | 1.5±0.2 | 0.13±0.05 | 14.4±4.2 | 0.27±0.06 |
| 50 μM GABA+40 μM EPA | 0.18±0.04 | 0.66±0.05 | 1.9±0.7 | 0.19±0.06 | 19.4±3.0 | 0.15±0.02* |
Abbreviation: EPA, eupalmerin acetate.
The mean durations (OT1-3, CT1-3; mean±s.d.) and relative contributions (fractions of OT1-3, fractions of CT1-3; mean±s.d.) for the intracluster open- and closed-time components are shown. The parameters are presented as mean±s.d. from four (GABA alone) or five patches (GABA+EPA). The total number of events was 23 523 under control conditions, and 31 734 for EPA. The control data (GABA alone) are from Li et al. (2007b). The presence of 40 μM EPA resulted in a statistically significant (*P<0.05; t-test) effect on the duration of OT1 and the fraction of CT3. Although the duration and fraction of OT3 were increased in the presence of EPA, the effect was not statistically significant. Cluster open probability, calculated as mean open time/(mean open time+mean closed time) was 0.42±0.04 in the presence of 50 μM GABA and 0.63±0.11 in the presence of GABA+40 μM EPA (P<0.01; t-test).
The single-channel experiments were not conducted at higher drug concentrations because of the limited water solubility of EPA. However, to examine the dose dependency of EPA-mediated modulation, we examined the effects of EPA at lower concentrations (10 and 20 μM). The concentration–effect relationships for the duration and prevalence of OT3 and the prevalence of CT3 are shown in Figure 3b. The OT3 duration was increased and the prevalence of CT3 decreased dose dependently by EPA. The effect on the fraction of OT3 was less straightforward, although in the presence of 10 and 40 μM EPA, the fraction of OT3 was higher than in control experiments.
We have previously associated the CT3 component of the closed-time histograms with activation-related processes, that is, agonist binding and channel opening (Steinbach and Akk, 2001). The lack of effect of EPA on the duration of CT3 suggests that the drug does not modulate receptor affinity to GABA or the channel-opening rate.
It is important to note that, even at 40 μM, EPA was a relatively weak modulator. Only the changes in the fraction of CT3 and the duration of OT1 were statistically significant. The duration and fraction of OT3 showed a tendency for increase in the presence of EPA; however, neither effect reached statistical significance. Although many potentiating neuroactive steroids efficaciously modulate the duration and fraction of OT3, and the fraction of CT3, previous studies have revealed neurosteroid analogues with only a subset of effects. For example, a neuroactive steroid (3α,5β,17β)-3-hydroxy-18-norandrostane-17-carbonitrile modifies the channel closed-time distributions and increases the prevalence of OT3 but is ineffective at increasing the duration of OT3 (Akk et al., 2004). The androgenic steroid aetiocholanolone increases the prevalence of OT3 but is without effect on other intracluster kinetic parameters (Li et al., 2007a). The underlying reason for the occurrence of only a subset of kinetic effects for some steroid analogues is unclear, but it has been proposed that non-ideal interactions with the steroid-binding site or (assuming that the kinetic effects are mediated by steroid interactions with separate sites) the ability of some analogues to interact with only some sites result in the modulation of some but not other kinetic parameters (Li et al., 2006, 2007a).
Effects of mutations to the neurosteroid site on potentiation by EPA
The results from the kinetic analysis of single-channel currents showed that EPA modifies GABAA receptor activation in a fashion mechanistically similarly to neurosteroids. Given the hydrophobic nature of the compound, it is not unreasonable to propose that EPA interacts with a steroid-binding site. A previous study identified several amino-acid residues that line the postulated neurosteroid-binding sites (Hosie et al., 2006). Residues α1Q241 and α1N407/Y410 are positioned at the opposite ends of a steroid-binding site, where they form hydrogen bonds with the A and D rings of the steroid molecule, respectively. Interaction of the steroid molecule with the site defined by these residues results in potentiation of receptor function. Another binding site, mediating direct activation by steroids, is formed, in part, by residues α1T236 and β2Y284. We have examined the effects of mutations to these sites on channel potentiation by EPA.
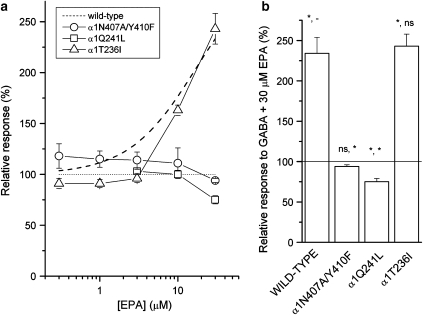
The α1Q241L mutation has been shown to prevent potentiation of the GABAA receptor by the neurosteroids allopregnanolone and THDOC (Hosie et al., 2006). Our findings demonstrate that this mutation also strongly affects potentiation by EPA. No potentiation was detected at up to 30 μM EPA (Figure 4). In contrast, 100 μM pentobarbital strongly potentiated GABA responses from the mutant receptor (data not shown), demonstrating that the mutant receptor retained the ability to be potentiated by other modulators. Lack of steroid potentiation in the α1Q241L mutant receptor has been accounted for by the inability of the leucine residue to form a hydrogen bond with the hydroxyl group of the A ring of the steroid. Lack of potentiation by EPA suggests that the residue α1Q241 may similarly interact with the cembranoid molecule.
