Abstract
The prostamides are part of a large and continually expanding series of pharmacologically unique neutral lipids. They are COX-2 derived oxidation products of the endocannabinoid/endovanniloid anandamide. Prostamide pharmacology is unique and, as in the case of the endocannabinoids anandamide and 2-arachidonylglycerol, bears little resemblance to that of the corresponding free acids. By virtue of its close relationship to the anti-glaucoma drug bimatoprost, prostamide F2α has received the greatest research attention. Prostamide F2α and bimatoprost effects appear independent of prostanoid FP receptor activation, according to a litany of agonist studies. Studies involving freshly isolated and separate feline iridial smooth muscle cells revealed that bimatoprost and FP receptor agonists stimulated different cells, without exception. This suggests the existence of receptors that preferentially recognize prostamide F2α. The recent discovery of prostamide antagonists has provided further support for prostamide receptors as discrete entities. The prototypical prostamide antagonists, AGN 204396 and 7, blocked the effects of prostamide F2α and bimatoprost but not those of PGF2α and FP receptor agonists in the feline iris. Second generation more potent prostamide antagonists, such as AGN 211334, should allow the role of prostamides in health and disease to be elucidated. From the therapeutics standpoint, the prostamide F2α analogue bimatoprost is the most efficacious ocular hypotensive agent currently available for the treatment of glaucoma.
Keywords: prostaglandins, prostaglandin-ethanolamides, FP receptor, prostaglandin glyceryl ester, anandamide, cyclo-oxygenase-2
Introduction
The origin of prostamide (prostaglandin-ethanolamide) research resides in two quite separate lines of investigation. Unrelated studies on endocannabinoid oxygenation by cyclo-oxygenase-2 (COX-2) and the pharmacology of neutral derivatives of prostaglandins (PGs) converged to reveal a close inter-relationship. The resultant concept was that the principal endocannabinoids are oxygenated by COX-2, with the formation of pharmacologically unique prostamides and PG-glyceryl esters. PGE2-ethanolamide was the first COX-2-derived product of anandamide to be described (Yu et al., 1997). Subsequent detailed analyses of endocannabinoid oxygenation by COX-2 revealed that anandamide and 2-arachidonylglyceryol ester (2-AG) are converted to a range of products that closely approaches those formed from arachidonic acid by COX-1 and COX-2 (Kozak et al., 2002; Koda et al., 2004). In addition to cell culture experiments, prostamides have also been shown to be formed from anandamide in living mice (Weber et al., 2004). The enzymatic formation of prostamides from anandamide was thereby established.
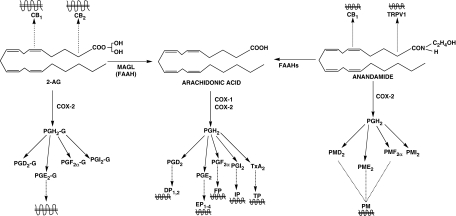
The PG-ethanolamides and glyceryl esters are members of an expanding network of neutral lipid mediators. The neutral lipids are already numerous but, in the majority of cases, the pharmacology is ill-defined or unknown. This is not the situation for the principal endocannabinoids anandamide and 2-AG, and rapid advances have recently occurred in the neutral PG area. From the perspective of the endocannabinoids anandamide and 2-AG, metabolic conversion equates with loss of primary activity but can result in altered pharmacology rather than de-activation, depending upon the metabolic pathway involved. For anandamide oxidation by COX-2 results in a loss of activity at cannabinoid-1 and TRPV1 receptors (De Petrocellis et al., 2004) and formation of the prostamides, which are in a quite separate pharmacological category. Enzymatic hydrolysis of anandamide by fatty acid amide hydrolase (Cravatt et al., 1996; Wei et al., 2006) leads to arachidonic acid formation, which may serve as a substrate for COX-1 and COX-2, lipo-oxygenases, or P450 enzymes and may also be re-incorporated into the lipid pool. A schematic showing the relationship of endocannabinoid metabolic pathways is provided in Figure 1. It follows that, in the context of the endocannabinoid system, prostamide formation may be viewed as both an inactivation mechanism for anandamide and a pathway leading to pharmacologically novel, PG-like substances. By the same token, inhibitors of COX-2 could partly attenuate anandamide breakdown and selectively prevent prostamide and PG-glyceryl ester formation. The relative importance of these events with respect to the anti-inflammatory and analgesic effects of COX-2 inhibitors remains to be elucidated. Under circumstances where COX-2 is induced and anandamide is present, substantial prostamide formation may occur (Glass et al., 2005).
Figure 1.
Pharmacology and metabolic conversion of endocannabinoids and neutral prostaglandin derivatives. G, glyceryl ester; PM, prostamide.
The prostamides have been pharmacologically characterized to a much greater extent than PGE2-glyceryl ester. Prostamide F2α has received the greatest attention, since the pharmacology of neutral PGF2α analogues is closely tied to the antiglaucoma drug bimatoprost (Woodward et al., 2003; Matias et al., 2004). An exhaustive series of agonist studies using prostamide F2α and bimatoprost have provided much evidence that their effects cannot be readily attributed to prostanoid FP receptor stimulation. In 2007, the first selective prostamide antagonist was reported, which provided further evidence for the prostamide receptor as a distinct entity (Woodward et al., 2003). This review provides an in-depth analysis of prostamide pharmacology, including a brief mention of more potent prostamide antagonists that have recently been discovered.
Prostamide biosynthesis
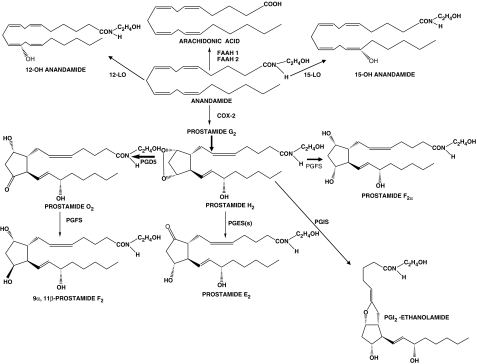
Anandamide may be metabolized by both hydrolytic and oxidative mechanisms. It is catabolized to arachidonic acid and ethanolamine by the fatty acid amide hydrolase enzymes. The first fatty acid amide hydrolase was discovered a decade ago (Cravatt et al., 1996), but a human-specific fatty acid amide hydrolase was reported recently (Wei et al., 2006). Anandamide is also oxidized by COX-2 and lipo-oxygenase pathways (Hampson et al., 1995; Ueda et al., 1995). A schematic for the biosynthesis of COX-2 and lipo-oxygenase products from the endocannabinoid anandamide is provided in Figure 2. The biological activity of the lipo-oxygenase products of anandamide is controversial and has received little attention, unlike the prostamides.
Figure 2.
Anandamide conversion pathways.
