Abstract
Drugs are named for their primary receptor target and overt action (agonism, antagonism) but the observation of multiple or collateral efficacies emanating from drugs activating a single receptor target is posing a challenge for drug classification and nomenclature. With increasing abilities to detect alteration in cellular function has come the identification of efficacies that are not necessarily manifest in obvious changes in cell response. Specifically, some agonists selectively activate cellular pathways, demonstrate phenotypic behaviour associated with cell type and some antagonists actively induce receptor internalization without activation. In addition, the effects of allosteric modulators can be linked to the nature of the co-binding ligand posing a similar complication in classification and naming. Thus, accurate labels for this new generation of selective drugs may require identification of receptor partners (G-protein type, β-arrestin) or pathway or, in the case of allosteric modulators, identification of co-binding ligands. The association of distinct phenotypic behaviours with molecules opens the opportunity to better associate clinical effects with distinct pharmacological properties.
Keywords: drug nomenclature, receptor agonists and antagonists, drug classification
Pharmacological onomastics: what's in a name?
A fundamental task of pharmacology is the taxonomy of biologically active molecules and with these classifications comes the task of nomenclature. How a drug is classified and named biases how it is used and misrepresentation of the activity of a drug through misnaming can cause confusion. Drugs are named with a combination of their primary target (for example, a histamine H1 receptor) and a description of either their directly observed effect (that is, agonist, inverse agonist) or interfering coeffect with another ligand, usually the endogenous ligand (that is, antagonist). In cases where a drug interacts with multiple targets (that is, amitriptylene with activity at histamine H1, H2, α1, muscarinic receptors, catecholamine uptake, phosphodiesterase), the most prominent mechanism (target for which the drug has the highest affinity and/or efficacy) or historically the first mechanism to be discovered often defines the default label for the drug. Multi-target activity also can be labelled for clinical application (that is, amitriptylene as an antidepressant). In cases where a collection of molecular mechanisms combine to produce a clinical phenotype, that may also become the label for the drug. For example, the class of drug atypical antipsychotics have a mixture of activities on dopamine D1, D2 receptors, histamine H1, 5-HT2A, 5HT1A, muscarinic m1 receptors and α1-adrenoceptors; these activities combine to determine a clinical phenotype (Goldstein, 2000).
A source of possible complication in nomenclature originates from system-dependent drug behaviour. For agonists, this can involve the strength of the observed agonist effect in any given system, that is, low-efficacy agonists may be antagonists, partial agonists or full agonists in various systems depending on the receptor density and/or efficiency of receptor coupling. This makes labels such as partial agonist and full agonist subjective and, at times, conflicting. System dependence also can be seen with antagonists in the observation of surmountable and insurmountable antagonism. The latter can be a reflection of the kinetics of offset of the antagonist and the temporal characteristics of the assay system. Thus most, if not all, slow offset orthosteric antagonists will demonstrate depression of maximal response (insurmountable apparently non-competitive behaviour) in short window response collections systems such as FLIPR. These differences highlight the capricious nature of naming drugs for a particular system behaviour.
In general, ambiguities arising from multiple target activity and cell- and assay-dependent variation in drug behaviour are known pharmacological phenomena and usually are dealt with on a case-by-case basis. However, a new aspect of multiple drug activity is emerging from single target-based molecules; the complications arise mainly because of drug efficacy. As a preface to discussion of nomenclature of such drugs, it is worth considering what is meant by the term efficacy and how it has expanded beyond the idea of the initiation of cellular response.
The expectation of efficacy
Historically, a judicious application of Occam's razor has led to a general expectation of zero efficacy in biologically active compounds as a first assumption, that is the default is to assume the most simple idea that a molecule only has affinity for a target. This view has been supported by historical data obtained from systems with limited windows into efficacy, namely overt tissue response observed in real time. When Stephenson (1956) defined efficacy, his indicator was guinea pig ileal contraction and the assumption was that molecules that did not produce contraction, but otherwise interfered with the production of contraction by agonists, were antagonists possessing affinity but not efficacy. The restricted means by which pharmacologists could detect receptor activation at the time limited the number of molecules thought to possess efficacy.
This view had to change with increasing experimental evidence as, for example, when Costa and Herz (1989) revealed an extensive ‘secret life' for antagonists as inverse agonists. As recombinant technology became more widespread and constitutively active assay systems became commonplace, the experimental finding was found to coincide with the theoretical prediction, namely that the predominance of antagonists are inverse agonists. For example, in a survey of 322 antagonists (that did not show positive partial agonist activity) for 73 receptors, 85% were found to be inverse agonists with only 15% producing no discernible change in constitutive activity (Kenakin, 2004). In this regard, the availability of assay technology to detect efficacy controls the perception of the presence of efficacy. In general, these data support the view that if a molecule binds to a biological target, there is likelihood that it also will have some sort of efficacy.