Figure 4.
The effects of mutations to the putative neurosteroid-binding site on channel modulation by eupalmerin acetate (EPA). (a) Modulation by EPA was tested in the presence of an EC25 concentration of GABA (10 μM for the α1N407A/Y410F double mutant, 20 μM for the α1Q241L receptor and 10 μM for the α1T236I receptor). The dashed line is reproduced from Figure 2 and shows potentiation in the wild-type receptor. The symbols represent mean values±s.e.mean. The findings demonstrate that mutations to the putative neurosteroid-binding site also affect channel potentiation by the cembranoid EPA. (b) The relative response to EC25 concentration of GABA+30 μM EPA. The columns show mean values±s.e.mean from three to six cells per construct. Statistical tests were carried out with respect to control (GABA alone) and to EPA-mediated potentiation of the wild-type receptor. *P<0.05; NS, not significant; –, not applicable.
We next examined the effect of the double mutation α1N407A/Y410F on receptor modulation by EPA. These residues are postulated to interact with the D ring of the steroid molecule by donating a pair of electrons to the carbonyl group (Hosie et al., 2006). Our experiments show that the double mutation also effectively blocks potentiation by EPA. In the presence of 30 μM EPA, the peak response was 94±2% of control (n=3 cells; Figure 4).
A previous study showed that the α1T236I mutation drastically diminishes direct activation by endogenous steroids allopregnanolone and THDOC but is essentially without effect on potentiation by these steroids (Hosie et al., 2006). We examined the effect of the α1T236I mutation on receptor modulation by EPA. The findings demonstrate that the mutation was essentially ineffective at modifying potentiation by EPA (Figure 4). The concentration–effect relationship was slightly shifted towards higher EPA concentrations in the mutant receptor, but potentiation by 30 μM EPA was indistinguishable from that in the wild-type receptor (243±15% of control; n=5 cells). The lack of effect of the α1T236I mutation on channel potentiation is consistent with studies on neuroactive steroids, and suggests that the site responsible for potentiation by steroids, but not the site responsible for direct activation by steroids, is involved in the potentiating actions of cembranoid EPA.
In summary, point mutations to the neurosteroid-binding site, previously shown to affect GABAA receptor modulation by neuroactive steroids (Hosie et al., 2006; Li et al., 2007a) and steroid analogues (Li et al., 2006), also reduce potentiation by the cembranoid EPA, suggesting the involvement of the putative neurosteroid-binding site in receptor modulation by EPA.
Competitive steroid antagonist (3α,5α)-17-phenylandrost-16-en-3-ol inhibits potentiation by EPA
The synthetic steroid (3α,5α)-17-phenylandrost-16-en-3-ol (17-PA) has previously been shown to antagonize potentiation by neurosteroids (Mennerick et al., 2004). The effect is specific to steroids in that potentiation by barbiturates and benzodiazepines is unaffected by 17-PA. Furthermore, the effect is selective for 5α-reduced steroids, for example, allopregnanolone, whereas 5β-reduced steroids are only weakly affected (Mennerick et al., 2004). Here, we examined whether 17-PA reduces potentiation by EPA.
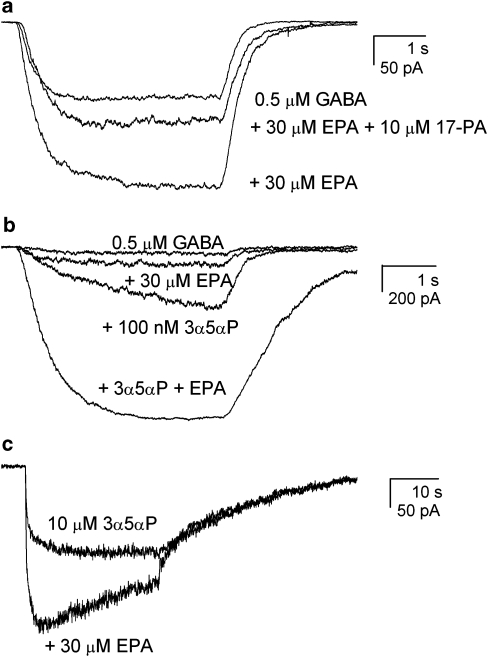
The experiments were carried out on wild-type α1β2γ2L receptors. The currents were elicited with 0.5 μM GABA, and the effect of 10 μM 17-PA on channel potentiation by 30 μM EPA was examined. Sample current traces are shown in Figure 5a. In seven cells, where the presence of EPA resulted in a 2.7±0.6-fold potentiation, coapplication of 17-PA with EPA reduced potentiation to 1.7±0.4 (P<0.01). Thus, 17-PA, a steroid antagonist, also reduced potentiation by the cembranoid EPA.
Figure 5.
