Abstract
Background and purpose:
Transgenesis of human paraoxonase 1 (PON1), a HDL-associated enzyme that destroys lipid peroxides, has been reported to reduce early atherogenesis in mice. The present study explored the therapeutic potential of human PON1 gene transfer in old apolipoprotein E-deficient (apoE−/−) mice with advanced atherosclerosis.
Experimental approach:
ApoE−/− mice (18 months, regular chow) were transfected with PON1 adenovirus (AdPON1, n=10) or control adenovirus (AdRR5, n=10). Non-transfected apoE−/− (n=9) and C57Bl/6J (WT, n=6) mice served as controls. Three weeks later, plaque size and composition, and endothelial cell (EC) and smooth muscle cell (SMC) function were assessed in the aorta.
Key results:
PON1 gene transfer raised total PON1 serum activity 13-15 fold during the 3-week study period, without affecting hypercholesterolaemia or lesion size. However, PON1 decreased the oxLDL content of the plaque. Plaque-free thoracic aorta rings from apoE−/− mice displayed, like rings from WT mice, complete relaxation to acetylcholine (ACh, 86±2%), ATP (90±2%) or UTP (83±3%). In contrast, in plaque-bearing segments amplitude (55±7%, 68±8%, 52±8% respectively) and sensitivity were decreased. EC function was completely (ATP, UTP) or largely (ACh) restored by AdPON1. Furthermore, apoE−/− SMCs released less intracellular calcium than WT upon sarco-endoplasmic reticulum calcium ATPase (SERCA) inhibition by cyclopiazonic acid. This defect was also restored by AdPON1 transfection.
Conclusions and implications:
These data indicate that AdPON1 gene transfer improved vascular wall oxidative stress, EC function, and SMC Ca2+ homeostasis in segments with pre-existing atherosclerosis, independently of an effect on plaque size.
Keywords: atherosclerosis, paraoxonase 1, oxidative stress, endothelial dysfunction, vascular smooth muscle cells, apoE
Introduction
During atherogenesis, oxidative stress modifies low-density lipoprotein (LDL) particles into minimally modified LDL and oxidized LDL (oxLDL). Both minimally modified LDL and oxLDL trigger expression of adhesion molecules on endothelial cells (ECs) and induce smooth muscle cell (SMC) migration and proliferation (Mertens and Holvoet, 2001). Epidemiological studies revealed that high-density lipoprotein (HDL) and its major structural protein apolipoprotein AI protect against atherosclerosis. HDL exerts its beneficial effects on plaque development partially through antagonism of oxLDL (Mertens and Holvoet, 2001).
Oxidative stress also plays a key role in endothelial dysfunction associated with cardiovascular disease, as superoxide inactivates nitric oxide (NO) and thereby interferes with the vasodilatation capacity of blood vessels. Moreover, this endothelial dysfunction is predictive of cardiovascular risk, and probably plays a key role in the pathology of atherosclerosis (Bonetti et al., 2003), and—in contrast to structural changes—is rapidly and profoundly improved by anti-atherogenic interventions (Heistad, 2006). Transgenesis of human apolipoprotein AI has been shown to improve the impaired endothelium-dependent relaxation of atherosclerotic aorta rings of apolipoprotein E-deficient (apoE−/−) mice (Deckert et al., 1999; Crauwels et al., 2003). The latter benefit was, however, secondary to the profound plaque reduction (Crauwels et al., 2003), since endothelial dysfunction is only apparent in segments with lesions, despite the hypercholesterolaemia (Deckert et al., 1999).
The atheroprotective properties of HDL are partly attributed to paraoxonase 1 (PON1). PON1 is an HDL-associated enzyme, which is known to prevent oxidation of LDL in vitro (Mackness et al., 1993) and in vivo (Aviram et al., 2000; Mackness et al., 2002) and is considered central to the antioxidative properties of HDL. Overexpression of human PON1 has been shown to reduce plaque progression in apoE−/− mice (Tward et al., 2002) and in mice with a combined leptin and LDL receptor deficiency (Mackness et al., 2006).
While the beneficial effects of PON1 transgenesis on early atherosclerosis are well documented, the therapeutic potential of PON1 in advanced atherosclerosis is not known. Therefore, we investigated the effects of PON1 gene transfer on plaque size and composition in 18 months old apoE−/− mice with pre-existing atherosclerosis. Since vasomotor function is a more sensitive marker of improvement than structural changes (Heistad, 2006), we assessed the activity of ECs in aorta segments with plaques and in adjacent rings without lesions. Finally, we studied SMC Ca2+ levels, since it has been shown that the sarco-endoplasmic reticulum Ca2+-ATPase (SERCA) activity decreases markedly in hypercholesterolaemic rabbit aorta SMCs (Adachi et al., 2001).
Methods
Animals
The studies were approved by the Ethical Committees of the Universities of Antwerp and Leuven, and conformed to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health. C57BL/6J mice (wild type (WT)) and apoE−/− mice, which had been back-crossed in the C57BL/6J background for more than 10 generations, were kept on a regular chow diet and given tap water ad libitum throughout the study. Plaque formation and vascular responses were studied in 18 months old apoE−/− and WT mice.
Adenovirus-mediated gene transfer of human paraoxonase 1
Human PON1 cDNA containing the Q/M polymorphism was subcloned in the shuttle plasmid pShuttle-CMV (Stratagene, La Jolla, CA, USA) downstream of the cytomegalovirus promoter. PON1 recombinant adenovirus (AdPON1) was generated as described (Mackness et al., 2006) using the AdEasy adenoviral vector system (Stratagene). The control recombinant adenovirus AdRR5 has been described elsewhere (Alcorn et al., 1993). A total of 5 × 108 plaque-forming units of AdPON1 or AdRR5 were injected into the tail vein of two groups (n=10) of apoE−/− mice. Another group of apoE−/− (n=9) and WT (n=6) mice did not receive virus particles and served as controls.
Blood parameters
Blood was collected before and 7 and 21 days after transfection (the end of the experiment). Serum PON1 activity was measured as described (Mackness et al., 1998) and expressed in nmol ml−1 min−1. The titres of Ig autoantibodies against malondialdehyde (MDA)-modified LDL were determined in individual plasma samples (1:500 dilutions). The amount of Ig bound to the MDA–LDL antigen was detected with alkaline phosphatases-labelled anti-mouse IgG, and data are expressed as relative absorbance units (Mertens et al., 2003; Mackness et al., 2006). Plasma total cholesterol and triglycerides were measured using standard enzymatic colorimetric assays (Boehringer Mannheim, Roche Diagnostics, Vilvoorde, Belgium).
