Abstract
Background and purpose:
Anatomical and pharmacological studies have demonstrated that the lower oesophageal sphincter (LES) is not a simple homogenous circular muscle with uniform innervation. Regional differences have been demonstrated in several species including humans. We investigated, for the first time in mice LES, regionally distinct physiological and pharmacological characteristics of the neuromusculature.
Experimental approach:
Conventional intracellular recordings and pharmacological techniques were employed to evaluate electrical properties and functional innervation of smooth muscle cells. Results from CD1 (control), nNOS(−/−) and eNOS(−/−) genetic knockout mice were compared.
Key results:
Smooth muscle of sling and clasp LES displayed unitary membrane potentials of 1– 4 mV. Transmural nerve stimulation produced a monophasic inhibitory junction potential (IJP) in the sling, whereas in the clasp a biphasic IJP, consisting of a brief IJP followed by a long-lasting slow IJP (lsIJP), was induced. Pharmacological interventions and genetically modified mice were used to demonstrate a monophasic apamin-sensitive (purinergic) component in both LES regions. However, the nitrergic IJP was monophasic in the sling and biphasic in the clasp. Unitary membrane potentials and IJPs were not different in CD1 and eNOS(−/−) mice, suggesting no involvement of myogenic NOS.
Conclusion and implications:
These data in mouse LES indicate that there are previously unreported regional differences in the IJP and that both the apamin-resistant monophasic and biphasic IJPs are mediated primarily by nitrergic innervation.
Keywords: ATP, niflumic acid, neuronal nitric oxide synthase, circular smooth muscle, long-lasting slow IJP
Introduction
The lower oesophageal sphincter (LOS) comprises specialized circular smooth muscle (CSM) that maintains myogenic tone at rest, thereby providing an antireflux pressure barrier at the gastroesophageal junction (Goyal and Paterson, 1989). The CSM of the LES is not simply a symmetrical ring. Rather, in most species it is composed of semicircular ‘clasp' muscle fibres on the right side, and obliquely oriented or ‘sling' muscle fibres on the left side (Liebermann-Meffert et al., 1979; Preiksaitis et al., 1994; Brookes et al., 1996; Yuan et al., 1998). Both fibres intermingle anteriorly and posteriorly. Recent investigations have not only shown functional differences, but also different distributions of motor neurons in the clasp and sling muscle fibres (Brookes et al., 1996; Diamant, 2005). The clasp fibres show greater basal tone than the sling fibres, but the latter are more sensitive to cholinergic stimulation and less responsive to nitrergic stimulation (Preiksaitis et al., 1994; Preiksaitis and Diamant, 1997; L'Heureux et al., 2006). These fibres also demonstrate differing responses to other pharmacological agents, altered expression of soluble N-ethylmaleimide-sensitive fusion attachment protein receptor (SNARE) proteins, L-type Ca2+ channels and K+ channels and diverse Ca2+ sources (Ji et al., 2000, 2002; Tian et al., 2004; Muinuddin et al., 2004a, 2004b).
Retrograde labelling and immunohistochemical studies have demonstrated a different distribution of motor neuron somata in guinea-pig LOS (Brookes et al., 1996). Clasp fibres receive nearly five times more motor neurons than sling fibres. Moreover, 33% of the neurons in the clasp stain positively for choline acetyl transferase, whereas 70% stain positively for NOS (Brookes et al., 1996). In contrast, the sling fibres are innervated predominantly by choline acetyl transferase-positive neurons (∼80%), with only 15% staining positively for NOS. (Yuan and Brookes, 1999). Together with the functional studies mentioned above, these findings strongly suggest that the inhibitory innervation predominates in the clasp, whereas excitatory innervation prevails in the sling.
Inhibitory neurotransmission plays a major role in regulation of LOS basal tone and in swallow-induced LOS relaxation (Goyal and Paterson, 1989). To date, relatively little is known about regional differences in inhibitory innervation to the LOS. Furthermore, although mice are increasingly used to study the physiology of gut motility because of the availability of gene knockout models, relatively little is known about the physiology of the LOS in this species. The goal of the current study was to use conventional intracellular recording methods to characterize the resting electrical properties and the inhibitory neurotransmission in clasp and sling muscle fibres from mouse LOS. The present data reveal previously unreported regional difference in nitrergic inhibitory junction potentials (IJP) in the CSM of the mouse LES.
Methods
Tissue preparation and conventional intracellular recordings
The protocol was approved by the Animal Care Committee of Queen's University. Adult mice (CD1, Charles River Laboratories, Montréal, Canada), neuronal NOS (nNOS)(−/−) knockout mice (B6.129S4-Nos1tm1Plh) (Jackson Laboratory, Bar Harbor, ME, USA) and endothelial NOS (eNOS)(−/−) knockout mice (B6.129P2-Nos2tm1Lau) (created and maintained at Harvard University) (Huang et al., 1995) of either of sex were killed by cervical dislocation after isoflurane anaesthesia. The abdominal cavity was then exposed via a mid-line incision. The stomach and part of attached oesophagus were dissected (Figure 1a). The LOS was then separated using a dissecting microscope. Similar to other species, the mouse LOS is not a simple concentric ring. Rather, the LOS clasp muscle (right) is distinctly thickened, but the LOS sling muscle (left) more resembles gastric muscle (Figure 1b). Strips (0.5–1 × 2–2.5 mm) of right and left LOS were pinned with mucosa side facing upward on the bottom of a recording chamber covered by Sylgard (Dow Corning) and perfused at 2 ml min−1 with pre-oxygenated Krebs' solution containing guanethidine (3 μM) and substance P (1 μM) at 36° C (Zhang and Paterson, 2001, 2005). Substance P was added to the bath to induce tachyphylaxis and thereby ensured that any neurokinin-mediated excitatory potentials were eliminated (Krysiak and Preiksaitis, 2001). The Krebs' solution consisted of (in mM) NaCl 118.07, NaHCO3 25.00, D(+)-glucose 11.10, KCl 4.69, CaCI2 2.52, MgSO4 1.00, NaH2PO4 1.01. Nerve stimulation using either 1- or 4-square wave pulses (20 Hz) with a duration of 0.3 ms and voltage of 70 V was delivered to the muscle preparations by a pair of silver wires, while electrical activity was recorded using conventional intracellular electrodes as previously described (Zhang and Lang, 1994; Zhang and Paterson, 2002). Previously defined electrical parameters were used to analyse quantitatively the electrical properties of the smooth muscle (Zhang and Paterson, 2002, 2003).
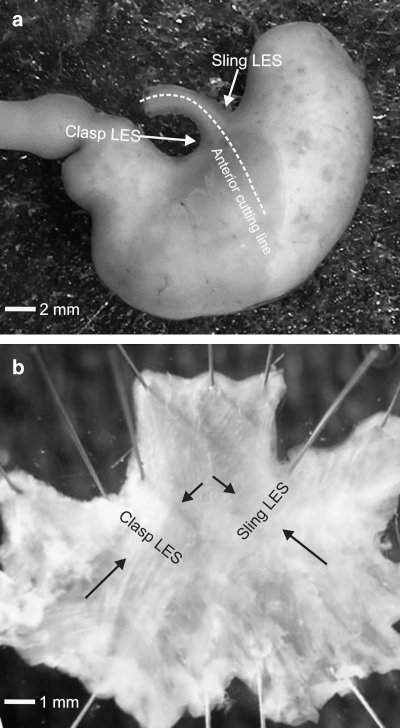
Figure 1.