Effects of coapplication of eupalmerin acetate (EPA) and steroids. (a) The effect of the specific steroid antagonist (3α,5α)-17-phenylandrost-16-en-3-ol (17-PA) on potentiation elicited by EPA. The cell was exposed to 0.5 μM GABA, GABA+30 μM EPA or GABA+EPA+10 μM 17-PA. The presence of 17-PA reduced the potentiating effect of EPA, suggesting that EPA acts on the receptor through the steroid site. The peak amplitudes were 163 pA (GABA alone), 344 pA (GABA+EPA) and 220 pA (GABA+EPA+17-PA). The averaged values from all experiments are given in the text. (b) The effect of coapplication of the potentiating neurosteroid allopregnanolone (3α5αP) and EPA on currents elicited by GABA. The cell was exposed to 0.5 μM GABA, GABA+30 μM EPA, GABA+100 nM allopregnanolone or GABA+EPA+allopregnanolone. The presence of EPA did not reduce the potentiation by allopregnanolone, suggesting that EPA is not a high-affinity, low-efficacy modulator acting through the steroid-binding site. The peak amplitudes were 77 pA (GABA alone), 186 pA (GABA+EPA), 551 pA (GABA+allopregnanolone) and 1506 pA (GABA+allopregnanolone+EPA). The averaged values from all experiments are given in the text. (c) The effect of EPA on receptors directly activated by allopregnanolone. The cell was exposed to 10 μM allopregnanolone (3α5αP), or allopregnanolone+30 μM EPA. The presence of EPA potentiated the current response, suggesting that the sites for allopregnanolone and EPA are non-overlapping. The peak amplitudes were 183 pA (allopregnanolone) and 324 pA (allopregnanolone+EPA). The averaged values from all experiments are given in the text. Exposure to 30 μM EPA alone did not elicit a discernible current (data not shown).
Is EPA a high-affinity, low-efficacy or a low-affinity, high-efficacy modulator of the GABAA receptor?
The low water solubility of EPA prevented us from using drug concentrations higher than 30–40 μM, whereas concentrations below 10 μM were relatively ineffective at producing modulation (Figures 2c, Figure 3b), thus limiting the studies of EPA effects to a narrow concentration range. In particular, the inability to examine EPA effects at higher concentrations prevented us from directly determining whether EPA is as efficacious as neurosteroids in modulating GABAA receptor activity.
However, we could indirectly estimate receptor affinity to EPA. In this experiment, we examined the effect of EPA on channel potentiation by 100 nM allopregnanolone. This is a steroid concentration that produces a smaller than half-maximal effect, but the extent of potentiation is still greater than potentiation by 30 μM EPA. We reasoned that if EPA is a high-affinity, low-efficacy modulator, acting through the molecular machinery utilized by neurosteroids, then its presence should reduce potentiation elicited by allopregnanolone. In contrast, if EPA is a low-affinity, high-efficacy modulator, then its addition to the bath solution is essentially equivalent to an increase in the potentiator concentration, and should result in enhanced potentiation.
In these experiments, the receptors were activated by 0.5 μM GABA (EC5), and the effect of 100 nM allopregnanolone alone or in the presence of 30 μM EPA measured. The lower GABA concentration (compared to previous potentiation experiments) was selected to allow for greater dynamic range in potentiation. In six cells, allopregnanolone potentiated the response to GABA by 4.7±0.9-fold (mean±s.e.mean), whereas 30 μM EPA enhanced the peak response by just 2.8±0.3-fold. In the same cells, when EPA and allopregnanolone were coapplied, the currents were potentiated by 13.9±2.9-fold. Sample currents are shown in Figure 5b.
The inability of EPA to reduce potentiation elicited by allopregnanolone clearly indicates that EPA is not a high-affinity, low-efficacy modulator competing with allopregnanolone for a common site. However, we were puzzled by the finding that the presence of EPA results in strong potentiation of the currents. Although a certain degree of additional potentiation was anticipated, due to an increase in the total concentration of positive modulators present in the bath, the multiplicative effects of EPA and allopregnanolone were unexpected.
EPA potentiates receptors directly activated by high concentrations of allopregnanolone
Several pieces of evidence suggest that EPA interacts with the site that mediates GABAA receptor potentiation by steroids: EPA potentiates the single-channel currents mechanistically similarly to neurosteroids, EPA effects are blocked by mutations to the site mediating potentiation by neurosteroids and the steroid antagonist 17-PA reduces the ability of EPA to enhance GABAA receptor currents. On the other hand, the finding that the potentiating effects of EPA and allopregnanolone, when coapplied, are multiplied is not in full agreement with such a simple model and is more indicative of independent actions of the two drugs, mediated by non-overlapping binding sites.
To address this issue more directly, we tested whether EPA potentiates receptors exposed to very high concentrations of allopregnanolone. The receptors were activated by 1 or 10 μM allopregnanolone, and the effect of 30 μM EPA on the responses was examined. It was hypothesized that exposure to such high concentrations of steroid fully saturates the steroid-binding sites disallowing the binding of EPA to these sites. Any further increase in the current response upon exposure to EPA would be suggestive of non-overlapping interaction sites for allopregnanolone and EPA. These experiments were carried out in the absence of GABA to avoid limitations imposed by dynamic range. Receptors activated by GABA and such high concentrations of steroid become fully active, and further modulation, by exposure to additional steroid or other modulators, is usually not observed.
