Abstract
Background and purpose:
Epidemiological data suggest that the risk of ethanol-associated cardiovascular disease is greater in men than in women. This study investigates the mechanisms underlying gender-specific vascular effects elicited by chronic ethanol consumption in rats.
Experimental approach:
Vascular reactivity experiments using standard muscle bath procedures were performed on isolated thoracic aortae from rats. mRNA and protein for inducible NO synthase (iNOS) and for endothelial NOS (eNOS) was assessed by RT-PCR or western blotting, respectively.
Key results:
In male rats, chronic ethanol consumption enhanced phenylephrine-induced contraction in both endothelium-intact and denuded aortic rings. However, in female rats, chronic ethanol consumption enhanced phenylephrine-induced contraction only in endothelium denuded aortic rings. After pre-incubation of endothelium-intact rings with L-NAME, both male and female ethanol-treated rats showed larger phenylephrine-induced contractions in aortic rings, compared to the control group. Acetylcholine-induced relaxation was not affected by ethanol consumption. The effects of ethanol on responses to phenylephrine were similar in ovariectomized (OVX) and intact (non-OVX) female rats. In the presence of aminoguanidine, but not 7-nitroindazole, the contractions to phenylephrine in rings from ethanol-treated female rats were greater than that found in control tissues in the presence of the inhibitors. mRNA levels for eNOS and iNOS were not altered by ethanol consumption. Ethanol intake reduced eNOS protein levels and increased iNOS protein levels in aorta from female rats.
Conclusions and implications:
Gender differences in the vascular effects elicited by chronic ethanol consumption were not related to ovarian hormones but seemed to involve the upregulation of iNOS.
Keywords: ethanol consumption, aorta, gender, nitric oxide, inducible nitric oxide synthase
Introduction
Chronic ethanol consumption has been implicated as a causative factor in a variety of cardiovascular abnormalities (Altura and Altura, 1982). Long-term ethanol intake is associated with hypertension in humans (Moore et al., 1990) and increased blood pressure in rats (Utkan et al., 2001; Resstel et al., 2006). Previous reports suggest that enhanced vascular reactivity to vasoconstrictor agents (Tirapelli et al., 2006a, 2006b) or impairment of vascular relaxation (Kahonen et al., 1999; Tirapelli et al., 2006b) contributes to cardiovascular complications associated with chronic ethanol consumption. For instance, enhanced vascular reactivity to α1-adrenoceptor agonists was demonstrated in different arteries from ethanol-treated rats (Chan and Sutter, 1983; Tirapelli et al., 2006a), but reduced (Strickland and Wooles, 1988) or unchanged (Utkan et al., 2001) responses have also been reported. It was previously suggested that the discrepancy among the above-mentioned results could be attributed to differences in treatment protocols, duration of ethanol consumption or gender (Utkan et al., 2001). It is interesting to note that epidemiological data show the risk of ethanol-associated cardiovascular disease to be greater in men than in women (Manolio et al., 1991). In addition, the relationship between ethanol and increased blood pressure is clearer in men than in women (Fortmann et al., 1983; Klatsky et al., 1986). The mechanisms responsible for such observations are poorly understood, although gender-related variation in vascular responsiveness may play a role. Stewart and Kennedy (1999) showed that male, but not female, rats demonstrated an ethanol-associated increase in the maximum response induced by phenylephrine in isolated aortic rings. The mechanisms underlying this proposed gender differences in vascular responsiveness to chronic ethanol consumption are yet unknown. Some reports suggest that sex hormones may play a role in the cardiovascular gender-related effects induced by ethanol (Fortmann et al., 1983; El-Mas and Abdel-Rahman, 1999). In fact, oestrogen has been reported to increase nitric oxide (NO) production (Weiner et al., 1994) and antagonize in vitro responses to noradrenaline and other vasoconstrictors (Cohen and Susemichel, 1996). As ethanol consumption enhances vascular reactivity to vasoconstrictor agents (Tirapelli et al., 2006a, 2006b), oestrogen-induced NO production could counteract the increased response to vasoconstrictor agents induced by ethanol.
The above-mentioned studies indicate that detailed description of the mechanisms underlying gender differences in the effect elicited by long-term ethanol consumption on the control of arterial tone remain still largely unknown. Thus, the purposes of this study were to compare the effects of ethanol consumption on phenylephrine-induced contraction of aortic rings from male and female rats in an attempt to understand the mechanism underlying gender-specific vascular response to chronic ethanol consumption. Our data showed that gender differences in the vascular effects elicited by chronic ethanol consumption are not related to ovarian hormones, but seemed to involve the upregulation of the inducible isoform of NO synthase (iNOS).
Methods
Experimental design
Housing conditions and experimental protocols are in accordance with the Ethical Animal Committee of the University of São Paulo, Campus of Ribeirão Preto. Female and male Wistar rats were housed under standard laboratory conditions with free access to food and water.
Animals with a body weight of 230–260 g (50–60 days old) were randomly divided into three groups at the start of the experiment: control, isocaloric and ethanol. Rats in the control group received tap water ad libitum; the isocaloric group received a solution containing an isocaloric amount of sucrose (290.5 g l−1) instead of ethanol; and rats in the ethanol group received 20% (v/v) ethanol in their drinking water (Tirapelli et al., 2006a, 2006b). The ethanol-treated group was submitted to a brief and gradual period adaptation: the animals received 5% ethanol in their drinking water in the first week, 10% in the second week and 20% in the third week (all values in volume ratios). At the end of the third week the experimental stage began. The same procedure was adopted for the isocaloric group. In these groups, the caloric content of the liquid diet was adjusted to match that of the ethanol-exposed group. The isocaloric animals were included in the study protocol to evaluate whether alterations in caloric intake following ethanol consumption would explain the possible influences of ethanol on arterial responses. The rats were treated for 4 weeks and weighed weekly.
Surgical ovariectomy
Female Wistar rats were anaesthetized with ketamine (46 mg kg−1) and xylazine (14 mg kg−1) i.p. and were subjected to bilateral ovariectomy (OVX), in which the ovaries were ligated and removed (Fukada et al., 2004). Seven days after OVX, the animals were divided into two groups, control and ethanol-treated, as mentioned above. To confirm whether OVX was successful, vaginal cells were smeared onto a slide with a drop of saline and observed under a light microscope for identification of cell shape. In ovariectomized rats, the vaginal contents consistently lacked cornified cells, whereas leukocytes were very abundant (Montes and Luque, 1988).
Blood ethanol levels
The ethanol content was measured as previously described (Resstel et al., 2006; Tirapelli et al., 2006a). Briefly, blood was collected from the aorta of anaesthetized rats using heparinzed syringes, and the samples were placed in 10-ml headspace vials by adding 1.0 g sodium chloride, 1.0 ml water, 100 μl of the internal standard (acetonitrile) solution and 1.0 ml of blood. Ethanol analysis was carried out using a CG-17A gas chromatograph (Shimadzu, Kyoto, Japan) equipped with a flame-ionization detector and an HSS-4A headspace sampler (Shimadzu). Calibrations standards were prepared in the same headspace vials (0.10–3.16 mg ml−1). The results are expressed as mg ethanol per ml blood.
Preparation of aortic rings
Animals were anaesthetized and the thoracic aorta was quickly removed and cut into rings (5–6 mm in length). The arteries were prepared for organ bath studies as described previously (Tirapelli et al., 2006a). The composition of the Krebs solution was as follows (in mmol l−1): NaCl, 118.0; KCl, 4.7; KH2PO4, 1.2; MgSO4, 1.2; NaHCO3, 15.0; glucose, 5.5; and CaCl2, 2.5. The rings were stretched until an optimal basal tension of 1.5 g, which was determined by length–tension relationship experiments. Endothelial integrity was assessed qualitatively by the degree of relaxation caused by ACh (1 μmol l−1) in the presence of contractile tone induced by phenylephrine (0.1 μmol l−1). For studies of endothelium-intact vessels, the ring was discarded if relaxation with ACh was not 80% or greater. For studies of endothelium-denuded vessels, rings were discarded if there was any measurable degree of relaxation.