Gross anatomy of mouse LES. (a) Stomach with attached oesophagus and duodenum. Arrows indicate location of right (clasp) and left (sling) LOS. (b) Oesophagus and stomach corpus were cut sagittally along the anterior wall as depicted by dotted line in (a). LOS muscle coat was pinned with serosal side facing downward after removal of mucosa. Black arrows point to LOS sling muscle and the distinctly thickened LES clasp muscle. The former resembles gastric sling muscle. LOS, lower oesophageal sphincter.
Statistical analysis
Data are shown as mean±s.e.mean, refer to number of animals. Only recordings in which a full protocol was completed in the same cell are included in the statistical analysis. Pre- and post-drug comparisons were made using the paired Student's t-test and a P-value <0.05 was considered statistically significant.
Drugs
All drugs were purchased from Sigma, except isoflurane (Baxter, Canada). The following drugs were used: nifedipine, atropine, guanethidine, apamin, substance P, NG-nitro-L-arginine methyl ester (L-NAME) NFA in DMSO and niflumic acid (NFA). Nifedipine was dissolved in alcohol, and others in distilled and de-ionized water. These were diluted to final concentrations with Krebs' solution. Final concentration of DMSO in Krebs' solution was not more than 1%, which did not produce any effect on the electrical activity of the tissue.
Results
General electrical properties of right (clasp) and left (sling) LOS
All of the conventional intracellular recordings were conducted in the presence of guanethidine (3 μM) and substance P (1 μM). Our earlier preliminary experiments suggested that there were no significant differences in electrical properties and neural responses among CD1 and true background wild types of nNOS(−/−) (B6x129SF2/J F1 strain) and eNOS(−/−) mice (C57BL/6J strain). Therefore, CD1 mice were used as controls for nNOS(−/−) and eNOS(−/−) strains in the current studies. LOS clasp muscle displayed on-going spontaneous action potentials with variable amplitudes of up to 10 mV. The spontaneous action potentials were usually superimposed on the upward deflections of membrane potential fluctuations. This is consistent with our previous observations in opossum (Zhang et al., 2000). Bath application of nifedipine (1 μM) abolished the spontaneous action potentials, but did not affect the underlying membrane potential fluctuations of 1–4 mV, which appear similar to the ‘unitary potentials' recorded in guinea-pig gastric smooth muscle (Edwards et al., 1999; Beckett et al., 2004) and CSM of opossum LOS (Zhang and Paterson, 2002, 2003). IJPs induced by four pulses (0.3 ms duration) at frequency of 20 Hz were not affected by nifedipine. Spontaneous action potentials were not observed consistently in LES sling muscle. To prevent frequent dislodgement of intracellular microelectrodes, nifedipine (1 μM) was routinely included in the bath perfusion solution in the remaining experiments.
No significant difference was observed in the resting membrane potential (RMP) between the clasp and sling fibres (Table 1). Nerve stimulation with parameters of one and four pulses (0.3 ms duration) at frequency of 10, 20 and 40 Hz was tested initially. Four pulses at frequency of 20 Hz evoked an IJP of maximal amplitude in both clasp and sling fibres (Figure 2) and these stimulus parameters were used in subsequent experiments. Nerve stimulation produced biphasic IJPs in clasp muscle, a brief IJP, followed by a long-lasting slow IJP (lsIJP). In sling muscle, nerve stimulation evoked a monophasic IJP.
Table 1.
Pharmacological properties of biphasic and monophasic IJPs in the LES from wild-type CD1 mice
|
LES clasp |
LES sling |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| RMP (mV) | IJP AMPL (mV) | IJP Duratn (ms) | lsIJP APL (mV) | lsIJP Duratn (ms) | RMP (mV) | IJP AMPL (ms) | IJP Duratn (mV) | |||
| Atropine | Control n=5 | −43.2±3.3 | 24.6±1.6 | 711±69 | 6.3±1.4 | 11 914±1825 | n=4 | −43.1±1.8 | 12.5±1.1 | 1033±91 |
| 3 μM | −42.3±3.5 | 29.4±1.5 | 720±49 | 9.5±1.8 | 14 485±2097 | −42.5±1.7 | 17.5±1.8 | 191±108 | ||
| Change | 0.9±0.4 | 4.7±1.1* | 9.6±42.8 | 3.2±0.6* | 2570±286* | 0.5±1.2 | 5.0±1.2* | 58±73 | ||
| Atropine+apamin | Control n=5 | −41.8±2.4 | 29.4±2.1 | 749±42 | 9.1±1.9 | 14 639±1977 | n=6 | −39.4±2.1 | 19.5±2.6 | 989±96 |
| 300 nM | −35.3±2.7 | 17.9±1.6 | 1087±110 | 7.6±1.6 | 12 197±1645 | −32.1±3.1 | 14.9±1.4 | 996±49 | ||
| Change | 6.5±1.2* | 11.5±1.5* | 339±80* | 1.5±1.3 | 2442±1698 | 7.3±1.8* | 4.6±1.6* | 7±76 | ||
| Atropine+apamin+L-NAME | Control n=5 | −37.8±1.6 | 19.9±2.5 | 1085±76 | 8.3±1.4 | 13 347±2683 | n=3 | −39.6±3.8 | 19.5±3.5 | 1049±92 |
| 200 μM | −33.3±1.0 | 8.1±1.5 | 469±36 | 0.3±0.2 | N/M | −34.5±2.9 | 2.5±1.5 | N/M | ||
| Change | 4.5±1.9 | 11.8±1.3* | 616±70* | 8.0±1.2* | N/M | 5.1±1.8 | 17.0±3.3* | N/M | ||
| Atropine+apamin+NFA | Control n=6 | −38.5±1.8 | 19.7±1.7 | 996±109 | 8.4±1.4 | 14 641±1633 | n=2 | −37.1 −40.8 |
16.6 22.9 |
1043 1385 |
| 200 μM | −48.9±3.5 | 6.2±1.0 | 1161±207 | 2.3±0.4 | N/M | −61.6 −47.1 |
2.8 1.4 |
N/M N/M |
||
| Change | 10.1±2.5* | 13.5±2.2* | 166±191* | 6.0±1.1 | N/M | 24.5 6.3 |
13.8 21.5 |
N/M N/M |
||
Abbreviations: AMPL, amplitude; Duratn, half-amplitude duration; IJP, monophasic IJP or initial phase of biphasic IJP; LES, lower oesophageal sphincter; L-NAME, N-nitro-L-arginine methyl ester; LSIJP, long-lasting slow IJP; N/M, not measured or not measurable; RMP, resting membrane potential.
*P<0.05.
