Abstract
Background:
Prostaglandin E2 (PGE2) suppresses, while indomethacin and aspirin enhance, eosinophil production in murine liquid bone-marrow cultures. Because cysteinyl leukotrienes (cys-LTs) enhance human eosinophil colony formation, we investigated whether the effects of indomethacin and aspirin on murine bone-marrow were due to blockade of PGE2 production alone, or involved further promotion of cys-LTs production/signalling.
Experimental approach:
BALB/c liquid bone-marrow cultures were established with IL-5, alone or associated with indomethacin, aspirin, or cys-LTs. The effects of preventing cys-LT production or signalling were assessed.
Key results:
Indomethacin and aspirin counteracted the suppression of eosinophil production by exogenous PGE2. LTD4, LTC4 and LTE4 enhanced IL-5-dependent eosinophil production and further counteracted the effect of exogenous PGE2. The 5-lipoxygenase activating protein (FLAP) inhibitor, MK886, a leukotriene synthesis inhibitor, zileuton, the CysLT1 receptor antagonists, MK571 and montelukast, or inactivation of the LTC4 synthase gene, abolished effects of indomethacin and aspirin. MK886 and zileuton were ineffective but MK571 and montelukast were effective, against LTD4. Indomethacin, aspirin and LTD4 failed to enhance eosinophil production in bone-marrow from CysLT1 receptor-deficient mice. Indomethacin, aspirin and LTD4 no longer counteracted the effects of exogenous PGE2 in the presence of MK571 and montelukast. MK886, MK571 and montelukast had no effect by themselves, or in association with PGE2.
Conclusions and implications:
Dependence on the FLAP/5-lipoxygenase/LTC4 synthase pathway and receptor signalling shows that cyclo-oxygenase inhibitors act here through endogenous cys-LTs. While PGE2 does not act by suppressing cys-LT production, cys-LTs override PGE2 signalling. Eosinophil production is therefore coordinately regulated by both pathways.
Keywords: NSAID, cysteinyl leukotriene, bone marrow, eosinophils, haematopoiesis, COX, 5-lipoxygenase
Introduction
Eosinophilic granulocytes are prominent in allergic inflammatory infiltrates and secrete numerous mediators of allergic inflammation and asthma (Rothenberg and Hogan, 2006). Maintenance of blood and tissue eosinophilia depends on the sustained upregulation of eosinophil production in the bone marrow (Sehmi et al., 2003), induced by allergen exposure as well as by stress hormones, drugs and cytokines (Elsas et al., 2003). The pathways through which these environmental influences are translated into cellular responses remain, however, largely undefined. We have provided evidence that prostaglandin E2 (PGE2) suppresses murine eosinophil production by inducing apoptosis in immature eosinophils (Jones et al., 2004). This effect depends on NO generation by inducible NOS and ultimately on interactions between the death receptor CD95 (Fas) and its ligand (CD154, Fas ligand). Furthermore, both indomethacin and aspirin, two nonsteroidal anti-inflammatory drugs (NSAIDs) which inhibit COX through distinct mechanisms, upregulate eosinophil production (Lintomen et al., 2002). The simplest explanation would be that both NSAIDs suppressed COX activity, thereby decreasing endogenous PGE2 production, with an ultimate decrease in apoptosis-inducing signals. However, there is evidence that the COX and the 5-lipoxygenase pathways interact, leading to an increased generation of cysteinyl leukotrienes (cys-LTs) in some asthmatic subjects exposed to NSAIDs (Szczeklik and Sanak, 2006). Such an interaction suggests other possible mechanisms to account for our observations, as cys-LTs are known to enhance eosinophil colony formation from human bone marrow (Braccioni et al., 2002).
The cys-LTs are central mediators of allergic reactions, where eosinophils are the most important leukocyte population (Boyce, 2007), and have stimulatory effects for various stages of the eosinophil lineage (Saito et al., 2004). Even though LTB4, the other major 5-lipoxygenase derivative released during allergic reactions, has chemoattractant activity for eosinophils, its predominant effects relate to neutrophil migration and activation. To our knowledge, no selective effect of LTB4 on eosinophil generation from human or murine bone marrow has been reported, even though this leukotriene has been described as a chemoattractant for mast cell progenitors (Weller et al., 2005; Boyce, 2007). For these reasons, we have focused on cys-LTs, evaluating whether they might influence eosinophil production in murine bone marrow, as one would expect from the existence of communication and cross-regulation between the COX and 5-lipoxygenase pathways.
To test this hypothesis, we initially assessed whether the effects of indomethacin and aspirin could be solely accounted for by prevention of PGE2 production, without any involvement of the 5-lipoxygenase pathway. Next, we assessed whether their effects would depend on endogenous cys-LTs and, finally, whether PGE2 would suppress this endogenous production of cys-LTs or, alternatively, be suppressed by it.
Methods
Animals and animal procedures
All animal housing and procedures followed the guidelines of and were approved by, the institutional Committee on Ethical Handling of Laboratory Animals (protocol CEUA no. P0107-02). Male and female BALB/c mice, bred at CECAL-FIOCRUZ, Rio de Janeiro (Brazil), were used at 6–8 weeks of age. For specific experiments, mice lacking (a) the cys-LT1 receptor, generated on both the BALB/c and C57BL/6 background (Maekawa et al., 2002), or (b) LTC4 synthase, generated in the BALB/c background (Kanaoka et al., 2001), bred at Brigham and Women's Hospital, Boston, were used. All transgenic animal studies were approved by the Animal Care and Use Committee of the Dana-Farber Cancer Institute (protocol no. 02-122).