The underlying mechanism of the increasing prevalence of efficacy in drug molecules is the obligatory energetic connection between affinity and efficacy, that is if a ligand binds to an ensemble of receptor conformations, it will bias the makeup of that set of conformations to a collection different from what it was before drug binding (Onaran and Costa, 1997; Onaran et al., 2000; Kenakin and Onaran, 2002). This necessarily opens the possibility that one or more of those conformations will interact with cellular components to produce a biological effect. Glimpses into this thermodynamic world have been gained through the study of fluorescently labelled receptors. Specifically, studies with a fluorescent label covalently linked to a β2-adrenoceptor (such that fluorescence is sensitive to the protein environment) indicate changes in receptor conformation with binding of different types of ligands (Gether et al., 1995; Ghanouni et al., 2001; Palanche et al., 2001; Swaminath et al., 2004). Other generic approaches to the detection of changes in protein conformation and interaction such as bioluminescent resonance energy transfer and fluorescent resonance energy transfer (Bouvier, 2001; Giepmans et al., 2006; Li et al., 2006; Pfleger and Eidne, 2006; Persani et al., 2007) have revealed ‘efficacies' not obvious from cellular assay systems. As discussed previously, the prevalence of inverse agonism among the ranks of antagonists also indicates that most antagonists produce changes in receptor conformation.
With increasing means to detect ligand-induced changes in receptor conformation, and cellular responses to those changes in conformation has become expanded definitions of efficacy beyond isolated organ responses (see Figure 1). Even efficacy as the simple definition of induction of cellular response needed to be redefined in light of evidence that receptors form active states in the absence of ligands (constitutive activity). This led to the definition of negative efficacy for inverse agonists and subsequently leads to a general definition of efficacy as the ‘property of a molecule that changes the behaviour of the receptor towards the cellular host'.
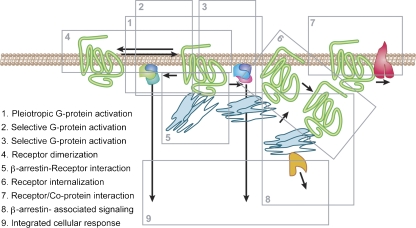
Figure 1.
A sample of interactions of seven transmembrane receptors with cellular components to generate phenotypes of efficacy. Specific assays are available to isolate these processes and define molecular activity associated with them. It should be noted that cellular response does not automatically indicate interactions of the ligand-bound receptor with some of these processes (that is, is not a universal indicator of molecular efficacy).
In general, the evidence supports the view that ligand binding goes hand-in-hand with target modification, that is the presence of efficacy resulting from the formation of a bias towards ligand-stabilized receptor conformations of a single receptor. Given this expanded view of efficacy for biologically active molecules, it is useful to discuss how efficacy relates to the phenomenon of cellular behaviours for multiple receptor conformations.
Single-target pathway pleiotropy
With increasing vantage points that pharmacologists have to view drug activity and dissect signalling pathways, the phenomenon of ‘single-target pathway pleiotropy' has been identified. This occurs when certain ligands stabilize different receptor active states which then go on to activate a number of different cellular processes (Kenakin, 1995a, 2002; Urban et al., 2007). These ligands become associated with biased activation of these processes and thus take on cell-specific phenotypic behaviour. The fact that some molecules in a general class (that is, agonists) take on specific behaviours in systems can lead to ambiguous nomenclature and the emergence of cell-specific aliases. These behaviours occur when the ligand-bound receptor activates some, but not all, of its repertoire of cellular pathways.
For receptors that interact with more than one G-protein in the cell, different agonists can produce selective G-protein activation. This phenomenon has been given various names from ‘stimulus trafficking', ‘biased agonism' to ‘functional selectivity'. For example, calcitonin receptor-activating ligands such as porcine calcitonin can preferentially activate Gs protein (over Gi proteins) when compared to agonist such as rat amylin (Watson et al., 2000), a bias in the cellular signal activation is produced. Pleiotropic G-protein interaction can be more pronounced as in the case of the CB1 cannabinoid ligand desacetyllevonantradol, which is a ‘positive' agonist for Gi1 and Gi2 but an ‘inverse' agonist for Gi3. Similarly, (R)-methanandamide is an ‘inverse' agonist for Gi1 and Gi2 and a ‘positive' agonist for Gi3 (Mukhopadhyay and Howlett, 2005). Therefore, the labels positive and inverse agonist are both associated with the ligand and CB1 receptor but for different G-protein pathways. A nomenclature based on simple target-centric behaviour for (R)-methanandamide as a cannabinoid receptor agonist breaks down; depending on the receptor coupling partner, this molecule can legitimately be called an agonist or an inverse agonist.