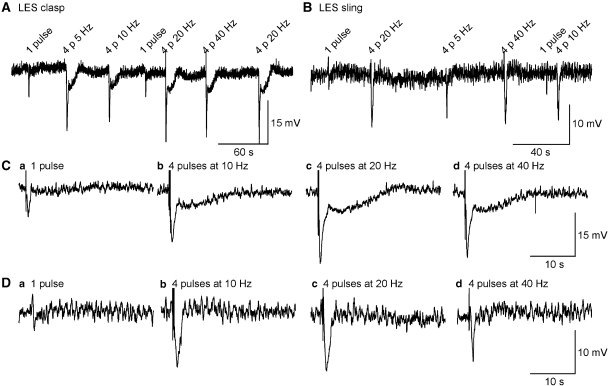
Figure 2.
Smooth muscle responses to transmural nerve stimulation of different intensity. Original recordings of IJPs induced by 1 or 4 pulses (p) of 0.3 ms duration, at frequency of 10, 20 and 40 Hz in LOS clasp (A) and sling muscle (B). (C and D) IJPs depicted in (A and B), respectively, at expanded time scale. Four pulses at 20 Hz appeared to evoke a maximal IJP. Resting membrane potential was ∼ −43 mV with membrane fluctuations of 1–5 mV, and was no different in clasp vs sling muscles. Nerve stimulation using 4 square wave pulses (20 Hz) to CSM of sling muscle (D) induced a monophasic IJP. However, nerve stimulation to CSM of clasp muscle (C) evoked a biphasic IJP consisting of an initial IJP, followed by a long-lasting slow IJP. These distinct regional differences in mouse LES IJPs have not previously been reported. CSM, circular smooth muscle; IJP, inhibitory junction potential; LOS, lower oesophageal sphincter.
Isolation of different IJP components
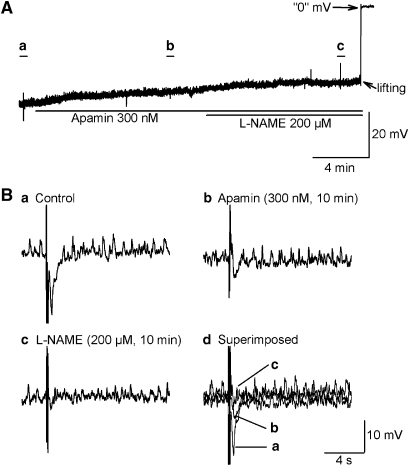
The following experiments were designed to isolate the different neural components of the evoked IJP, with results summarized in Table 1. In the clasp muscle, atropine (3 μM) increased the amplitude of the biphasic IJP and widened the half-amplitude duration of the lsIJP over control (Figure 3a). In the sling muscle, atropine potentiated the amplitude of the monophasic IJP over control (Figure 3b). The IJP induced by ATP is known to be due to the opening of small conductance ca++-activated K+channels (SK) channels, which are specifically apamin-sensitive. Thus, apamin was used to identify the purinergic component of the evoked IJPs. Apamin's onset of action was rapid, reaching a maximum value in 5–10 min (Figure 4), but was not reversed even 60 min after wash out. In the presence of atropine (3 μM), apamin (300 nM, 10 min) depolarized RMP in the clasp and in the sling muscle, and markedly inhibited the amplitude of the first phase of the biphasic IJP in clasp muscle and monophasic IJP in sling muscle (Table 1).
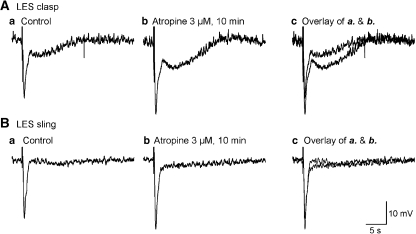
Figure 3.
Effect of atropine (3 μM) on IJPs. (A and B) Original recordings of IJPs induced by 4 pulses of nerve stimulation (0.3 ms duration, 20 Hz) in clasp and sling muscle, respectively. (a) Represents control tracing, (b) following administration of atropine and (c) overlap of control and post-atropine tracings. Atropine significantly increased the amplitudes of biphasic and monophasic IJPs, implying that cholinergic neurotransmission is present in both sides of LES. IJP, inhibitory junction potential; LOS, lower oesophageal sphincter.
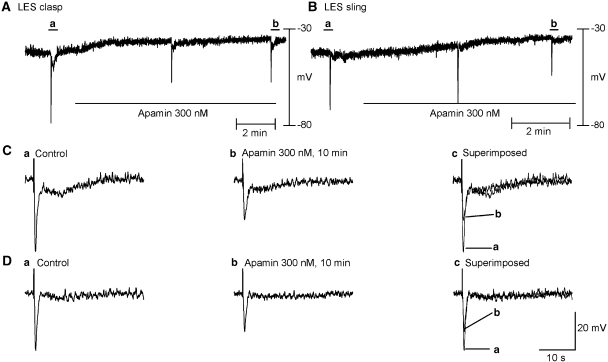
Figure 4.
Effect of apamin (300 nM) in the presence of atropine. (A and B) Time course of effects of apamin, a small conductance K+ channel blocker, on the clasp and sling muscle, respectively. (C(a) and (b) and D(a) and (b)) Snapshots of IJPs before and 10 min after apamin in clasp and sling muscle, respectively. (C(c) and D(c)) Overlay of IJPs before and after apamin. Apamin depolarized resting membrane potential and decreased the IJP amplitudes in both clasp and sling muscles. However, no significant effect was observed on the long-lasting phase of the biphasic IJP in clasp muscle. IJP, inhibitory junction potential.
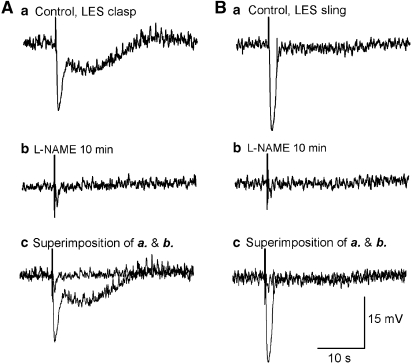
In the presence of muscarinic and purinergic blockade, nerve stimulation still evoked a biphasic IJP in clasp muscle (Figure 5a) (n=5) and a monophasic IJP in sling muscle (Figure 5b; Table 1). L-NAME, an NOS inhibitor, was used to identify a nitrergic component of the IJP. L-NAME had a maximal effect in 10 min and in keeping with our previous publication (Zhang and Paterson, 2002), its effect was not reversible with washout. Application of L-NAME abolished the lsIJP (Figure 5a) and significantly inhibited the amplitude of the first phase of the apamin-resistant biphasic IJP (clasp; Figure 5a) and monophasic IJP (sling; Figure 5b).
Figure 5.
Characterization of apamin-resistant biphasic and monophasic IJPs in LES clasp (A) and sling (B) muscles before (a) and 10 min after (b) application of L-NAME (200 μM), an NOS inhibitor. Experiments were performed in the presence of atropine, apamin, guanethidine and substance P. (c) Superimposed IJPs before and 10 min after L-NAME. L-NAME inhibited IJP amplitude in sling muscle by about 90%, while it decreased the amplitude of the initial phase of the biphasic IJP by 60% and abolished the long-lasting slow IJP in clasp muscle, suggesting that nitric oxide mediates the apamin-resistant IJP and the biphasic IJP in both sides of the LOS. IJP, inhibitory junction potential; LOS, lower oesophageal sphincter; L-NAME, N-nitro-L-arginine methyl ester.