Bone marrow cell studies
Bone marrow cells were obtained by flushing the two femurs of BALB/c mice with RPMI-1640 medium containing 1% fetal calf serum. Liquid bone marrow cultures were established as described (Gaspar Elsas et al., 2000b). Briefly, 106 bone marrow cells were seeded in 1 ml of RPMI-1640 medium, with 10% fetal calf serum, in the presence of rmIL-5 (1 ng ml−1), and incubated at 37 °C, 5% CO2/95% air for 7 days, before counting total cells in a haemocytometer and determining the frequency of cells positive for eosinophil peroxidase (EPO) after staining of cytocentrifuge smears by the method of Ten et al. (1989). Eosinophil numbers were calculated from total and differential counts. For the entire series of experiments with BALB/c bone marrow, the yield at day 7 was 10.3(±4.0) × 104 eosinophils per culture (mean±s.d., n=35) established in the presence of interleukin (IL)-5 alone (control cultures). In a much smaller series, C57BL/6 mice presented slightly higher counts of bone marrow cells per femur and eosinophils produced in culture than BALB/c mice. The functional significance of these differences, if any, has not been established. Comparisons between wild-type and knockout mice were made within the same genetic background (C57BL/6 or BALB/c), not between strains. Our experimental conditions were previously shown to allow detection of both enhancing and suppressive effects (Gaspar Elsas et al., 2000a, 2000b). This was always done by adding various drugs, alone or in combination, to cultures already established in the presence of IL-5, at the beginning of the culture, without replenishment (as indicated in the figures by ‘+', followed by specification of the drug). Hence, unless otherwise stated, IL-5 was present all the time in the experimental conditions used throughout the study.
Statistical analysis
Data were analysed with Systat for Windows version 4 from (Systat Inc., Evanston, IL, USA), using factorial analysis of variance with the Tukey (HSD) correction for multiple comparisons of means observed with different treatments.
Reagents
Heat-inactivated fetal calf serum and culture media were from Hyclone (Logan, UT, USA); L-glutamine, penicillin, streptomycin, aspirin, indomethacin and PGE2 were from Sigma (St Louis, MO, USA); recombinant rmIL-5 was from Pharmingen (San Diego, CA, USA); MK886 and MK571 were from Calbiochem (Merck KgaA affiliated, Darmstadt, Germany); LTD4, LTC4, LTE4 and montelukast were from Cayman Chemical Company (Ann Arbor, MI, USA). Purified zileuton was a kind gift from Dr Artur Kummerle (LASSBIO, UFRJ, Rio de Janeiro, Brazil). Optimal concentrations for MK886, MK571, zileuton and montelukast were established on the basis of dose–response studies in preliminary experiments. Optimal doses were effective against the intended agonists (see Results section) but had no effect by themselves.
Results
Blockade of the effects of exogenous PGE2 by NSAIDs
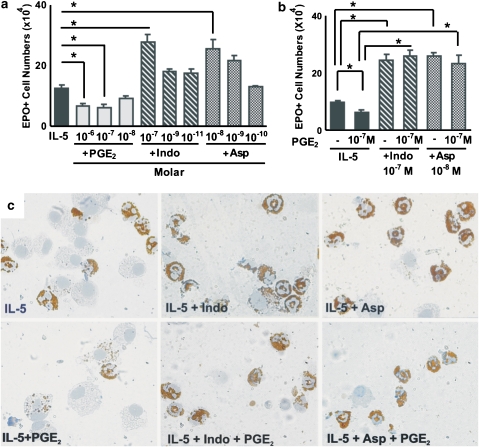
We hypothesized that, if NSAIDs were acting exclusively by preventing the COX-dependent generation of PGE2, which in turn induces apoptosis in developing eosinophils, they would be unable to counteract the suppressive actions of exogenously administered, preformed PGE2. To test this hypothesis, we evaluated the response to IL-5 in the presence of COX inhibitors, PGE2 or both. Figure 1a confirms that the response to IL-5 is suppressed by PGE2 (10−6 and 10−7 M, but not 10−8 M); by contrast, it is enhanced by both indomethacin (10−7 M but not 10−9 or 10−11 M) and aspirin (10−8 M but not 10−9 or 10−10 M). Figure 1b shows that PGE2 (10−7 M) is able to suppress the response to IL-5 only in the absence, but not in the presence, of COX inhibitors. This shows that both COX inhibitors act through a mechanism that cannot be entirely accounted for by the inhibition of PGE2 synthesis, since addition of the end product does not overcome their effects. The photomicrographs shown in the upper row of Figure 1c illustrate representative views of the eosinophils generated in the presence of IL-5 alone (left) or associated with indomethacin (centre) and aspirin (right). In the presence of COX inhibitors, the numbers of mature, well-preserved EPO+ cells are increased and EPO− cells (mostly macrophages) are proportionately decreased. The lower row of Figure 1c shows the eosinophils generated in the presence of IL-5 and PGE2 (left), or of both IL-5 and PGE2 associated with either indomethacin (centre) or aspirin (right). PGE2 drastically reduced the number of intact EPO+ cells, leading to a predominance of macrophages at the end of the culture, some of which have ingested EPO+ cellular debris. By contrast, this effect was totally abolished in the presence of the COX inhibitors. Because these COX inhibitors blocked the actions of preformed PGE2, it was necessary to define a mechanism of action, distinct from inhibition of COX alone, to account for their effects.