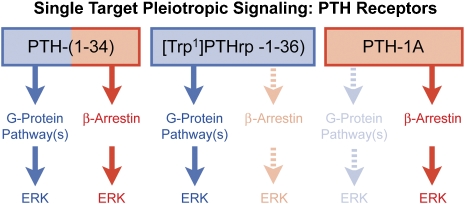
Selective pathway interaction goes beyond the G-protein level. As assays enable observation of activities deeper into the cytoplasm, other 7TM receptor ligand-induced signalling differences are observed. The discovery that β-arrestins bind to phosphorylated receptors to function as signalling scaffolds for kinases (Lefkowitz, 2004; Terrillon and Bouvier, 2004; Lefkowitz and Shenoy, 2005; Luttrell, 2005, Lefkowitz et al., 2006) opens a new G-protein-independent signalling paradigm for 7TM receptors. It can now be shown that many receptors activate cellular kinases through separate pathways. For example, parathyroid hormone (PTH) activates extracellular signal-related kinase through separate G-protein-related and G-protein-independent pathways. The intriguing and pharmacologically relevant aspect to this effect is that the chemical structure of the agonist may be a control point for the selective activation of these processes. Thus, analogues of PTH can induce separate activation of each pathway with the selective stimulation of G-protein-mediated extracellular signal related kinase (ERK)1/2 stimulation by [Trp1]PTHrp-(1–36) and selective stimulation of β-arrestin-dependent stimulation of ERK1/2 by PTH-1A ([D-Trp12,Tyr34]PTH-(7–34); Gesty-Palmer et al., 2006, see Figure 2). Similarly in another receptor system, the Substance P analogue SpD ([D-Arg1,D-Phe5,D-Trp7,9,Leu11]Substance P) and bombesin are both agonists of the bombesin/gastrin-releasing peptide receptor producing ERK1/2 activation. However, the effects of SpD are pertussis sensitive (indicating a Gi-protein dependence of the effect) while the response due to bombesin is not. This latter agonist traffics stimulus directly to the ERK1/2 pathway through a G-protein-independent β-arrestin effect (Mackinnon et al., 2001). Thus, while both SpD and bombesin are agonists of the same receptor, they induce response through different pathways.
Figure 2.
Schematic diagram of parathyroid hormone (PTH) and analogues as activators of two separate pathways for extracellular signal kinase activation, namely G-protein-dependent (blue) and β-arrestin G-protein-independent (red). PTH-1A=[D-Trp12,Tyr34]PTH-(7–34). Data from Gesty-Palmer et al. (2006).
New cellular eyes to see also have revealed efficacies not immediately evident, such as the active internalization of receptors by antagonists. For a number of years it had been observed that serotonin antagonists produce effects consistent with the active internalization of receptors (Gray and Roth, 2001). The advent of technology enabling the visualization of receptor internalization has since definitively shown that antagonists, for a number of different receptors, produce no measurable receptor activation but still actively internalize receptors. Like inverse agonism, this is another hidden efficacy of antagonists, made evident by the appropriate assay. In the case of antagonists, added properties currently not explicitly associated with certain molecules, can result in cellular and clinical phenotypes. For example, the known ability of inverse agonists to induce cell surface upregulation (Milligan and Bond, 1997) can lead to tolerance as in the treatment of ulcer with clinically used histamine H2 antagonists (Smit et al., 1996). Thus, a case could be made for differentiating antagonists on the basis of efficacy that causes changes in cell surface receptor regulation. For example, 5-HT2A antagonists that induce receptor internalization (that is, clozapine: Willins et al., 1999; mianserin, ritanserin: Bhatnagar et al., 2001) should be differentiated from those that induce receptor upregulation (MDL 11939 [α-phenyl-2-(2-phenylmethyl)-4-piperidinemethanol], Aloyo et al., 2001).
