Abstract
The perceived speed of motion in one part of the visual field is influenced by the speed of motion in its surrounding fields. Little is known about the cellular mechanisms causing this phenomenon. Recordings from mammalian visual cortex revealed that speed preference of the cortical cells could be changed by displaying a contrast speed in the field surrounding the cell’s classical receptive field. The neuron’s selectivity shifted to prefer faster speed if the contextual surround motion was set at a relatively lower speed, and vice versa. These specific center–surround interactions may underlie the perceptual enhancement of speed contrast between adjacent fields.
Keywords: visual cortex, cat, surround effect
Many psychophysical experiments (1–6) have demonstrated that the perceived speed of motion in one part of the visual field is dependent on the speed of motion in its surrounding fields. The moon appears to move when clouds pass through it, a phenomenon known as “induced motion” (7). Viewing two adjacent areas of random dot patterns moving at different speeds, the observers reported that the difference in speed of the dots next to the discontinuity appeared enhanced (2). Little is known about the cellular mechanisms causing these phenomena. Recent neurophysiological studies indicated that regions beyond the classical receptive field (CRF) of visual cortex cells, although alone unresponsive to visual stimuli, could modulate the cell’s response (8–14). This modulatory effect can be facilitatory or inhibitory, and its extent can be assessed from area summation characteristics (9). It has been demonstrated that the selectivity of visual attributes (orientation, spatial frequency, and speed of motion) of the surround fields are similar to those of the CRF (9), and the inhibitory interactions between the two fields enable cortical neurons to analyze feature contrast between neighboring fields (10, 11). Modulatory effects of moving textured background on neuronal responses have been described for the striate cortex of cat (12–14). Here we report center–surround interactions for speed of motion. We found that the speed selectivity for a given cell was not fixed, but could be dynamic, adapting to the contextual speed in the surround field. The changes in speed tuning at the single cell level may underlie the perceptual phenomenon in which the difference in speed between neighboring fields appears exaggerated.
METHODS
Using electrophysiologic techniques, single neurons were recorded extracellularly from area 18 in paralyzed and anesthetized cats. A detailed description of conventional procedures for animal surgery, anesthesia, recording technique, and stimulus generation is given in Li and Li (9). Briefly, experiments were performed on adult cats, weighing between 2 and 3 kg. Anesthesia was induced with an i.m. injection of ketamine hydrochloride (30 mg/kg of body weight). Anesthesia and paralysis were maintained for the rest of the experiment with an i.v. infusion of gallamine triethiodide (10 mg/kg per hr), d-tubocurarine chloride (0.25 mg/kg per hr), urethane (20 mg/kg per hr), and glucose (200 mg/kg per hr) in Ringer’s solution (3 ml/hr). End-tidal CO2, body temperature, electroencephalogram, and electrocardiogram were monitored and maintained at normal levels. Drifting sine-wave gratings were displayed on an oscilloscope (Tektronix 608, P31 phosphor) with the use of a Picasso stimulus generator (Innisfree, Cambridge, MA). The contrast of the gratings was 40%, and the mean luminance was 8.3 cd/m2. Under computer control, the grating orientation, spatial frequency, movement direction, and size of the window were matched to those preferred by the cell under study, and real-time analyses of the responses were performed. All measurements were made during stimulation of the neuron’s dominant eye with the other eye occluded. All recorded cells were obtained from the cortex area representing the central 15° (radius) of the visual field.
The CRF profiles of the cell were tested by placing an optimal grating patch at successive positions across the screen and measuring the response to its drift. Area summation tests were made using a set of circular windows of various diameters that contained a sine-wave grating moving in the cell’s optimal direction. The nature and extent of center–surround interactions were evaluated from the shape of area summation curves. Procedures for characterizing the surround have been described (9). Two major classes of surround effects were identified. Cells with a facilitatory surround responded more vigorously as the stimulus size was extended well beyond the borders of the CRF. Cells with an inhibitory surround responded more weakly to large field stimuli than to small field.