Figure 1.
Effect of PGE2 and NSAIDs on eosinophil production. (a and b) The data are mean±s.e.mean of the number of EPO+ cells in liquid cultures established from BALB/c bone marrow, in the presence of IL-5 alone, or associated with the following: PGE2, indomethacin (Indo) or aspirin (Asp) (a), or the indicated combinations of these agents (b). Data are derived from 4 to 5 experiments. *P<0.05; significant differences relative to the indicated controls. (c) Morphological features of EPO+ cells (bearing brown cytoplasmic granules, and donut-, circle- or C-shaped nuclei) generated in the various conditions described above counterstained with Harris' haematoxylin and photographed under immersion (× 1000). Counterstaining was kept to a minimum to allow easier visualization of individual EPO+ debris inside macrophages. EPO, eosinophil peroxidase; IL, interleukin; NSAID, nonsteroidal anti-inflammatory drug; PGE2, prostaglandin E2.
Effect of exogenous cys-LTs on eosinophil differentiation
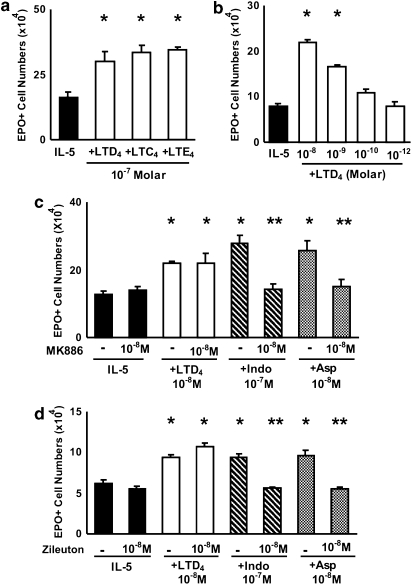
LTD4 has been reported to enhance colony formation by human bone marrow cells (Braccioni et al., 2002). We evaluated the effects of LTD4 and other cys-LTs on murine eosinophil production in our system. Figure 2a shows that LTD4, LTC4 or LTE4, all tested at 10−7 M, strongly enhanced IL-5-dependent eosinophil differentiation in bone marrow liquid cultures, as compared to control cultures with IL-5 alone. As further shown in Figure 2b, the response to IL-5 was increased (P⩽0.01) by LTD4 at 10−8 and 10−9 M, but not 10−10 or 10−12 M); LTD4, at the indicated effective concentrations, did not support eosinophil survival or differentiation by itself. LTD4 was most effective if added at the beginning of the culture, but still had a significant enhancing effect if added up to day 2, although not at later times (data not shown). This shows that cys-LTs can duplicate the enhancing effects of indomethacin and aspirin on eosinophil production at concentrations that occur in vivo. Furthermore, the effect of cys-LTs is to modulate the responses to IL-5, not to substitute for IL-5 as a growth stimulus.
Figure 2.
Effects of cys-LTs and MK886 on murine eosinophil production. The data are mean±s.e.mean of the number of EPO+ cells in liquid cultures established from BALB/c bone marrow, in the presence of IL-5 alone, or associated with the following: LTD4, LTC4 or LTE4 (10−7 M) (a); LTD4 (10−8 to 10−12 M) (b); FLAP inhibitor MK886 (c) and 5-lipoxygenase inhibitor zileuton (d), with or without LTD4, indomethacin or aspirin at the indicated concentrations. Data are derived from 3 to 5 experiments. *P<0.05 relative to IL-5 controls. **P<0.05 relative to positive controls without MK886 or zileuton. cys-LT, cysteinyl-leukotriene; IL, interleukin.
Mediation of the effects of COX inhibitors by endogenous cys-LTs
Because exogenous cys-LTs duplicated the enhancement of eosinophil production observed with COX inhibitors, we next evaluated whether endogenous cys-LTs might mediate this enhancement as well. To test this hypothesis, we initially investigated whether blocking the generation of endogenous cys-LTs would affect the effectiveness of COX inhibitors. Figure 2c shows that MK886, a 5-lipoxygenase activating protein (FLAP) inhibitor, completely inhibited the effects of both indomethacin and aspirin on eosinophilopoiesis in bone marrow culture. By contrast, MK886 had no effect on the response to IL-5 alone, nor on the effectiveness of preformed LTD4. Furthermore, a comparable blockade was achieved with a specific leukotriene biosynthesis inhibitor, zileuton (Figure 2d). Again, zileuton had no effect on the response to IL-5 alone, or on the effectiveness of LTD4. Taken together, these findings indicate that the effects of COX inhibitors are dependent on FLAP and ultimately on leukotriene biosynthesis. Importantly, the lack of effect of MK886 and zileuton by themselves indicates that leukotriene biosynthesis is not required for the response to IL-5 alone, only for its upregulation by NSAIDs.
However, because the effects of these biosynthesis inhibitors would not be restricted to cys-LTs, it is necessary to demonstrate further a specific link between the production of cys-LTs and the effects of COX inhibitors. We therefore evaluated whether specifically targeting production of cys-LTs and their function would affect the effectiveness of COX inhibitors. This was done through both pharmacological (Figure 3) and genetic (Figure 4) approaches.