In three cells tested, exposure to 30 μM EPA enhanced currents elicited by 1 μM allopregnanolone to 189±19% of control (P<0.05). Further, the application of EPA potentiated the peak current elicited by 10 μM allopregnanolone to 179±15% of control (n=7 cells, P<0.002). A sample recording is shown in Figure 5c. Given that the apparent affinity of the potentiation site to allopregnanolone is approximately 100–200 nM (Weir et al., 2004; Akk et al., 2005; Hosie et al., 2006) and that EPA has a relatively low affinity for the GABAA receptor (see above), it is highly unlikely that the potentiating effect of EPA under these conditions resulted from cembranoid interactions with the allopregnanolone-binding site. We note a caveat, however, that the affinity of the steroid potentiation site to allopregnanolone is measured in the presence of GABA, whereas the lack of GABA in our experiments may have affected the affinity of the steroid site for allopregnanolone. Taken together, the observations suggest that EPA and neurosteroids may be interacting with the same key loci within the steroid-binding cavity and/or share transduction elements so that mutations to the neurosteroid-binding site affect modulation by both drugs. However, it is unlikely that the bound EPA occludes access of allopregnanolone to its site of action.
Comparison of neurosteroid and eupalmerin acetate structures
The electrophysiological data provide strong evidence for similarities in potentiation produced by neurosteroids and EPA, including actions on channel kinetics, the effects of mutations and the block by 17-PA, although there are also indications of differences in binding sites for the two classes of compound. Accordingly, we wanted to determine whether the molecular structures of EPA and allopregnanolone showed significant similarity. Neurosteroids have two structural features found in all effective steroidal GABAA receptor potentiators, a hydroxyl group on C3 of the steroid, and a hydrogen bond acceptor at C17, typically a ketone. It is a reasonable assumption that some portion of EPA mimics these hydrogen-bonding features.
We used a programme (ROCS) that is a shape-based superposition method (Bender and Glen, 2004). Molecules are aligned by a solid-body optimization process that maximizes the overlap volume between them. Although ROCS is primarily a shape-based method, user-specified definitions of chemistry such as hydrogen-bond donor or acceptor, rings, cations and anions can be included into the superposition and similarity analysis process, facilitating the identification of compounds that are similar both in shape and chemical properties.
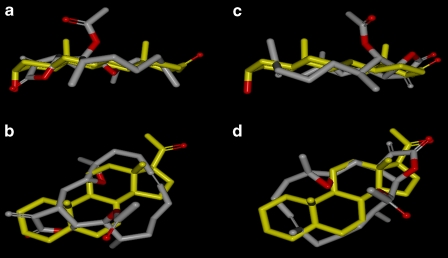
We generated and minimized a library of low-energy conformations of EPA (see Methods). A set of low-energy conformations for allopregnanolone was obtained in a similar fashion. The programme ROCS was then used to find the most reasonable superposition between these molecules. Figure 6 shows the two best mutual superpositions found between EPA and allopregnanolone. Figures 6a and b show the possible match in which the γ-lactone carbonyl group of EPA aligns to the C3 hydroxyl of allopregnanolone, whereas Figures 6c and d show the best match of the γ-lactone carbonyl group to the C17 ketone. The programme ROCS was unable to superpose the molecules in a manner that could match both of the steroid features simultaneously. In terms of molecular volume, allopregnanolone (329 Å3) compares quite favourably to the EPA conformation matching to C3 (367 Å3) as well as the conformation matching to C17 (382 Å3).
Figure 6.
Comparison of chemical structures for eupalmerin acetate (EPA) and allopregnanolone. In all four figures, EPA is shown in grey with oxygens in red, whereas the allopregnanolone is shown in yellow. Hydrogens are removed from both molecules to provide a clearer view of the superposition. (a) A side view of the best superposition of EPA onto allopregnanolone matching the γ-lactone carbonyl to the C3 hydroxyl. (b) A top-down view of the same alignment. (c) A side view of EPA aligned with the C17 ketone. (d) A top-down view of this superposition.
Using the proposed steroid-binding site of Hosie et al. (2006) as a reference for understanding the possible binding interactions, the C3 hydroxyl group of allopregnanolone hydrogen bonds to α1Q241. On the basis of the ROCS superposition, this interaction would be replaced by the interaction of the γ-lactone carbonyl of EPA with Q241. EPA would be expected to have a weaker interaction with the binding site as only one hydrogen-bonding interaction could occur, as unlike allopregnanolone, there is no possible D ring H-bonding interaction with α1N407 or Y410. Alternatively, EPA could interact with N407/Y410, and thereby lose the interactions to Q241. In terms of energetic penalties for adopting these conformations, the EPA conformation aligning the γ-lactone carbonyl with ketone group of the allopregnanolone is favoured by 15.07 kJ mol−1.