RESULTS AND DISCUSSION
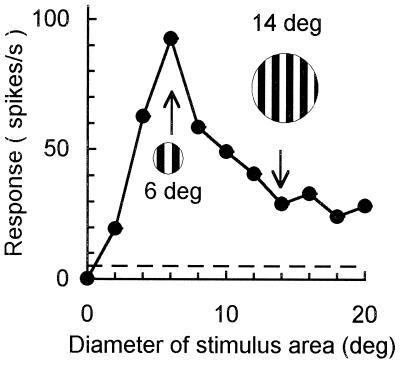
We chose the cells with inhibitory surround for our study. Inhibition evoked by the surround stimulus for these cells was ≥25%. One such example is illustrated in Fig. 1. CRF of this cell is 6° (diameter), and its surround is 14° (diameter). The full-screen grating (20° in diameter) evoked an inhibition of 74%.
Figure 1.
Area summation curve showing the inhibitory surround effect of an area 18 cell. A circular grating patch located at the center of the CRF was systematically varied in size. With increasing stimulus size, a maximum response was reached at a point (arrow on the left) indicating the limit of the CRF. Then the response decreased with further increasing size. The point at which the response decreased to an asymptote (arrow on the right) indicates the extent of the inhibitory surround. Size of the small and large gratings (Insets) represents the relative size of the CRF and the inhibitory surround, respectively. Responses were accumulated for 10 cycles, and SEs were calculated over five repetitions. Bars = ±1 SE.
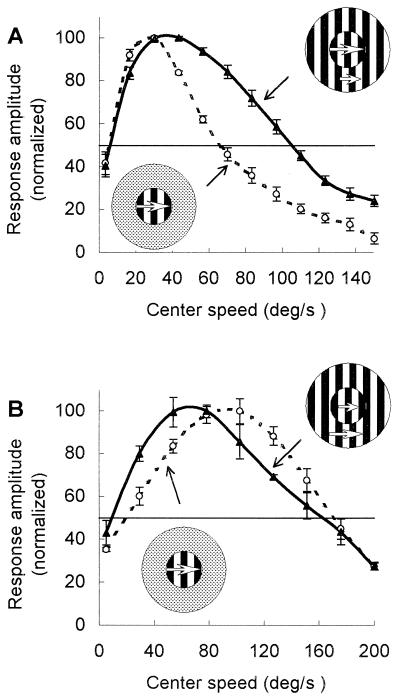
To test the influence of surround speed on CRF speed selectivity, we used a concentric stimulus pattern consisting of a central and an annular surround grating. For each cell, we first measured the speed tuning repeatedly, using only the central grating (whose size was matched to the CRF) while the surround field was uniformly illuminated (Fig. 2, Lower Left Insets). Then the same measurements were repeated when a surround grating was concurrently presented at a higher or lower speed (Fig. 2, Upper Right Insets). An example showing the changes of speed tuning caused by presenting a slower speed in the surround is depicted in Fig. 2A. The solid curve in Fig. 2A was obtained with the presence of a surround grating drifted in the same direction as in the center, but at a slower speed (13.3°/s, 12.7°/s lower than the optimal speed). Compared with the “center-only” tuning curve (dashed line), the peak of the “center-plus-surround” curve shows an 11.0°/s shift toward the high-speed side. The high cut-off speed, measured as the half-height point on the curve, shifted from 66°/s to 104°/s for this cell. In addition, the bandwidth of the tuning curve (computed as the difference between the high cut-off and the low cut-off speeds) broadened from 61 to 99°/s (P < 0.01).
Figure 2.