Isolation of the aorta
Three weeks after gene transfer, the mice were anaesthetized with sodium pentobarbital (Nembutal, 75 mg kg−1, intraperitoneally) and the aorta was carefully removed and cleaned of adherent tissue. The thoracic aorta was systematically divided into five 2 mm wide rings (TA1–5) (Crauwels et al., 2003; Guns et al., 2005). The first four rings of the thoracic aorta (TA1, -2, -3 and -4) were used for measuring EC-dependent relaxations. ECs of TA5 were removed by perfusion with 3 ml of 0.01% Triton X-100 in Krebs–Ringer solution. TA5 was used to measure isometric force development and intracellular calcium ([Ca2+]i) simultaneously (Van Assche et al., 2007).
Relaxation studies
Rings were mounted between two parallel tungsten wire hooks in 10 ml organ baths with a Krebs–Ringer solution (37 °C and continuously aerated with a 95% O2, 5% CO2 gas mixture, pH 7.4) containing (mM) NaCl 118, KCl 4.7, CaCl2 2.5, KH2PO4 1.2, MgSO4 1.2, NaHCO3 25, CaEDTA 0.025 and glucose 11.1. Tension was measured isometrically with a force transducer (Statham UC2, Gould, Cleveland, OH, USA) connected to a data acquisition system (Moise 3, EMKA Technologies, Paris, France). Rings were gradually stretched until a stable loading tension of 20 mN to bring the segments to their optimal length–tension relationship. Indomethacin (10 μM) was always present to avoid vasomotor interferences due to contractile prostanoids (Crauwels et al., 2003; Guns et al., 2005). Rings were first contracted with 50 mM KCl. After three washing steps, a cumulative concentration–response curve was made for phenylephrine (3 nM–30 μM) and the EC50 was assessed for each vessel segment. Thereafter, vessels were contracted with their individual EC50 of phenylephrine followed by cumulative concentration–response curves for ACh (all segments), ATP and UTP (TA1, TA2), and diethylamine NONOate (TA3, TA4).
Smooth muscle cell [Ca2+]i and isometric tension recording
Owing to strong autofluorescence, plaque-bearing segments could not be used for calcium measurements. Plaque-free rings of the distal thoracic aorta (TA5) were mounted in a wire (40 μm) myograph (Danish Myotechnology A/S, Aarhus, Denmark), set to their normalized diameter according to Mulvany and Halpern (1977) and incubated for 2.5 h in Krebs–Ringer with 0.02% pluronic F-127 and 10 μM Fura2-AM (Van Assche et al., 2007). Thereafter, the tissue was rinsed with Krebs–Ringer for 0.5 h at 5 ml min−1. Excitation wavelengths (340 and 380 nm) were delivered at 1 Hz with a DeltaRam Multiwavelength Illuminator (Photon Technology International (PTI), Birmingham, NJ, USA). Emission (dichroic mirror, emission filter XF2031, XF3007, Omega Optical Inc., Laser Components GmbH, Olching, Germany) was measured with a microscope photometer (D-104, PTI). The emission at both excitation wavelengths was analysed with Felix software (PTI). Before loading with Fura2-AM, the mean background emission values for excitation at 340 and 380 nm were determined over a period of 60 s. These values were real-time subtracted from the emission values during the experiment. Force was reported in mN mm−1, and the emission ratio 340/380 nm was used as a relative measure of free [Ca2+]i. Absolute levels of [Ca2+]i are not reported due to the uncertainty of the conventional calibration method in intact tissues (Lalli et al., 1999; Van Assche et al., 2007).
TA5 was stimulated with 50 mM KCl, 10 μM phenylephrine and finally 10 μM cyclopiazonic acid (CPA), a blocker of SERCA. The segment was thoroughly washed between each stimulus.
Real-time reverse transcription-PCR
RNA was isolated from aorta arches using a Microprep kit (Stratagene). The mRNA expression was evaluated using the Two-step RT qPCR core kit (Eurogentec, Seraing, Belgium) on an ABIPrism 7300 (Applied Biosystems, Lennik, Belgium; 40 cycles consisting of 15 s at 95 °C and 1 min at 60 °C). The expression of P2Y2 receptors, P2Y6 receptors, caveolin-1, endothelial nitric oxide synthase (eNOS), p47phox and superoxide dismutase (SOD) 1 and SOD3 was expressed relative to β-actin. Primers and probes for all genes were obtained from assays on demand from Applied Biosystems.
In pilot experiments, we first determined the stability of potential reference genes by measuring mRNA of β-actin, hypoxanthine guanine phosphoribosyl transferase, succinate dehydrogenase complex, subunit A and YWHAZ (tyrosine 3-monooxygenase/trytophan 5-monooxygenase activation protein, zeta polypeptide) in segments with atherosclerosis (aortic arch) and without lesions (central thoracic aorta) from five 18 months old apoE−/− mice. Geometric averaging of the threshold cycles (CT) with GeNorm (Vandesompele et al., 2002) demonstrated that β-actin and succinate dehydrogenase complex, subunit A were the most stable control genes (M-value 0.269) in murine arteries with or without atherosclerosis, YWHAZ was less stable (M-value 0.379) and hypoxanthine guanine phosphoribosyl transferase was not suitable (M-value 0.664). For practical reasons, β-actin was selected as reference gene, since it is more frequently used in the literature than succinate dehydrogenase complex, subunit A.
Assessment of lesion size and plaque composition
At the end of the functional study, each segment was placed in paraformaldehyde (4%, 24 h), embedded in optimum compound temperature (OCT) and kept at −80 °C. Transverse sections (6 μm) were stained with haematoxylin and eosin. The cross-sectional area of the intima was measured using a computer-assisted image analysis system (Image-Pro Plus, EpixVISION). Additionally, composition and size of the plaques were determined in the aortic root at the level of the aortic valves, since immunohistochemistry was not feasible in thoracic aorta rings that had been studied in organ baths. Therefore, hearts were fixed 24 h in 4% paraformaldehyde and embedded in OCT. Sections were stained with oil-red O, with a rat anti-mouse Mac-3 monoclonal antibody (mAb, Pharmingen, San Diego, CA, USA) and with mAb4E6 to visualize oxLDL (Verreth et al., 2004). The blinded analysis of positive immunostaining was also performed with Image-pro Plus. An intensity threshold mask for positive colour was defined by sampling, and the same threshold was applied to all specimens. Results are expressed as per cent positive area oil-red O, Mac-3 and oxLDL in the intima.