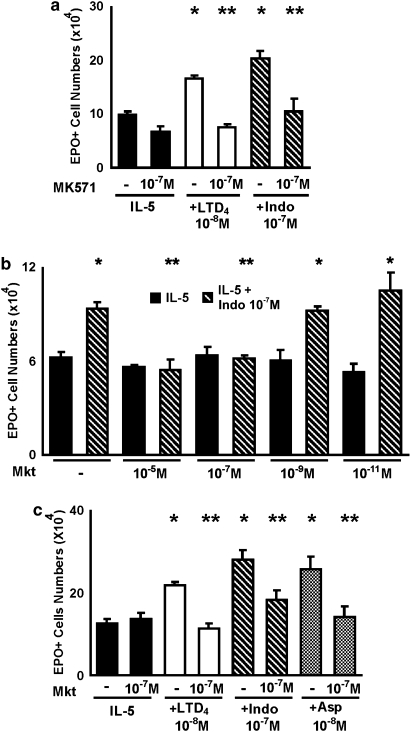
Figure 3.
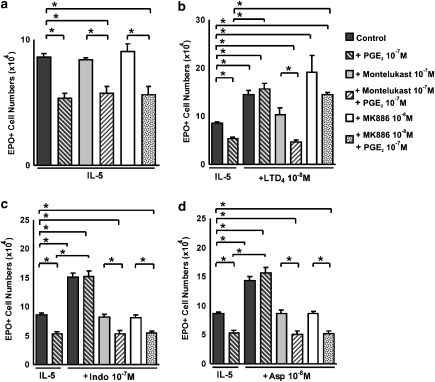
Effects of cys-LT1 receptor antagonists on the responses to indomethacin, aspirin and LTD4. The data are mean±s.e.mean of the number of EPO+ cells in liquid cultures established from BALB/c bone marrow, in the presence of IL-5 alone, or associated with the following: LTD4 (10−8 M), indomethacin (10−7 M) or aspirin (10−8 M), in the absence or in the presence of MK571 (a), 10−7 M, or montelukast (Mkt; b and c), at the indicated concentrations. Data are derived from 3 to 5 experiments. *P<0.05 relative to IL-5 controls. **P<0.05 relative to positive controls without MK571 or Mkt. cys-LT, cysteinyl-leukotriene; EPO, eosinophil peroxidase; IL, interleukin.
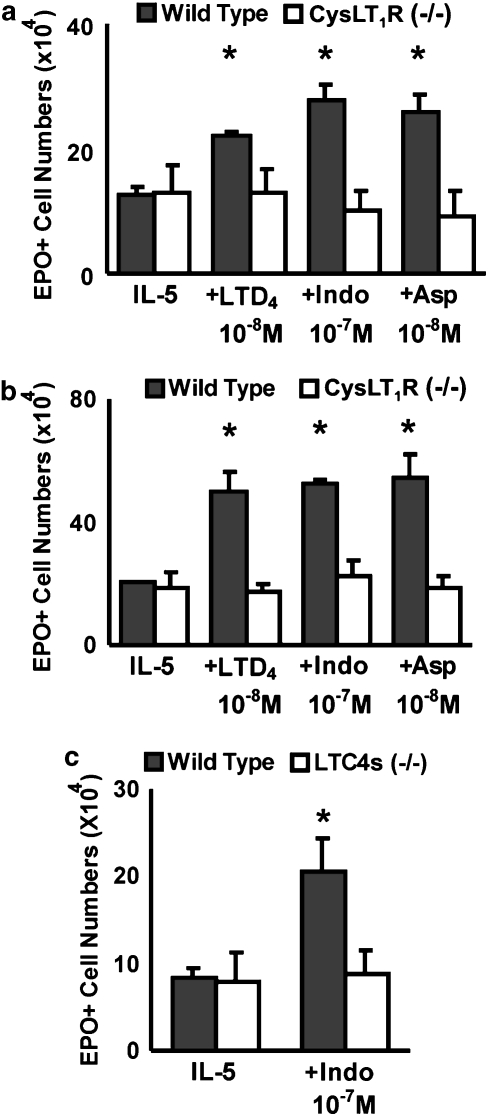
Figure 4.
Inability of indomethacin and aspirin to enhance eosinophil production in bone marrow from cys-LT1 receptor- or LTC4 synthase-deficient mice. The data are mean±s.e.mean of the number of EPO+ cells in liquid cultures established from bone marrow of control (Wild Type) or mutant (cys-LT1R−/− (a and b); LTC4s−/− (c)) mice from the BALB/c (a and c) or the C57BL/6 strains (b), in the presence of IL-5 alone, or associated with the following: LTD4, indomethacin or aspirin, at the indicated molar concentrations (10−7 M in (c)). Data are derived from 3 to 4 experiments. *P<0.01 relative to the IL-5 control. cys-LT, cysteinyl-leukotriene; EPO, eosinophil peroxidase; IL, interleukin.
Figure 3a shows that the selective cys-LT1 receptor antagonist, MK571, inhibits the effects of both LTD4 and indomethacin on eosinophil production in bone marrow culture. In the absence of LTD4 or indomethacin, MK571 had no significant effect. Figure 3b further shows that montelukast, a clinically effective cys-LT1 receptor antagonist, equally antagonized the actions of indomethacin and aspirin. Montelukast blocked, as expected, the effects of LTD4, but had no effect by itself. These results indicate that the upregulation of eosinophilopoiesis observed in the presence of indomethacin, aspirin or LTD4 is dependent on the intact function of the cys-LT1 receptor. Again, blocking the cys-LT1 receptor in the absence of NSAIDs had no effect, showing the dependence of this mechanism on the inhibition of the COX pathway. Taken together, these observations are consistent with the hypothesis that the effects of both NSAIDs are mediated by the production of cys-LTs that takes place in the culture as a result of blocking the COX pathway.