Speed tuning curves of two cells from area 18 were tested in the presence and absence of a surround grating moving at a different speed. Dashed lines represent the tuning curve measured with the central grating alone. Solid lines represent the tuning curve tested in the presence of the surround grating. (A) The surround grating was set at a speed (13.3°/s) lower than the optimal speed (26°/s) of the CRF. The central grating was 8.0° (the same size as the CRF) and the surround grating was 20° in diameter. The spatial frequency was 0.15 cycles/°, contrast 0.4, and mean luminance 8.3 cd/m2. (B) The surround grating was set at a speed (149.8°/s) faster than the optimal speed (92.6°/s) of the CRF. The central grating was 12.5° in diameter (the same size as the CRF). The spatial frequency, mean luminance, and contrast were the same as in A. Responses were normalized to the maximum at the optimal speed. (Insets) The stimulus patterns for the center-only and the center-plus-surround conditions. In the latter case, the center and surround gratings were both set at the CRF’s optimal values of orientations, spatial frequency, and movement direction, but moving at different speeds (represented by the length of the arrows). Each point is the mean of 10 trials. The lines represent the best fit of a sixth-order polynomial function to the data. SEs (±1 SE) have been plotted about the mean of the normalized response.
Fig. 2B shows another example. For this cell, the optimal speed was 92.6°/s when tested under a uniform background. With the presence of a surround grating drifted at a faster speed (149.8°/s), the tuning peak shifted to 65.8°/s, and at the same time, both the high cut-off and low cut-off of the tuning curve also shifted toward the low-speed side.
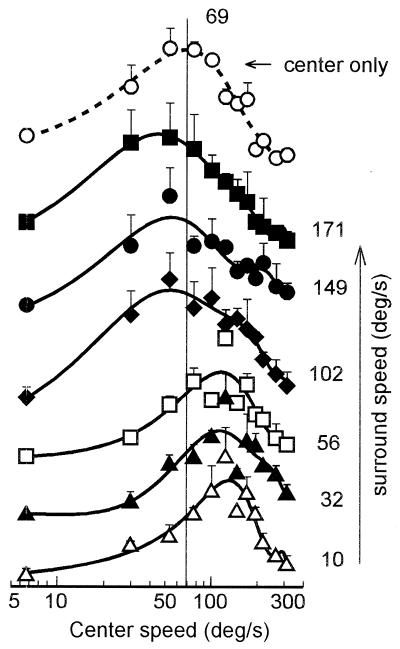
In Fig. 3 the shift in speed selectivity of a neuron was tested by measuring multiple speed tuning curves of the cell in the presence of a surround grating moving at systematically varied speeds. The uppermost curve (dashed line) shows the speed tuning tested with the center-only stimulus; it peaks at 69°/s (indicated by a vertical line). The tuning peak shifted to the right side of the vertical line (to 124°/s, ≈2× faster than the original speed), when a slower speed surround grating was presented concurrently (see the three curves at bottom, surround grating drifted at 10°, 32°, and 56°/s, respectively). On the contrary, the peak shifted to the left when the surround motion was set at relatively higher speeds (the three upper solid curves, surround grating set at 102°, 149°, and 171°/s, respectively). Similar results were obtained for two other cells tested in the same way as in Fig. 3. The most effective surround speeds for causing the shift were within the range between the high cut-off and the low cut-off points. When the surround speed was too much different in magnitude from the center speed, the shifts of center speed tuning were not found.
Figure 3.
Speed tuning curves of an area 18 cell measured in the presence of a surround grating drifted at systematically varying speeds. Numerals on the right represent the surround speed (in °/s) used to obtain each curve. The dashed curve on the top was obtained with the center-only grating; vertical line indicates location of the optimal speed (69°/s) tested under this condition. Each point is the mean of five trials. The curves are the best fitting polynomial functions of the sixth order. Bars = 1 SE; in some cases, the bar is too small to be seen.