IJP in clasp muscle of nNOS(−/−) mouse
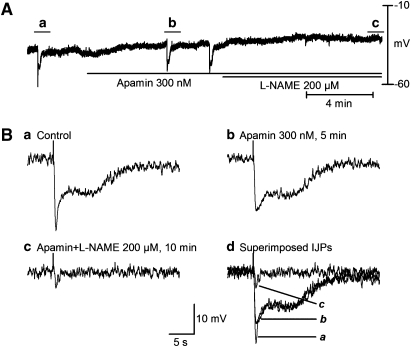
To confirm the presence of nitrergic inhibitory neurotransmission in mouse LOS, similar experiments were performed using clasp LOS muscle from nNOS(−/−) mice (Figure 6 and Table 2.). In the presence of atropine, nerve stimulation produced only a monophasic IJP with a lower amplitude and shorter duration (Figure 6b; Table 2), which were markedly different from the corresponding values in CD1 mice (Figure 2c; Table 1). However, RMP (Table 2) and associated unitary potentials were no different from control mice. Administration of apamin (300 nM) significantly suppressed the amplitude of the monophasic IJP (Figure 6b). Concomitant application of L-NAME (200 μM) did not significantly decrease the amplitude of the monophasic IJP (Figure 6b), in keeping with a lack of nitrergic IJP in nNOS(−/−) mice.
Figure 6.
Properties of the IJP recorded in clasp muscle of nNOS(−/−) mouse in the presence of atropine, guanethidine and substance P. (A) Recording of membrane potentials. (B) IJPs depicted in (A) at expanded time scale. Only a monophasic IJP (B(a)) was recorded, which was essentially abolished by apamin (300 nM, 10 min) (B(b)). Further application of L-NAME (200 μM, 10 min) did not have any significant effect (B(c) and (d)). The finding of only an apamin-sensitive IJP in nNOS(−/−) mice suggests that nitrergic innervation mediates a component of the initial IJP and all of the long-lasting slow IJP in clasp muscle. IJP, inhibitory junction potential; L-NAME, N-nitro-L-arginine methyl ester.
Table 2.
Pharmacological properties of IJPs in LOS clasp of nNOS knockout mice
| RMP (mV) | IJP amplitude (mV) | IJP duration (ms) | |
|---|---|---|---|
| Control n=4 | −41.0±3.3 | 6.6±1.5 | 366±61 |
| Apamin (300 nM) | −38.4±3.0 | 2.4±0.5* | 518±97 |
| Apamin (300 nM)+ L-NAME (200 μM) | −35.0±3.9 | 1.8±0.4 | N/M |
Abbreviations: LES, lower oesophageal sphincter; L-NAME, N-nitro-L-arginine methyl ester; N/M, not measured or not measurable; RMP, resting membrane potential.
*P<0.05 before and after drug application.
‘Control' represents results obtained in the presence of atropine (3 μM), guanethidine (3 μM) and substance P (1 μM).
IJP in LES clasp muscle of eNOS(−/−) mouse
It has been proposed that myogenic NOS is involved in downstream neuronal VIP signalling and neuronal nitric oxide (NO) amplification in gastrointestinal smooth muscle (Murthy et al., 1993; Murthy and Makhlouf, 1994; Sanders, 1996; Makhlouf and Murthy, 1997; Goyal, 2000; Murthy, 2006). In addition, the unique two-component nitrergic IJP noted in mouse clasp LES CSM raised the possibility that one of the IJP components could involve myogenic NOS. This was tested using eNOS(−/−) mice, which have been reported to lack myogenic NOS in gut smooth muscle (Huang et al., 1995). Nerve stimulation produced a biphasic IJP in clasp LOS CSM of eNOS(−/−) mice that was no different from that recorded in CD1 control mice (Figure 7; Table 3 vs Table 1). Application of apamin (300 nM) depolarized RMP and inhibited amplitude of IJPs, but did not affect the lsIJP (Figure 7b). Subsequent application of L-NAME suppressed the IJP amplitude and abolished the lsIJP (Figure 7b; Table 3). These data do not support a role for eNOS in either purinergic or nitrergic inhibitory neurotransmission in mouse LOS.
Figure 7.
Properties of the IJP recorded in clasp muscle of eNOS(−/−) mouse in the presence of atropine, guanethidine and substance P. (A) Raw recording of membrane potential. (B) IJPs depicted in (A) at expanded time scale. Clasp muscle in eNOS(−/−) mouse had a normal response to apamin (300 nM) and L-NAME (200 μM). Unitary membrane potentials and biphasic IJPs were not different in CD1 and eNOS(−/−) mice, suggesting that myogenic eNOS is not involved in the events in clasp muscle. IJP, inhibitory junction potential; L-NAME, N-nitro-L-arginine methyl ester.
Table 3.
Pharmacological properties of biphasic IJPs in LES clasp of eNOS knockout mice
| RMP (mV) | IJP amplitude (mV) | IJP duration (ms) | lsIJP amplitude (mV) | lsIJP duration (ms) | |
|---|---|---|---|---|---|
| Control n=4 | −43.7±0.90 | 20.3±3.50 | 953±750 | 5.9±2.1 | 9509±1232 |
| Apamin (300 nM) | −35.8±1.5* | 14.5±2.3* | 1593±392 | 6.0±2.1 | 11 283±1173 |
| Change | 7.9±1.4* | 5.8±1.2* | 639±467 | 0.1±0.7 | 1775±710 |
| Apamin (300 nM)+L-NAME (200 μM) | −33.3±1.30 | 2.1±0.1* | N/M | N/M | N/M |
| Change | 2.5±1.50 | 12.4±2.4* | N/M | N/M | N/M |
Abbreviations: eNOS, endothelial NOS; IJP, monophasic IJP or initial phase of biphasic IJP; LOS, lower oesophageal sphincter; L-NAME, N-nitro-L-arginine methyl ester; LSIJP, long-lasting slow IJP; N/M, not measured or not measurable; RMP, resting membrane potential.
*P<0.05.
‘Control' represents results obtained in the presence of atropine (3 μM), guanethidine (3 μM) and substance P (1 μM).
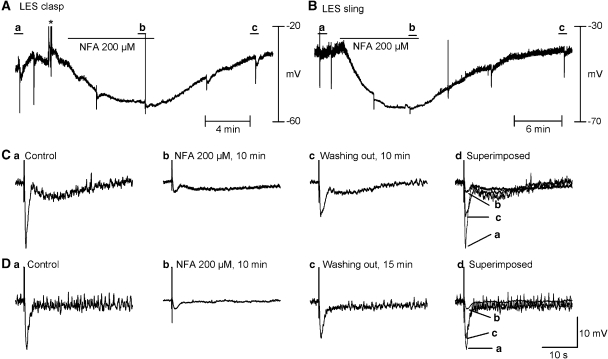
Effects of NFA on nitrergic IJP
We previously reported that NFA, an antagonist of ClCa, abolished the nitrergic IJP in opossum oesophagus and guinea-pig ileum, while leaving the purinergic IJP in guinea-pig ileum unaffected. However, such an effect has not been investigated in other species, including mice. In the presence of atropine and apamin, NFA (200 μM) hyperpolarized RMP, abolished unitary potentials and inhibited the nitrergic component of the biphasic IJP in the clasp and monophasic IJP in the sling (Figure 8; statistical data summarized in Table 1). The inhibitory effects of NFA on RMP, unitary potentials and nitrergic IJPs reversed after wash out. Figure 9 summarizes the relative contributions of nitrergic and purinergic neurotransmission in the mouse clasp and sling on the basis of the current studies.