Anaesthetic effects of eupalmerin acetate
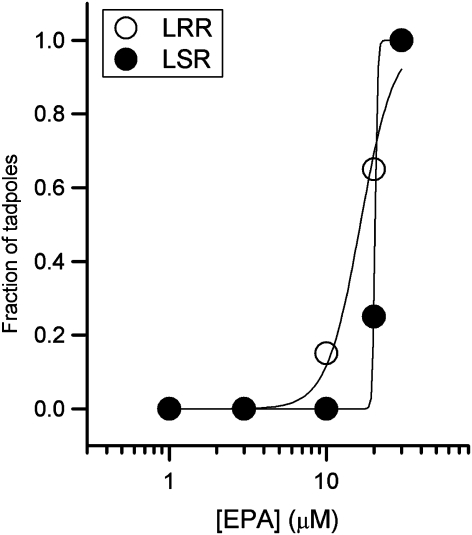
To evaluate the physiological effects of EPA, we examined the ability of EPA to induce the LRR and LSR using X. laevis tadpoles. The tadpoles were incubated in beakers containing various concentrations of EPA, and the righting and swimming reflexes were estimated at the end of a 3-h exposure period. The number of tadpoles with LRR and LSR were plotted as a fraction of the total, and the EC50 values for the LRR and LSR curves were estimated from fits to the Hill equation (Figure 7). The EC50 for the LRR curve was 16.4±0.9 μM, and the EC50 for the LSR curve was 20.4±0.01 μM. For comparison, the EC50 for LRR is 0.39 μM for allopregnanolone (Wittmer et al., 1996).
Figure 7.
Eupalmerin acetate (EPA) causes anaesthesia in Xenopus tadpoles. Concentration–response curves for loss of righting reflex (LRR) and loss of swimming reflex (LSR) in tadpoles using EPA as an anaesthetic. The curves were fitted to Hill equation yielding, for LRR, an EC50 of 16.4±0.9 μM and nH of 4.1±0.7, and, for LSR, an EC50 of 20.4±0.01 μM and nH of 58.3±1.4. Each point represents the fraction of 10 animals that met the criteria for LRR or LSR. The points for 1, 3 and 30 μM EPA are the same for LRR and LSR.
Discussion and conclusions
Eupalmerin acetate is a diterpene isolated from the gorgonian octocorals Eunicea succinea and Eunicea mammosa. Extensive previous studies have shown that EPA modulates the activation of the muscle- and neuronal-type nicotinic receptors (Eterovic et al., 1993; Hann et al., 1998; Ferchmin et al., 2001). To the best of our knowledge, this is the first study to examine the effects of EPA on GABAA receptor function. Our findings demonstrate that, at micromolar concentrations, EPA potentiates the α1β2γ2L GABAA receptor. Further, in X. laevis tadpole behavioural assays, exposure to EPA causes LRR and LSR, effects that are generally considered to be mediated by GABAA receptors (Reith and Sillar, 1999; Belelli et al., 2003).
Several lines of evidence suggest that EPA acts via interactions with the site that mediates receptor potentiation by neurosteroids. Mutations to residues α1Q241 and α1N407/Y410 diminished potentiation by EPA. These residues have been previously shown to line the neurosteroid-binding site, and specific mutations to these sites reduce potentiation by steroids (Hosie et al., 2006). In contrast, a mutation to the site that mediates direct activation by steroids (α1T236I) did not reduce potentiation by EPA. Additionally, potentiation by EPA was reduced upon exposure to a steroid antagonist, 17-PA. Previous work has shown that this compound interferes specifically with potentiation caused by steroids, but not by barbiturates or benzodiazepines (Mennerick et al., 2004). Finally, the kinetic mode of action of EPA is similar to that of neuroactive steroids. Kinetic analysis of single-channel currents recorded in the presence of EPA shows that the drug acts by decreasing the prevalence of the longest intracluster closed-time component. In addition, EPA caused a slight, although statistically insignificant, increase in the mean duration and prevalence of the longest open-time component. These three intracluster kinetic parameters, or subsets of them, are affected during channel exposure to many neuroactive steroids (Akk et al., 2004, 2005; Li et al., 2007a).
It should be pointed out that another potentiator of the GABAA receptor, pentobarbital, shares some of the kinetic mechanisms through which neurosteroids and EPA modulate the GABAA receptor (Steinbach and Akk, 2001). However, the mutations to the putative neurosteroid-binding site diminish whole-cell potentiation by steroids (Hosie et al., 2006) and EPA (Figure 4) but not by pentobarbital (Hosie et al., 2006). Further, 17-PA diminishes potentiation elicited by allopregnanolone (Mennerick et al., 2004) and EPA (Figure 5) but not by pentobarbital (Mennerick et al., 2004), thus placing the modulatory effects of EPA and neurosteroids into a common category that is distinct from that of barbiturates.
Despite the many lines of evidence suggesting that EPA acts through the neurosteroid site, the results from the experiments where EPA was coapplied with allopregnanolone indicate that the binding sites for this steroid and EPA are not overlapping. Examination of potentiation of receptor activity elicited by GABA demonstrated that EPA and allopregnanolone act essentially independently with augmented effects on channel activity, whereas exposure to EPA of receptors directly activated by high concentrations of allopregnanolone resulted in potentiation. The high-steroid concentration (10 μM) used in the latter set of experiments essentially guaranteed that the steroid potentiation site was fully occupied with allopregnanolone. Thus, potentiation of currents elicited by allopregnanolone, by EPA, suggests that the sites for EPA and allopregnanolone are not identical.