The first prostamide to be discovered was PGE2-ethanolamide. It was identified as the major product when anandamide was incubated with human recombinant COX-2 or human foreskin fibroblasts cells expressing COX-2 (Yu et al., 1997). In marked contrast, COX-1 failed to bind and oxidize anandamide (Yu et al., 1997). Formation of prostamide E2 from anandamide was subsequently confirmed in RAW 264.7 cells (Burstein et al., 2000). These earliest findings provided a basis for much more detailed and extensive studies, which revealed that COX-2 converted the endocannabinoids anandamide and 2-AG to a range of oxidation products that closely approached those formed from arachidonic acid (Kozak et al., 2002). COX-2 oxidizes anandamide to the endoperoxide intermediates prostamide G2 and prostamide H2 (Kozak et al., 2002; Koda et al., 2004; Yang et al., 2005), which are then converted by specific PG synthases to the various prostamides. Thus, it was demonstrated in RAW264.7 cells that exogenous anandamide is converted to prostamide D2 by prostaglandin D synthase-directed isomeration of PGH2-ethanolamide (Kozak et al., 2002). Similarly, anandamide was converted to prostamide E2 and F2α in HCA-7 cells (Kozak et al., 2002). Moreover, it appears that prostamide H2 and prostamide D2 are substrates for prostaglandin F synthase, with the resultant formation of prostamide F2α and 11β-prostamide F2α (Koda et al., 2004; Yang et al., 2005). Using coupled assays involving COX-2 and prostacyclin synthase, evidence was provided for the formation of PGI2-ethanolamide (Kozak et al., 2002). Finally, prostamide formation from exogenously administered anandamide has been demonstrated in living animals (Weber et al., 2004).
Prostamide pharmacology
Agonists
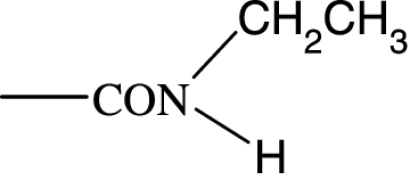
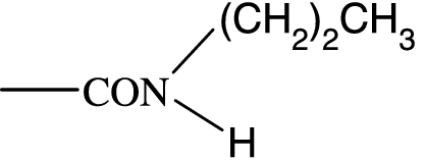
At the time when the prostamides were discovered as COX-2-derived products of anandamide, virtually nothing was known about their pharmacology. Neutral PGF2α analogues were known to exert little or no agonist activity in PGF2α-sensitive preparations (Maddox et al., 1978; Schaaf and Hess, 1979). Replacement of the charged carboxylate group of PGF2α by a -CONH2 group reduced activity at FP receptors by more than two orders of magnitude. Monomethyl and dimethyl functionalities produced a further reduction in agonist potency, to the point where PGF2α-CON(CH3)2 was purported to behave as an antagonist (Maddox et al., 1978). This activity could not be confirmed in more recent studies (Sharif et al., 2000; Table 1). What is more important, however, was an absence of meaningful agonist activity at prostanoid FP receptors. This has been confirmed on numerous occasions (Woodward et al., 2000, 2003; Liang et al., 2003, 2004; Krauss and Woodward, 2004; Matias et al., 2004; Chen et al., 2005). Given the structural similarity of PGs and prostamides, interaction with PG receptors was an obvious line of investigation for elucidating prostamide pharmacology but it was not the only one to be pursued.
Table 1.
Pharmacology of PGF2α-amides
| PGF2α C1-substituent |
Feline iris |
Swiss 3T3 cell |
Rabbit jugular vein |
Guinea-pig ileum |
Guinea-pig vas deference |
Human platelets (aggregation) |
Human platelets |
|---|---|---|---|---|---|---|---|
| •Prostamide | •FP | •FP •EP4 •DP1 | •EP1 | •EP3 | •TP | •DP1, •IP | |
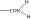 |
21 | 18 000 | 643 | NA | 794 | >10 000 | — |
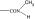 |
28 | 13 300 | — | — | — | — | — |
 |
35 | — | 250 | >10 000 | >10 000 | — | — |
 |
19 | — | 281 | NA | >10 000 | — | — |
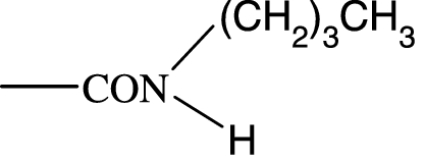 |
60 | — | 17 800 | NA | >10 000 | — | NA |
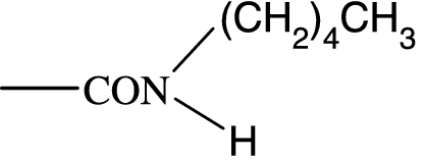 |
158 | — | 17 800 | NA | >10 000 | — | NA |
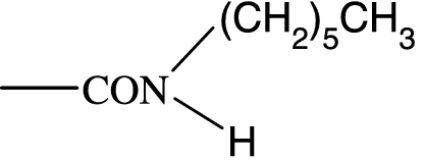 |
258 | — | 20 734 | — | — | — | — |
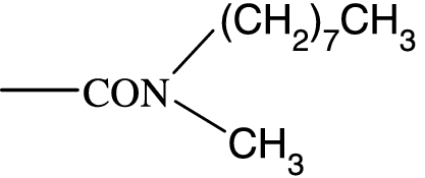 |
771 | — | 3649 | — | — | — | — |
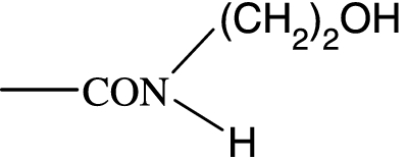 |
57 | 4285 | 1277 | >10 000 | 1590 | NA | NA |
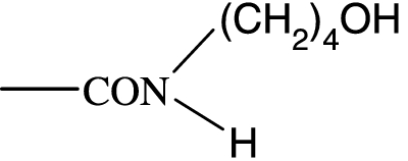 |
158 | — | 2860 | — | — | — | — |
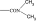 |
4500 | >10 000 | 3649 | — | — | — | — |
Abbreviations: NA, not active; prostamide, prostaglandin-ethanolamide.
Values are EC50 (nM). Experiments involved at least four replicates. Receptor subtype involvement is given (•) under the description of each preparation. Physiological responses are as follows: feline iris=contraction; Swiss 3T3 cells=Ca2+ ↑; rabbit jugular vein=relaxation; guinea-pig ileum=contraction; guinea pig vas deferens=inhibition of field stimulated contraction; human platelets (TP)=aggregation; human platelets (DP, IP)=inhibition of ADP induced aggregation.
The natural mammalian endocannabinoids are neutral arachidonic acid derivatives, and the possibility that prostamides behaved as cannabimimetics (Pinto et al., 1994; Berglund et al., 1999) or were similar to anandamide with respect to vanilloid receptor stimulation (Matias et al., 2004) was investigated. No cannabinoid or transient receptor potential vanilloid type 1 channels activity was observed until a 10−6 M concentration was exceeded and it was concluded that prostamides do not possess cannabimimetic and related activity. It should be noted that although supra-μM activity is often dismissed as pharmacologically meaningful, there is not a general consensus on this issue (Sharif et al., 2001). The possibility that high local concentrations of prostamides may stimulate cannabinoid receptors cannot be dismissed. Other hypotheses have been advanced to explain prostamide activity. These include activation of the peroxisome proliferation-activated receptor-γ (Rockwell and Kaminski, 2004), prostaglandin F synthase inhibition (Koda et al., 2004; Komoto et al., 2006) and substrates that indirectly reduce anandamide metabolism (Matias et al., 2004).