Figure 4 shows that neither indomethacin nor aspirin had any enhancing effect on the response to IL-5 in bone marrow culture from cys-LT1 receptor-deficient mice, regardless of whether the bone marrow came from donors bearing the BALB/c (Figure 4a) or C57BL/6 (Figure 4b) genetic background. As expected, cys-LT1 receptor-deficient mice of both strains were unresponsive to LTD4, confirming that the enhancement of eosinophil production by cys-LTs depends on the cys-LT1 receptor class. By contrast, wild-type controls of the BALB/c (Figure 4a) or C57BL/6 (Figure 4b) strains were fully responsive to indomethacin, aspirin and LTD4, as were CysLT2 receptor deficient mice, which have functional cys-LT1 receptors (data not shown). Further evidence was provided by the inability of LTC4 synthase-deficient mice to respond to indomethacin, unlike the wild-type control mice of the same genetic background (BALB/c) (Figure 4c). These findings demonstrate that the link between NSAID effects and intact production and signalling of cys-LTs can be established on the basis of gene inactivation approaches as well as on the use of specific receptor antagonists.
Finally, we addressed the issue of whether the proposed mechanism could account for the ability of both NSAIDs to counteract the actions of preformed PGE2. To do so, bone marrow cultures were established in the presence of the following: (a) the suppressive agonist, PGE2; (b) the modulators of its activity (namely indomethacin, aspirin or LTD4) and (c) the inhibitors of either the production (MK886) or action (MK571, montelukast) of cys-LTs. Controls included each of these reagents in isolation, as well as their combinations, in the absence of COX inhibitors.
Figure 5a shows that PGE2 suppressed eosinophil production as well in the absence as in the presence, of montelukast or MK886, thereby confirming that these two inhibitors have no effect by themselves, and that the presence of PGE2 does not change this situation.
Figure 5.
The cys-LTs and NSAIDs depend on cys-LT1 receptors to override the PGE2 suppressive signalling. The data are mean±s.e.mean of the number of EPO+ cells in liquid cultures established from BALB/c bone marrow, in the indicated conditions, which include the presence of an agonist (PGE2, 10−7 M), of modulators of its activity (LTD4, indomethacin or aspirin) and of inhibitors of cys-LT-dependent pathways (MK886, montelukast) as well as the appropriate combinations of these substances. Asterisks indicate significant differences between the indicated groups. cys-LT, cysteinyl-leukotriene; NSAID, nonsteroidal anti-inflammatory drug; PGE2, prostaglandin E2.
Figure 5b addresses the ability of LTD4 to modulate eosinophil production by itself, as well as to counteract the suppressive effects of PGE2 as follows: (a) PGE2 suppressed, and LTD4 enhanced, eosinophil production relative to the baseline (IL-5 control); (b) LTD4 could completely override PGE2 signalling; (c) montelukast, as expected, completely blocked both the direct enhancing effect of LTD4 and its ability to counteract the effects of PGE2 and (d) MK886, as expected, failed to prevent either effect of preformed LTD4.
By contrast, both inhibitors were seen to abolish the effectiveness of indomethacin (Figure 5c) and aspirin (Figure 5d), in the absence as well as in the presence of PGE2: (a) PGE2 suppressed, and indomethacin (or aspirin) enhanced eosinophil production; (b) both indomethacin and aspirin were able to override PGE2 signalling; (c) montelukast completely blocked the direct effects of the NSAIDs, as well as their ability to counteract the effects of PGE2, thereby confirming an essential role for cys-LT1 receptors in both experimental conditions and (d) MK886 similarly abolished both the direct effects of NSAIDs and their ability to override signalling by PGE2, confirming that in both experimental conditions the mechanism depends on endogenous leukotriene biosynthesis.
Discussion
The findings reported here concern the pathways through which external signals are translated into opposing cellular responses—namely apoptosis or increased differentiation and survival—in developing murine eosinophils. PGE2 induces apoptosis through a novel pathway, involving induction of NO production through iNOS, followed by activation of the death receptor CD95 by its ligand (Jones et al., 2004). However, most regulators studied so far, increased, rather than suppressed, eosinophil production, (Elsas et al., 2003). For dexamethasone, enhancement was paralleled by acquisition of resistance to the pro-apoptotic actions of PGE2 and downregulation of iNOS (Jones et al., 2004). Hence, it is necessary to further define the relationship between the following: (a) the signalling pathways involved in enhancing eosinophil production and (b) the mechanisms that counteract the actions of PGE2. The enhancing effects of NSAIDs offer a unique experimental probe for dissecting these issues.
Below, we address in sequence the following central issues: (a) why the experimental findings support a role for cys-LTs in regulating eosinophil production; (b) in what specific circumstances this role can be demonstrated, and what it implies for the relationships between the COX and 5-lipoxygenase pathways; (c) why we have followed pharmacological and genetic, rather than biochemical, approaches and (d) the potential implications of these findings.