Earlier findings have demonstrated that the cavity, defined by the α1Q241 and α1N407/Y410 residues and implicated in neurosteroid actions, is able to accommodate structurally distinct compounds. Besides neuroactive steroids of various structures (Hosie et al., 2006; Li et al., 2007a), it has been shown that three-ringed benz[e]indene neurosteroid analogues (Li et al., 2006) interact with the site. We earlier found that the α1N407A/Y410F double mutation was capable of blocking steroid actions mediated by what appeared as separate, non-overlapping sites and proposed that the two residues control steroid access to multiple sites (Li et al., 2007a). If applicable to EPA, the α1N407A/Y410F double mutation may prevent EPA access to the cavity where both steroids and EPA bind, even if the two drugs have non-overlapping interaction sites. Thus, the site is conceptually similar to another crevice in the GABAA receptor, formed between the M1, M2 and M3 transmembrane domains, which has been implicated in the binding of general anaesthetics of widely differing sizes and structures (Jenkins et al., 2001). Alternatively, it is possible that the steroid-binding site is located downstream from the EPA interaction site, and the latter utilizes (some of) the structural elements involved in steroid potentiation so that mutations to the steroid site also affect channel modulation by EPA. Recent modelling studies have demonstrated the presence of multiple water-accessible pockets within the GABAA receptor, which probably serve as binding sites for receptor modulators (Ernst et al., 2005).
It is of interest that many cembranoids, at micromolar concentrations, reversibly inhibit the closely related nicotinic receptor. Although the structural details of the binding site for many of the cembranoids, including EPA, are unknown, there is evidence that the site sterically overlaps the site in the channel to which the non-competitive inhibitor [piperidyl-3,4,-3H]-phencyclidine binds (Pagan et al., 2001). In addition, there is a correlation between the cembranoid affinity to the receptor and its hydrophobicity; this also suggests that the site of action is within the hydrophobic transmembrane region of the receptor (Hann et al., 1998). However, we note that there is not necessarily any structural relationship between the sites of action in the nicotinic receptor and the GABAA receptor.
With the exception of lophotoxin, cembranoids do not affect the binding of α-bungarotoxin to the nicotinic receptor (Abramson et al., 1991; Eterovic et al., 1993; Hann et al., 1998), thereby ruling out cembranoid interactions with the nicotinic agonist-binding site. Similarly, we found that the presence of EPA did not affect GABAA receptor affinity to GABA nor did exposure to 30 μM EPA lead to channel activation in the absence of GABA or pentobarbital.
Besides its effects on transmitter-gated ion channels, EPA and several other marine cembranoids have been shown to induce apoptosis in human malignant glioma cells, thereby presenting as potentially useful anticancer agents (Iwamaru et al., 2007). Interestingly, the concentration range at which EPA acted against the tumours (EC50 at 5–7 μM) is close to the range at which its effects on transmitter-gated ion channels were observed. Detailed studies in both systems are therefore warranted for the selection of the most specific and suitable drugs.
Acknowledgments
We thank John Bracamontes for help with molecular biology and Steve Mennerick for comments on the work. This work was supported by National Institutes of Health Grants GM08102 (to ADR), GM47969 (to ASE and JHS), NS39408 (to VAE) and AA14707 (to GA). JHS is the Russell and Mary Shelden Professor of Anesthesiology.
Abbreviations
- Allopregnanolone
3α-hydroxy-5α-pregnan-20-one
- EPA
eupalmerin acetate
- 17-PA
(3α,5α)-17-phenylandrost-16-en-3-ol
Conflict of interest
The authors state no conflict of interest.
References
- Abramson SN, Li Y, Culver P, Taylor P. An analog of lophotoxin reacts covalently with Tyr190 in the α-subunit of the nicotinic acetylcholine receptor. J Biol Chem. 1989;264:12666–12672. [PubMed] [Google Scholar]
- Abramson SN, Trischman JA, Tapiolas DM, Harold EE, Fenical W, Taylor P. Structure/activity and molecular modeling studies of the lophotoxin family of irreversible nicotinic receptor antagonists. J Med Chem. 1991;34:1798–1804. doi: 10.1021/jm00110a007. [DOI] [PubMed] [Google Scholar]