PGE2-ethanolamide was the first prostamide to be discovered (Yu et al., 1997) and was the first subjected to pharmacological characterization as a prostanoid receptor ligand. The effects of PGE2-ethanolamide in the guinea-pig trachea could not be readily explained by interaction with prostanoid EP receptors (Ross et al., 2002). The most extensive comparative pharmacological studies with prostanoids have been conducted for prostamide F2α and its analogue bimatoprost. In fact, bimatoprost has been the focus of many studies by virtue of its clinical status as an antiglaucoma agent (Dubiner et al., 2001; Higginbotham et al., 2002; Noecker et al., 2003; Parrish et al., 2003; Woodward et al., 2004). The evolution of prostamide pharmacology is very much rooted in the pharmacological characterization of bimatoprost, with a continual cross-reference.
As in many previous cases, the elucidation of prostamide pharmacology originated from agonist studies. The earliest neutral PGF2α analogues were PGF2α 1-OH and PGF2α 1-OCH3, and their pharmacology was extensively characterized using recombinant prostanoid receptors and native receptors in cells and isolated tissues (Woodward et al., 2000). A number of C-1 modifications were made, most resulted in uninteresting compounds. The amido substituents provided the most satisfactory compounds in terms of both pharmacological novelty and ophthalmological profile. The activity of PGF2α 1-amides is provided in Table 1. The primary amide (-CONH2) and N-monosubstituted analogues provided potent and selective prostamide agonists. A variety of monosubstituents provided compounds with similar activity, linear and branched alkyl substituents and ethanolamide being well tolerated. Di-substitution caused a dramatic reduction in prostamide activity. All of these prostamides exhibited no meaningful activity at prostanoid receptors, including the FP receptor. None were FP receptor antagonists. Based on the fundamental PGF2α 1-amide structure a wide range of analogues was prepared, which included the antiglaucoma drug bimatoprost (Woodward et al., 2004).
Although the pharmacology of bimatoprost and other PGF2α amides appeared unique (Woodward et al., 1994), the biological significance was obscure until the first prostamide was discovered in 1997 by Yu et al. At this juncture PGF2α 1-ethanolamide was elevated from being one in an expansive collection of PGF2α amides to a key comparator for bimatoprost. A series of comparative experiments were conducted over several years to address key questions and hypotheses that were raised. These questions/hypotheses are listed as follows, with the background, key experiments and outcome provided.
(a) Does prostamide pharmacology reflect differences between isolated tissue and cell studies? The unique pharmacology of PGF2α amides was originally revealed by comparing responses in the isolated feline iris with Ca2+ signalling in Swiss 3T3 cells, which is an FP receptor-mediated event (Woodward and Lawrence, 1994). The rank orders of potency were as follows:
- Feline iris
17-phenyl PGF2α=fluprostenol⩾PGF2α=prostamide F2α=bimatoprost>PGD2>PGE2>U-46619>sulprostone.
- Swiss 3T3 cells
17-phenyl PGF2α=fluprostenol⩾PGF2α>PGD2>PGE2>U-46619>sulprostone⩾prostamide F2α=bimatoprost.
Several isolated tissue preparations known to constitutively express FP receptors were found to be essentially insensitive to prostamide F2α and bimatoprost. These included the gerbil colon, intact rabbit jugular vein, mouse uterus, rat uterus and human uterus (Woodward et al., 2001, 2003; Matias et al., 2004; Chen et al., 2005). They behaved in a similar manner to that observed in Swiss 3T3 cells (Woodward and Lawrence, 1994). It was concluded that only certain isolated tissue preparations uniquely recognize prostamide F2α and its congeners: feline iris (Woodward et al., 2001; Matias et al., 2004), feline lung parenchyma (Woodward et al., 2003) and rabbit uterus (Chen et al., 2005).
(b) Is bimatoprost an FP receptor agonist? The prostanoid receptor classification designates receptors according to the ligands with which they preferentially interact (Coleman et al., 1984). This is a key concept for avoiding confusion, as many compounds that preferentially interact with a single receptor have off-target activity at much higher concentrations. For example, fluprostenol is widely and correctly regarded as a selective FP receptor agonist but it has measurable EP3 activity at concentrations that exceed 10−6 M (Hellberg et al., 2001). Similarly, U-46619 is widely viewed as a selective TP receptor agonist but it also stimulates FP receptors at high concentrations (Woodward and Lawrence, 1994). It has been postulated that bimatoprost is an FP receptor agonist per se (Sharif et al., 2001, 2002, 2003). This is based on the weak interaction with FP receptors that occurs at supra-μM concentrations (Sharif et al., 2001; Woodward et al., 2001, 2003, 2004). Such a definition of bimatoprost as an FP receptor agonist does not take into account the prostamide-like activity that is observed at concentrations of at least 100-fold lower in prostamide-sensitive preparations (Liang et al., 2003, 2004; Woodward et al., 2003, 2004; Matias et al., 2004; Chen et al., 2005). Finally, bimatoprost does not block FP receptor stimulation, indicating a lack of FP receptor interaction (Woodward et al., 2003).
(c) Does prostamide activity result from enzymatic conversion to the free acid (FP agonist)? Conversion of bimatoprost and prostamide F2α in prostamide-sensitive preparations has been investigated by both indirect (bioassay) and direct methods. Using anandamide as a positive control, no detectable conversion of prostamide F2α or bimatoprost was detected in the isolated feline iris and ciliary body (Matias et al., 2004). This corresponds to the low bimatoprost conversion rates observed in isolated bovine and human ocular tissues (Maxey et al., 2002; Davies et al., 2003; Krauss and Woodward, 2004). Even the ester latanoprost undergoes low enzymatic hydrolysis in certain tissues, as indicated by the very weak activity of latanoprost in the isolated feline (Resul et al., 1997) or porcine iris (Hasegawa et al., 2006). Finally, potent stimulation of the isolated rabbit uterus was attributed to the activity of intact bimatoprost, since bioassay indicated no enzymatic hydrolysis to the free acid metabolite (Chen et al., 2005).
(d) Is prostamide activity species specific? Since prostamide activity was first discovered in feline tissues, it was originally suggested that such activity was species specific. Studies comparing responses at feline and human recombinant FP receptors demonstrated a single pharmacological identity, with no meaningful interaction with bimatoprost (Woodward et al., 2003) or prostamide F2α (Matias et al., 2004). The rabbit uterus was later identified as exquisitely sensitive to bimatoprost but no such activity was observed in the intact rabbit jugular vein (Chen et al., 2005). Further confirmation that prostamide activity is species independent was provided by gene regulation studies (Liang et al., 2003). Comparing cysteine-rich angiogenic protein 61 (Cyr 61) and connective tissue growth factor expression in human ciliary smooth muscle cells, PGF2α was shown to upregulate connective tissue growth factor and Cyr 61. In contrast to PGF2α and at concentrations that do not stimulate FP receptors, bimatoprost upregulated Cyr 61 but not connective tissue growth factor. The feline iris was used as a positive control and responded in an identical manner with respect to Cyr 61 and connective tissue growth factor expression (Liang et al., 2003).
Issues relating to species, tissue and metabolism were thereby addressed. One salient feature of these studies was that whenever prostamide F2α effects in cells and tissues were manifest, a response to PGF2α was also apparent, albeit not necessarily identical. This comparative agonist activity profile for FP receptor stimulation and prostamide F2α mimetics made further pharmacological analysis extremely difficult. Prostamide pharmacology could be explained in two ways: (1) a receptor population that preferentially recognizes prostamide F2α and coexists with FP receptors (2) an FP receptor subclass that equally recognizes both PGF2α and prostamide F2α. To address the latter hypothesis, one further series of agonist studies was performed in the feline iris. These involved isolated feline iris cells, with Ca2+ signalling monitored by fluorescence confocal microscopy (Spada et al., 2005). These studies revealed that bimatoprost and FP receptor agonists (PGF2α, 17-phenyl PGF2α) stimulated entirely different cells (Spada et al., 2005). No overlap occurred. These studies provided evidence for the existence of a population of receptors that exclusively recognize prostamides. These studies also provided the impetus to search for an antagonist.