Statistical analysis
All results are expressed as mean±s.e.mean; n represents the number of mice, S refers to the number of segments. Area under the curve was analysed using Graphpad software (San Diego, CA, USA). Concentration–response curves were fitted with a sigmoid function to determine Emax and pD2 (−log EC50) for each segment. Differences among groups were evaluated using the non-parametric (Kruskal–Wallis) test (plaque size, the only variable without normal distribution) or parametric ANOVA and Bonferroni post hoc tests (SPSS release 12, SPSS Inc., Chicago, IL, USA). A 5% level of significance was selected.
Chemicals
Sodium pentobarbital (Nembutal) was obtained from Sanofi (Brussels, Belgium), indomethacin from Federa (Brussels, Belgium), OCT from Klinipath (Duiven, The Netherlands) and ACh from Sterop (Brussels, Belgium). Phenylephrine hydrochloride, ATP, UTP and CPA were purchased from Sigma (Bornem, Belgium).
Results
Weight and blood parameters
The body weight and triglycerides levels (P=0.062) were comparable between all groups (Table 1). Plasma cholesterol was increased in apoE−/− mice, but was not affected by gene transfer of AdRR5 or AdPON1 (Table 1).
Table 1.
Characteristics of WT mice, untreated apoE−/− mice and apoE−/− mice transfected with AdRR5 or AdPON1
| WT | apoE−/− | AdRR5 | AdPON1 | |
|---|---|---|---|---|
| n=6 | n=9 | n=10 | n=10 | |
| Body weight (g) | 32.3±0.6 | 28.6±1.3 | 28.4±1.6 | 31.8±1.7 |
| Cholesterol (g l−1) | 1.07±0.17 | 3.48±0.22* | 3.44±0.31* | 4.17±0.33* |
| Triglycerides (g l−1) | 0.77±0.08 | 1.35±0.20 | 1.13±0.14 | 1.45±0.21 |
Abbreviations: AdPON1, paraoxonase 1 recombinant adenovirus; AdRR5, control recombinant adenovirus; apoE−/−, apolipoprotein E-deficient (mice).
Results are expressed as mean±s.e.mean, n=number of mice; *P<0.05, versus WT.
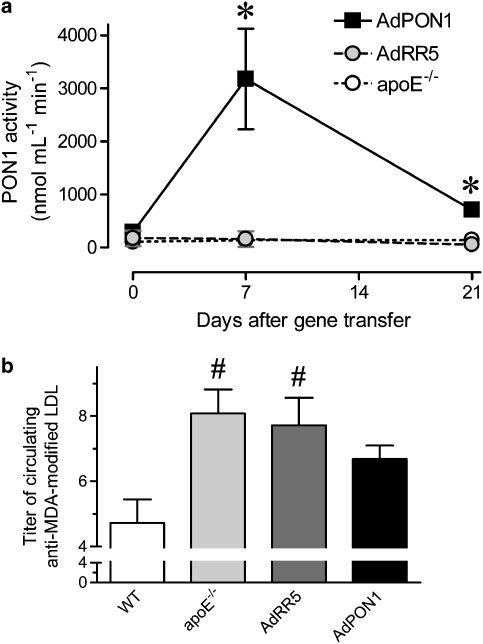
Seven days after AdPON1 gene transfer, total serum PON1 activity was 10-fold higher in comparison with day 0 (Figure 1). At 21 days, PON1 activity was 2.5-fold higher. Moreover, the area under the curve of PON1 activity during 3 weeks was 13- to 15-fold higher in AdPON1 than in untreated or AdRR5 apoE−/− mice (Figure 1a).
Figure 1.
Total PON1 activities in serum of untreated, AdRR5-treaed and AdPON1-treated apoE−/− mice before (day 0) and 7 and 21 days after transfection (a). Titres of circulating autoantibodies against MDA-modified LDL in WT mice, untreated apoE−/−mice, and apoE−/−mice treated with AdRR5 or AdPON1 21 days after gene transfer (b). Results are expressed as mean±s.e.mean. #P<0.05 versus WT; *P<0.05 AdPON1 versus apoE−/− and AdRR5.
At the start of the experiment, the titres of circulating autoantibodies against MDA-modified LDL in the three groups were the same: untreated apoE−/− (7.4±0.9, n=9), AdRR5 (7.1±1.0, n=10) and AdPON1 mice (8.5±1.2, n=10). In the following 3 weeks, the titres did not change in apoE−/− (mean difference 0.6±1.5, n=9) or AdRR5 mice (mean difference 0.6±1.0, n=10), but tended to decrease in AdPON1 mice (−21%, mean difference −1.8±1.2, n=10). In comparison to WT mice, plasma of untreated and RR5-treated apoE−/−mice contained elevated titres of autoantibodies against MDA-modified LDL on day 21, whereas in the AdPON1 group they were not different from the levels in WT mice (Figure 1b).
Plaque size and composition
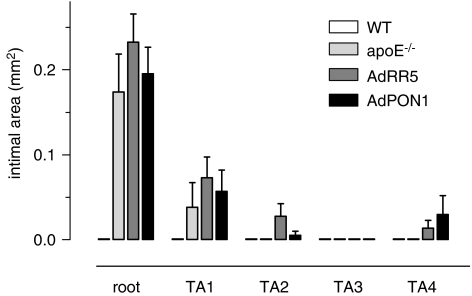
Wild-type mice were free of atherosclerosis. In the apoE−/− strain, plaques were found in the aortic root and to a lesser extent in the proximal thoracic aorta (Figure 2). Central thoracic aorta segments (TA2–4) contained few and small lesions. Transfer of AdRR5 or AdPON1 did not affect plaque size or distribution along the aorta (Figure 2). Furthermore, plaque size and relative lipid (oil-red O) and macrophage (Mac-3) content of lesions at the aortic valves were comparable for AdPON1, AdRR5 and untreated apoE−/− groups (Table 2). However, at the latter site, the immunoreactive area of oxLDL was decreased in AdPON1-treated mice compared to AdRR5-treated or untreated apoE−/− mice.
Figure 2.
Distribution and cross-sectional size of atherosclerotic plaques along the mouse aorta. The apoE−/− strain predominantly developed atherosclerotic plaques in the aortic root and in the most proximal part (TA1) of the thoracic aorta. Short-term PON1 gene transfer did not affect plaque size or distribution in comparison to untreated or AdRR5-treated apoE−/− mice. Aorta segments of WT mice were all plaque-free. Results are expressed as mean±s.e.mean.
Table 2.