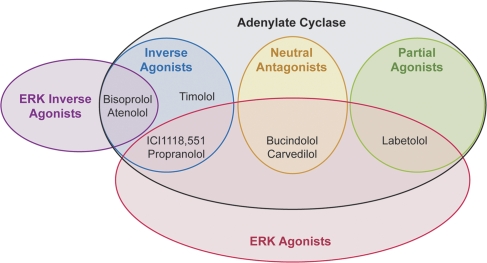
With the introduction of new technologies that enable viewing of drug-induced biological activity from numerous vantage points, multiple behaviours are increasingly being discovered leading to a log jam in nomenclature. For example, the β-blocker propranolol reveals different activities when tested in different systems being an inverse agonist with respect to Gs-protein activation and a positive agonist with respect to ERK1/2 activation (Azzi et al., 2003; Baker et al., 2003). These discoveries of multiple behaviours bring complications in nomenclature, that is propranolol lays legitimate claim to being a β-blocker, inverse agonist for Gs-protein and and ERK1/2 agonist. An extensive examination of eight ligands on β1- and β2-mediated activation of adenylate cyclase and mitogen-activated protein kinase activity reveals a rich texture of positive, and inverse agonism as well as neutral antagonism for the various pathways (Galandrin and Bouvier, 2006). These data and data for propranolol were used to construct Figure 3 to show how these various ligands can be distinguished through their varying efficacies for these pathways. The activity of interest is in the eye of the beholder and the beholder then chooses the alias for the drug.
Figure 3.
The classification of β2-adrenoceptor ligands. Eight β2-adrenoceptor ligands can be distinguished as positive and inverse agonists and neutral antagonists for adenylate cyclase (Gs-protein-mediated response) and agonists or inverse agonists for ERK1/2 signalling. Data from Galandrin and Bouvier (2006), Azzi et al. (2003) and Baker et al. (2003).
The stabilization of different receptor conformations, or ensemble of conformations of receptor, by molecules forms the basis of a system where molecules may not have a single efficacy, but rather possibly numerous efficacies depending on the biological activity of the various conformations enriched by the molecule. This has been referred to as ‘collateral efficacy', meaning effects produced in parallel and belonging to the same but not in a direct line of descent and not secondary in nature (Kenakin, 2006). Another description used for ligands involved with β-arrestin ERK and G-protein signalling is ‘pluridimensional efficacy' (Galandrin and Bouvier, 2006) suggesting multiple efficacies for a single molecule activating a single target. In light of these multiple behaviours, efficacy may require qualification and a formalized convention for nomenclature. In addition to efficacy-based complications in drug nomenclature, the same problem arises with molecules that are permissive in their receptor effects with other molecules, namely, allosteric modulators.
Allosteric modulators as pharmacologic chameleons
Allosteric modulators are molecules that co-bind to the receptor with other molecules and, as such, their effects can be probe-dependent. For example, the m2 muscarinic receptor allosteric ligand eburnamonine produces potentiation of affinity to pilocarpine, no change in the affinity of arecaidine propyl ester and reduction of affinity to arecoline (Jakubic et al., 1997). What is such a ligand to be called? In this case, the name is dependent on the co-interacting molecule. Another unique aspect of allosteric molecules is that, since their effects emanate from an interaction at a separate site on the receptor, they have saturable effect when the allosteric site is completely occupied. This results in finite effects on the affinity and efficacy of the co-binding ligand. Whereas competitive antagonists completely block the effects of agonists, if present in adequate concentrations, an allosteric modulator may produce only a mild effect on agonist effect allowing the tissue to still respond but at a lower intensity. For example, an allosteric modulator with a co-operative constant α of 0.3 produces a threefold decrease in agonist affinity and this will be the maximal effect on the agonist. In these cases, the term modulator is an important distinction from the term antagonist.
How important is descriptive nomenclature?
Over the past 15 years, evidence has accumulated to show that molecules can have efficacies not evident from studies in conventional biological response-orientated assays and that these efficacies are often pluridimensional. The corollary to this idea is that not all possible efficacies reside in every molecule for a given target. This results in a texture for agonism, antagonism, modulation and potentiation for a range of cellular processes; the question then is, should names be associated with these molecules to identify pharmacological uniqueness? An analogy can be drawn to describing a person as a musician in a general sense and then progressively defining more accurately that person's function with successive modifiers such as stringed instrumentalist then a guitarist, then electric guitarist and so on.
There are tangible benefits from uniquely identifying drugs that do different things to biological systems. Specifically, this can assist in the process of linking therapeutic value to pharmacological properties through translational medicine (Littman et al., 2007). One line of reasoning for this approach argues that if a molecule is biologically active, it is evident that it changes the ensemble of receptor conformations. Therefore it follows that there is a likelihood that it may have efficacies for other systems associated with that target. The value of characterizing this efficacy would be evident in the association of the collection of activities with a clinical phenotype.