Antagonist pharmacology
Studies with agonists were pursued to the point where it appeared that the putative prostamide receptor was dedicated to selectively interact with neutral PGs. Definitive pharmacological characterization required, however, a selective antagonist that blocked either (1) prostanoid FP receptor or (2) prostamide activity.
Several drugs have been claimed to block prostanoid FP receptors, but subsequent experiments have failed to provide confirmation (Table 1; Sharif et al., 2001). AL-8810 has been reported to be a selective FP receptor antagonist (Griffin et al., 1999) and accordingly, its utility was investigated. The results were mixed. On studying the effects of AL-8810 on FP receptor-mediated upregulation of the orphan nuclear receptor Nur 77, it behaved as a satisfactory antagonist with little or no residual agonist activity (Liang et al., 2004). These results did not transition into Ca2+ signalling studies in cells stably expressing human recombinant FP receptors. Careful analysis of other reports on AL-8810 indicates measurable FP receptor stimulation (Griffin et al., 1999; Hutchinson et al., 2003). Unfortunately, AL-8810 behaved as a weak but high efficacy agonist in the feline iris (Woodward et al., 2007). AL-8810 was, therefore, unsuitable for delineating prostanoid FP and prostamide receptor pharmacology in the feline iris. This obligated the discovery of a prostamide antagonist.
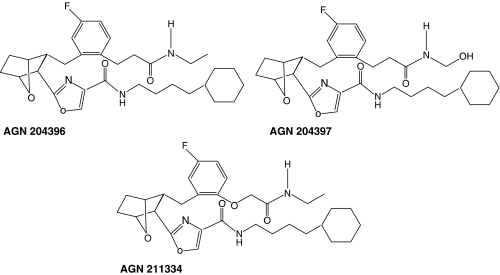
The strategy for identifying a prostamide antagonist was to use antagonists for receptors in cluster 2 of the molecular evolution classification (Narumiya et al., 1999) and then derivatize them to the C-1 amide analogue. Cluster 2 comprises EP1, FP and TP receptors. The TP receptor antagonists were considered as particularly useful as several potent and structurally diverse TP receptor antagonist series have been designed. Moreover, antagonists for other prostanoid receptors have originated from a TP antagonist as the starting template. The prostamide receptor antagonists were discovered based on the oxabicycloheptane analogue BMS 180,291 (Webb et al., 1993) and were AGN 204396 and AGN 204397 (Krauss and Woodward 2006; Woodward et al., 2007). Structures AGN 204396 and 204397 are 3(-2-[(1R,2R,3S,4R)-3-[-4-cyclohexyl-butylcarbarnoyl)-oxazol-2-yl]-7-oxa-bicyclo[2.2.1]hept-2-ylmethyl)-4-fluro-phenyl)-propyl-ethylamide and hydroxyethylamide, respectively. The pharmacological activity profile is provided in Table 2. They are highly selective prostamide antagonists, with no meaningful off-target activity at prostanoid receptors, including FP receptors constitutively expressed in the feline iris. AGN 204396 and 204397 are also TP antagonists but no functional TP receptors are present in most prostamide preparations, and, therefore, no complicating pharmacology exists.
Table 2.
Pharmacology of prostamide antagonists AGN 204396 and AGN 204397
| Receptor | 204396 Kb (nM) | AGN 204397 Kb (nM) |
|---|---|---|
| Prostamide | 2635 | 3099 |
| DP1 | NA | NA |
| EP1 | NA | NA |
| EP2 | NA | NA |
| EP3 | NA | NA |
| EP4 | NA | NA |
| FP | NA | NA |
| IP | NA | NA |
| TP | 1.5 | 1.5 |
Abbreviation: prostamide, prostaglandin-ethanolamide.
Prostamide refers to activity in the feline iris sphincter preparation; other preparations are human recombinant prostanoid receptors.
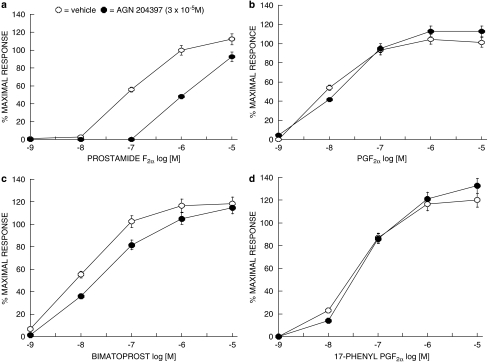
It should be noted that the feline iris is highly responsive to both prostamides and prostanoid FP receptor agonists, which makes the feline iris a particularly useful preparation for investigating prostamide pharmacology and its relationship to prostanoid pharmacology. AGN 204396 (Woodward et al., 2007) and AGN 204397 (Figure 3) antagonized the contractile effects of prostamide F2α and its analogue bimatoprost, but not those of PGF2α and synthetic FP agonists in the feline iris (Figure 3). Consistent with previous feline iris studies, where Ca2+ signalling was monitored in individual cells (Spada et al., 2005), antagonist studies also suggest that the prostamide receptor does not meaningfully interact with PGF2α but preferentially recognizes prostamides. The contractile effects of AL-8810 were not affected by AGN 204396 pretreatment, providing further evidence for prostamide and prostanoid FP receptors as distinct entities. Finally, the prostamide antagonist AGN 204396 did not affect contraction produced by PGE2-glyceryl ester, a COX-2-derived metabolite of the endocannabinoid 2-AG (Kozak et al., 2002; Nirodi et al., 2004).
Figure 3.
Effect of AGN 204397 (3 × 10−5 M) on contraction of the feline iris produced by (a) prostamide F2α (b) PGF2α (c) bimatoprost (d) and 17-phenyl PGF2α. Open symbols represent vehicle treated preparations, closed symbols represent preparations that received AGN 204397. Values are mean±s.e.m.; n=4.
AGN 204396 and AGN 204397 are considered the first prototypical prostamide antagonists but are not very potent. AGN 204396 has a pA2 of 5.64 (Woodward et al., 2007). Very recently, more potent antagonists have been designed (Selcia Ltd, Ongar, Essex, England). These are typified by AGN 211334 (Wan et al., 2007), which is more than one order of magnitude more potent than AGN 204396 and AGN 204397. AGN 211334 was used to provide more definitive evidence that the effects of bimatoprost on conventional aqueous humour outflow are prostamide receptor mediated (Wan et al., 2007). For the future, AGN 211334 and its congeners are likely to be sufficiently potent to be useful for pharmacological studies in living animals. The structures of the prostamide antagonists AGN 204396, AGN 204397 and AGN 211334 are provided in Figure 4.
Figure 4.
Structure of prostamide antagonists.