Cross-sectional area and composition of plaques at aortic valves in untreated apoE−/−, and in apoE−/− mice transfected with AdRR5 or AdPON1
| apoE−/− | AdRR5 | AdPON1 | |
|---|---|---|---|
| n=9 | n=9 | n=10 | |
| Area (mm2) | 0.23±0.03 | 0.31±0.02 | 0.30±0.01 |
| Oil-red O (%) | 5.9±0.8 | 8.6±1.1 | 8.1±1.4 |
| Mac-3 (%) | 3.7±1.7 | 3.3±1.3 | 3.7±1.0 |
| oxLDL (%) | 43.3±2.0 | 48.5±2.7 | 37.9±1.9* |
Abbreviations: AdPON1, paraoxonase 1 recombinant adenovirus; AdRR5, control recombinant adenovirus; apoE−/−, apolipoprotein E-deficient (mice); oxLDL, oxidized low-density lipoprotein.
Results are expressed as mean±s.e.mean, n=number of mice; *P<0.05, versus apoE−/− and AdRR5.
There were no differences between untreated and AdRR5-treated mice for systemic parameters (Table 1 and Figure 1) or for markers of atherosclerosis (Table 2 and Figure 2). Therefore, both groups were combined for further analysis and referred to as control apoE−/− mice.
Relaxation studies
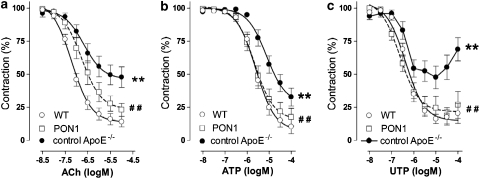
Since the presence of plaques has a dominant effect on vasomotor responses in apoE−/− mice (Crauwels et al., 2003), segments with and without lesions were analysed separately. The plaque-load of atherosclerotic segments was identical in control apoE−/− (mean intimal area 0.10±0.02 mm2, S=14) and AdPON1 mice (mean intimal area 0.10±0.03 mm2, S=9). In the plaque-bearing segments, the endothelium-dependent agonists (ACh, ATP and UTP) all evoked impaired relaxations in control apoE−/− mice, compared to WT mice, as indicated by a decreased sensitivity or Emax (Figure 3 and Table 3). AdPON1 gene transfer restored the endothelium-dependent relaxation almost completely (ATP, UTP) or partially (ACh) by raising amplitude (ACh, UTP) or normalizing the pD2 value (ATP). Moreover, the EC50 of ACh showed a linear association with the plasma anti-MDA titres on day 21 (R=0.451, P=0.031, S=23).
Figure 3.
Relaxation induced by ACh (a), ATP (b) and UTP (c) in phenylephrine-constricted segments of the thoracic aorta of WT mice (S=11–15) and in plaque-bearing segments of control apoE−/− (S=12–14; pooled data from untreated and RR5-treated apoE−/− mice) and AdPON1 mice (S=7–9, PON1). Aorta rings of control apoE−/− mice displayed impaired endothelial-dependent relaxations, which were restored by AdPON1 treatment. Results are expressed as mean±s.e.mean, S is number of segments, **P<0.01 control apoE−/− versus WT mice, ##P<0.01 AdPON1 versus control apoE−/− mice.
Table 3.
Sensitivity (pD2) and maximum response (Emax) of vasoactive agonists in segments with atherosclerotic plaques from control apoE−/− (untreated and RR5-treated apoE−/− mice combined) and AdPON1 mice in comparison with plaque-free segments from WT mice
| Parameter |
WT |
Control apoE−/− |
AdPON1 |
|||
|---|---|---|---|---|---|---|
| Mean±s.e.mean | S | Mean±s.e.mean | S | Mean±s.e.mean | S | |
| pD2 | ||||||
| ACh (−log EC50) | 7.16±0.08 | 22 | 6.53±0.15* | 14 | 6.96±0.13 | 9 |
| ATP (−log EC50) | 5.63±0.09 | 11 | 5.17±0.10** | 12 | 5.65±0.11†† | 7 |
| UTP (−log EC50) | 6.27±0.09 | 11 | 6.35±0.13 | 12 | 6.67±0.06* | 7 |
| DEANO (−log EC50) | 7.74±0.23 | 11 | 7.02±0.49 | 2 | 7.82±0.07 | 2 |
| Emax | ||||||
| ACh (%) | 86±4 | 22 | 55±7** | 14 | 77±6†† | 9 |
| ATP (%) | 89±5 | 11 | 69±7** | 12 | 82±6 | 7 |
| UTP (%) | 84±4 | 11 | 52±8** | 12 | 82±6†† | 7 |
| DEANO (%) | 95±2 | 11 | 96±1 | 2 | 95±2 | 2 |
Abbreviations: AdPON1, paraoxonase 1 recombinant adenovirus; apoE−/−, apolipoprotein E-deficient (mice); DEANO, diethylamine NONOate; WT, wild type.
S=number of segments; *P<0.05, **P<0.01 versus WT; ††P<0.01 versus control apoE−/−.
Furthermore, plaque-bearing segments from control apoE−/− mice tended to be less sensitive to diethylamine NONOate in comparison to segments from WT (P=0.053, Table 3); the sensitivity normalized after AdPON1 gene transfer.
In segments without plaques, responses to endothelium-dependent vasodilators (ACh, ATP and UTP) or the NO donor diethylamine NONOate were not different between WT and control apoE−/− mice with respect to sensitivity or amplitude (Table 4). AdPON1 gene transfer did not affect responses to ACh or ATP, while slightly increasing sensitivity to UTP in plaque-free segments from apoE−/− mice.
Table 4.
Sensitivity (pD2) and maximum response (Emax) of vasoactive agonists in segments without atherosclerotic plaques from WT, control apoE−/− (untreated and RR5-treated apoE−/− mice combined) and AdPON1 mice
| Parameter |
WT |
Control apoE−/− |
AdPON1 |
|||
|---|---|---|---|---|---|---|
| Mean±s.e.mean | S | Mean±s.e.mean | S | Mean±s.e.mean | S | |
| pD2 | ||||||
| ACh (−log EC50) | 7.16±0.08 | 22 | 7.15±0.04 | 52 | 7.19±0.10 | 31 |
| ATP (−log EC50) | 5.63±0.09 | 11 | 5.61±0.05 | 24 | 5.84±0.08 | 13 |
| UTP (−log EC50) | 6.27±0.09 | 11 | 6.40±0.05 | 24 | 6.67±0.06* | 13 |
| DEANO (−log EC50) | 7.74±0.23 | 11 | 7.77±0.06 | 27 | 7.43±0.10 | 18 |
| Emax | ||||||
| ACh (%) | 86±4 | 22 | 86±2 | 52 | 88±2 | 31 |
| ATP (%) | 89±5 | 11 | 90±2 | 24 | 93±1 | 13 |
| UTP (%) | 84±4 | 11 | 83±3 | 24 | 91±2 | 13 |
| DEANO (%) | 95±2 | 11 | 98±0.5 | 27 | 98±0.4 | 18 |
Abbreviations: AdPON1, paraoxonase 1 recombinant adenovirus; apoE−/−, apolipoprotein E-deficient (mice); DEANO, diethylamine NONOate; WT, wild type.