Protocols for vascular reactivity assays
After equilibration for 60 min in the Krebs solution, each aortic ring was exposed twice to phenylephrine (0.1 μmol l−1) to assess its maximum contractility. Each ring was sequentially washed and re-equilibrated and was allowed to relax to baseline. After 30 min, cumulative concentration–response curves for phenylephrine (0.0001–10 μmol l−1) were determined on endothelium-intact or -denuded aortic rings from male and female rats. The vascular responsiveness to phenylephrine was studied in tissues from the control, isocaloric and ethanol-treated groups. In another set of experiments, aortic rings from female rats from the control and the ethanol-treated groups were stimulated with angiotensin II (0.0001–0.3 μmol l−1) or 90 mmol l−1 KCl.
To further investigate the putative protective effect of the endothelium in female rats, concentration–response curves for phenylephrine were obtained from the control and ethanol-treated OVX rats. The curves were obtained on endothelium-intact and -denuded rings from control and ethanol-treated female rats and subsequently compared to those obtained from non-OVX (intact) female rats.
Contribution of NO in modulating the response to phenylephrine
Cumulative concentration–response curves for phenylephrine were obtained on endothelium-intact aortic rings from control and ethanol-treated male or female rats. The curves were performed in the absence or after incubation for 30 min with the non-selective NO synthase (NOS) inhibitor NG-nitro-L-arginine methyl ester (L-NAME) (100 μmol l−1) (Rees et al., 1990).
Effect of ethanol consumption on basal NO production
To determine the contribution of basal or spontaneous production and release of NO, concentration–response curves for L-NAME (0.1–300 μmol l−1) were obtained on endothelium-intact aortic rings of male and female animals that were pre-contracted submaximally with phenylephrine 0.01 μmol l−1 (that is, to induce 10–20% maximal response). These experiments were performed in aortic rings from control and 4-week ethanol-treated rats. In another set of experiments, increase in tension induced by L-NAME was obtained after pre-incubation of the rings from female rats with aminoguanidine (100 μmol l−1) for 30 min.
Effect of ethanol consumption on ACh and sodium nitroprusside-induced relaxation
The concentration–response curves for ACh were determined on endothelium-intact rings, whereas the relaxation induced by sodium nitroprusside (SNP) was determined on endothelium-denuded tissues. The aortic rings from male or female rats were pre-contracted with phenylephrine (0.1 μmol l−1) and when the contraction reached a plateau, ACh (0.0001–10 μmol l−1) or SNP (0.0001–0.01 μmol l−1) was added cumulatively. The magnitude of contraction induced by phenylephrine did not differ between the experimental groups at 0.1 μmol l−1. The curves for SNP, an NO donor, were performed to test the responsiveness of the NO pathway in the vascular smooth muscle. In another set of experiments, concentration–response curves for ACh were performed after incubation with L-NAME (30 min, 100 μmol l−1). Relaxation was expressed as a percentage change from phenylephrine-contracted levels.
Contribution of NOS isoforms in modulating the response to phenylephrine in female rats
Cumulative concentration–response curves for phenylephrine were obtained in endothelium-intact aortic rings from control and ethanol-treated female rats in the absence and presence of either aminoguanidine at 100 μmol l−1 (Joly et al., 1994; Tokuno et al., 2002)—a selective inducible NOS (iNOS) inhibitor—or 7-nitroindazole (7-NI) at 100 μmol l−1 (Chinellato et al., 1998)—a selective neuronal NOS (nNOS) inhibitor.
Reverse transcriptase-PCR
Reverse transcriptase (RT)-PCR was carried out using extracts of female rat aortae with intact endothelium from control or ethanol-treated rats. Total cell RNA was isolated from aorta arteries using Trizol Reagent (Gibco BRL, Life Technologies, Rockville, MD, USA). After DNA digestion (RQ1 DNAse RNAse-free; Promega Corporation, Madison, WI, USA), total RNA (20 ng per sample) was used for RT in the presence of an RNase inhibitor (RnasIn; Promega Corporation), 200 U of Moloney murine leukaemia virus RT (Gibco BRL) and 1 μg of oligo (dT) 12–18 primers at 37 °C for 60 min, according to the specifications from the manufacturer. The cDNA products were isolated by phenol–chloroform extraction, precipitated with ethanol, resuspended in 120 μl TE (10 mmol l−1 Tris-HCl and 1 mmol l−1 EDTA, pH 7.5) and stored at −20 °C until required for PCR. PCR primers were designed on the basis of published rat cDNA sequences: glyceraldehyde 3-phosphate dehydrogenase (GAPDH), antisense primer CACCACCCTGTTGCTGTA and sense primer TATGATGACATCAAGAAGGTGG (219-bp fragment); endothelial NOS (eNOS), antisense primer CTGGCCTTCTGCTCATTTTC and sense primer TGACCCTCACCGATACAACA (210-bp fragment); and iNOS, antisense primer CTCCTGGGAGCTTTGTTGAG and sense primer TGGTGGTGACAAGCACATTT (418-bp fragment). GAPDH was used as an internal control for the co-amplification. To identify the optimal amplification conditions, a series of pilot studies were performed using a thermal cycler with a temperature gradient in the annealing step (Eppendorf Mastercycler gradient; Eppendorf-Netheler-Hinz, Hamburg, Germany), various amounts of RT products from 2 to 200 ng RNA and 20–35 cycles of PCR amplification. The following conditions were selected for PCR in a volume of 50 μl: RT products from 20 ng of RNA, 2.5 U Taq polymerase (Gibco BRL), 26 cycles for the eNOS gene, 34 cycles for the iNOS gene and 24 cycles for the GAPDH gene. Amplification was carried out using an initial denaturing cycle at 94 °C for 5 min and the subsequent cycles were as follows: denaturation, 30 s at 94 °C; annealing, 30 s at 60 °C for eNOS, 60 °C for iNOS, 62 °C for GAPDH; and extension, 45 s at 72 °C. PCR products (10 μl per lane) were electrophoresed using 1% agarose gel containing ethidium bromide (0.5 μg ml−1) and the identity of cDNA products was confirmed by DNA sequence analysis. The gel was subjected to ultraviolet light and photographed. The band intensities were measured using a software package (Kodak Digital Science, Eastman Kodak Company, New Haven, CT, USA), and the signals are reported relatively to the intensity of GAPDH amplification in each co-amplified sample.
Western immunoblotting
Aortic samples from female rats were homogenized in 50 mmol l−1 Tris-HCl lysis buffer containing 2 mmol l−1 EDTA, 5 mmol l−1 EGTA, 1 mmol l−1 PMSF (phenylmethylsulphonyl fluoride), 1 mmol l−1 Na3VO4 and 2 μl protease inhibitor cocktail (P-8340; Sigma, St Louis, MO, USA) per ml of reaction. Equal amounts of protein (50 μg per sample) were separated on 7.5% polyacrylamide gels and transferred to polyvinylidene difluoride membranes. Membranes were blocked with Tris-buffered saline and 0.1% Tween-20 containing 5% non-fat dry milk for 1 h at room temperature. Membranes were then incubated with polyclonal eNOS or iNOS antibodies (1:1000; BD Transduction Laboratories, San Diego, CA, USA) overnight at 4 °C. After incubation with secondary antibodies, signals were revealed with chemiluminescence, visualized by autoradiography and quantified by densitometry.
Statistical analysis
Two pharmacological parameters were obtained from concentration–response curves: the maximal effect generated by the agonist (or Emax) and −log EC50 (or pD2). Results were expressed as mean±s.e.mean. Statistical analyses of Emax and pD2 values were performed using one-way ANOVA or Student's t-test. Post hoc comparisons were performed using Bonferroni's multiple comparison test as indicated in the text, legends and tables. A P-value below 0.05 was considered significant.
Drugs
The following drugs were used: phenylephrine hydrochloride, ACh hydrochloride, 7-NI, aminoguanidine, SNP (Sigma), L-NAME (Sigma/RBI, Natick, MA, USA). 7-NI was prepared as a stock solution in dimethylsulphoxide (DMSO). The other drugs were dissolved in distilled water. The bath concentration of DMSO did not exceed 0.5%, which was shown to have no effect per se on the basal tonus of the preparations or on the agonist-mediated contraction.