Therapeutics
Prostamide research is in its infancy and the direct involvement of prostamides and PG-glyceryl esters in disease processes remains to be determined. Upregulation of COX-2, coupled to increased anandamide levels, may lead to the formation of prostamides as major products at sites of inflammation and infection (Glass et al., 2005). In previous studies using antibody-based methods, prostamides may have been misidentified as PGs, since commercial antibodies to PGE2 and PGF2α exhibited major cross-reactivity with the corresponding prostamides (Glass et al., 2005). It is possible that the therapeutic benefit of COX-2 inhibitors is, at least in part, derived from a reduction in prostamide and PG-glyceryl ester levels. The newly developed prostamide antagonists may be very helpful in testing this concept.
The prostamide area of research has, however, already yielded one successful therapeutic. This is the antiglaucoma drug bimatoprost. The unique pharmacology of bimatoprost was recognized some time ago (Woodward et al., 1994) but was not elucidated as a prostamide F2α mimetic until recently. The pharmacological distinction between bimatoprost and latanoprost has been made at the clinical level. Glaucoma patients refractory to latanoprost treatment were found to be susceptible to bimatoprost, which produced a pronounced lowering of intraocular pressure (Williams, 2002; Gandolfi and Cimino, 2003). In ‘glaucomatous' monkeys that responded to latanoprost, travoprost and bimatoprost, a combination of bimatoprost and latanoprost produced a greater lowering of trough and peak intraocular pressure than a latanoprost/travoprost combination (Gagliuso et al., 2004). Given these results, combined latanoprost/bimatoprost therapy could even be considered as a glaucoma treatment regimen.
Bimatoprost is a potent and highly efficacious ocular hypotensive. Topical application to the ocular surface produces significant decreases in intraocular pressure at doses as low as 0.001% in dogs and monkeys (Woodward et al., 2004). Clinical effects are similar, although the dose–response relationship is steeper in glaucomatous patients (Laibovitz et al., 2001). Randomized, controlled clinical studies have shown that bimatoprost is a safe, well-tolerated and highly effective antiglaucoma drug (Dubiner et al., 2001; Sherwood and Brandt, 2001; Higginbotham et al., 2002; Noecker et al., 2003; Parrish et al., 2003). Taken together, clinical evidence and experience indicate that bimatoprost is the most efficacious antiglaucoma drug currently available. The difference in absolute mm Hg is only about 0.5–1 but, when intraocular pressure approaches the normal range, each mm Hg of additional ocular hypotensive efficacy is of significant value in preventing visual field loss (Woodward and Chen, 2007). Bimatoprost efficacy may be related to a dual mechanism of action on aqueous humour outflow that involves both uveoscleral and trabecular meshwork/Schlemm's canal pathways (Brubaker et al., 2001; Christiansen et al., 2004; Wan et al., 2007). Effects on uveoscleral outflow have been studied directly in monkeys (Woodward et al., 2001) and have been correlated with morphological changes in the anterior portion of the ciliary body (Richter et al., 2003). In brief, bimatoprost causes a controlled remodelling of the anterior third of the ciliary body resulting in new, organized drainage channels that are partially lined with endothelial cells. This mechanism of action is common to all receptor selective ocular hypotensive PG analogues (Richter et al., 2003), with the exception of EP4 receptor agonists. At the gene regulation level, Cyr 61 appears to provide a common upstream starting point for prostamides, FP agonists and EP2 agonists (Liang et al., 2003). Studies in human models of the trabecular meshwork/Schlemm's canal outflow pathways have demonstrated that bimatoprost produces marked increases in hydraulic conductivity that are prostamide receptor mediated (Wan et al., 2007).
Generally regarded as very safe drugs, bimatoprost and prostanoid FP receptor agonists also produce ocular side effects. It should be stressed that these side effects are mostly cosmetic in quality (Stjernschantz, 2001; Hollo, 2007). They are reversible side effects (Hollo, 2007), with the exception of iridial hyperpigmentation. Again, there is pharmacological differentiation between bimatoprost and latanoprost. There is a high incidence of iridial hyperpigmentation associated with latanoprost therapy (Alm et al., 2004; Kitazawa, 2006), but this is a rare occurrence with bimatoprost (Sherwood and Brandt, 2001; Cohen et al., 2004). Side effects common to bimatoprost, latanoprost and related compounds are ocular surface hyperaemia, eyelash hypertrichosis and periorbital hyperpigmentation, albeit with differing incidence and severity (Hollo, 2007; Woodward and Chen, 2007). It should be noted that not all of these side effects are undesirable.
Luxuriant eyelash growth is regarded as a bonus that such antiglaucoma drugs provide. It is also envisaged that application of, for example, bimatoprost to the cutaneous surface and margins of the eyelids may provide a semipermanent substitute for ‘eye-liner' and ‘eye-shadow'. Not to mention a permanent replacement for mascara. Drugs related to bimatoprost may provide the impetus for an era of pharmacocosmetics.
Cloning the prostamide receptor
The next step, now that reasonably potent and selective prostamide antagonists have been invented, is to clone the prostamide receptor. The antagonist direction (Wan et al., 2007; Woodward et al., 2007) could be further pursued to obtain very high affinity ligands that could be used to isolate the receptor protein. This still remains a difficult and long-term strategy.
It is pertinent to note that, to date, prostamide activity has never been reported as a phenomenon completely independent of prostanoid FP receptor stimulation. Both activities are present in prostamide-sensitive preparations such as the feline lung parenchyma (Woodward et al., 2003), feline iris (Liang et al., 2003; Matias et al., 2004; Spada et al., 2005; Woodward et al., 2007), rabbit uterus (Chen et al., 2005) and human ciliary smooth muscle cells (Liang et al., 2003). In addition, reports from studies involving prostanoid FP receptor knockout mice claim that bimatoprost-induced ocular hypotension is attenuated (Crowston et al., 2005; Ota et al., 2005). Taken together, these results suggest that prostamide and FP receptors may be encoded by the same gene. Following this line of reasoning, the possibility that FP receptor mRNA splicing variants (Fujino et al., 2000; Sakamoto et al., 2002; Vielhauer et al., 2004) may account for prostamide activity appears attractive. This is an important hypothesis to test and, if correct, would unify disparate studies of mixed origin.
Acknowledgments
We thank Lisa L Rubin for preparing the manuscript.
Abbreviations
- 2-AG
2-arachidonylglycerol
- COX-1
cyclo-oxygenase 1
- COX-2
cyclo-oxygenase 2
- Cyr 61
cysteine-rich angiogenic protein 61
- PG
prostaglandin
- prostamide
prostaglandin-ethanolamide
Conflict of interest
The authors state no conflict of interest.