We studied in detail the effects of surround speed on the speed tuning properties of the center in 41 cells. Out of the sample, 22 cells were tested with slower surround speeds and 19 cells were tested with faster surround speeds, relative to the optimal speed of each individual cell. The scatter plots in Fig. 4 show the alterations in speed tuning properties caused by slower surround motion. Each point represents measurements from one neuron. Fig. 4A plots the optimal speed tested with the center-only grating (abscissa) against the optimal speed of the same cell tested in the presence of a surround grating (ordinate) drifted at a relatively lower speed (the low cut-off speed of each cell) for the individual cells. Fig. 4B shows the relevant changes in high cut-off speed, and Fig. 4C, in bandwidth for the same sample of cells. The summary graphs illustrate that most of the points clustered above the line of unit slope. In other words, for the majority of the cells recorded (17 of 22), when a surround grating was presented concurrently at a slower speed, the tuning peak (Fig. 4A) and the high cut-off (Fig. 4B) shifted, and the bandwidth (Fig. 4C) broadened, all significantly toward the high-speed side (Student’s t test, P < 0.05). Only five cells (22.7%) from this sample did not have significant shifts.
Figure 4.
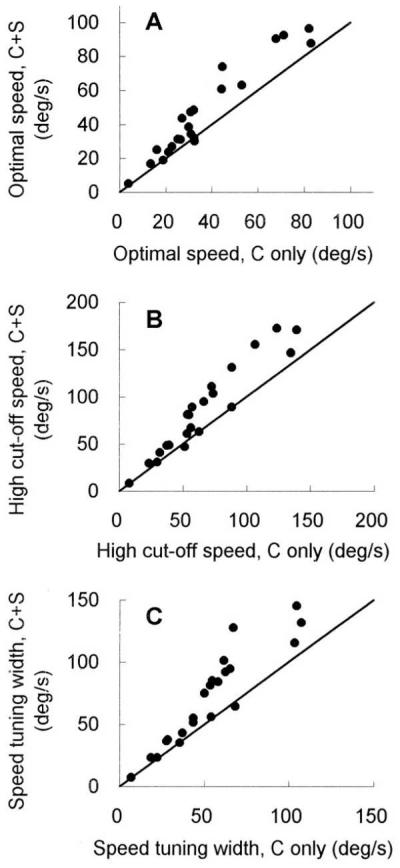
The summary plots of 22 cells, showing the alterations in speed tuning properties caused by a surround grating moving at a slower speed relative to the optimal speed. (A) The optimal speed measured with the center-only grating (abscissa) is plotted against the optimal speed of the same cell tested in the presence of a surround grating (ordinate) drifted at a relatively lower speed (the low cut-off speed for each cell). (B and C) The same comparison for high cut-off speed (B) and for tuning bandwidth (C). In the axis labels, C represents the center-only condition, and C+S represents the center-plus-surround condition.
The summary graphs in Fig. 5 show the variations of speed tuning properties caused by surround speeds faster than the optimal speed. Of the 19 cells tested, significant shifts were found in 16 cells (84.2%). Fig. 5 A, B, and C illustrates the shift in optimal speed, high cut-off, and tuning bandwidth, respectively, for the sample of cells. Contrary to the changes caused by slower movement in the surround (Fig. 4), most of the points in Fig. 5 are below the diagonal line, indicating a slowdown in the speed preference when a surround grating was presented concurrently at a faster speed.
Figure 5.
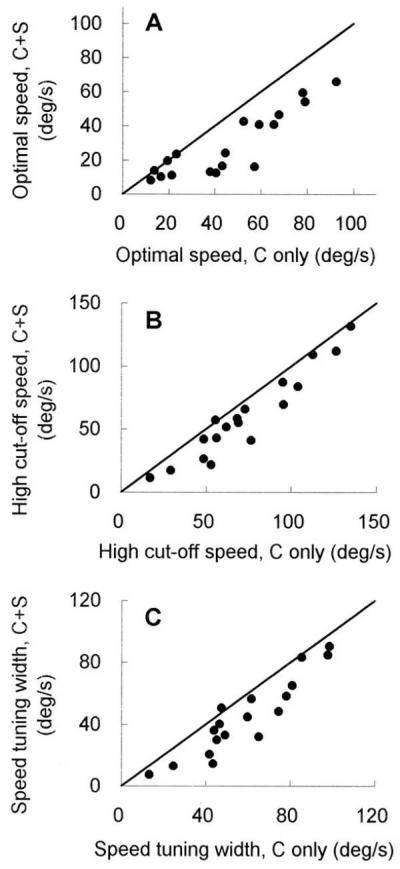
The summary plots of 19 cells, showing the alterations in speed tuning properties caused by a surround grating moving at a faster speed relative to the optimal speed. (A–C) The variation in optimal speed (A), high cut-off (B), and tuning bandwidth (C) is illustrated. Axis labels are similar to those described in Fig. 4.