Results
Body mass and blood ethanol levels
Before treatment, female rats showed mean body masses of 226±3 g (control group), 230±10 g (isocaloric) and 229±3 g (ethanol-treated). After treatment for 4 weeks, no variation in body mass was observed in animals across the three experimental groups: 305±7 g (control), 318±16 g isocaloric) and 291±7 g (ethanol-treated). Body mass of the male rats averaged 240±9 g in the control rats, 236±10 g in isocaloric rats and 245±12 g in ethanol-treated rats before treatment. After 4 weeks of ethanol administration, male rats from the ethanol-treated group showed a significant reduction in body mass (310±8 g) when compared to the control rats (349±11 g) and the isocaloric-treated animals (364±12 g) (P<0.05; ANOVA).
Blood ethanol levels in the ethanol-treated female animals averaged 1.4±0.1 mg ml−1 (n=7) and 1.7±0.2 mg ml−1 in male rats (n=8). No ethanol was detectable in the blood of control or isocaloric animals. The blood ethanol levels were not significantly different between male and female animals (Student's t-test).
Effect of chronic ethanol consumption on phenylephrine-induced contraction
Initially, we investigated a possible influence of the female reproductive cycle on the phenylephrine-induced contraction. Identification of the cycle was performed as previously described by Freeman (1994) and no differences were found in phenylephrine-induced contraction of endothelium-intact aorta from female rats during oestrus (Emax=17.8±2.3 mN; pD2=7.27±0.12, n=9), dioestrus (Emax=17.6±2.6 mN; pD2=7.25±0.14, n=10) or proestrus (Emax=16.2±1.4 mN; pD2=7.20±0.15, n=8) (ANOVA).
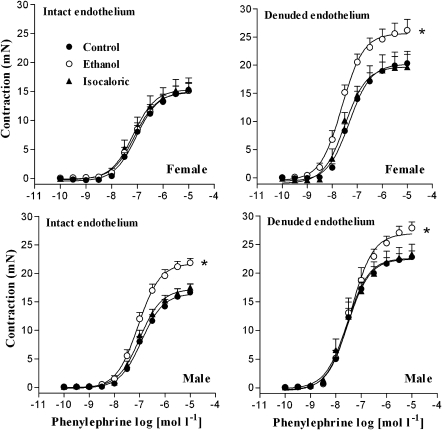
In addition, the Emax and pD2 values for phenylephrine did not differ significantly among endothelium-intact rings from the control, isocaloric or ethanol-treated female rats. After endothelial removal, the Emax values for phenylephrine were significantly greater in rings from ethanol-treated rats than in rings from control or isocaloric animals (Figure 1, Table 1). Conversely, in male rats, chronic ethanol consumption enhanced phenylephrine-induced contraction in both endothelium-intact and -denuded rings. However, no differences were observed in the pD2 values among the three experimental groups. It was also noticeable that mechanical removal of the endothelium significantly increased both Emax and pD2 values for phenylephrine in arteries from the control and isocaloric rats (Figure 1, Table 1).
Figure 1.
Effect of chronic ethanol consumption on phenylephrine-induced contraction in aortic rings from male and female rats. Concentration–response curves for phenylephrine were determined in endothelium-intact or endothelium-denuded aortic rings from control, isocaloric and ethanol-treated rats. *Compared to control and isocaloric groups (P<0.05; ANOVA).
Table 1.
Effect of chronic ethanol consumption on the Emax (mN) and pD2 values for phenylephrine in endothelium-intact (E+) or -denuded (E−) aortic rings from male and female rats
|
Control |
Isocaloric |
Ethanol |
||||
|---|---|---|---|---|---|---|
| Emax | pD2 | Emax | pD2 | Emax | pD2 | |
| Female (E+) | 15.2±1.3 (12) | 7.00±0.06 | 15.5±1.8 (8) | 7.09±0.18 | 15.1±1.2 (14) | 7.02±0.09 |
| Female (E−) | 20.3±1.6a (11) | 7.43±0.09 | 19.7±1.9a (6) | 7.54±0.12 | 27.2±1.8a,b (11) | 7.67±0.09 |
| Male (E+) | 16.6±1.4 (8) | 6.94±0.05 | 17.3±0.7 (6) | 6.98±0.07 | 22.4±1.0b (7) | 7.05±0.07 |
| Male (E−) | 22.5±1.0a (7) | 7.53±0.09 | 23.3±1.7a (7) | 7.50±0.16 | 27.8±1.3a,b (7) | 7.44±0.12 |
The number in parentheses indicates the number of animals per group. Values are means±s.e.mean.
Compared to corresponding E− group (ANOVA followed by Bonferroni's multiple comparison test, P<0.05).
Compared to respective control and isocaloric groups.
Angiotensin II-induced contraction in aortic rings from female rats was not altered after treatment with ethanol in either endothelium-intact (control: Emax=3.5±0.5 mN; pD2=7.59±0.07, n=5; ethanol: Emax=2.5±0.7 mN; pD2=7.48±0.20, n=4) or -denuded rings (control: Emax=8.0±1.3 mN; pD2: 7.55±0.06, n=5; ethanol: Emax=7.1±1.0 mN; pD2=7.74±0.10, n=5). Additionally, KCl-induced contraction of endothelium-intact rings was not different between control (19.7±1.9 mN; n=8) and ethanol-treated tissues (21±4.9 mN; n=4). Conversely, the contraction induced by KCl in endothelium-denuded rings was increased after the treatment with ethanol (31±4.0 mN; n=4) when compared to the control group (20±2.0 mN; n=4) (P<0.05; ANOVA).
Contribution of NO in modulating the response to phenylephrine
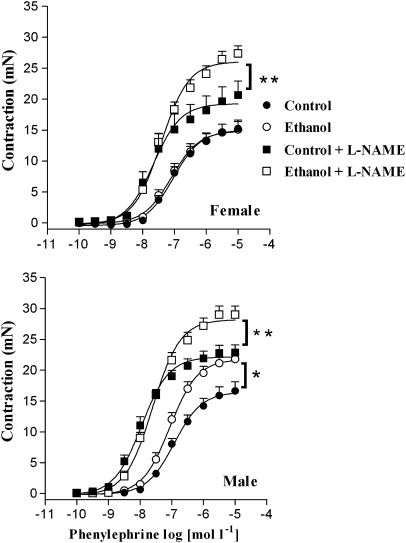
In both male and female rats, pre-incubation with L-NAME increased phenylephrine-induced contraction in endothelium-intact rings from control rats when compared to contraction obtained in the absence of the inhibitor. Similarly, L-NAME enhanced contraction induced by phenylephrine in tissues from the ethanol-treated rats from both sexes when compared to the group without the inhibitor (Figure 2, Table 2).
Figure 2.
Effect of L-NAME on phenylephrine-induced contraction of endothelium-intact aortic rings from male and female rats. Concentration–response curves for phenylephrine were determined in endothelium-intact aortic rings from control, isocaloric and ethanol-treated rats. The curves were determined in the absence or after 30-min incubation with L-NAME (100 μmol l−1). *Compared to the control group; **compared to control+L-NAME (P<0.05; ANOVA). L-NAME, NG-nitro-L-arginine methyl ester.
Table 2.
Effect of L-NAME (100 μmol l−1) on the Emax (mN) and pD2 values for phenylephrine in endothelium-intact aortic rings from male and female rats
|
Control |
Ethanol |
|||
|---|---|---|---|---|
| Emax | pD2 | Emax | pD2 | |
| Female | 15.2±1.3 (12) | 7.00±0.06 | 15.1±1.2 (14) | 7.02±0.09 |
| Female+ L-NAME | 20.7±2.2a (8) | 7.56±0.11a | 27.4±1.2b (9) | 7.41±0.07b |
| Male | 16.6±1.4 (8) | 6.94±0.05 | 22.4±1.0a (7) | 7.05±0.07 |
| Male+ L-NAME | 22.8±1.2 (11) | 7.98±0.08 | 29.0±1.3 (8) | 7.63±0.07 |
Abbreviations: L-NAME, NG-nitro-L-arginine methyl ester.
The number in parentheses indicates the number of animals per group. Values are means±s.e.mean.
Compared to control in the absence of the inhibitors.
Compared to ethanol in the absence of the inhibitors (ANOVA followed by Bonferroni's multiple comparison test, P<0.05).