S=number of segments; *P<0.05 versus WT.
Smooth muscle cell function
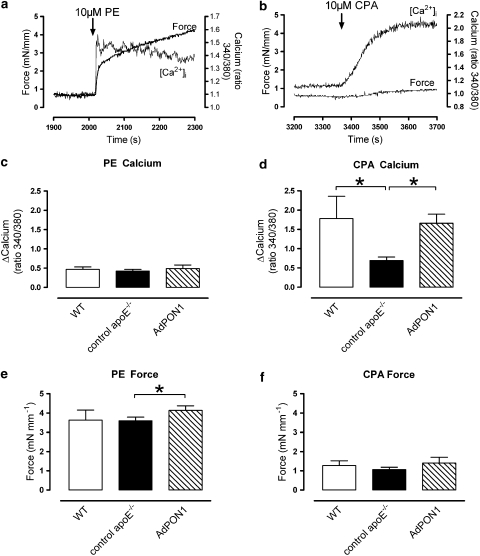
Smooth muscle cell basal [Ca2+]i levels were lower in control apoE−/− mice than in WT mice (ratio 340/380 nm: 0.97±0.03 and 1.13±0.07, respectively, P=0.028), whereas AdPON1 mice (ratio 340/380 nm: 1.03±0.06) displayed intermediate basal [Ca2+]i levels. Phenylephrine evoked biphasic responses for [Ca2+]i and force with a fast rise of [Ca2+]i and contraction followed by a slow decrease of [Ca2+]i and increase of force (Figure 4). In these de-endothelialized segments, phenylephrine caused comparable contractions in WT and control apoE−/− mice, but AdPON1 segments developed slightly, but significantly more force than those from control apoE−/− mice. The increase of [Ca2+]i induced by phenylephrine was not different between the different groups (Figure 4). Analogous findings were observed for [Ca2+]i increase and force development elicited by 50 mM KCl (data not shown).
Figure 4.
Representative examples of simultaneous measurement of force and [Ca2+]i in endothelium-denuded aortic rings during stimulation with phenylephrine (10 μM, PE) (a) or CPA (10 μM) (b). The amplitude of the phenylephrine-induced Ca2+ release was not changed in control apoE−/− (c). The mean amplitude of the CPA-induced Ca2+ release was, however, attenuated in control apoE−/− mice and largely restored by AdPON1 gene transfer (d). Force development after phenylephrine administration was slightly elevated by AdPON1 (e). Finally, CPA-induced contractions were statistically not different between groups (f). *P<0.05, results expressed as mean±s.e.mean.
The SERCA inhibitor CPA evoked a monophasic increase in [Ca2+]i, which was on average threefold higher than that evoked by KCl or phenylephrine (Figure 4). Control apoE−/− mice showed significantly less [Ca2+]i release after stimulation with CPA than WT. The CPA-sensitive [Ca2+]i release was largely restored in AdPON1 mice. The CPA-induced contractions were small and comparable for all groups.
Gene expression
To further unravel the mechanisms underlying the improved EC and SMC function, we evaluated the effect of AdPON1 on the mRNA expression of genes involved in NO production and in the regulation of oxidative stress. AdPON1 gene transfer did not affect the expression of P2Y2 or P2Y6 receptors, eNOS, caveolin-1, the p47phox subunit of NAD(P)H oxidase, SOD1 or SOD3 (Table 5).
Table 5.
Gene expression in the aortic arch of control apoE−/− (untreated and RR5-treated apoE−/− mice combined) and AdPON1 mice
| ΔCT | Control apoE−/− | AdPON1 |
|---|---|---|
| Mean±s.e.mean | Mean±s.e.mean | |
| n=5 | n=6 | |
| P2Y2 | 9.34±0.40 | 9.79±0.31 |
| P2Y6 | 6.87±0.16 | 6.76±0.11 |
| eNOS | 7.33±0.46 | 6.97±0.18 |
| Caveolin-1 | 2.66±0.31 | 3.12±0.32 |
| SOD1 | 2.57±0.25 | 3.02±0.21 |
| SOD3 | 2.60±0.32 | 3.21±0.18 |
| p47phox | 7.00±0.22 | 7.42±0.17 |
Abbreviations: AdPON1, paraoxonase 1 recombinant adenovirus; apoE−/−, apolipoprotein E-deficient (mice); eNOS, endothelial nitric oxide synthase; SOD1, superoxide dismutase 1; SOD3, superoxide dismutase 3.
ΔCT=CT of gene for interest−CT for β-actin (CT=threshold cycle); n=number of mice. Gene expression did not differ between control apoE−/− and AdPON1 mice.
Discussion and conclusion
Previously, human PON1 transgenesis has been found to reduce plaque formation in young apoE−/− mice (Tward et al., 2002). The effect of PON1 gene transfer on preformed lesions was, however, not known. The present study was aimed at exploring the therapeutic potential of PON1 gene transfer.
Transfection efficiency
Gene transfer with AdPON1 resulted in a 13- to 15-fold increase in PON1 activity for 3 weeks (area under the curve). Furthermore, the titres of autoantibodies against MDA-modified LDL, which were used as a proxy for circulating oxLDL in mice (Tsimikas et al., 2001; Mertens et al., 2003), since the assay to measure oxLDL directly in blood is based on a mouse monoclonal antibody, were higher in control apoE−/− compared to WT mice. This indicates that in control apoE−/− the systemic oxidative stress was the highest on the day of the vasomotor studies. At that time, the anti-MDA-LDL titres showed a tendency to decline 21% in the AdPON1 group. This value was equivalent to the 23% reduction previously seen in mice 3 weeks after AdPON1 administration (Mackness et al., 2006). It is expected that the actual drop of circulating oxLDL peaks earlier due to the delay of the immune response, and later time points are required to see a more prominent decline of the anti-MDA-LDL titres (Mackness et al., 2006).
Plaque size and composition
Short-term PON1 gene transfer did not interfere with the distribution and cross-sectional area of the lesions along the aorta. Also lipid load or macrophage density remained unaltered after AdPON1 transfection. A reduction of the physical dimensions of the plaques was not expected in view of the short intervention period and the unaffected hypercholesterolaemia. Even prolonged, aggressive lipid lowering is often insufficient to reduce the size of plaques induced by feeding rabbits (Kockx et al., 1998) or mice (Tsimikas et al., 2001; MacDougall et al., 2006) high-fat diets.