Figure 8.
Niflumic acid (NFA, 200 μM), a ClCa channel blocker, inhibited IJPs induced by nitrergic neurons in the presence of atropine and apamin. (A and B) Continuous recording of membrane electrical activity in clasp and sling muscle for up to 25 min. (C(a–c) and D(a–c)) IJPs before, 10 min after application of NFA and 10 min after wash out in clasp and sling muscles, respectively The effects of NFA were reversible. NFA almost completely abolished membrane potential fluctuations and the apamin-resistant component in both the clasp and sling muscles. The concentration of NFA used was previously found by us to maximally inhibit the nitrergic IJP in opossum LES. *Denotes artifacts resulting from perfusion bubbles. IJP, inhibitory junction potential; LES, lower oesophageal sphincter.
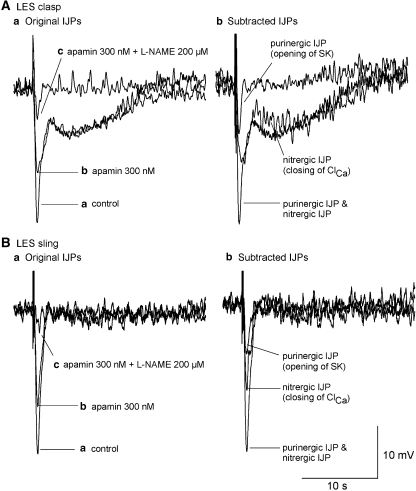
Figure 9.
Summary of ionic mechanisms underlying monophasic and biphasic IJPs recorded in mouse clasp (A) and sling (B) muscles. (A(a) and B(a)) Digitally recorded IJPs after cumulative application of apamin (300 nM) and L-NAME (200 μM). (A(b) and B(b)) Purinergic (monophasic) and nitrergic (biphasic) IJPs were obtained after digital subtraction of the original IJPs. The data suggest that the opening of SK by ATP and the closing of Clca by NO are responsible for the monophasic IJP and initial component of the biphasic IJP, while closing of ClCa channels by NO underlies the long-lasting slow IJP. Note the subtle change in the rate of the upstroke of the initial phase of the IJP (A(a)), in keeping with it comprising both purinergic and nitrergic components. IJP, inhibitory junction potential; L-NAME, N-nitro-L-arginine methyl ester; NO, nitric oxide.
Discussion
In the current study, we have demonstrated previously unreported electrophysiological differences in the clasp vs sling muscle of the mouse LOS. In the clasp muscle, nerve stimulation produced a biphasic IJP, consisting of a relatively brief initial hyperpolarization followed by an unusual long-lasting hyperpolarization, whereas in the sling muscle, only a monophasic IJP was observed. Using pharmacological antagonists and genetically altered nNOS(−/−) and eNOS(−/−) mice, we have also demonstrated that nitrergic innervation was responsible for the entire long-lasting IJP, as well as a component of the initial IJP. Consistent with our previous reports in smooth muscle of opossum oesophagus (Zhang et al., 1998; Zhang and Paterson, 2002, 2003) and guinea-pig ileum (Zhang and Paterson, 2002), NFA hyperpolarized the RMP and abolished unitary membrane potentials and the nitrergic components of the biphasic and monophasic IJPs, suggesting that the nitrergic IJP is due to the closing of ClCa channels.
Maintenance of basal tone in opossum LOS is mainly dependent on influx of extracellular Ca2+ via nifedipine-sensitive (L-type) Ca2+ channels (Fox and Daniel, 1979; Zhang et al., 2000). Furthermore, the RMP of LES muscle is relatively more positive (∼ −41 mV) compared to the adjacent oesophageal body (∼ −54 mV) (Daniel et al., 1976; Jury et al., 1992; Conklin et al., 1993; Zhang et al., 2000; Zhang and Paterson, 2003), which results in on-going spike-like action potentials, due to continuous Ca2+ influx through voltage-sensitive Ca2+ channels (Kubota et al., 1998; Zhang et al., 2000). In the current study, we recorded an RMP of ∼ −43 mV, which is comparable to that recorded in guinea-pig (Yuan et al., 1998; Yuan and Brookes, 1999) and opossum LES (Daniel et al., 1976; Zhang et al., 2000). It is also similar to that recorded in mouse LES by Imaeda and Cunnane (2003), but quite different from that reported by Ward et al. (1998) (RMP ∼ −57 mV). The reason for the more negative RMP in the latter study is unclear.
Inhibitory innervation to LES muscle is of critical importance in swallow-induced LES relaxation (Goyal and Paterson, 1989). In the current studies, the NOS inhibitor L-NAME blocked the apamin-resistant biphasic and monophasic IJPs, indicating that nitrergic innervation plays a dominant role in the inhibitory innervation to the LES. This conclusion is confirmed by recordings from the clasp LES of nNOS(−/−) mouse, in which only apamin-sensitive monophasic IJPs were recorded. Thus, the combined studies in CD1 and nNOS(−/−) mice provide compelling evidence that purinergic and nitrergic innervations are involved in the monophasic IJP and the first phase of the biphasic IJP. Moreover, the second, long-lasting phase of the biphasic IJP appears to be exclusively mediated by nitrergic neurotransmission. It should be noted that on close inspection of our tracings, a subtle change in the rate of rise of the upstroke of the initial component of the biphasic IJP in the LES clasp as well as the monophasic IJP in the LES sling is apparent. This is consistent with the initial phase being comprised of both purinergic and nitrergic components. Indeed, this resembles the classic combined fast and slow IJP recorded in guinea-pig ileum (Crist and He, 1991; Bennett, 1997; Zhang and Paterson, 2002), which are mediated by purinergic and nitrergic innervations, respectively. In this tissue, however, there is no long-lasting nitrergic IJP.
Interestingly, previous studies in the opossum, guinea-pig and cat showed no evidence for an ATP-mediated fast IJP in the smooth muscle of LES and adjacent oesophageal body (Crist et al., 1991a; Yuan et al., 1998; Yuan and Brookes, 1999; Zhang and Paterson, 2002, 2003; L'Heureux et al., 2006). On the other hand, we found clear evidence of an apamin-sensitive component to the IJP in mouse LES, similar to that observed in mouse gastric fundus (Mashimo et al., 1996). Although sensitivity to apamin does not necessarily equate with purinergic neurotransmission, previous investigators have shown that the apamin-sensitive component of the IJP in mouse LES is also inhibited by purinergic antagonists (Imaeda and Cunnane, 2003). Similar to the opossum model, application of atropine increased the amplitudes of the biphasic and monophasic IJPs, suggesting the presence of intrinsic cholinergic innervation, which serves to dampen the amplitude of the IJP when all nerves are activated simultaneously.