Regulation by cys-LT
The demonstration that NSAIDs could counteract the effects of preformed PGE2 shows that their mechanism of action does not conform, in this case, to the general mode of action for this class of drugs, namely preventing the generation of COX end-products. Indeed, our data concerning the effects of MK886 suggest that COX inhibition is important, not because it prevents the generation of any specific COX product, but because it enhances generation of 5-lipoxygenase products. Specifically, cys-LTs are responsible, because two distinct antagonists of cys-LT1 receptors (MK571 and montelukast; Bäck, 2002) completely blocked the actions of NSAIDs in the system, and because mice deficient in either LTC4 synthase or in cys-LT1 receptors were insensitive to these agents (Kanaoka and Boyce, 2004). This mechanism is consistent with previous studies suggesting that inhibition of COX by NSAIDs diverts arachidonate into the 5-lipoxygenase pathway, with a resulting generation of cys-LTs (Bertolini et al., 2002; Obase et al., 2005). This variously termed shunting, diversion or COX hypothesis requires the presence, in the system studied, of the key enzymes involved in both pathways, as well as the NSAIDs themselves. COX-1 is expressed in most cells in baseline conditions, and is effectively inhibited by both indomethacin and aspirin; by contrast, COX-2 is induced by cytokines and other inflammatory stimuli, and almost undetectable in baseline conditions (Charlier and Michaux, 2003; Amman and Peskar, 2004). We assume, therefore, that the primary target in our experiments is COX-1. The key enzymes involved in cys-LT synthesis have a more restricted distribution; nevertheless, 5-lipoxygenase is expressed in myeloid cells (Charlier and Michaux, 2003), and both macrophages and eosinophils, which are present in our culture conditions, express LTC4 synthase (Lam, 2003) and secrete LTC4 (Rothenberg and Hogan, 2006). Finally, the response to cys-LTs depends on the cys-LT1 receptor, which is present in eosinophils (Kanaoka and Boyce, 2004).
Conditions and directionality for cys-LT-dependent regulation
Most importantly, MK886, MK571 and montelukast had no effect by themselves, nor in the presence of PGE2. This shows that, when COX is not inhibited, no 5-lipoxygenase- and cys-LT1 receptor-dependent activity can be demonstrated. This clearly establishes a relationship between blockade of COX and functioning of the 5-lipoxygenase pathway. Furthermore, a directionality in the mechanism can be defined: while PGE2 could not prevent functioning of the 5-lipoxygenase pathway and generation of the endogenous cys-LTs that determine the final outcome, cys-LTs, by contrast, could totally block the effects of PGE2. Downregulation of eosinophil production depends therefore on the presence of PGE2, a ubiquitous COX product; by contrast, upregulation through this mechanism depends on several factors, namely (a) a constitutively active arachidonate turnover, (b) an effective blockade of COX and (c) the local expression of 5-lipoxygenase, FLAP and LTC4 synthase (Lam, 2003; Peters-Golden and Brock, 2003; Brock, 2005). Hence, the effects of PGE2 are likely to reflect the primary mechanism, while the effects of NSAIDs may reflect a superimposable control pathway. Accordingly, the PGE2-dependent pathway acts on the target cell itself, via iNOS-CD95 (Jones et al., 2004). By contrast, the NSAID-dependent pathway, which overrides the PGE2-dependent pathway, may require other cells in the environment, as transcellular synthesis is a major factor in production of cys-LTs in tissues (Folco and Murphy, 2006).
Justification and limits of our approach
A study of cultured bone marrow in which the response to NSAIDs or cys-LTs is evaluated by haematological methods is not a physiological system, but enables us to analyse the relationship between external signals and cellular responses, which would be extremely difficult to determine in vivo, as discussed in detail elsewhere (Xavier Elsas and Gaspar Elsas, 2007). Any factors released from cells closely packed in the bottom of the culture well and attached to a variable extent to an adherent stromal layer, would be necessarily be more concentrated in their vicinity than in the culture supernatants. Such factors might be effective where they are produced, even though they might suffer dilution beyond the detection range in culture supernatants. Cultures take place over a week and the exact timing of release of the mediators, which is critical for their detection, is unknown. We consider therefore a biochemical approach less effective than the one we have taken, where disruption of the relevant mechanism is reliably achieved throughout the culture period, and regardless of the concentration of factors anywhere in the culture, or of the rate and duration of their release. The success of this approach is documented by the striking agreement between all of the disruptive strategies taken: the use of MK886, zileuton, MK571 and montelukast, and disruption of cys-LT1 receptor function or of LTC4 synthase activity have all led to an identical blockade of the effects of NSAIDs.