References
- Alm A, Schoenfelder J, McDermott J. A 5-year, multicenter, open-label, safety study of adjunctive latanoprost therapy for glaucoma. Arch Ophthalmol. 2004;122:957–965. doi: 10.1001/archopht.122.7.957. [DOI] [PubMed] [Google Scholar]
- Berglund BA, Boring DL, Howlett AC. Investigation of structural analogs of prostaglandin amides for binding to and activation of CB1 and CB2 cannabinoid receptors in rat brain and human tonsils. Adv Exp Med Biol. 1999;469:527–533. doi: 10.1007/978-1-4615-4793-8_77. [DOI] [PubMed] [Google Scholar]
- Brubaker RF, Schoff EO, Nau CB, Carpenter SP, Chen K, Van Den Burgh AM. Effect of AGN 192024, a new ocular hypotensive agent, on aqueous dynamics. Am J Ophthalmol. 2001;131:19–24. doi: 10.1016/s0002-9394(00)00843-6. [DOI] [PubMed] [Google Scholar]
- Burstein SH, Rossetti RG, Yagen B, Zurier RB. Oxidative metabolism of anandamide. Prostaglandins Other Lipid Mediat. 2000;61:29–41. doi: 10.1016/s0090-6980(00)00053-8. [DOI] [PubMed] [Google Scholar]
- Chen J, Senior J, Marshall K, Abbas F, Dinh H, Dinh T, et al. Studies using isolated uterine and other preparations show bimatoprost and prostanoid FP agonists have different activity profiles. Br J Pharmcol. 2005;144:493–501. doi: 10.1038/sj.bjp.0706044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christiansen GA, Nau CB, McLaren JW, Johnson DH. Mechanism of ocular hypotensive action of bimatoprost (Lumigan) in patients with ocular hypertension or glaucoma. Ophthalmology. 2004;111:1658–1662. doi: 10.1016/j.ophtha.2004.02.006. [DOI] [PubMed] [Google Scholar]
- Cohen JS, Gross RL, Cheetham JK, VanDenburgh AM, Bernstein P, Whitcup SM. Two-year double-masked comparison of bimatoprost with timolol in patients with glaucoma or ocular hypertension. Surv Ophthalmol. 2004;49:S45–S52. doi: 10.1016/j.survophthal.2003.12.019. [DOI] [PubMed] [Google Scholar]
- Coleman RA, Humphrey PPA, Kennedy I, Lumley P. Prostanoid receptors: the development of a working classification. Trends Pharmacol Sci. 1984;5:303–306. [Google Scholar]
- Cravatt BF, Giang DK, Mayfield SP, Boger DL, Lerner RA, Gilula NB. Molecular characterization of an enzyme that degrades neuromodulatory fatty-acid amides. Nature. 1996;384:83–87. doi: 10.1038/384083a0. [DOI] [PubMed] [Google Scholar]
- Crowston JG, Lindsey JD, Morris CA, Wheeler LA, Medeiros FA, Weinreb RN. Effect of bimatoprost on intraocular pressure in prostaglandin FP receptor knockout mice. Invest Ophthalmol Vis Sci. 2005;46:4571–4577. doi: 10.1167/iovs.05-0723. [DOI] [PubMed] [Google Scholar]
- Davies SS, Ju WK, Neufeld AH, Abran D, Chemtob S, Roberts LJ. Hydrolysis of bimatoprost (Lumigan) to its free acid by ocular tissue in vitro. J Ocular Pharmacol Ther. 2003;19:45–54. doi: 10.1089/108076803762718105. [DOI] [PubMed] [Google Scholar]
- De Petrocellis L, Cascio MG, Di Marzo V. The endocannabinoid system: a general view and latest additions. Br J Pharmacol. 2004;141:765–774. doi: 10.1038/sj.bjp.0705666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubiner H, Cooke D, Dirks M, Stewart W, Vandenburg AM, Felix C. Efficacy and safety of bimatoprost in patients with elevated intraocular pressure. Surv Opthalmol. 2001;45:S353–S360. doi: 10.1016/s0039-6257(01)00212-0. [DOI] [PubMed] [Google Scholar]
- Fujino H, Pierce KL, Srinivasan D, Protzman CE, Krauss AH-P, Woodward DF, et al. Delayed reversal of shape change in cells expressing FPβ prostanoid receptor, possible role of receptor resensitization. J Biol Chem. 2000;275:29907–29914. doi: 10.1074/jbc.M003467200. [DOI] [PubMed] [Google Scholar]
- Gagliuso DJ, Wang RF, Mittag TW, Podos SM. Additivity of bimatoprost or travoprost to latanoprost in glaucomatous monkey eyes. Arch Ophthalmol. 2004;122:1342–1347. doi: 10.1001/archopht.122.9.1342. [DOI] [PubMed] [Google Scholar]
- Gandolfi SA, Cimino L. Effect of bimatoprost on patients with primary open-angle glaucoma on ocular hypertension who are nonresponders to latanoprost. Ophthalmology. 2003;110:609–614. doi: 10.1016/S0161-6420(02)01891-2. [DOI] [PubMed] [Google Scholar]
- Glass M, Hong J, Sato TA, Mitchell MD. Misidentification of prostamides as prostaglandins. J Lipid Res. 2005;46:1364–1368. doi: 10.1194/jlr.C500006-JLR200. [DOI] [PubMed] [Google Scholar]
- Griffin BW, Klimko P, Crider JY, Sharif NA. AL-8810: a novel prostaglandin F2α analog with selective antagonist effects at the prostaglandin F2α (FP) receptor. J Pharmacol Exp Ther. 1999;290:1278–1284. [PubMed] [Google Scholar]
- Hampson AJ, Hill WA, Zan-Philips M, Makriyannis A, Leung E, Eglen RM, et al. Anandamide hydroxylation by brain lipoxyenase: metabolite structures and potencies at the cannabinoid receptor. Biochem Biophys Acta. 1995;1259:173–179. doi: 10.1016/0005-2760(95)00157-8. [DOI] [PubMed] [Google Scholar]
- Hasegawa Y, Nishimura J, Niiro N, Hirano K, Ishibashi T, Kanaide H. Prostaglandin F2α, but not latanoprost, increases the Ca2+ sensitivity of the pig iris sphincter muscle. Invest Ophthalmol Vis Sci. 2006;47:4865–4871. doi: 10.1167/iovs.05-1518. [DOI] [PubMed] [Google Scholar]
- Hellberg MR, Sallee VL, McLaughlin MA. Preclinical efficacy of travoprost, a potent and selective FP prostaglandin receptor agonist. J Ocul Pharmacol Ther. 2001;17:421–432. doi: 10.1089/108076801753266802. [DOI] [PubMed] [Google Scholar]
- Higginbotham EJ, Schuman JS, Goldberg I, Gross RL, Vandenburgh A, Chen K, et al. One-year, randomized study comparing bimatoprost and timolol in glaucoma and ocular hypertension. Arch Ophthalmol. 2002;120:1286–1293. doi: 10.1001/archopht.120.10.1286. [DOI] [PubMed] [Google Scholar]
- Hollo G. The side effects of the prostaglandin analogues. Expert Opin Drug Saf. 2007;6:45–52. doi: 10.1517/14740338.6.1.45. [DOI] [PubMed] [Google Scholar]
- Hutchinson J, Marshall K, Senior J. Preliminary studies using a putative FP-receptor antagonist, AL-8810, on isolated mouse uterus. pA2 Online. 2003;1:038P. [Google Scholar]
- Kitazawa Y. An open-label multicenter study on the efficacy and safety of topical use of latanoprost for 156 weeks. Jap J Clin Ophthalmol. 2006;60:2047–2054. [Google Scholar]