- Akk G. Contributions of the non-α subunit residues (loop D) to agonist binding and channel gating in the muscle nicotinic acetylcholine receptor. J Physiol. 2002;544:695–705. doi: 10.1113/jphysiol.2002.029413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akk G, Bracamontes J, Steinbach JH. Pregnenolone sulfate block of GABAA receptors: mechanism and involvement of a residue in the M2 region of the α subunit. J Physiol. 2001;532:673–684. doi: 10.1111/j.1469-7793.2001.0673e.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akk G, Bracamontes JR, Covey DF, Evers A, Dao T, Steinbach JH. Neuroactive steroids have multiple actions to potentiate GABAA receptors. J Physiol. 2004;558:59–74. doi: 10.1113/jphysiol.2004.066571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akk G, Shu HJ, Wang C, Steinbach JH, Zorumski CF, Covey DF, et al. Neurosteroid access to the GABAA receptor. J Neurosci. 2005;25:11605–11613. doi: 10.1523/JNEUROSCI.4173-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atchison WD, Narahashi T, Vogel SM. Endplate blocking actions of lophotoxin. Br J Pharmacol. 1984;82:667–672. doi: 10.1111/j.1476-5381.1984.tb10805.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belelli D, Muntoni AL, Merrywest SD, Gentet LJ, Casula A, Callachan H, et al. The in vitro and in vivo enantioselectivity of etomidate implicates the GABAA receptor in general anaesthesia. Neuropharmacology. 2003;45:57–71. doi: 10.1016/s0028-3908(03)00144-8. [DOI] [PubMed] [Google Scholar]
- Bender A, Glen RC. Molecular similarity: a key technique in molecular informatics. Org Biomol Chem. 2004;2:3204–3218. doi: 10.1039/B409813G. [DOI] [PubMed] [Google Scholar]
- Culver P, Fenical W, Taylor P. Lophotoxin irreversibly inactivates the nicotinic acetylcholine receptor by preferential association at one of the two primary agonist sites. J Biol Chem. 1984;259:3763–3770. [PubMed] [Google Scholar]
- Einhauer A, Jungbauer A. The FLAG peptide, a versatile fusion tag for the purification of recombinant proteins. J Biochem Biophys Methods. 2001;49:455–465. doi: 10.1016/s0165-022x(01)00213-5. [DOI] [PubMed] [Google Scholar]
- Ernst M, Bruckner S, Boresch S, Sieghart W. Comparative models of GABAA receptor extracellular and transmembrane domains: important insights in pharmacology and function. Mol Pharmacol. 2005;68:1291–1300. doi: 10.1124/mol.105.015982. [DOI] [PubMed] [Google Scholar]
- Eterovic VA, Hann RM, Ferchmin PA, Rodriguez AD, Li L, Lee YH, et al. Diterpenoids from Caribbean gorgonians act as noncompetitive inhibitors of the nicotinic acetylcholine receptor. Cell Mol Neurobiol. 1993;13:99–110. doi: 10.1007/BF00735367. [DOI] [PubMed] [Google Scholar]
- Ferchmin PA, Hao J, Perez D, Penzo M, Maldonado HM, Gonzalez MT, et al. Tobacco cembranoids protect the function of acute hippocampal slices against NMDA by a mechanism mediated by α4β2 nicotinic receptors. J Neurosci Res. 2005;82:631–641. doi: 10.1002/jnr.20666. [DOI] [PubMed] [Google Scholar]
- Ferchmin PA, Lukas RJ, Hann RM, Fryer JD, Eaton JB, Pagan OR, et al. Tobacco cembranoids block behavioral sensitization to nicotine and inhibit neuronal acetylcholine receptor function. J Neurosci Res. 2001;64:18–25. doi: 10.1002/jnr.1049. [DOI] [PubMed] [Google Scholar]
- Gutierrez M, Capson TL, Guzman HM, Gonzalez J, Ortega-Barria E, Quinoa E, et al. Leptolide, a new furanocembranolide diterpene from Leptogorgia alba. J Nat Prod. 2005;68:614–616. doi: 10.1021/np049745z. [DOI] [PubMed] [Google Scholar]
- Hann RM, Pagan OR, Gregory L, Jacome T, Rodriguez AD, Ferchmin PA, et al. Characterization of cembranoid interaction with the nicotinic acetylcholine receptor. J Pharmacol Exp Ther. 1998;287:253–260. [PubMed] [Google Scholar]
- Hosie AM, Wilkins ME, da Silva HM, Smart TG. Endogenous neurosteroids regulate GABAA receptors through two discrete transmembrane sites. Nature. 2006;444:486–489. doi: 10.1038/nature05324. [DOI] [PubMed] [Google Scholar]
- Iwamaru A, Iwado E, Kondo S, Newman RA, Vera B, Rodriguez AD, et al. Eupalmerin acetate, a novel anticancer agent from Caribbean gorgonian octocorals, induces apoptosis in malignant glioma cells via the c-Jun NH2-terminal kinase pathway. Mol Cancer Ther. 2007;6:184–192. doi: 10.1158/1535-7163.MCT-06-0422. [DOI] [PubMed] [Google Scholar]
- Jenkins A, Greenblatt EP, Faulkner HJ, Bertaccini E, Light A, Lin A, et al. Evidence for a common binding cavity for three general anesthetics within the GABAA receptor. J Neurosci. 2001;21:RC136. doi: 10.1523/JNEUROSCI.21-06-j0002.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li P, Bracamontes J, Katona BW, Covey DF, Steinbach JH, Akk G. Natural and enantiomeric etiocholanolone interact with distinct sites on the rat α1β2γ2L GABAA receptor. Mol Pharmacol. 2007a;71:1582–1590. doi: 10.1124/mol.106.033407. [DOI] [PubMed] [Google Scholar]