As reported previously (Mackness et al., 2006), human PON1 gene transfer did not decrease total plasma cholesterol or triglyceride levels, but the immunoreactive area of oxLDL in the plaques was significantly lower in AdPON1 mice. This pointed to an intravascular antioxidative effect, although it is unlikely that PON1 was expressed by cells of the arterial wall, since adenoviral vectors are trapped by the liver after systemic application (Beck et al., 2004). In the liver, the human PON1 is incorporated in HDL particles by which it is distributed to the vessel wall. Previously PON1 gene transfer has been shown to inhibit LDL oxidation in vivo (Mackness et al., 2006) and in vitro (Rozenberg et al., 2005). Moreover, experiments with peritoneal macrophages from PON1 transgenic mice clearly illustrated the antioxidative effects of PON1 on the oxidative status of macrophages (Rozenberg et al., 2005).
Endothelium-dependent relaxations
In agreement with previous reports, atherosclerotic aorta rings of apoE−/− mice showed a pronounced endothelial dysfunction in response to ACh (Deckert et al., 1999; d'Uscio et al., 2001; Laursen et al., 2001). The defect was, however, only observed in segments with lesions, despite the pronounced hypercholesterolaemia (Deckert et al., 1999; Crauwels et al., 2003). Therefore, it was essential to analyse segments with and without plaques separately.
Endothelium-dependent relaxations induced by ATP and UTP were impaired in plaque-bearing segments of control apoE−/− mice as well. The dysfunction was, however, more pronounced for ACh than for the nucleotides, as indicated by the greater desensitization for ACh (4.3-fold rightward shift of the EC50) than for ATP (2.9-fold) or UTP (−0.85-fold, that is, no shift). This is presumably explained by the fact that ACh activates fewer ECs (33%) than ATP (82%) (Marie and Beny, 2002). Therefore, ACh-induced relaxations are probably affected earlier than those evoked by nucleotides.
AdPON1 gene transfer in apoE−/− mice restored these relaxations, for the nucleotides even to the level of WT mice. The latter benefit was apparently not due to the upregulation of nucleotide receptors by PON1 gene transfer, in view of the unaltered mRNA levels of the P2Y2 and P2Y6 receptors, which mediate the vasodilator effects of ATP and UTP, respectively (Guns et al., 2005, 2006). Since endothelial dysfunction in apoE−/− mice is strictly correlated with plaque size (Crauwels et al., 2003), it is also important to note that lesion dimensions remained unaltered in AdPON1 mice. Endothelium-dependent relaxations in the aorta of WT and apoE−/− mice are solely mediated by NO, without participation of prostacyclin or endothelium-derived hyperpolarizing factor (Crauwels et al., 2003; Guns et al., 2005). However, gene transfer of AdPON1 did not raise mRNA expression of eNOS or reduce the expression of its suppressor caveolin-1 (Michel et al., 1997). The mRNA expression of the SOD1 and SOD3, or the p47phox subunit of the most prominent superoxide anion-forming enzyme NAD(P)H-oxidase, was not affected by AdPON1 either. Therefore, the effects of AdPON1 gene transfer appeared to be due to the antioxidative capacity of PON1 itself: attenuation of LDL oxidation, as shown by the reduced oxLDL content of the plaques after PON1 gene transfer. Hence, it seems reasonable to assume that overexpression of PON1 decreased the scavenging of NO by superoxide anion by reducing oxidative stress in the vascular wall. In accordance with this, the limited number of plaque-bearing segments that could be exposed to diethylamine NONOate also showed an augmented responsiveness to this donor molecule of exogenous NO.
Furthermore, oxLDL has been proposed to deplete caveolae of cholesterol, resulting in the displacement of eNOS from caveolae and impaired eNOS activation (Uittenbogaard et al., 2000). In this view, the reduction of oxLDL in circulation and in the plaque of AdPON1 mice would favour the activation of eNOS. In support of this, the sensitivity to ACh in plaque-bearing segments increased as the circulating anti-MDA titres decreased.
The remarkable recovery of EC function, only 3 weeks after the adenoviral gene transfer of human PON1, suggests that oxidative stress-lowering strategies may be a promising approach to treat endothelial dysfunction that occurs during atherosclerosis. Although the beneficial effects of antioxidative vitamin supplementation on cardiovascular disease are rather controversial (Morris and Carson, 2003), a recent publication reported improvement of the impaired endothelial function, assessed in the brachial artery of patients with coronary artery disease upon diet supplementation with isoflavonoids (Widlansky et al., 2007). The benefit from these antioxidative molecules in humans is compatible with our PON1 findings in apoE−/− mice.
Smooth muscle cell function
Little information is available on SMC function in arteries from hypercholesterolaemic mice. Although force development was unchanged, the Ca2+ homeostasis of SMC in old hypercholesterolaemic apoE−/− mice was impaired as assessed by the attenuated Ca2+ release from the sarcoplasmic reticulum evoked by CPA. This is in line with the decreased SERCA activity in vascular SMCs from hypercholesterolaemic rabbits (Adachi et al., 2001) and the inhibition of SERCA-mediated Ca2+ uptake by peroxynitrite (Grover et al., 2003). PON1 gene transfer restored the impaired CPA-induced Ca2+ release. Previously, it has been shown that the antioxidant t-butylhydroxytoluene reversed the impaired smooth muscle SERCA function in hypercholesterolaemic rabbits (Adachi et al., 2002). Our results are in accordance with those findings and indicate that sarcoplasmic reticulum Ca2+ handling in the SMCs of old apoE−/− mice is decreased and that gene transfer of AdPON1 restored sarcoplasmic reticulum function, presumably by its intra-arterial antioxidative properties (see above).
Finally, it has been proposed that the decreased SERCA activity in hypercholesterolaemic SMCs contributes to the impaired responses to NO, and both were improved by antioxidant treatment (Adachi et al., 2001). Hence, the enhanced SERCA function observed in the SMCs of AdPON1 mice is a third mechanism that could have played a role in the ameliorated vasodilatation in those mice and in the increased sensitivity to UTP of plaque-free segments.
Conclusion
These results demonstrate, for the first time, that short-term PON1 gene transfer in old apoE−/− mice greatly improved EC function in arteries with pre-existing atherosclerosis and normalized SERCA function in the underlying SMCs without effects on plaque size. The first finding was striking, since endothelial dysfunction in mice is strongly determined by the plaque size. Both benefits are probably explained by the reduced oxidative stress in the vascular wall, as indicated by the decreased immunoreactive area of oxLDL in the plaques during AdPON1 gene transfer. This proves that PON1, besides inhibiting early atherogenesis (Tward et al., 2002; Mackness et al., 2006), also lowers oxidative stress and improves vasomotor function during established atherosclerosis. The impressive recovery of EC function, only 3 weeks after the adenoviral gene transfer of human PON1, suggests that oxidative stress-lowering strategies may be a promising approach for the treatment of atherosclerosis.