- Koda N, Tsutsui Y, Niwa H, Ito S, Woodward DF, Watanabe K. Synthesis of prostaglandin F ethanolamide by prostaglandin F synthase and identification of bimatoprost as a potent inhibitor of the enzyme. New enzyme method by LC/GSI/MS. Arch Biochem Biophys. 2004;424:128–136. doi: 10.1016/j.abb.2004.02.009. [DOI] [PubMed] [Google Scholar]
- Komoto J, Yamada T, Watanabe K, Woodward DF, Takusaga F. Prostaglandin F2α formation from prostaglandin H2 by prostaglandin F synthase (PGFS): crystal structure of PGFS containing bimatoprost. Biochem. 2006;45:1987–1996. doi: 10.1021/bi051861t. [DOI] [PubMed] [Google Scholar]
- Kozak KR, Crews BC, Morrow JD, Wang LH, Ma YH, Weinander R, et al. Metabolism of the endocannabinoids, 2-arachidonylglycerol and anandamide, into prostaglandin, thromboxane and prostacyclin glycerol esters and ethanolamides. J Biol Chem. 2002;277:44877–44885. doi: 10.1074/jbc.M206788200. [DOI] [PubMed] [Google Scholar]
- Krauss AH-P, Woodward DF. Update on the mechanism of action of bimatoprost: a review and discussion of new evidence. Surv Ophthalmol. 2004;49:S5–S11. doi: 10.1016/j.survophthal.2003.12.014. [DOI] [PubMed] [Google Scholar]
- Krauss AH-P, Woodward DF.Prostamide receptor antagonists 2006. US Patent 7,045,634
- Laibovitz RA, Van Den Burgh AM, Felix C, David R, Batoosingh A, Rosenthal A, et al. Comparison of the ocular hypotensive lipid AGN 192024 with timolol. Dosing, efficacy and safety evaluation of a novel compound for glaucoma management. Arch Ophthalmol. 2001;199:994–1060. doi: 10.1001/archopht.119.7.994. [DOI] [PubMed] [Google Scholar]
- Liang Y, Li C, Guzman VM, Chang WW, Evinger A, Pablo JV, et al. Upregulation of orphan nuclear receptor Nur 77 following PGF2α, bimatoprost and butaprost treatments. Essential role of a protein kinase C pathway involved in the EP2 receptor activated Nur 77 gene transcription. Br J Pharmacol. 2004;142:737–748. doi: 10.1038/sj.bjp.0705829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang Y, Li C, Guzman VM, Evinger AJ, Protzman CE, Krauss AH-P, et al. Comparison of PGF2α, bimatoprost (prostamide) and butaprost (EP2 agonist) on Cyr 61 and CTGF gene expression. J Biol Chem. 2003;278:27267–27277. doi: 10.1074/jbc.M301009200. [DOI] [PubMed] [Google Scholar]
- Maddox YT, Ramwell PW, Shiner CS, Corey EJ. Amide and 1-amino derivatives of prostaglandins as prostaglandin antagonists. Nature. 1978;273:549–552. doi: 10.1038/273549a0. [DOI] [PubMed] [Google Scholar]
- Matias I, Chen J, De Petrocellis L, Bisogno T, Ligresti A, Fezza F, et al. Prostaglandin ethanolamides (prostamides): in vitro pharmacology and metabolism. J Pharmacol Exp Ther. 2004;309:745–757. doi: 10.1124/jpet.103.061705. [DOI] [PubMed] [Google Scholar]
- Maxey KM, Johnson JL, Labreque J. The hydrolysis of bimatoprost in corneal tissue generates a potent prostanoid FP receptor agonist. Surv Ophthalmol. 2002;47:34–40. doi: 10.1016/s0039-6257(02)00323-5. [DOI] [PubMed] [Google Scholar]
- Narumiya S, Sugimoto Y, Ushikubi F. Prostanoid receptors: structure, properties, and functions. Pharmacol Rev. 1999;79:1193–1226. doi: 10.1152/physrev.1999.79.4.1193. [DOI] [PubMed] [Google Scholar]
- Nirodi CS, Crews BC, Kozak KR, Morrow JD, Marnett LJ. The glyceryl ester of prostaglandin E2 mobilizes calcium and activates signal transduction in RAW 264.7 cells. Proc Nat Acad Sci USA. 2004;101:1840–1845. doi: 10.1073/pnas.0303950101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noecker RS, Dirks MS, Choplin NT, Bernstein P, Batoosongh AL, Whitcup SM. A six-month randomized clinical trail comparing the intraocular pressure lowering efficacy of bimatoprost and latanoprost in patients with ocular hypertension or glaucoma. Am J Ophthalmol. 2003;135:55–63. doi: 10.1016/s0002-9394(02)01827-5. [DOI] [PubMed] [Google Scholar]
- Ota T, Aibara M, Narumiya S, Araie J. The effects of prostaglandin analogues on IOP in prostanoid FP receptor-deficient mice. Invest Ophthalmol Vis Sci. 2005;46:4159–4163. doi: 10.1167/iovs.05-0494. [DOI] [PubMed] [Google Scholar]
- Parrish RK, Palmberg P, Sheu W-P, for the XLT Study Group A comparison of latanoprost, bimatoprost, and travoprost in patients with elevated intraocular pressure: a 12-week randomized, masked evaluation multicenter study. Am J Ophthalmol. 2003;135:688–703. doi: 10.1016/s0002-9394(03)00098-9. [DOI] [PubMed] [Google Scholar]
- Pinto JC, Potié F, Rice KC, Boring D, Johnson MR, Evans DM, et al. Cannabinoid receptor binding and agonist activity of amides and esters of arachidonic acid. Mol Pharmacol. 1994;46:516–522. [PubMed] [Google Scholar]
- Resul B, Stjernschantz J, Selen G, Bito L. Structure–activity relationships and receptor profiles of some ocular hypotensive prostanoids. Surv Ophthalmol. 1997;41:S47–S52. doi: 10.1016/s0039-6257(97)80007-0. [DOI] [PubMed] [Google Scholar]
- Richter M, Krauss AH-P, Woodward DF, Lütjen-Drecoll E. Morphological changes in the anterior eye segment after long term treatment with different receptor selective prostaglandin agonists and a prostamide. Invest Ophthalmol Vis Sci. 2003;44:4419–4426. doi: 10.1167/iovs.02-1281. [DOI] [PubMed] [Google Scholar]
- Rockwell CE, Kaminski NE. A cyclooxygenase metabolite of anandamide causes inhibition of interleukin-2 secretion in murine splenocytes. J Pharmacol Exp Ther. 2004;34:683–690. doi: 10.1124/jpet.104.065524. [DOI] [PubMed] [Google Scholar]
- Ross RA, Craib SJ, Stevenson LA, Pertwee RG, Henderson A, Toole J, et al. Pharmacological characterization of the anandamide cyclooxygenase metabolite: prostaglandin E2 ethanolamide. J Pharmacol Exp Ther. 2002;301:900–907. doi: 10.1124/jpet.301.3.900. [DOI] [PubMed] [Google Scholar]
- Sakamoto K, Ishii Y, Onodera T, Sugano T. Cloning and characterization of the novel isoforms for PGF2α receptor in the bovine corpus luteum. DNA Seq. 2002;13:307–311. doi: 10.1080/1042517021000011645. [DOI] [PubMed] [Google Scholar]
- Schaaf TK, Hess H-J. Synthesis and biological activity of carboxyl-terminus modified prostaglandin analogs. J Med Chem. 1979;22:1340–1346. doi: 10.1021/jm00197a012. [DOI] [PubMed] [Google Scholar]