Effect of ethanol consumption on basal NO production
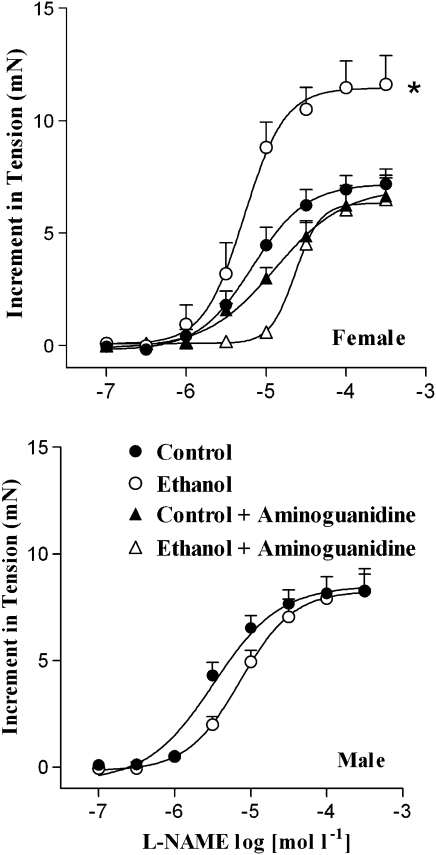
L-NAME, a non-selective NOS inhibitor, induced a concentration-dependent increase in the tension of endothelium-intact aortic rings from male and female rats that were submaximally pre-contracted with phenylephrine (Figure 3). In female rats, the increase in tension was significantly higher in rings from the ethanol-treated animals (Emax=11.6±1.2 mN; n=8) than in rings from the control animals (Emax=7.2±0.6 mN; n=10) (P<0.05; ANOVA). However, the pD2 values were not significantly different between tissues from the control (5.20±0.12) and the ethanol-treated rats (5.32±0.11). Pre-incubation of control rings from female rats with aminoguanidine, a selective iNOS inhibitor, induced a slight decrease in the pD2 value (4.95±0.06) but not in the Emax (6.6±0.8 mN; n=8) when compared to the control group in the absence of the inhibitor (P<0.05; ANOVA). Interestingly, in the presence of aminoguanidine, increase in ring tension in ethanol-treated female rats induced by L-NAME (Emax=6.5±1.1 mN; n=8) was reduced to values similar to the ones observed in control tissues. Moreover, there was a reduction in the pD2 value (4.61±0.12) after incubation with aminoguanidine when compared to the ethanol group in the absence of the inhibitor (P<0.05; ANOVA). L-NAME-induced increase in tension in male aortic rings was not different between control (Emax=7.7±1.0 mN; n=5) and ethanol-treated rats (Emax=7.3±1.3 mN; n=5). Additionally, no difference was found for the pD2 values from control (5.16±0.05) and ethanol-treated rats (5.14±0.07). When the endothelium was removed, L-NAME failed to increase the tension in aortic rings from both control and ethanol-treated rats (data not shown).
Figure 3.
Effect of ethanol consumption on L-NAME-evoked increases in isometric tension of endothelium-intact aortic rings. Arteries from male or female rats were submaximally contracted with phenylephrine (0.01 μmol l−1) and when the contraction reached a plateau, L-NAME (0.1–100 μmol l−1) was added cumulatively. In the arteries from female rats, the curves were determined in the absence or after 30-min incubation with aminoguanidine (100 μmol l−1). *Compared to the other groups (P<0.05; ANOVA). L-NAME, NG-nitro-L-arginine methyl ester.
Effect of chronic ethanol consumption on ACh and SNP-induced vasorelaxation
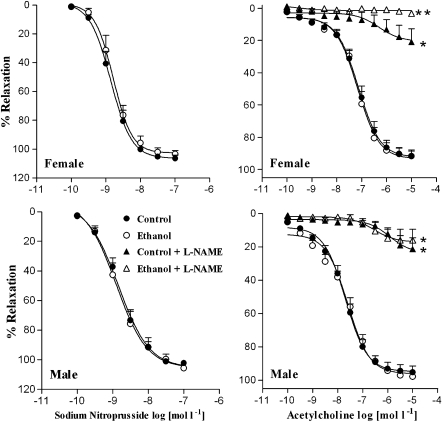
Figure 4 shows that the endothelium-dependent relaxation induced by ACh did not significantly differ between the tissues from control (Emax=90.13±3.79%; pD2=7.06±0.12, n=8) and ethanol-treated female rats (Emax=91.62±3.64%; pD2=6.99±0.11, n=9). Pre-incubation with L-NAME strongly inhibited ACh-induced relaxation in the rings from control (Emax=20.85±8.10%; pD2=6.60±0.15, n=5) or ethanol-treated female rats (Emax=3.16±1.5%, n=8). However, the inhibitory action displayed by L-NAME was more pronounced in the rings from ethanol-treated rats when compared to the control group (P<0.05; ANOVA). In male rats, ACh-induced relaxation was not different between the control (Emax=95.21±3.80%; pD2=7.70±0.10, n=10) and the ethanol groups (Emax=97.80±1.20%; pD2=7.67±0.11, n=5). L-NAME inhibited the ACh-induced relaxation in rings from the ethanol-treated rats (Emax=16.10±6.7%; pD2=6.49±0.11, n=5) to the same extent as the control (Emax=21.35±6.8%; pD2=6.45±0.17, n=6) (P<0.05; ANOVA).
Figure 4.
Effect of ethanol consumption on ACh- and SNP-induced relaxation. The concentration–response curves for ACh and sodium nitroprusside (SNP) were obtained in endothelium-intact and -denuded aortic rings, respectively. ACh-induced relaxation was determined in the absence or after 30-min incubation with L-NAME (100 μmol l−1). *Compared to control and ethanol-treated groups; **compared to control, ethanol-treated and control+L-NAME (P<0.05; ANOVA). L-NAME, NG-nitro-L-arginine methyl ester.
SNP-induced arterial relaxation of pre-contracted rings with phenylephrine did not significantly vary between the control (Emax=105.48±2.18%; pD2=8.85±0.10, n=7) and ethanol-treated female rats (Emax=104.68±2.11%; pD2=8.78±0.10, n=6). Similarly, SNP-induced endothelium-independent relaxation in the rings from male rats did not differ between the control (Emax=103.20±1.78%; pD2=8.79±0.09, n=7) and the ethanol-treated animals (Emax=114.56±9.03%; pD2=8.72±0.20, n=8) (Student's t-test).
Effect of chronic ethanol consumption on ovariectomized rats
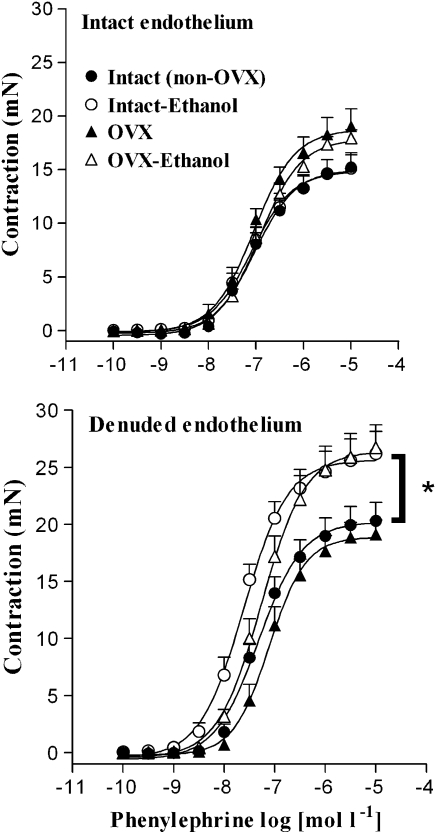
Body mass of female rats before OVX averaged 233±2 g in control animals and 234±2.2 g in ethanol-treated group. On the experimental day, body mass of control OVX rats averaged 387±12 g, with this value being significantly higher than that found in non-OVX control rats (305±7 g) (P<0.05; ANOVA). Similarly, body mass of ethanol OVX female rats averaged 324±8 g, in contrast with that found for non-OVX ethanol-treated rats (291±7 g) (P<0.05; ANOVA). Endothelium-intact aortic rings from non-OVX (intact) or OVX rats showed no differences in the Emax or pD2 values for phenylephrine between the control and ethanol-treated rats (Figure 5, Table 3). After endothelial removal, the Emax values for phenylephrine were significantly greater in rings from intact or OVX rats treated with ethanol when compared to the respective control group (Figure 5, Table 3).