Implications of these findings
The cellular mechanisms described here resemble those which have been proposed to explain the pathogenesis of aspirin-exacerbated respiratory disease. This condition is associated with bronchoconstriction, rhinorrhea, conjunctival irritation and scarlet flush, ocurring within 3 h of ingestion of aspirin or other NSAIDs capable of inhibiting COX-1 (Obase et al., 2005). Prominent eosinophilia is a hallmark of the disease (Kim and Park, 2006; Stevenson and Szczeklik, 2006). Several lines of evidence involve cys-LTs in its pathogenesis, including the elevated levels of cys-LTs in the urine, sputum and bronchoalveolar lavage fluid of these subjects, even in the absence of NSAID provocation, and further increases following administration of NSAIDs, along with a favourable clinical response to zileuton and montelukast (Obase et al., 2005; Stevenson and Szczeklik, 2006). Even though a wide variety of abnormalities were described in affected subjects, the shunting hypothesis defines cys-LTs production in the lungs as the central pathogenetic event (Kim and Park, 2006; Szczeklik and Sanak, 2006). It is clear that our observations do not concern lung tissue from human asthmatic subjects, but bone marrow taken from nonsensitized mice; and therefore our findings do not automatically translate into increased understanding of aspirin-exacerbated respiratory disease. With these qualifications in mind, bone marrow might still provide a useful model for those wishing to explore the biochemical mechanisms of aspirin-exacerbated respiratory disease. An important difference, however, may be that PGE2 downregulates cys-LT production in the lungs of aspirin-exacerbated respiratory disease patients (Szczeklik and Sanak, 2006), contrasting with our observations in murine bone marrow. In our case, caution should be exercised in advancing a shunting hypothesis, because there is evidence that COX and 5-lipoxygenase utilize distinct arachidonate pools (Peters-Golden and Brock, 2000), which would preclude this mechanism. Definitive demonstration of a shunting mechanism would require evidence that in bone marrow of wild-type mice the radiolabelled precursor (that is, arachidonate) is quantitatively converted to cys-LTs as a function of NSAID concentration, through a mechanism that would be missing from bone marrow deficient in 5-lipoxygenase or LTC4 synthase. This is a technically challenging approach which is presently beyond our reach. Nevertheless, no such experiment is required to conclude that the mechanism of action of NSAIDs in murine bone marrow cultured with IL-5 requires both production of cys-LTs and signalling by cys-LT1 receptors. Our findings suggest that in bone marrow, at least, activation of the cys-LTs-generating enzymes does not require external stimuli other than (perhaps) IL-5 (which is indispensable for eosinophil production).
Acknowledgments
This work supported by CNPq (Grant 474979/2004-0, Fellowships to PXE, MIGE and TQ), CAPES (Travel Grant to PXE), and FIOCRUZ.
Abbreviations
- cys-LT
cysteinyl-leukotriene
- NSAID
nonsteroidal anti-inflammatory drug
Conflict of interest
The authors state no conflict of interest.
References
- Amman R, Peskar BA. Anti-inflammatory effects of aspirin and sodium salicylate. Eur J Pharmacol. 2004;447:1–9. doi: 10.1016/s0014-2999(02)01828-9. [DOI] [PubMed] [Google Scholar]
- Bäck M. Functional characteristics of cysteinyl-leukotriene receptor subtypes. Life Sci. 2002;71:611–622. doi: 10.1016/s0024-3205(02)01733-2. [DOI] [PubMed] [Google Scholar]
- Bertolini A, Ottani A, Sandrini M. Selective COX-2 inhibitors and dual acting anti-inflammatory drugs: critical remarks. Curr Med Chem. 2002;9:1033–1043. doi: 10.2174/0929867024606650. [DOI] [PubMed] [Google Scholar]
- Boyce JA. Mast cells and eicosanoid mediators: a system of reciprocal paracrine and autocrine regulation. Immunol Rev. 2007;217:168–185. doi: 10.1111/j.1600-065X.2007.00512.x. [DOI] [PubMed] [Google Scholar]
- Braccioni F, Dorman SC, O'Byrne PM, Inman MD, Denburg JA, Parameswaran K, Baatjes AJ, Foley R, Gauvreau GM. The effect of cysteinyl leukotrienes on growth of eosinophil progenitors from peripheral blood and bone marrow of atopic subjects. J Allergy Clin Immunol. 2002;110:96–101. doi: 10.1067/mai.2002.125000. [DOI] [PubMed] [Google Scholar]
- Brock TG. Regulating leukotriene synthesis: the role of nuclear 5-lipoxygenase. J Cell Biochem. 2005;96:1203–1211. doi: 10.1002/jcb.20662. [DOI] [PubMed] [Google Scholar]
- Charlier C, Michaux C. Dual inhibition of cyclooxygenase-2 (COX-2) and 5-lipoxygenase (5-LOX) as a new strategy to provide safer non-steroidal anti-inflammatory drugs. Eur J Med Chem. 2003;38:645–659. doi: 10.1016/s0223-5234(03)00115-6. [DOI] [PubMed] [Google Scholar]
- Elsas PX, Maximiano ES, Vargaftig BB, Elsas MI. The effects of allergen and anti-allergic drugs on murine hemopoietic cells: moving targets, unusual mechanisms, and changing paradigms. Curr Drug Targets Inflamm Allergy. 2003;2:329–337. doi: 10.2174/1568010033484089. [DOI] [PubMed] [Google Scholar]
- Folco G, Murphy RC. Eicosanoid transcellular biosynthesis: from cell–cell interactions to in vivo tissue responses. Pharmacol Rev. 2006;58:375–388. doi: 10.1124/pr.58.3.8. [DOI] [PubMed] [Google Scholar]