Our observations demonstrate that inhibitory neurotransmission predominates in both clasp and sling muscles of mouse. This is different from tension-recording studies in cat and human in which excitatory cholinergic innervation to the LES sling is predominant (Preiksaitis et al., 1994; Preiksaitis and Diamant, 1997; L'Heureux et al., 2006). The biphasic IJPs in the clasp LES of mouse also differ from the monophasic IJPs in the LES of guinea-pig and opossum, for which nitrergic innervation is exclusively responsible (Yuan et al., 1998; Yuan and Brookes, 1999; Zhang and Paterson, 2003). The functional consequences of these interspecies differences remain to be determined. Our preliminary muscle strip studies confirm the presence of both nitrergic and purinergic components to LES relaxation. Unfortunately, due to the very small size of this muscle, it has not been technically possible to compare nerve-mediated mechanical responses between the clasp and sling components.
A decade ago, it was proposed that neuronal VIP acts as an inhibitory neurotransmitter to gastrointestinal smooth muscle by activating myogenic NOS, which then generates NO within the muscle cells (Murthy et al., 1993; Murthy and Makhlouf, 1994; Makhlouf and Murthy, 1997; Murthy, 2006). In addition, there are reports that interstitial cells of Cajal amplify neuronal NO signalling by producing more NO through activation of myogenic NOS. In those studies, eNOS was reported as functioning as the myogenic NOS. Daniel et al. (2001) subsequently reported that in some smooth muscles, myogenic NOS may actually be a variant of nNOS. Our studies do not support a role for myogenic NOS in the nitrergic IJP. Not only was the nitrergic IJP absent in nNOS(−/−) mice, but normal IJPs, indistinguishable from those of wild-type mice, were observed in eNOS(−/−) mice.
The ionic mechanisms underlying the nitrergic IJP in digestive smooth muscle have been debated for more than a decade. Initially, it was proposed that this resulted from opening of K+ channels via a cGMP-mediated signal pathway (Jury et al., 1985; Sanders and Ozaki, 1994). However, the inability of specific K+ channel blockers to abolish the nitrergic IJP has called this hypothesis into question (Crist et al., 1991a; Zhang and Paterson, 2002, 2003). Crist et al. (1991a, 1991b) first suggested that NO hyperpolarized intestinal smooth muscle by closing ClCa channels, and several recent publications have lent support to this mechanism (Zhang and Paterson, 2002, 2003; Craven et al., 2004). We previously demonstrated that ClCa channel blockers abolished both unitary potentials and nitrergic IJP in opossum LES and guinea-pig ileum, without affecting the fast purinergic IJP in the latter tissue (Zhang and Paterson, 2002, 2003). In the current studies, NFA also abolished the nitrergic component of the IJP and markedly attenuated the unitary potentials. Recently, Craven et al. (2004) have reported that NO blocked spontaneous transient inward currents via a cGMP-dependent pathway in isolated cells in rabbit corpus cavernosum smooth muscle, providing further support for closure of ClCa channels as the mechanism underlying the nitrergic IJP.
In contrast, Imaeda and Cunnane (2003) have reported that glibenclamide, a blocker of KATP channels, partially inhibited the nitrergic IJP in murine LES, suggesting that activation of KATP channels by the NO-cGMP pathway plays some role in the generation of the nitrergic IJP. However, the degree of attenuation of the IJP by glibenclamide was quite small in comparison to NFA and unitary potentials appeared unaffected by glibenclamide.
Attributing the nitrergic IJP to closing of ClCa channels implies that ClCa channels have basal activity in the resting state. Recent publications have proposed that unitary membrane potentials (Edwards et al., 1999) can be regarded as a marker of ClCa channel activity (Zhang and Paterson, 2002, 2003). The basal activation of ClCa channels results from spontaneous Ca2+ release from sarcoplasmic reticulum (Hogg et al., 1994; Janssen and Sims, 1994; Large and Wang, 1996; Zhang and Paterson, 2003) and involves a complicated signal cascade, the details of which remain unclear. We have found that drugs interfering with sarcoplasmic reticulum function (for example caffeine), myosin light-chain kinase inhibitors or ClCa channel blockers hyperpolarize RMP and abolish the unitary potentials and the nitrergic IJP in opossum oesophageal smooth muscle (Zhang and Paterson, 2002, 2003) and mouse LES. This suggests that spontaneous release of Ca2+ from sarcoplasmic reticulum activates ClCa channel via Ca2+-dependent activation of myosin light-chain kinase, and that the nitrergic IJP results from the closing of ClCa channels. Therefore, any intervention that blocks the unitary potentials is theoretically expected to inhibit nitrergic IJP as well. These results also suggest that myosin light-chain kinase is an important cross-link between ClCa channel activity and contractile protein regulation.
The mechanisms underlying the unusual long-lasting component of the nitrergic IJP observed in the clasp muscle are uncertain, but clearly this involves a more complicated mechanism in the clasp compared to the sling muscles. NFA blocks both the initial and long-lasting components of this nitrergic IJP, suggesting that Clca channels are involved in both phases of the IJP. Further physiological and anatomical studies are required to determine whether the biphasic IJP on the right side is due to pre- or post-synaptic factors, differences in intracellular signalling pathways or possibly regional differences in interstitial cells of Cajal, which are believed to be involved in coupling neural signals to gastro-intestinal smooth muscle (Ward and Sanders, 2001; Hirst and Ward, 2003; Hirst and Edwards, 2004; Sanders et al., 2004; Ward et al., 2004).
Acknowledgments
Supported by Canadian Institutes of Health Research Grant no. MT 9978 (WP) and a VA Merit Award (HM). The authors thank Dr Michael Blennerhassett for his assistance with the photography of the mouse gastroesophageal junction.
Abbreviations
- ClCa
calcium-activated chloride channels
- CSM
circular smooth muscle
- eNOS
endothelial NOS
- LES
lower oesophageal sphincter
- lsIJP
long-lasting slow IJP
- L-NAME
N-nitro-L-arginine methyl ester
- NFA
niflumic acid
- nNOS
neuronal NOS
Conflict of interest
The authors state no conflict of interest.