Figure 5.
Effect of chronic ethanol consumption on phenylephrine-induced contraction in aortic rings from female intact (non-OVX) and OVX rats. Concentration–response curves for phenylephrine were determined in endothelium-intact or endothelium-denuded aortic rings from control and ethanol-treated female rats. *Compared to intact (non-OVX) and OVX groups (P<0.05; ANOVA). OVX, ovariectomy.
Table 3.
Effect of chronic ethanol consumption on the Emax (mN) and pD2 values for phenylephrine in endothelium-intact (E+) or denuded (E−) aortic rings from intact (non-OVX) and OVX female rats
| Groups |
Control |
Ethanol |
||
|---|---|---|---|---|
| Emax | pD2 | Emax | pD2 | |
| Non-OVX (E+) | 15.2±1.3a (12) | 7.00±0.06a | 15.1±1.2a (14) | 7.02±0.09a |
| Non-OVX (E−) | 20.3±1.6 (11) | 7.43±0.09 | 27.2±1.8b (11) | 7.67±0.09b |
| OVX (E+) | 19.0±1.6c (9) | 7.10±0.13 | 17.9±0.9a,c (7) | 6.90±0.10a |
| OVX (E−) | 19.2±1.2 (8) | 7.10±0.10 | 26.7±1.9 (9) | 7.27±0.07 |
Abbreviations: OVX, ovariectomy.
The number in parentheses indicates the number of animals per group. Values are means±s.e.mean.
Compared to corresponding E− group; compared to non-OVX (E+) group (ANOVA followed by Bonferroni's multiple comparison test, P<0.05).
Compared to respective control group.
Compared to non-OVX (Et) group.
Contribution of NOS isoforms in modulating the response to phenylephrine in female rats
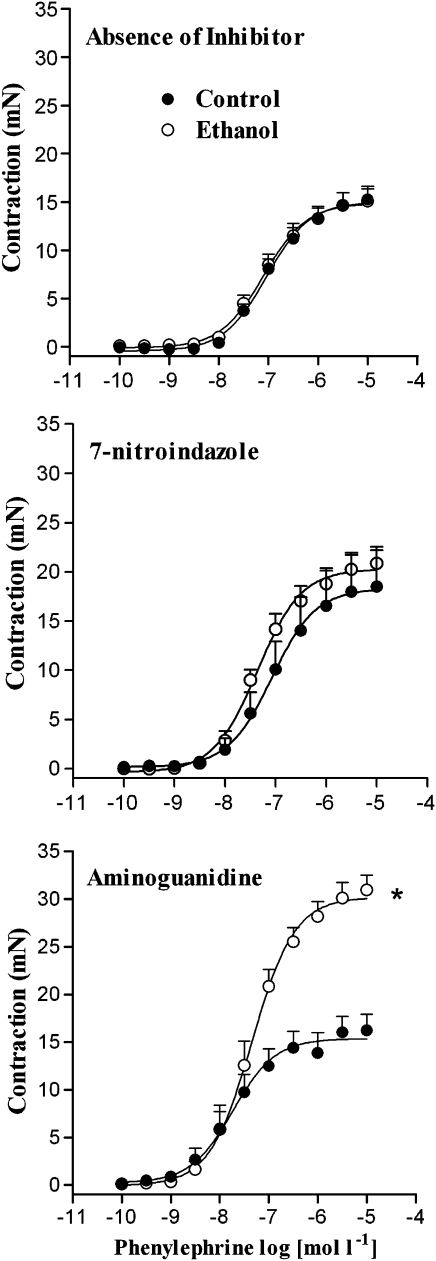
After pre-incubation with aminoguanidine, phenylephrine-induced contraction was greater in endothelium-intact rings from ethanol-treated rats. Conversely, no differences in the Emax or pD2 values between tissues from control and ethanol-treated rats were found after incubation with 7-NI (Figure 6, Table 4).
Figure 6.
Effect of 7-nitroindazole or aminoguanidine on phenylephrine-induced contraction of female rat aortic rings. Concentration–response curves for phenylephrine were determined in endothelium-intact aortic rings from control and ethanol-treated rats. The curves were determined in the absence (control) or after 30-min incubation with 7-nitroindazole (100 μmol l−1) or aminoguanidine (100 μmol l−1). *Compared to respective control group (P<0.05; Student's t-test).
Table 4.
Effect of 7-nitroindazole (100 μmol l−1) or aminoguanidine (100 μmol l−1) on the Emax (mN) and pD2 values for phenylephrine in endothelium-intact aortic rings from female rats
| Inhibitor |
Control |
Ethanol |
||
|---|---|---|---|---|
| Emax | pD2 | Emax | pD2 | |
| None | 15.2±1.3 (12) | 7.00±0.06 | 15.1±0.12 (14) | 7.02±0.09 |
| 7-Nitroindazole | 18.4±3.4 (5) | 6.98±0.14 | 20.8±0.16a,b (8) | 7.35±0.71a,b |
| Aminoguanidine | 16.2±1.8 (8) | 7.12±0.13 | 30.9±1.5a,b (8) | 7.40±0.13a,b |
The number in parentheses indicates the number of animals per group. Values are means±s.e.mean.
Compared to control in the absence of the inhibitors.
Compared to ethanol in the absence of the inhibitors. (ANOVA followed by Bonferroni's multiple comparison test, P<0.05).
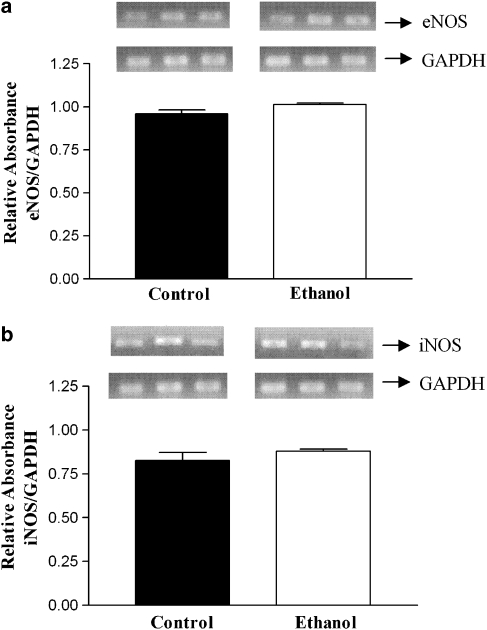
Effect of ethanol on eNOS and iNOS mRNA levels in female rats
The results obtained by RT-PCR show that there is no difference in the expression of mRNA for either eNOS or iNOS between the arteries from the control and ethanol-treated rats (Figure 7).
Figure 7.
Representative RT-PCR products of 20 ng total RNA extracted from endothelium-intact female rat aortae from control or ethanol-treated rats. The bar graphs show the relative absorbance values of eNOS (a) and iNOS (b) bands. Values were normalized by the corresponding GAPDH amplicons used as internal standard. Results are reported as mean±s.e.mean and are representative of three experiments. eNOS, endothelial nitric oxide synthase; iNOS, inducible nitric oxide synthase; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; RT-PCR, reverse transcriptase-PCR.
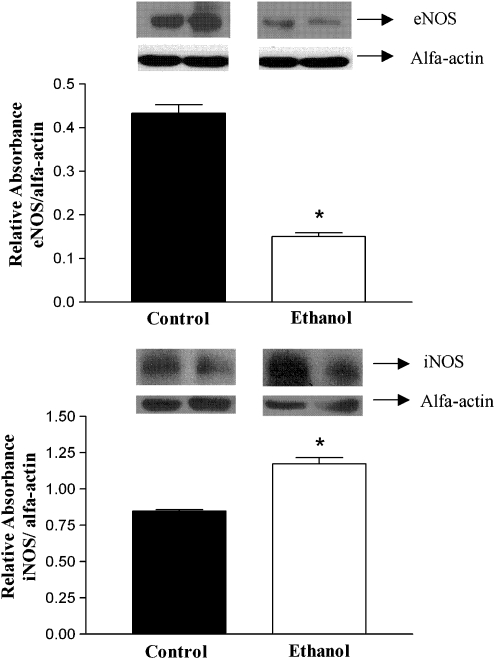
Effect of ethanol on the protein levels of eNOS and iNOS in female rats
Western immunoblotting assays showed that the treatment with ethanol reduced the levels of rat aorta eNOS protein when compared to control tissues (Figure 8). Conversely, the protein levels of iNOS in arteries from ethanol-treated rats were significantly increased when compared to control tissues (Figure 8).