- Gaspar Elsas MI, Joseph D, Lintomen L, Maximiano ES, Bodstein M, Xavier Elsas P, Vargaftig BB. Murine myeloid progenitor responses to GM-CSF and eosinophil precursor responses to IL-5 represent distinct targets for downmodulation by prostaglandin E(2) Br J Pharmacol. 2000a;130:1362–1368. doi: 10.1038/sj.bjp.0703403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaspar Elsas MI, Maximiano ES, Joseph D, Alves L, Topilko A, Vargaftig BB, Xavier Elsas P. Upregulation by glucocorticoids of responses to eosinopoietic cytokines in bone-marrow from normal and allergic mice. Br J Pharmacol. 2000b;129:1543–1552. doi: 10.1038/sj.bjp.0703145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones CP, Paula Neto HA, Assreuy J, Vargaftig BB, Gaspar Elsas MI, Elsas PX. Prostaglandin E2 and dexamethasone regulate eosinophil differentiation and survival through a nitric oxide- and CD95-dependent pathway. Nitric Oxide. 2004;11:184–193. doi: 10.1016/j.niox.2004.08.001. [DOI] [PubMed] [Google Scholar]
- Kanaoka Y, Boyce JA. Cysteinyl leukotrienes and their receptors: cellular distribution and function in immune and inflammatory responses. J Immunol. 2004;173:1503–1510. doi: 10.4049/jimmunol.173.3.1503. [DOI] [PubMed] [Google Scholar]
- Kanaoka Y, Maekawa A, Penrose JF, Austen KF, Lam BK. Attenuated zymosan-induced peritoneal vascular permeability and IgE-dependent passive cutaneous anaphylaxis in mice lacking leukotriene C4 synthase. J Biol Chem. 2001;276:22608–22613. doi: 10.1074/jbc.M103562200. [DOI] [PubMed] [Google Scholar]
- Kim SH, Park HS. Pathogenesis of nonsteroidal antiinflammatory drug-induced asthma. Curr Opin Allergy Clin Immunol. 2006;6:17–22. doi: 10.1097/01.all.0000199794.79551.ec. [DOI] [PubMed] [Google Scholar]
- Lam BK. Leukotriene C(4) synthase. Prostaglandins, Leukotrienes and Essential Fatty Acids. 2003;69:111–116. doi: 10.1016/s0952-3278(03)00071-1. [DOI] [PubMed] [Google Scholar]
- Lintomen L, Gaspar-Elsas MI, Maximiano ES, de Paula Neto HA, Joseph D, Vargaftig BB, Elsas PX. Allergenic sensitization prevents upregulation of haemopoiesis by cyclo-oxygenase inhibitors in mice. Br J Pharmacol. 2002;135:1315–1323. doi: 10.1038/sj.bjp.0704580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maekawa A, Austen KF, Kanaoka Y. Targeted gene disruption reveals the role of cysteinyl leukotriene 1 receptor in the enhanced vascular permeability of mice undergoing acute inflammatory responses. J Biol Chem. 2002;277:20820–20824. doi: 10.1074/jbc.M203163200. [DOI] [PubMed] [Google Scholar]
- Obase Y, Matsuse H, Shimoda T, Haahtela T, Kohno S. Pathogenesis and management of aspirin-intolerant asthma. Treat Respir Med. 2005;4:325–336. doi: 10.2165/00151829-200504050-00004. [DOI] [PubMed] [Google Scholar]
- Peters-Golden M, Brock TG. Intracellular compartmentalization of leukotriene biosynthesis. Am J Respir Crit Care Med. 2000;161:S36–S40. doi: 10.1164/ajrccm.161.supplement_1.ltta-8. [DOI] [PubMed] [Google Scholar]
- Peters-Golden M, Brock TG. 5-lipoxygenase and FLAP. Prostaglandins, Leukotrienes and Essential Fatty Acids. 2003;69:99–109. doi: 10.1016/s0952-3278(03)00070-x. [DOI] [PubMed] [Google Scholar]
- Rothenberg ME, Hogan SP. The eosinophil. Annu Rev Immunol. 2006;24:147–174. doi: 10.1146/annurev.immunol.24.021605.090720. [DOI] [PubMed] [Google Scholar]
- Saito H, Morikawa H, Howie K, Crawford L, Baatjes AJ, Denburg E, Cyr MM, Denburg JA. Effects of a cysteinyl-leukotriene receptor antagonist on eosinophil recruitment in experimental allergic rhinitis. Immunology. 2004;113:246–252. doi: 10.1111/j.1365-2567.2004.01944.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sehmi R, Baatjes AJ, Denburg JA. Hemopoietic progenitor cells and hemopoietic factors: potential targets for treatment of allergic inflammatory diseases. Curr Drug Targets Inflamm Allergy. 2003;2:271–278. doi: 10.2174/1568010033484007. [DOI] [PubMed] [Google Scholar]
- Stevenson DE, Szczeklik A. Clinical and pathologic perspectives on aspirin sensitivity and asthma. J Allergy Clin Immunol. 2006;118:773–786. doi: 10.1016/j.jaci.2006.07.024. [DOI] [PubMed] [Google Scholar]
- Szczeklik A, Sanak M. The broken balance in aspirin hypersensitivity. Eur J Pharmacol. 2006;553:145–155. doi: 10.1016/j.ejphar.2005.12.053. [DOI] [PubMed] [Google Scholar]
- Ten RM, Pease RL, McKean DJ, Bell MP, Gleich GJ. Molecular cloning of the human eosinophil peroxidase. Evidence for the existence of a peroxidase multigene family. J Exp Med. 1989;169:1757–1769. doi: 10.1084/jem.169.5.1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weller CL, Collington SJ, Brown JK, Miller HR, Al-Kashi A, Clark P, Jose PJ, Hartnell A, Williams TJ. Leukotriene B4, an activation product of mast cells, is a chemoattractant for their progenitors. J Exp Med. 2005;201:1961–1971. doi: 10.1084/jem.20042407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xavier Elsas P, Gaspar Elsas MI. Eosinophilopoiesis at the cross-roads of research on development, immunity and drug discovery. Curr Med Chem. 2007;14:1925–1939. doi: 10.2174/092986707781368487. [DOI] [PubMed] [Google Scholar]