Acknowledgments
The research was supported by the Interuniversity Attraction Poles Programme—Belgian State—Federal Office for Scientific, Technical and Cultural Affairs (Grant P5/02 to HB and PH) and FWO Grants (G.0276.04 to PH, G.0627.06 to HB). P-JG is a research assistant of the FWO.
Abbreviations
- AdPON1
paraoxonase 1 recombinant adenovirus
- AdRR5
control recombinant adenovirus
- apoE−/−
apolipoprotein E-deficient (mice)
- CPA
cyclopiazonic acid
- EC
endothelial cell
- eNOS
endothelial nitric oxide synthase
- HDL
high-density lipoprotein
- LDL
low-density lipoprotein
- MDA
malondialdehyde
- OCT
optimal compound temperature
- oxLDL
oxidized low-density lipoprotein
- PON1
paraoxonase 1
- SERCA
sarco-endoplasmic reticulum Ca2+-ATPase
- SMC
smooth muscle cell
- SOD
superoxide dismutase
- WT
wild type
Conflict of interest
The authors state no conflict of interest.
References
- Adachi T, Matsui R, Weisbrod RM, Najibi S, Cohen RA. Reduced sarco/endoplasmic reticulum Ca2+ uptake activity can account for the reduced response to NO, but not sodium nitroprusside, in hypercholesterolemic rabbit aorta. Circulation. 2001;104:1040–1045. doi: 10.1161/hc3501.093798. [DOI] [PubMed] [Google Scholar]
- Adachi T, Matsui R, Xu S, Kirber M, Lazar HL, Sharov VS, et al. Antioxidant improves smooth muscle sarco/endoplasmic reticulum Ca2+-ATPase function and lowers tyrosine nitration in hypercholesterolemia and improves nitric oxide-induced relaxation. Circ Res. 2002;90:1114–1121. doi: 10.1161/01.res.0000019757.57344.d5. [DOI] [PubMed] [Google Scholar]
- Alcorn JL, Gao E, Chen Q, Smith ME, Gerard RD, Mendelson CR. Genomic elements involved in transcriptional regulation of the rabbit surfactant protein-A gene. Mol Endocrinol. 1993;7:1072–1085. doi: 10.1210/mend.7.8.8232306. [DOI] [PubMed] [Google Scholar]
- Aviram M, Hardak E, Vaya J, Mahmood S, Milo S, Hoffman A, et al. Human serum paraoxonases (PON1) Q and R selectively decrease lipid peroxides in human coronary and carotid atherosclerotic lesions: PON1 esterase and peroxidase-like activities. Circulation. 2000;101:2510–2517. doi: 10.1161/01.cir.101.21.2510. [DOI] [PubMed] [Google Scholar]
- Beck C, Uramoto H, Boren J, Akyurek LM. Tissue-specific targeting for cardiovascular gene transfer. Potential vectors and future challenges. Curr Gene Ther. 2004;4:457–467. doi: 10.2174/1566523043346138. [DOI] [PubMed] [Google Scholar]
- Bonetti PO, Lerman LO, Lerman A. Endothelial dysfunction: a marker of atherosclerotic risk. Arterioscler Thromb Vasc Biol. 2003;23:168–175. doi: 10.1161/01.atv.0000051384.43104.fc. [DOI] [PubMed] [Google Scholar]
- Crauwels HM, Van Hove CE, Holvoet P, Herman AG, Bult H. Plaque-associated endothelial dysfunction in apolipoprotein E-deficient mice on a regular diet. Effect of human apolipoprotein AI. Cardiovasc Res. 2003;59:189–199. doi: 10.1016/s0008-6363(03)00353-5. [DOI] [PubMed] [Google Scholar]
- Deckert V, Lizard G, Duverger N, Athias A, Palleau V, Emmanuel F, et al. Impairment of endothelium-dependent arterial relaxation by high-fat feeding in ApoE-deficient mice: toward normalization by human ApoA-I expression. Circulation. 1999;100:1230–1235. doi: 10.1161/01.cir.100.11.1230. [DOI] [PubMed] [Google Scholar]
- d'Uscio LV, Baker TA, Mantilla CB, Smith L, Weiler D, Sieck GC, et al. Mechanism of endothelial dysfunction in apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol. 2001;21:1017–1022. doi: 10.1161/01.atv.21.6.1017. [DOI] [PubMed] [Google Scholar]
- Grover AK, Kwan CY, Samson SE. Effects of peroxynitrite on sarco/endoplasmic reticulum Ca2+ pump isoforms SERCA2b and SERCA3a. Am J Physiol Cell Physiol. 2003;285:C1537–C1543. doi: 10.1152/ajpcell.00299.2003. [DOI] [PubMed] [Google Scholar]
- Guns PJ, Korda A, Crauwels HM, Van Assche T, Robaye B, Boeynaems JM, et al. Pharmacological characterization of nucleotide P2Y receptors on endothelial cells of the mouse aorta. Br J Pharmacol. 2005;146:288–295. doi: 10.1038/sj.bjp.0706326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guns PJ, Van Assche T, Fransen P, Robaye B, Boeynaems JM, Bult H. Endothelium-dependent relaxation evoked by ATP and UTP in the aorta of P2Y2-deficient mice. Br J Pharmacol. 2006;147:569–574. doi: 10.1038/sj.bjp.0706642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heistad DD. Oxidative stress and vascular disease: 2005 Duff lecture. Arterioscler Thromb Vasc Biol. 2006;26:689–695. doi: 10.1161/01.ATV.0000203525.62147.28. [DOI] [PubMed] [Google Scholar]
- Kockx MM, De Meyer GRY, Buyssens N, Knaapen MWM, Bult H, Herman AG. Cell composition, replication, and apoptosis in atherosclerotic plaques after 6 months of cholesterol withdrawal. Circ Res. 1998;83:378–387. doi: 10.1161/01.res.83.4.378. [DOI] [PubMed] [Google Scholar]
- Lalli MJ, Shimizu S, Sutliff RL, Kranias EG, Paul RJ. [Ca2+]i homeostasis and cyclic nucleotide relaxation in aorta of phospholamban-deficient mice. Am J Physiol. 1999;277:H963–H970. doi: 10.1152/ajpheart.1999.277.3.H963. [DOI] [PubMed] [Google Scholar]
- Laursen JB, Somers M, Kurz S, McCann L, Warnholtz A, Freeman BA, et al. Endothelial regulation of vasomotion in apoE-deficient mice: implications for interactions between peroxynitrite and tetrahydrobiopterin. Circulation. 2001;103:1282–1288. doi: 10.1161/01.cir.103.9.1282. [DOI] [PubMed] [Google Scholar]