- Sharif N, Crider JY, Davis TL. AL-3138 antagonizes FP prostanoid receptor-mediated inositol phosphates generation: comparison with some purported FP antagonists. J Pharm Pharmacol. 2000;52:1529–1539. doi: 10.1211/0022357001777586. [DOI] [PubMed] [Google Scholar]
- Sharif N, Kelly CR, Crider JY. Agonist activity of bimatoprost, travoporst, latanoprost, unoprostone isopropyl ester and other prostaglandin analogs at the cloned human ciliary body FP prostaglandin receptor. J Ocular Pharmacol Ther. 2002;18:313–324. doi: 10.1089/10807680260218489. [DOI] [PubMed] [Google Scholar]
- Sharif NA, Kelly CR, Williams GW. Bimatoprost (Lumigan) is an agonist at the cloned human ocular FP prostaglandin receptor: real-time FLIPR-based intracellular Ca2+ mobilization studies. Prostaglandins Leukot Essent Fatty Acids. 2003;68:27–33. doi: 10.1016/s0952-3278(02)00232-6. [DOI] [PubMed] [Google Scholar]
- Sharif NA, Williams GW, Kelly CB. Bimatoprost and its free acid are prostaglandin FP receptor agonists. Eur J Pharmacol. 2001;432:211–213. doi: 10.1016/s0014-2999(01)01486-8. [DOI] [PubMed] [Google Scholar]
- Sherwood M, Brandt J. Six-month comparison of bimatoprost once-daily and twice daily with timolol twice-daily in patients with elevated intraocular pressure. Surv Ophthalmol. 2001;45:S361–S368. doi: 10.1016/s0039-6257(01)00219-3. [DOI] [PubMed] [Google Scholar]
- Spada CS, Krauss AH-P, Woodward DF, Chen J, Protzman CE, Nieves AL, et al. Bimatoprost and prostaglandin F2α selectively stimulate intracellular calcium signaling in different cat iris sphincter cells. Exp Eye Res. 2005;80:135–145. doi: 10.1016/j.exer.2004.08.019. [DOI] [PubMed] [Google Scholar]
- Stjernschantz JW. From PGF2α-isopropyl ester to latanoprost: a review of the development of Xalatan. Invest Ophthalmol Vis Sci. 2001;42:1134–1145. [PubMed] [Google Scholar]
- Ueda N, Yamamoto K, Yamamoto S, Tokunaga T, Shirakawa E, Shinaki H, et al. Lipoxygenase-catalyzed oxygenation of arachidonylethanolamide, a cannabinoid receptor agonist. Biochem Biophys Acta. 1995;1254:127–134. doi: 10.1016/0005-2760(94)00170-4. [DOI] [PubMed] [Google Scholar]
- Vielhauer GA, Fujino H, Regan JW. Cloning and localization of hFPs: a six-transmembrane mRNA splice variant of the human FP prostanoid receptor. Arch Biochem Biophys. 2004;421:175–185. doi: 10.1016/j.abb.2003.10.021. [DOI] [PubMed] [Google Scholar]
- Wan Z, Woodward DF, Cornell C, Fliri H, Martos J, Pettit S, et al. Bimatoprost and conventional drainage Invest Ophthalmol Vis Sci 200748(in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb ML, Liu ECK, Monschizadegan H, Hedberg A, Misra RN, Goldenberg H, et al. Binding and function of a potent new thromboxane receptor antagonist, BMS 180,291, in human platelets. J Pharmacol Exp Ther. 1993;264:1387–1394. [PubMed] [Google Scholar]
- Weber A, Ni J, Ling KH-J, Acheampong A, Tang-Liu DD-S, Cravatt BF, et al. Formation of prostaglandin 1-ethanolamides (prostamides) from anandamide in fatty acid amide hydrolose knockout (FAAH −/−) mice analyzed by high performance liquid chromatography with tandem mass spectrometry. J Lipid Res. 2004;45:757–763. doi: 10.1194/jlr.M300475-JLR200. [DOI] [PubMed] [Google Scholar]
- Wei BQ, Mikkelsen TJ, McKinney MK, Lander ES, Cravatt BF. A second fatty acid amide hydrolase with variable distribution among placental mammals. J Biol Chem. 2006;281:36569–36578. doi: 10.1074/jbc.M606646200. [DOI] [PubMed] [Google Scholar]
- Williams RD. Efficacy of bimatoprost in glaucoma and ocular hypertension unresponsive to latanoprost. Adv Ther. 2002;19:275–281. doi: 10.1007/BF02853173. [DOI] [PubMed] [Google Scholar]
- Woodward DF, Andrews SW, Burk RM, Garst ME.Non-acidic cyclopentane heptanoic acid, 2 cycloalkyl or arylalkyl derivatives as therapeutic agents 1994. US Patent 5,352,708
- Woodward DF, Chen J. Fixed-combination and emerging glaucoma therapies. Expert Opin Emerging Drugs. 2007;12:313–327. doi: 10.1517/14728214.12.2.313. [DOI] [PubMed] [Google Scholar]
- Woodward DF, Krauss AH-P, Chen J, Gil DW, Kedzie KM, Protzman CE, et al. Replacement of the carboxylic acid group of prostaglandin F2α with a hydroxyl or methoxy substituent provides biologically unique compounds. Br J Pharmacol. 2000;130:1933–1943. doi: 10.1038/sj.bjp.0703462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward DF, Krauss AH-P, Chen J, Lai RK, Spada CS, Burk RM, et al. The pharmacology of bimatoprost (LumiganTM) Surv Ophthalmol. 2001;45:S337–S345. doi: 10.1016/s0039-6257(01)00224-7. [DOI] [PubMed] [Google Scholar]
- Woodward DF, Krauss AH-P, Chen J, Liang Y, Li C, Protzman CE, et al. Pharmacological characterizations of a novel antiglaucoma agent, bimatoprost (AGN 192024) J Pharmacol Exp Ther. 2003;305:772–785. doi: 10.1124/jpet.102.047837. [DOI] [PubMed] [Google Scholar]
- Woodward DF, Krauss AH-P, Wang JW, Protzman CE, Nieves AL, Liang Y, et al. Identification of an antagonist that selectively blocks the activity of prostamides (prostaglandin-ethanolamides) in the feline iris. Br J Pharmacol. 2007;150:342–352. doi: 10.1038/sj.bjp.0706989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward DF, Lawrence RA. Identification of a single (FP) receptor associated with prostanoid-induced Ca2+ signals in Swiss 3T3 cells. Biochem Pharmacol. 1994;47:1567–1574. doi: 10.1016/0006-2952(94)90533-9. [DOI] [PubMed] [Google Scholar]
- Woodward DF, Phelps RL, Krauss AH-P, Weber A, Short B, Chen J, et al. Bimatoprost: a novel anti-glaucoma agent. Cardiovasc Drug Rev. 2004;22:103–120. doi: 10.1111/j.1527-3466.2004.tb00134.x. [DOI] [PubMed] [Google Scholar]
- Yang W, Ni J, Woodward DF, Tang-Lui DD-S, Ling J. Enzymatic formation of prostamide F2α from anandamide involves as newly identified intermediate metabolite, prostamide H2. J Lipid Res. 2005;46:2745–2751. doi: 10.1194/jlr.M500374-JLR200. [DOI] [PubMed] [Google Scholar]
- Yu M, Ives D, Ramesha CS. Synthesis of prostaglandin E2 ethanolamide from anandamide by cyclooxygenase-2. J Biol Chem. 1997;272:21181–21186. doi: 10.1074/jbc.272.34.21181. [DOI] [PubMed] [Google Scholar]