Figure 8.
Representative western immunoblotting products of 50 μg total protein extracted from endothelium-intact female rat aortic arteries from control or ethanol-treated rats. The bar graphs show the relative absorbance values of eNOS and iNOS bands. Values were normalized by the corresponding α-actin bands used as internal standard. Results are reported as mean±s.e.mean and are representative of four experiments. *Compared to control group (P<0.05; Student's t-test). eNOS, endothelial nitric oxide synthase; iNOS, inducible nitric oxide synthase.
Discussion
Chronic ethanol consumption produced an enhanced responsiveness to phenylephrine in endothelium-intact aortae from male but not female rats. In contrast, after endothelial removal, the hyper-reactivity to phenylephrine was observed either in male or female rats, further suggesting a protective role for the endothelium against ethanol-induced altered responsiveness in aorta from female rats. In addition, endothelial denudation of arterial rings from male and female control rats enhanced the contractile response of aortic rings to phenylephrine, indicating that the endothelium partially counteracts the phenylephrine-mediated vasoconstriction. Enhanced responsiveness to KCl was also observed in the rings from female rats after endothelial denudation, supporting the idea that the endothelium has a protective role against the effects of ethanol. As KCl-induced contraction of rat aortic rings rely almost exclusively on Ca2+ influx through activation of voltage-sensitive channels (Hudgins and Weiss, 1968), it is suggested that ethanol consumption increases Ca2+ influx through these channels. Interestingly, the hyper-reactivity to phenylephrine induced by ethanol consumption was not the result of a nonspecific increase in the reactivity of the rat aorta, because the contractile response of these arteries to angiotensin II was not affected by ethanol intake. However, the result observed with angiotensin II does not rule out the possibility that ethanol consumption could affect the contraction induced by other vasoactive agents. In fact, we have recently observed that the effect of ethanol on vascular reactivity is dependent on the blood vessel studied and on the agonist tested (Tirapelli et al., 2007). Moreover, we observed that sucrose feeding did not have influence on the vascular reactivity to phenylephrine, suggesting that the caloric content of the ethanol diet did not play a significant role in the present findings.
As our results showed an endothelium-dependent protective effect on female rats, we studied the possibility that the modulatory action of the endothelium could be achieved, at least partially, through the release of NO. In control aortae from male and female rats, incubation of the rings with L-NAME significantly enhanced the maximal contraction induced by phenylephrine, indicating an inhibitory role for NO in the modulation of the contractile response of the aorta to this agonist. Similarly, incubation of arteries from male ethanol-treated rats with L-NAME significantly enhanced the maximal contraction induced by phenylephrine, suggesting that, in male ethanol-treated rats, the inhibitory role of NO in the response of aorta to phenylephrine did not differ from that seen in control rats. In contrast, incubation of endothelium-intact arteries from female ethanol-treated rats with L-NAME significantly enhanced the maximal contraction induced by phenylephrine, suggesting that, in female ethanol-treated rats, NO displays a protective effect as it counteracts the hyper-reactivity induced by chronic ethanol consumption, which was observed after endothelial removal.
The increase in tension induced by an NOS inhibitor reflects the amount of constitutively available NO, which modulates contractile responses of the vascular smooth muscle (Hayashi et al., 1992). The current findings show that the additional endothelium-dependent increase of the phenylephrine-induced contraction produced by L-NAME is greater in the aortae from ethanol-treated female rats than those of the control group, although not significantly different between the groups in the rings from male rats. Interestingly, we found that the iNOS inhibitor aminoguanidine reduced the maximum increase in tension evoked by L-NAME in aortic rings from ethanol-treated female rats. This observation suggests that the increased production/release of NO in the aorta from female rats chronically treated with ethanol is mediated by iNOS. Additionally, our results for the relaxation induced by SNP indicated no differences in the sensitivity to NO in the smooth muscle cells of the aortae from ethanol-treated rats. These results combined indicate the involvement of NOS-derived NO in the gender-related response of vascular tone to chronic ethanol consumption.
Gender-associated differences are often mediated by sex hormones, and gender differences in the regulation of vascular tone have been reported (for review see Thompson and Khalil, 2003). For example, ovarian hormones can improve NO bioavailability by enhancing eNOS activity (MacRitchie et al., 1997) and inhibiting NO degradation through their antioxidant properties (Subbiah et al., 1993). Moreover, ethanol increases oestrogen levels (Gavaler and Rosenblum, 1987), which in turns increases iNOS expression (Nuedling et al., 1999). Thus, we hypothesized that ovarian sex hormones could mediate the gender-related differences observed in the present study. Our data in OVX rats is in accordance with previous observations (Crews and Khalil, 1999; Castillo et al., 2005) that the vascular contraction induced by phenylephrine is significantly enhanced in OVX when compared to intact (non-OVX) females. Surprisingly, the effect of ethanol consumption on phenylephrine-induced contraction was not different in the aorta from intact or OVX female rats, further discarding the participation of ovarian sex hormones in the gender differences in the response of vascular tone to chronic ethanol consumption. This observation suggests the participation of other hormones such as progesterone, as ethanol was described to increase serum progesterone levels in female rats (Budec et al., 1996) by activating adrenal glands (Budec et al., 2002; Khisty et al., 2003). At physiological concentrations, this steroid increases NOS activity in the rat aorta (Selles et al., 2002). Moreover, progesterone increases endothelial NO release and induces rat aorta relaxation (Zhang et al., 2002). Thus, progesterone released from adrenal glands could counteract the hyper-reactivity to phenylephrine by increasing the production of endothelial NO. However, this is a hypothesis that requires further investigation.
To identify the NOS isoform(s) involved in the protective response of female aortas to ethanol, we found that the selective iNOS inhibitor aminoguanidine significantly enhanced the maximal contraction induced by phenylephrine. This result is in agreement with our initial hypothesis that the activation of iNOS plays a role in the endothelial protective effect in female ethanol-treated rats. In contrast, there was no evidence for the involvement of nNOS in this response, as 7-NI did not alter the response of the aortae from ethanol-treated rats. The protein levels of iNOS, but not its mRNA levels, were increased by the treatment, suggesting that ethanol consumption upregulates iNOS at the post-transcriptional level. This finding is in accordance with earlier reports describing that ethanol increases iNOS expression in the female rat liver (Yuan et al., 2006) and ovarian tissues (Srivastava et al., 1999). Moreover, recent observations of El-Mas et al. (2006) showed that upregulation of iNOS in the aorta mediates the hypotensive effect of acute ethanol administration in conscious female rats. Therefore, the functional and molecular biology results described here support the hypothesis that changes in vascular iNOS levels is part of the cellular mechanism related to the protective effect of the endothelium against the effects of ethanol consumption on the vascular responsiveness. Nevertheless, the mechanisms by which ethanol upregulates iNOS expression and whether it involves direct or indirect pathways are not yet clear. One possible explanation may involve the ethanol-induced enhancement in the intestinal absorption and plasma levels of endotoxin (Kono et al., 2000), which enhances iNOS expression (Forstermann et al., 1998, Isobe et al., 2001). Our findings provide the first experimental evidence of a relationship between chronic ethanol consumption and the upregulation of iNOS in vascular tissues from female rats.