- Li P, Covey DF, Steinbach JH, Akk G. Dual potentiating and inhibitory actions of a benz[e]indene neurosteroid analog on recombinant α1β2γ2 GABAA receptors. Mol Pharmacol. 2006;69:2015–2026. doi: 10.1124/mol.106.022590. [DOI] [PubMed] [Google Scholar]
- Li P, Shu HJ, Wang C, Mennerick S, Zorumski CF, Covey DF, et al. Neurosteroid migration to intracellular compartments reduces steroid concentration in the membrane and diminishes GABA-A receptor potentiation. J Physiol. 2007b;584:789–800. doi: 10.1113/jphysiol.2007.142794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maia LF, Epifanio RA, Eve T, Fenical W. New fish feeding deterrents, including a novel sesquiterpenoid heterogorgiolide, from the Brazilian gorgonian Heterogorgia uatumani (octocorallia, gorgonacea) J Nat Prod. 1999;62:1322–1324. doi: 10.1021/np990138z. [DOI] [PubMed] [Google Scholar]
- Mennerick S, He Y, Jiang X, Manion BD, Wang M, Shute A, et al. Selective antagonism of 5α-reduced neurosteroid effects at GABAA receptors. Mol Pharmacol. 2004;65:1191–1197. doi: 10.1124/mol.65.5.1191. [DOI] [PubMed] [Google Scholar]
- Mohamadi F, Richards NGJ, Guida WC, Liskamp R, Lipton M, Caulfield C, et al. Macromodel—an integrated software system for modeling organic and bioorganic molecules using molecular mechanics. J Comput Chem. 1990;11:440. [Google Scholar]
- Pagan OR, Eterovic VA, Garcia M, Vergne D, Basilio CM, Rodriguez AD, et al. Cembranoid and long-chain alkanol sites on the nicotinic acetylcholine receptor and their allosteric interaction. Biochemistry. 2001;40:11121–11130. doi: 10.1021/bi0112255. [DOI] [PubMed] [Google Scholar]
- Pawlik JR. Marine invertebrate chemical defenses. Chem Rev. 1993;93:1911–1922. [Google Scholar]
- Qin F, Auerbach A, Sachs F. Estimating single-channel kinetic parameters from idealized patch-clamp data containing missed events. Biophys J. 1996;70:264–280. doi: 10.1016/S0006-3495(96)79568-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reith CA, Sillar KT. Development and role of GABAA receptor-mediated synaptic potentials during swimming in postembryonic Xenopus laevis tadpoles. J Neurophysiol. 1999;82:3175–3187. doi: 10.1152/jn.1999.82.6.3175. [DOI] [PubMed] [Google Scholar]
- Reyes F, Arda A, Martin R, Fernandez R, Rueda A, Montalvo D, et al. New cytotoxic cembranes from the sea pen Gyrophyllum sibogae. J Nat Prod. 2004;67:1190–1192. doi: 10.1021/np049903m. [DOI] [PubMed] [Google Scholar]
- Rodríguez AD, Piña IC, Acosta AL, Ramírez C, Soto JJ. Synthesis of analogues of Eunicea γ-cembranolides containing cyclic ethers via saponification. J Org Chem. 2001;66:648–658. doi: 10.1021/jo001025j. [DOI] [PubMed] [Google Scholar]
- Sanchez MC, Ortega MJ, Zubia E, Carballo JL. Cembrane diterpenes from the gorgonian Lophogorgia peruana. J Nat Prod. 2006;69:1749–1755. doi: 10.1021/np060388x. [DOI] [PubMed] [Google Scholar]
- Sawant SS, Youssef DT, Reiland J, Ferniz M, Marchetti D, El Sayed KA. Biocatalytic and antimetastatic studies of the marine cembranoids sarcophine and 2-epi-16-deoxysarcophine. J Nat Prod. 2006;69:1010–1013. doi: 10.1021/np050527v. [DOI] [PubMed] [Google Scholar]
- Sorenson EM, Culver P, Chiappinelli VA. Lophotoxin: selective blockade of nicotinic transmission in autonomic ganglia by a coral neurotoxin. Neuroscience. 1987;20:875–884. doi: 10.1016/0306-4522(87)90248-x. [DOI] [PubMed] [Google Scholar]
- Steinbach JH, Akk G. Modulation of GABAA receptor gating by pentobarbital. J Physiol. 2001;537:715–733. doi: 10.1111/j.1469-7793.2001.00715.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueno S, Zorumski C, Bracamontes J, Steinbach JH. Endogenous subunits can cause ambiguities in the pharmacology of exogenous γ-aminobutyric acidA receptors expressed in human embryonic kidney 293 cells. Mol Pharmacol. 1996;50:931–938. [PubMed] [Google Scholar]
- Ulrich H, Akk G, Nery AA, Trujillo C, Rodriguez AD, Eterovic VA.Mode of cembranoid action on embryonic muscle acetylcholine receptor J Neurosci Res 2007(in press) [DOI] [PubMed]
- Weir CJ, Ling AT, Belelli D, Wildsmith JA, Peters JA, Lambert JJ. The interaction of anaesthetic steroids with recombinant glycine and GABAA receptors. Br J Anaesth. 2004;92:704–711. doi: 10.1093/bja/aeh125. [DOI] [PubMed] [Google Scholar]
- Wittmer LL, Hu Y, Kalkbrenner M, Evers AS, Zorumski CF, Covey DF. Enantioselectivity of steroid-induced γ-aminobutyric acidA receptor modulation and anesthesia. Mol Pharmacol. 1996;50:1581–1586. [PubMed] [Google Scholar]