For all the cells showing a significant shift, the tuning peak and the cut-off speed shifted away from the surround speed. This repulsive effect between center and surround acted to enhance the speed contrast if the surround speed was relatively close to (within the limits of the cell’s cut-off speed) the optimum speed of the cells.
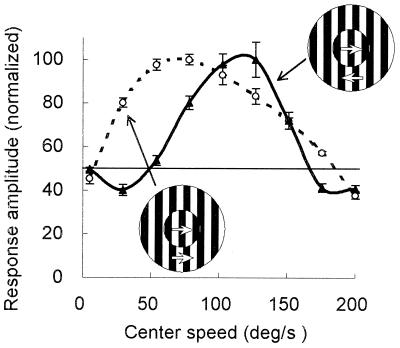
To convince that the shifts were not caused by temporal frequency interactions, we compared the effects of surround grating moving at the optimal speed, but in two opposite directions. One example of such an experiment is shown in Fig. 6. The dotted line represents the speed tuning tested when the surround grating moved in the preferred direction of the cell. The solid line, the tuning obtained when the surround grating moved in the opposite direction. In the later case, the tuning peak, as well as the low cut-off, shifted considerably toward the high-speed side (the peak shifted from 69.3°/s to 120.2/s; and the low cut-off, from 8.5°/s to 49.5°/s). Because the drifting speed of the surround gratings were identical (92.6°/s) in the two cases (so was the temporal frequency, 9.26 cycles/s), the shift seen in Fig. 6 could not result from any temporal–frequency-dependent effect. In fact, the direction of the shift observed in the single cell responses is consistent with what one would expect from the psychophysical phenomenon on speed contrast. We have compared the effects induced by the opposite movements for a population of 19 cells. In Fig. 7 the optimal speed of each of these cells tested in the preferred direction is compared with that obtained in the opposite direction. The scatter plot illustrates that all the points but two (one is on the line, the other is below) are above the diagonal, indicating that an opposite movement in the surround elicited a rise of the preferred speed for most (17/19) of the cells.
Figure 6.
Comparison of the speed tuning of an area 18 cell tested with surround gratings moving at the same speed but in two opposite directions. The dotted line represents the speed tuning tested when the surround grating moved in the preferred direction; the solid line, the speed tuning obtained when the surround grating moved in the opposite direction. The drifting speed of the surround gratings was set at the optimal speed (92.6°/s) in both cases.
Figure 7.
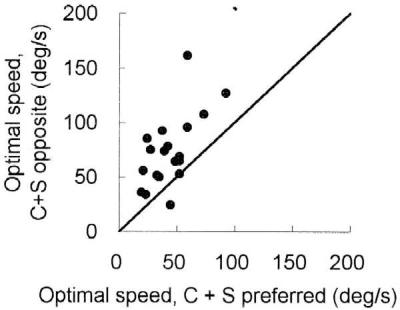
Comparison of the speed tuning of 19 cells tested with surround grating moving in the preferred and the opposite directions. For each of these cells, the optimal speed tested with the preferred direction of surround movement is plotted against that measured with the surround grating moving in the opposite direction. Speed for the preferred and the opposite movement was set at the optimal speed of each of the individual cells.