Chronic ethanol consumption strongly reduced eNOS protein levels, although we did not observe any alteration on the mRNA levels for this isoform. The endothelium-dependent relaxation induced by ACh was not affected by ethanol consumption. However, in female rats a significant difference in ACh-induced relaxation became evident under eNOS blockage with L-NAME. This functional observation suggests a reduced eNOS expression in the rings from female rats, as L-NAME promoted a more pronounced blockage of ACh-induced relaxation, compared to rings from male rats. Ethanol-induced decrease in eNOS expression and activity was previously described by Karaa et al. (2005) on the rat liver. More recently, Krecsmarik et al. (2006) demonstrated that chronic ethanol consumption has a differential effect on the NOS isoforms in the rat gastrointestinal tract, where it induces an increase in iNOS activity and a decrease in nNOS expression. Moreover, eNOS-dependent relaxation of pial arterioles is reduced by long-term ethanol consumption in male and female rats (Sun and Mayhan, 2005). These observations support the notion that ethanol consumption affects eNOS-derived NO in different tissues. It is not possible to derive from our data a mechanism related to ethanol-induced reduction of eNOS expression. However, the reduced expression of eNOS and the increased expression of iNOS described here could be the result of a compensatory mechanism. Thus, increased iNOS expression could induce a substantial and sustained release of NO to compensate for the reduction of eNOS expression.
The present study offers insights into the role of the NOS–NO pathways in the vascular reactivity of the rat aorta in response to ethanol treatment according to gender. In female rats, the endothelium displays a protective role against the effects induced by ethanol consumption that is not related to the ovarian hormones, but seemed to involve the increased expression of iNOS.
Acknowledgments
We thank Juliana A Vercesi, Mirian CC Melo and Mayara Gomes for technical support. This work was supported by FAPESP (Fundação de Amparo à Pesquisa do Estado de São Paulo).
Abbreviations
- eNOS
endothelial nitric oxide synthase
- iNOS
inducible nitric oxide synthase
- L-NAME
NG-nitro-L-arginine methyl ester
- 7-NI
7-nitroindazole
- nNOS
neuronal nitric oxide synthase
- NOS
nitric oxide synthase
- OVX
ovariectomy
- SNP
sodium nitroprusside
Conflict of interest
The authors state no conflict of interest.
References
- Altura BM, Altura BT. Microvascular and vascular smooth muscle actions of ethanol, acetaldehyde, and acetate. Fed Proc. 1982;41:2447–2451. [PubMed] [Google Scholar]
- Budec M, Koko V, Milovanovic T, Balint-Peric L, Petkovic A. Acute ethanol increases level of progesterone in ovariectomized rats. Alcohol. 2002;26:173–178. doi: 10.1016/s0741-8329(02)00197-0. [DOI] [PubMed] [Google Scholar]
- Budec M, Trtic T, Balint-Peric L, Bugarski D. Increased levels of progesterone in rats following acute ethanol treatment. Acta Vet. 1996;46:293–298. [Google Scholar]
- Castillo C, Ariznavarreta MC, Lahera V, Cachofeiro V, Gil-Loyzaga P, Tresguerres JA. Effects of ovariectomy and growth hormone administration on body composition and vascular function and structure in old female rats. Biogerontology. 2005;6:49–60. doi: 10.1007/s10522-004-7383-x. [DOI] [PubMed] [Google Scholar]
- Chan TC, Sutter MC. Ethanol consumption and blood pressure. Life Sci. 1983;33:1965–1973. doi: 10.1016/0024-3205(83)90734-8. [DOI] [PubMed] [Google Scholar]
- Chinellato A, Froldi G, Caparrotta L, Ragazzi E. Pharmacological characterization of endothelial cell nitric oxide synthase inhibitors in isolated rabbit aorta. Life Sci. 1998;62:479–490. doi: 10.1016/s0024-3205(97)01144-2. [DOI] [PubMed] [Google Scholar]
- Cohen ML, Susemichel AD. Effects of 17 β-estradiol and the nonsteroidal benzothiophene, LY117018 on in vitro rat aortic responses to norepinephrine, serotonin, U46619 and BAYK 8644. Drug Dev Res. 1996;37:97–104. [Google Scholar]
- Crews JK, Khalil RA. Gender-specific inhibition of Ca2+ entry mechanisms of arterial vasoconstriction by sex hormones. Clin Exp Pharmacol Physiol. 1999;26:707–715. doi: 10.1046/j.1440-1681.1999.03110.x. [DOI] [PubMed] [Google Scholar]
- El-Mas MM, Abdel-Rahman AA. Oestrogen-dependent hypotensive effects of ethanol in conscious female rats. Alcohol Clin Exp Res. 1999;23:624–632. [PubMed] [Google Scholar]
- El-Mas MM, Zhang J, Abdel-Rahman AA. Upregulation of vascular inducible nitric oxide synthase mediates the hypotensive effect of ethanol in conscious female rats. J Appl Physiol. 2006;100:1011–1018. doi: 10.1152/japplphysiol.01058.2005. [DOI] [PubMed] [Google Scholar]
- Forstermann U, Boissel JP, Kleinert H. Expressional control of the ‘constitutive' isoforms of nitric oxide synthase (NOS I and NOS III) FASEB J. 1998;12:773–790. [PubMed] [Google Scholar]
- Fortmann SP, Haskell WL, Vranizan K, Brown BW, Farquhar JW. The association of blood pressure and dietary alcohol: differences by age, sex, and oestrogen use. Am J Epidemiol. 1983;118:497–507. doi: 10.1093/oxfordjournals.aje.a113655. [DOI] [PubMed] [Google Scholar]
- Freeman ME.The neuroendrocrine control of ovarian cycle of the rat The Physiology of Reproduction 1994Raven Press Ltd: New York; 613–658.In: Knobil E, Neil JD (eds) [Google Scholar]
- Fukada SY, Iyomasa MM, Cunha FQ, Correa FM, de Oliveira AM. Mechanisms of impaired vascular response to ANG II in perivascular injured carotid arteries of ovariectomized rat. J Cardiovasc Pharmacol. 2004;44:393–400. doi: 10.1097/01.fjc.0000138162.03988.86. [DOI] [PubMed] [Google Scholar]
- Gavaler JS, Rosenblum ER. Exposure-dependent effects of ethanol administered in drinking water on serum estradiol and uterus mass in sexually mature oophorectomized rats: a rat model for bilaterally ovariectomized/postmenopausal women. J Stud Alcohol. 1987;48:295–303. doi: 10.15288/jsa.1987.48.295. [DOI] [PubMed] [Google Scholar]
- Hayashi T, Fukuto JM, Ignarro LJ, Chaudhuri G. Basal release of nitric oxide from aortic rings is greater in female rabbits than in male rabbits: implications for atherosclerosis. Proc Natl Acad Sci USA. 1992;89:11259–11263. doi: 10.1073/pnas.89.23.11259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudgins A, Weiss G. Effects of Ca2+ removal upon vascular smooth muscle contraction induced by noripenephrine, histamine and potassium. J Pharm Exp Ther. 1968;159:91–97. [PubMed] [Google Scholar]
- Isobe H, Okajima K, Uchiba M, Mizutani A, Harada N, Nagasaki A, et al. Activated pretein C prevents endotoxin-induced hypotension in rats by inhibiting excessive production of nitric oxide. Circulation. 2001;104:1171–1175. doi: 10.1161/hc3501.093799. [DOI] [PubMed] [Google Scholar]
- Joly GA, Ayres M, Chelly F, Kilbourn RG. Effects of NG-methyl-L-arginine, and aminoguanidine on constitutive and inducible nitric oxide synthase in rat aorta. Biochem Biophys Res Commun. 1994;199:147–154. doi: 10.1006/bbrc.1994.1207. [DOI] [PubMed] [Google Scholar]