References
- Beckett EA, Bayguinov YR, Sanders KM, Ward SM, Hirst GD. Properties of unitary potentials generated by intramuscular interstitial cells of Cajal in the murine and guinea-pig gastric fundus. J Physiol. 2004;559:259–269. doi: 10.1113/jphysiol.2004.063016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett MR.Non-adrenergic non-cholinergic (NANC) transmission to smooth muscle: 35 years on Prog Neurobiol 199752159–195.[Review] [261 refs] [DOI] [PubMed] [Google Scholar]
- Brookes SJ, Chen BN, Hodgson WM, Costa M. Characterization of excitatory and inhibitory motor neurons to the guinea-pig lower esophageal sphincter. Gastroenterology. 1996;111:108–117. doi: 10.1053/gast.1996.v111.pm8698189. [DOI] [PubMed] [Google Scholar]
- Conklin JL, Du C, Murray JA, Bates JN. Characterization and mediation of inhibitory junction potentials from opossum lower esophageal sphincter. Gastroenterology. 1993;104:1439–1444. doi: 10.1016/0016-5085(93)90353-e. [DOI] [PubMed] [Google Scholar]
- Craven M, Sergeant GP, Hollywood MA, McHale NG, Thornbury KD. Modulation of spontaneous Ca2+-activated Cl− currents in the rabbit corpus cavernosum by the nitric oxide–cGMP pathway. J Physiol (Lond) 2004;556:495–506. doi: 10.1113/jphysiol.2003.058628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crist JR, He XD. Noncholinergic membrane potential responses to transmural nerve stimulation in the guinea-pig ileum. Am J Physiol. 1991;260:G240–G249. doi: 10.1152/ajpgi.1991.260.2.G240. [DOI] [PubMed] [Google Scholar]
- Crist JR, He XD, Goyal RK. Chloride-mediated inhibitory junction potentials in opossum esophageal circular smooth muscle [see comments] Am J Physiol. 1991a;261:G752–G762. doi: 10.1152/ajpgi.1991.261.5.G752. [DOI] [PubMed] [Google Scholar]
- Crist JR, He XD, Goyal RK. Chloride-mediated junction potentials in circular muscle of the guinea-pig ileum. Am J Physiol. 1991b;261:G742–G751. doi: 10.1152/ajpgi.1991.261.5.G742. [DOI] [PubMed] [Google Scholar]
- Daniel EE, Jury J, Wang YF. nNOS in canine lower esophageal sphincter: colocalized with Cav-1 and Ca2+-handling proteins. Am J Physiol. 2001;281:G1101–G1114. doi: 10.1152/ajpgi.2001.281.4.G1101. [DOI] [PubMed] [Google Scholar]
- Daniel EE, Taylor GS, Holman ME. The myogenic basis of active tension in the lower esophageal sphincter. Gastroenterology. 1976;70:874. [Google Scholar]
- Diamant NE. Gastrointestinal sphincters: up and down and ups and downs. Neurogastroenterol Motil. 2005;17 Suppl 1:1–2. doi: 10.1111/j.1365-2982.2005.00674.x. [DOI] [PubMed] [Google Scholar]
- Edwards FR, Hirst GD, Suzuki H. Unitary nature of regenerative potentials recorded from circular smooth muscle of guinea-pig antrum. J Physiol. 1999;519 Part 1:235–250. doi: 10.1111/j.1469-7793.1999.0235o.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox JA, Daniel EE. Role of Ca2+ in genesis of lower esophageal sphincter tone and other active contractions. Am J Physiol. 1979;237:E163–E171. doi: 10.1152/ajpendo.1979.237.2.E163. [DOI] [PubMed] [Google Scholar]
- Goyal RK. Targets of enteric motor neurones: smooth muscle cells. Gut. 2000;47 Suppl 4:iv38–iv39. doi: 10.1136/gut.47.suppl_4.iv38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goyal RK, Paterson WG.Esophageal motility Hand Book of Physiology 1989Oxford University Press: Bethesda, MD; 865–908.In Schultz SG, Wood JD and Rauner BB (eds). [Google Scholar]
- Hirst GD, Edwards FR. Role of interstitial cells of Cajal in the control of gastric motility. J Pharmacol Sci. 2004;96:1–10. doi: 10.1254/jphs.crj04002x. [DOI] [PubMed] [Google Scholar]
- Hirst GD, Ward SM. Interstitial cells: involvement in rhythmicity and neural control of gut smooth muscle. J Physiol. 2003;550:337–346. doi: 10.1113/jphysiol.2003.043299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogg RC, Wang Q, Large WA. Action of niflumic acid on evoked and spontaneous calcium-activated chloride and potassium currents in smooth muscle cells from rabbit portal vein. Br J Pharmacol. 1994;112:977–984. doi: 10.1111/j.1476-5381.1994.tb13177.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang PL, Huang Z, Mashimo H, Bloch KD, Moskowitz MA, Bevan JA, et al. Hypertension in mice lacking the gene for endothelial nitric oxide synthase. Nature. 1995;377:239–242. doi: 10.1038/377239a0. [DOI] [PubMed] [Google Scholar]
- Imaeda K, Cunnane TC. Electrophysiological properties of inhibitory junction potential in murine lower oesophageal sphincter. J Smooth Muscle Res. 2003;39:119–133. doi: 10.1540/jsmr.39.119. [DOI] [PubMed] [Google Scholar]
- Janssen LJ, Sims SM. Spontaneous transient inward currents and rhythmicity in canine and guinea-pig tracheal smooth muscle cells. Pflugers Arch. 1994;427:473–480. doi: 10.1007/BF00374263. [DOI] [PubMed] [Google Scholar]
- Ji J, Lau H, Sheu L, Diamant NE, Gaisano HY. Distinct regional expression of SNARE proteins in the feline oesophagus. Neurogastroenterol Motil. 2002;14:383–394. doi: 10.1046/j.1365-2982.2002.00343.x. [DOI] [PubMed] [Google Scholar]
- Ji J, Salapatek AM, Diamant NE. Inwardly rectifying K+ channels in esophageal smooth muscle. Am J Physiol Gastrointest. 2000;279:G951–G960. doi: 10.1152/ajpgi.2000.279.5.G951. [DOI] [PubMed] [Google Scholar]
- Jury J, Ahmedzadeh N, Daniel EE. A mediator derived from arginine mediates inhibitory junction potentials and relaxations in lower esophageal sphincter: an independent role for vasoactive intestinal peptide. Can J Physiol Pharmacol. 1992;70:1182–1189. doi: 10.1139/y92-164. [DOI] [PubMed] [Google Scholar]
- Jury J, Jager LP, Daniel EE. Unusual potassium channels mediate nonadrenergic noncholinergic nerve-mediated inhibition in opossum esophagus. Can J Physiol Pharmacol. 1985;63:107–112. doi: 10.1139/y85-020. [DOI] [PubMed] [Google Scholar]
- Krysiak PS, Preiksaitis HG. Tachykinins contribute to nerve mediated contractions in the human esophagus. Gastroenterology. 2001;120:39–48. doi: 10.1053/gast.2001.20910. [DOI] [PubMed] [Google Scholar]
- Kubota M, Suita S, Szurszewski JH. Membrane properties and the neuro-effector transmission of smooth muscle cells in the canine internal anal sphincter. J Smooth Muscle Res. 1998;34:173–184. doi: 10.1540/jsmr.34.173. [DOI] [PubMed] [Google Scholar]
- Large WA, Wang Q. Characteristics and physiological role of the Ca2+-activated Cl− conductance in smooth muscle. Am J Physiol. 1996;271:C435–C454. doi: 10.1152/ajpcell.1996.271.2.C435. [DOI] [PubMed] [Google Scholar]