These results demonstrate that a context motion of lower speed pushes the speed preference of visual cortex cells toward the high-speed side, and vice versa. A similar dependency of the functional properties of single cells on visual context has been observed for orientation selectivity (15). These observations reveal that the filtering properties of visual cortical cells are not fixed, but can be dynamic, to a certain extent, according to the visual context. In our experiments, the effect of the surround was always repulsive, with the tuning curve shifting in a direction away from the speed of the surround stimulus. These specific interactions between the CRF and its surround demonstrate a special form of lateral inhibition phenomenon (16), which exaggerates the difference not in luminance, but in a visual attribute (speed) between adjacent visual regions. The alterations in speed tuning properties observed at the single cell level are in good agreement with the perceptual acceleration and/or deceleration of motion when an object is shown against a moving background of slightly lower or higher speed.
Acknowledgments
We thank Drs. C. L. Baker and Y. C. Diao for helpful comments on the manuscript, and Mrs. X. Z. Xu for technical assistance. This work was supported by grants from the National Natural Science Foundation of China and the Laboratory of Visual Information Processing, Chinese Academy of Sciences, to C.-Y.L.
ABBREVIATION
- CRF
classical receptive field
References
- 1.Loomis J M, Nakayama K. Perception. 1973;2:425–427. doi: 10.1068/p020425. [DOI] [PubMed] [Google Scholar]
- 2.Walker P, Powell D J. Nature (London) 1974;252:732–733. doi: 10.1038/252732a0. [DOI] [PubMed] [Google Scholar]
- 3.Tynan P, Sekuler R. Vision Res. 1975;15:1231–1238. doi: 10.1016/0042-6989(75)90167-4. [DOI] [PubMed] [Google Scholar]
- 4.Marshak W, Sekuler R. Science. 1979;205:1399–1401. doi: 10.1126/science.472756. [DOI] [PubMed] [Google Scholar]
- 5.Mather G, Moulden B. Q J Exp Psychol. 1980;32:325–333. doi: 10.1080/14640748008401168. [DOI] [PubMed] [Google Scholar]
- 6.Levi D M, Schor C M. Vision Res. 1984;24:1189–1196. doi: 10.1016/0042-6989(84)90174-3. [DOI] [PubMed] [Google Scholar]
- 7.Dunker K. In: Source Book of Gestalt Psychology. Ellis W, editor. New York: Humanities Press; 1938. pp. 161–172. [Google Scholar]
- 8.Allman J, Miezin F, McGuinness E. Annu Rev Neurosci. 1985;8:407–430. doi: 10.1146/annurev.ne.08.030185.002203. [DOI] [PubMed] [Google Scholar]
- 9.Li C Y, Li W. Vision Res. 1994;34:2337–2355. doi: 10.1016/0042-6989(94)90280-1. [DOI] [PubMed] [Google Scholar]
- 10.Li C Y. News Physiol Sci. 1996;11:181–186. [Google Scholar]
- 11.Sillito A M, Grieve K L, Jones H E, Cudeiro J, Davis J. Nature (London) 1995;378:492–496. doi: 10.1038/378492a0. [DOI] [PubMed] [Google Scholar]
- 12.Gulyás B, Orban G A, Duysens J, Maes H. J Neurophysiol. 1987;57:1767–1791. doi: 10.1152/jn.1987.57.6.1767. [DOI] [PubMed] [Google Scholar]
- 13.Hammond P, MacKay D M. J Physiol (London) 1981;319:431–442. doi: 10.1113/jphysiol.1981.sp013919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hammond P, Kim J N. Proc R Soc London B. 1994;257:179–184. doi: 10.1098/rspb.1994.0113. [DOI] [PubMed] [Google Scholar]
- 15.Gilbert C D, Wiesel T N. Vision Res. 1990;30:1689–1701. doi: 10.1016/0042-6989(90)90153-c. [DOI] [PubMed] [Google Scholar]
- 16.Ratliff F. Mach Bands: Quantitative Studies on Neuronal Networks in the Retina. San Francisco: Holden-Day; 1965. [Google Scholar]