- Kahonen M, Karjala K, Hutri-Kahonen N, Wu X, Jaatinen P, Riihioja P, et al. Influence of chronic ethanol consumption on arterial tone in young and aged rats. Am J Physiol. 1999;276 2 Part 2:H464–H471. doi: 10.1152/ajpheart.1999.276.2.H464. [DOI] [PubMed] [Google Scholar]
- Karaa A, Kamoun WS, Clemens MG. Chronic ethanol sensitizes the liver to endotoxin via effects on endothelial nitric oxide synthase regulation. Shock. 2005;24:447–454. doi: 10.1097/01.shk.0000180616.13941.7d. [DOI] [PubMed] [Google Scholar]
- Khisty RT, Kumar S, Morrow AL. Ethanol rapidly induces steroidogenic acute regulatory protein expression and translocation in rat adrenal gland. Eur J Pharmacol. 2003;473:223–225. doi: 10.1016/s0014-2999(03)01969-1. [DOI] [PubMed] [Google Scholar]
- Klatsky AL, Friedman GD, Armstrong MA. The relationships between alcoholic beverage use and other traits to blood pressure: a new Kaiser Permanent study. Circulation. 1986;73:628–636. doi: 10.1161/01.cir.73.4.628. [DOI] [PubMed] [Google Scholar]
- Kono H, Wheeler MD, Rusyn I, Lin M, Seabra V, Rivera CA, et al. Gender differences in early alcohol-induced liver injury: role of CD14, NF-κB, and TNFα. Am J Physiol Gastrointest Liver Physiol. 2000;278:G625–G661. doi: 10.1152/ajpgi.2000.278.4.G652. [DOI] [PubMed] [Google Scholar]
- Krecsmarik M, Izbeki F, Bagyanszki M, Linke N, Bodi N, Kaszaki J, et al. Chronic ethanol exposure impairs neuronal nitric oxide synthase in the rat intestine. Alcohol Clin Exp Res. 2006;30:967–973. doi: 10.1111/j.1530-0277.2006.00110.x. [DOI] [PubMed] [Google Scholar]
- MacRitchie AN, Jun SS, Chen Z, German Z, Yuhanna IS, Sherman TS, et al. Oestrogen up regulates endothelial nitric oxide synthase gene expression in fetal pulmonary artery endothelium. Circ Res. 1997;81:355–362. doi: 10.1161/01.res.81.3.355. [DOI] [PubMed] [Google Scholar]
- Manolio TA, Levy D, Garrison RJ, Castelli WP, Kannel WB. Relation of alcohol intake to left ventricular mass: the Framingham study. J Am Coll Cardiol. 1991;17:717–721. doi: 10.1016/s0735-1097(10)80189-5. [DOI] [PubMed] [Google Scholar]
- Montes GS, Luque EH. Effects of ovarian steroids on vaginal smears in the rat. Acta Anat (Basel) 1988;133:192–199. doi: 10.1159/000146639. [DOI] [PubMed] [Google Scholar]
- Moore RD, Levine DM, Southard J, Entwisle G, Shapiro S. Alcohol consumption and blood pressure in the 1982 Maryland hypertension survey. Am J Hypertens. 1990;3:1–7. doi: 10.1093/ajh/3.1.1. [DOI] [PubMed] [Google Scholar]
- Nuedling S, Kahlert S, Loebbert K, Doevendans PA, Meyer R, Vetter H, et al. 17 Beta-estradiol stimulates expression of endothelial and inducible NO synthase in rat myocardium in-vitro and in-vivo. Cardiovasc Res. 1999;43:666–674. doi: 10.1016/s0008-6363(99)00093-0. [DOI] [PubMed] [Google Scholar]
- Rees DD, Palmer RM, Schulz R, Hodson HF, Moncada S. Characterization of three inhibitors of endothelial nitric oxide synthase in vitro and in vivo. Br J Pharmacol. 1990;101:746–752. doi: 10.1111/j.1476-5381.1990.tb14151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resstel LB, Tirapelli CR, Lanchote VL, Uyemura SA, de Oliveira AM. Chronic ethanol consumption alters cardiovascular functions in conscious rats. Life Sci. 2006;78:2179–2187. doi: 10.1016/j.lfs.2005.09.021. [DOI] [PubMed] [Google Scholar]
- Selles J, Polini N, Alvarez C, Massheimer V. Nongenomic action of progesterone in rat aorta: role of nitric oxide and prostaglandins. Cell Signal. 2002;14:431–436. doi: 10.1016/s0898-6568(01)00265-0. [DOI] [PubMed] [Google Scholar]
- Srivastava VK, Hiney JK, Rettori V, Dees WL. Effects of ethanol on intraovarian nitric oxide production in the prepubertal rat. J Endocrinol. 1999;161:69–75. doi: 10.1677/joe.0.1610069. [DOI] [PubMed] [Google Scholar]
- Stewart CW, Kennedy RH. Effects of chronic ethanol consumption on aortic constriction in male and female rats. Eur J Pharmacol. 1999;366:55–60. doi: 10.1016/s0014-2999(98)00900-5. [DOI] [PubMed] [Google Scholar]
- Strickland JA, Wooles WR. Effect of acute and chronic ethanol on the agonist responses of vascular smooth muscle. Eur J Pharmacol. 1988;152:83–91. doi: 10.1016/0014-2999(88)90838-2. [DOI] [PubMed] [Google Scholar]
- Subbiah MT, Kessel B, Agrawal M, Rajan R, Abplanalp W, Rymaszevisk Z. Antioxidant potential of specific oestrogens on lipid peroxidation. J Clin Endocrinol Metab. 1993;77:1095–1097. doi: 10.1210/jcem.77.4.8408459. [DOI] [PubMed] [Google Scholar]
- Sun H, Mayhan WG. Sex difference in nitric oxide synthase-dependent dilatation of cerebral arterioles during long-term alcohol consumption. Alcohol Clin Exp Res. 2005;29:430–436. doi: 10.1097/01.alc.0000156117.87892.22. [DOI] [PubMed] [Google Scholar]
- Thompson J, Khalil RA. Gender differences in the regulation of vascular tone. Clin Exp Pharmacol Physiol. 2003;30:1–15. doi: 10.1046/j.1440-1681.2003.03790.x. [DOI] [PubMed] [Google Scholar]
- Tirapelli CR, Al-Khoury J, Bkaily G, D'Orleans-Juste P, Lanchote VL, Uyemura SA, et al. Chronic ethanol consumption enhances phenylephrine-induced contraction in the isolated rat aorta. J Pharmacol Exp Ther. 2006a;316:233–241. doi: 10.1124/jpet.105.092999. [DOI] [PubMed] [Google Scholar]
- Tirapelli CR, Casolari DA, Montezano AC, Yogi A, Tostes RC, Legros E, et al. Ethanol consumption enhances endothelin-1-induced contraction in the isolated rat carotid. J Pharmacol Exp Ther. 2006b;318:819–827. doi: 10.1124/jpet.106.103010. [DOI] [PubMed] [Google Scholar]
- Tirapelli CR, Leone FCA, Coelho EB, Resstel LB, Corrêa FMA, Lanchote VL, et al. Effect of ethanol consumption on blood pressure and rat mesenteric arterial bed, aorta and carotid responsiveness. J Pharm Pharmacol. 2007;59:985–993. doi: 10.1211/jpp.59.7.0011. [DOI] [PubMed] [Google Scholar]
- Tokuno S, Chen F, Pernow J, Jiang J, Valen G. Effects of spontaneous or induced brain ischemia on vessel reactivity: the role of inducible nitric oxide synthase. Life Sci. 2002;71:679–692. doi: 10.1016/s0024-3205(02)01711-3. [DOI] [PubMed] [Google Scholar]
- Utkan T, Yildiz F, Ilbay G, Ozdemirci S, Erden BF, Gacar N, et al. Blood pressure and vascular reactivity to endothelin-1, phenylephrine, serotonin, KCl and acetylcholine following chronic alcohol consumption in vitro. Fundam Clin Pharmacol. 2001;15:157–165. doi: 10.1046/j.1472-8206.2001.00024.x. [DOI] [PubMed] [Google Scholar]
- Weiner CP, Lizasoain I, Baylis SA, Knowles RG, Charles IG, Moncada S. Induction of calcium-dependent nitric oxide syntheses by sex hormones. Proc Natl Acad Sci USA. 1994;91:5212–5216. doi: 10.1073/pnas.91.11.5212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan GJ, Zhou XR, Gong ZJ, Zhang P, Sun XM, Zheng SH. Expression and activity of inducible nitric oxide synthase and endothelial nitric oxide synthase correlate with ethanol-induced liver injury. World J Gastroenterol. 2006;12:2375–2381. doi: 10.3748/wjg.v12.i15.2375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M, Wang GJ, Benishin CG, Pang PKT. Rapid effect of progesterone on the contraction of rat aorta in-vitro. J Pharm Pharmacol. 2002;54:1529–1534. doi: 10.1211/00223570263. [DOI] [PubMed] [Google Scholar]