- L'Heureux MC, Muinuddin A, Gaisano HY, Diamant NE. Feline lower esophageal sphincter sling and circular muscles have different functional inhibitory neuronal responses. Am J Physiol. 2006;290:G23–G29. doi: 10.1152/ajpgi.00303.2005. [DOI] [PubMed] [Google Scholar]
- Liebermann-Meffert D, Allgower M, Schmid P, Blum AL. Muscular equivalent of the lower esophageal sphincter. Gastroenterology. 1979;76:31–38. [PubMed] [Google Scholar]
- Makhlouf GM, Murthy KS. Signal transduction in gastrointestinal smooth muscle. Cell Signal. 1997;9:269–276. doi: 10.1016/s0898-6568(96)00180-5. [DOI] [PubMed] [Google Scholar]
- Mashimo H, He XD, Huang PL, Fishman MC, Goyal RK. Neuronal constitutive nitric oxide synthase is involved in murine enteric inhibitory neurotransmission. J Clin Invest. 1996;98:8–13. doi: 10.1172/JCI118781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muinuddin A, Kang Y, Gaisano HY, Diamant NE. Regional differences in L-type Ca2+ channel expression in feline lower esophageal sphincter. Am J Physiol. 2004a;287:G772–G781. doi: 10.1152/ajpgi.00102.2004. [DOI] [PubMed] [Google Scholar]
- Muinuddin A, Neshatian L, Gaisano HY, Diamant NE. Calcium source diversity in feline lower esophageal sphincter circular and sling muscle. Am J Physiol. 2004b;286:G271–G277. doi: 10.1152/ajpgi.00291.2003. [DOI] [PubMed] [Google Scholar]
- Murthy KS. Signaling for contraction and relaxation in smooth muscle of the gut. Annu Rev Physiol. 2006;68:345–374. doi: 10.1146/annurev.physiol.68.040504.094707. [DOI] [PubMed] [Google Scholar]
- Murthy KS, Makhlouf GM. Vasoactive intestinal peptide/pituitary adenylate cyclase-activating peptide-dependent activation of membrane-bound NO synthase in smooth muscle mediated by pertussis toxin-sensitive Gi1−2. J Biol Chem. 1994;269:15977–15980. [PubMed] [Google Scholar]
- Murthy KS, Zhang KM, Jin JG, Grider JR, Makhlouf GM. VIP-mediated G protein-coupled Ca2+ influx activates a constitutive NOS in dispersed gastric muscle cells. Am J Physiol. 1993;265:G660–G671. doi: 10.1152/ajpgi.1993.265.4.G660. [DOI] [PubMed] [Google Scholar]
- Preiksaitis HG, Diamant NE. Regional differences in cholinergic activity of muscle fibers from the human gastroesophageal junction. Am J Physiol. 1997;272:G1321–G1327. doi: 10.1152/ajpgi.1997.272.6.G1321. [DOI] [PubMed] [Google Scholar]
- Preiksaitis HG, Tremblay L, Diamant NE. Cholinergic responses in the cat lower esophageal sphincter show regional variation. Gastroenterology. 1994;106:381–388. doi: 10.1016/0016-5085(94)90596-7. [DOI] [PubMed] [Google Scholar]
- Sanders KM. A case for interstitial cells of Cajal as pacemakers and mediators of neurotransmission in the gastrointestinal tract. Gastroenterology. 1996;111:492–515. doi: 10.1053/gast.1996.v111.pm8690216. [DOI] [PubMed] [Google Scholar]
- Sanders KM, Koh SD, Ordog T, Ward SM. Ionic conductances involved in generation and propagation of electrical slow waves in phasic gastrointestinal muscles. Neurogastroenterol Motil. 2004;16 Suppl 1:100–105. doi: 10.1111/j.1743-3150.2004.00483.x. [DOI] [PubMed] [Google Scholar]
- Sanders KM, Ozaki H.Excitation-contraction coupling in gastrointestinal smooth muscles Pharmacology of Smooth Muscle 1994Springer-Verlag: Berlin; 331–404.In: Szekeres L and Papp JG (eds). [Google Scholar]
- Tian ZQ, Liu JF, Wang GY, Li BQ, Wang FS, Wang QZ, et al. Responses of human clasp and sling fibers to neuromimetics. J Gastroenterol Hepatol. 2004;19:440–447. doi: 10.1111/j.1440-1746.2003.03307.x. [DOI] [PubMed] [Google Scholar]
- Ward SM, Morris G, Reese L, Wang XY, Sanders KM. Interstitial cells of Cajal mediate enteric inhibitory neurotransmission in the lower esophageal and pyloric sphincters. Gastroenterology. 1998;115:314–329. doi: 10.1016/s0016-5085(98)70198-2. [DOI] [PubMed] [Google Scholar]
- Ward SM, Sanders KM. Interstitial cells of Cajal: primary targets of enteric motor innervation. Anat Rec. 2001;262:125–135. doi: 10.1002/1097-0185(20010101)262:1<125::AID-AR1017>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Ward SM, Sanders KM, Hirst GD. Role of interstitial cells of Cajal in neural control of gastrointestinal smooth muscles. Neurogastroenterol Motil. 2004;16 Suppl 1:112–117. doi: 10.1111/j.1743-3150.2004.00485.x. [DOI] [PubMed] [Google Scholar]
- Yuan S, Brookes SJ. Neuronal control of the gastric sling muscle of the guinea-pig. J Comp Neurol. 1999;412:669–680. doi: 10.1002/(sici)1096-9861(19991004)412:4<669::aid-cne8>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Yuan S, Costa M, Brookes SJ. Neuronal pathways and transmission to the lower esophageal sphincter of the guinea-pig. Gastroenterology. 1998;115:661–671. doi: 10.1016/s0016-5085(98)70145-3. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Lang RJ. Effects of intrinsic prostaglandins on the spontaneous contractile and electrical activity of the proximal renal pelvis of the guinea-pig. Br J Pharmacol. 1994;113:431–438. doi: 10.1111/j.1476-5381.1994.tb17007.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Miller DV, Paterson WG. Opposing roles of K+ and Cl− channels in maintenance of opossum lower esophageal sphincter tone. Am J Physiol. 2000;279:G1226–G1234. doi: 10.1152/ajpgi.2000.279.6.G1226. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Paterson WG. Nitric oxide contracts longitudinal smooth muscle of opossum oesophagus via excitation–contraction coupling. J Physiol. 2001;536:133–140. doi: 10.1111/j.1469-7793.2001.00133.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Paterson WG. Role of Ca2+-activated Cl− channels and MLCK in opossum esophageal smooth muscle. Am J Physiol. 2002;283:G104–G114. doi: 10.1152/ajpgi.00052.2002. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Paterson WG. Role of sarcoplasmic reticulum in control of membrane potential and nitrergic response in opossum lower esophageal sphincter. Br J Pharmacol. 2003;140:1097–1107. doi: 10.1038/sj.bjp.0705537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Paterson WG. Excitatory purinergic neurotransmission in smooth muscle of guinea-pig taenia caeci. J Physiol. 2005;563:855–865. doi: 10.1113/jphysiol.2004.077636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Vogalis F, Goyal RK. Nitric oxide suppresses a Ca2+-stimulated Cl− current in smooth muscle cells of opossum esophagus. Am J Physiol. 1998;274:G886–G890. doi: 10.1152/ajpgi.1998.274.5.G886. [DOI] [PubMed] [Google Scholar]