- MacDougall ED, Kramer F, Polinsky P, Barnhart S, Askari B, Johansson F, et al. Aggressive very low-density lipoprotein (VLDL) and LDL lowering by gene transfer of the VLDL receptor combined with a low-fat diet regimen induces regression and reduces macrophage content in advanced atherosclerotic lesions in LDL receptor-deficient mice. Am J Pathol. 2006;168:2064–2073. doi: 10.2353/ajpath.2006.051009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackness B, Durrington PN, Mackness MI. The paraoxonase gene family and coronary heart disease. Curr Opin Lipidol. 2002;13:357–362. doi: 10.1097/00041433-200208000-00002. [DOI] [PubMed] [Google Scholar]
- Mackness B, Mackness MI, Arrol S, Turkie W, Julier K, Abuasha B, et al. Serum paraoxonase (PON1) 55 and 192 polymorphism and paraoxonase activity and concentration in non-insulin dependent diabetes mellitus. Atherosclerosis. 1998;139:341–349. doi: 10.1016/s0021-9150(98)00095-1. [DOI] [PubMed] [Google Scholar]
- Mackness B, Quarck R, Verreth W, Mackness M, Holvoet P. Human paraoxonase-1 overexpression inhibits atherosclerosis in a mouse model of metabolic syndrome. Arterioscler Thromb Vasc Biol. 2006;26:1545–1550. doi: 10.1161/01.ATV.0000222924.62641.aa. [DOI] [PubMed] [Google Scholar]
- Mackness MI, Arrol S, Abbott C, Durrington PN. Protection of low-density lipoprotein against oxidative modification by high-density lipoprotein associated paraoxonase. Atherosclerosis. 1993;104:129–135. doi: 10.1016/0021-9150(93)90183-u. [DOI] [PubMed] [Google Scholar]
- Marie I, Beny JL. Calcium imaging of murine thoracic aorta endothelium by confocal microscopy reveals inhomogeneous distribution of endothelial cells responding to vasodilator agents. J Vasc Res. 2002;39:260–267. doi: 10.1159/000063691. [DOI] [PubMed] [Google Scholar]
- Mertens A, Holvoet P. Oxidized LDL and HDL: antagonists in atherothrombosis. FASEB J. 2001;15:2073–2084. doi: 10.1096/fj.01-0273rev. [DOI] [PubMed] [Google Scholar]
- Mertens A, Verhamme P, Bielicki JK, Phillips MC, Quarck R, Verreth W, et al. Increased low-density lipoprotein oxidation and impaired high-density lipoprotein antioxidant defense are associated with increased macrophage homing and atherosclerosis in dyslipidemic obese mice: LCAT gene transfer decreases atherosclerosis. Circulation. 2003;107:1640–1646. doi: 10.1161/01.CIR.0000056523.08033.9F. [DOI] [PubMed] [Google Scholar]
- Michel JB, Feron O, Sacks D, Michel T. Reciprocal regulation of endothelial nitric-oxide synthase by Ca2+-calmodulin and caveolin. J Biol Chem. 1997;272:15583–15586. doi: 10.1074/jbc.272.25.15583. [DOI] [PubMed] [Google Scholar]
- Morris CD, Carson S. Routine vitamin supplementation to prevent cardiovascular disease: a summary of the evidence for the US Preventive Services Task Force. Ann Intern Med. 2003;139:56–70. doi: 10.7326/0003-4819-139-1-200307010-00014. [DOI] [PubMed] [Google Scholar]
- Mulvany MJ, Halpern W. Contractile properties of small arterial resistance vessels in spontaneously hypertensive and normotensive rats. Circ Res. 1977;41:19–26. doi: 10.1161/01.res.41.1.19. [DOI] [PubMed] [Google Scholar]
- Rozenberg O, Shih DM, Aviram M. Paraoxonase 1 (PON1) attenuates macrophage oxidative status: studies in PON1 transfected cells and in PON1 transgenic mice. Atherosclerosis. 2005;181:9–18. doi: 10.1016/j.atherosclerosis.2004.12.030. [DOI] [PubMed] [Google Scholar]
- Tsimikas S, Palinski W, Witztum JL. Circulating autoantibodies to oxidized LDL correlate with arterial accumulation and depletion of oxidized LDL in LDL receptor-deficient mice. Arterioscler Thromb Vasc Biol. 2001;21:95–100. doi: 10.1161/01.atv.21.1.95. [DOI] [PubMed] [Google Scholar]
- Tward A, Xia YR, Wang XP, Shi YS, Park C, Castellani LW, et al. Decreased atherosclerotic lesion formation in human serum paraoxonase transgenic mice. Circulation. 2002;106:484–490. doi: 10.1161/01.cir.0000023623.87083.4f. [DOI] [PubMed] [Google Scholar]
- Uittenbogaard A, Shaul PW, Yuhanna IS, Blair A, Smart EJ. High density lipoprotein prevents oxidized low density lipoprotein-induced inhibition of endothelial nitric-oxide synthase localization and activation in caveolae. J Biol Chem. 2000;275:11278–11283. doi: 10.1074/jbc.275.15.11278. [DOI] [PubMed] [Google Scholar]
- Van Assche T, Fransen P, Guns PJ, Herman AG, Bult H. Altered Ca2+ handling of smooth muscle cells in aorta of apolipoprotein E-deficient mice before development of atherosclerotic lesions. Cell Calcium. 2007;41:295–302. doi: 10.1016/j.ceca.2006.06.010. [DOI] [PubMed] [Google Scholar]
- Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, et al. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002;3:34.1–34.11. doi: 10.1186/gb-2002-3-7-research0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verreth W, De Keyzer D, Pelat M, Verhamme P, Ganame J, Bielicki JK, et al. Weight-loss-associated induction of peroxisome proliferator-activated receptor-alpha and peroxisome proliferator-activated receptor-gamma correlate with reduced atherosclerosis and improved cardiovascular function in obese insulin-resistant mice. Circulation. 2004;110:3259–3269. doi: 10.1161/01.CIR.0000147614.85888.7A. [DOI] [PubMed] [Google Scholar]
- Widlansky ME, Hamburg NM, Anter E, Holbrook M, Kahn DF, Elliott JG, et al. Acute EGCG supplementation reverses endothelial dysfunction in patients with coronary artery disease. J Am Coll Nutr. 2007;26:95–102. doi: 10.1080/07315724.2007.10719590. [DOI] [PMC free article] [PubMed] [Google Scholar]