The need to identify special ligand activity is balanced by the need to simplify behaviour-rooted systems of nomenclature. One approach would be to label according to observed biological phenotype, that is the previously mentioned typical and atypical antipsychotics. For example, agonists activating ERK pathways through G-proteins and independently through β-arrestin can be separated in terms of the observed response patterns. The former demonstrates intense but short-lived response while the latter produce lower intensity more prolonged responses. However, the problem with these phenotypes is the possibility of mixed actions (that is, PTH does both and shows an intense but prolonged response).
Another problem with phenotype-based labelling is the possible divergence from target dependence and emergence of cellular dependence of these phenotypes. For example, receptor desensitization and internalization is an important issue for opioid analgesia (some opioid agonists desensitize receptors more than others) and in some cases the differences between opioid agonists is profound as in the clear inability of the μ-opioid receptor agonist herkinorin to cause the receptor to interact with β-arrestin or to internalize (Groer et al., 2007). However, there is cell type dependence for desensitization and internalization observed for many other opioid agonists (Marie et al., 2006) and this would lead to confusion in phenotype-based classification. Similarly, while some CCR5 ligands produce receptor internalization, an activity that may be relevant in protection against HIV-1 entry, CCR5 internalization varies with the variety of cells used for the experiments therefore the phenotype of receptor internalization is variable with cell type. Cell-specific phenotypic behaviour is fragile and different phenotypes could emerge from something as simple as receptor density. For example, the opioid agonist [D-Ala2-D-Leu5]-enkephalin produces inhibition of adenylate cyclase and stimulation of high-affinity GTPase in NG 108–115 cells. However, diminution of receptor number through alkylation soon removes the more sensitive GTPase response and changes the phenotype to only adenylate cyclase inhibition (Costa et al., 1988).
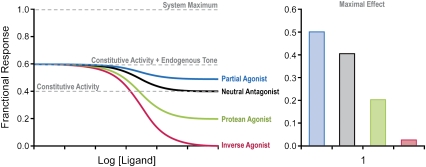
There are cases where a term has been used to identify unique activity. For example, protean agonists, defined in theoretical terms, are molecules that produce an active state of the receptor that is of lower efficacy than the state formed constitutively in the absence of agonists (Kenakin, 1995b, 2001). Under these circumstances, they are partial agonists in non-constitutively active systems and inverse agonists in constitutively active systems; these molecules would function as the ultimate normalizers in vivo. Thus, dichloroisoproterenol is a β-adrenoceptor partial agonist with the legitimate alias protean agonist as well (Chidiac et al., 1996). Such molecular-mechanism-based monikers can be useful differentiators for unique activity. Figure 4 shows the effect of four different molecular types of ligand on an in vivo constitutively active system with endogenous tone. It can be seen that different levels of effect are seen with a partial agonist, neutral antagonist, protean agonist and inverse agonist. In an in vitro non-constitutively active system, the partial and protean agonists and, separately, the inverse agonists and neutral antagonists would give similar profiles and be non-differentiable. This highlights the importance of using appropriate assays to characterize ligand activity.
Figure 4.
Hypothetical dose–response curves for four molecules with different patterns of efficacy. The system is constitutively active (fractional effect=0.4) and then placed under an additional endogenous agonist tone to a level of 0.6 fractional effect. A partial agonist decreases the endogenous tone but imposes its own intrinsic activity on the system, a neutral competitive antagonist abolishes the endogenous tone but does not eliminate constitutive activity, a protean agonist reduces the constitutive activity somewhat but imposes its own intrinsic effect and an inverse agonist abolishes both the endogenous tone and the constitutive activity.
At present, there is no systematic naming convention for distinguishing single-target uniqueness; this may be a topic for future consideration. Attempts have been made in some cases, such as protean agonism and ago-antagonism. This latter term, which has been previously used to label opposing muscle spindle inputs in physiology (Ribot-Ciscar and Roll, 1998) and separately as opposing ‘ying yang' hormones such as vasopressin and oxytocin (Legros, 2001), for single targets refers to molecules that are positive agonists for one pathway (that is, G-protein) and antagonists (or inverse agonists) at another (Beyermann et al., 2007). Similarly, ago-allosteric modulators refer to agonists that also potentiate endogenous agonist action (Schwartz and Holst, 2006). While these terms suffer from the same shortcoming as other system-based labels (the bias in the cellular pleiotropic G-protein content may define the primary observed activity), it also identifies ligands as being different from standard agonists.
Naming biased-agonists and other pluridimensional ligands becomes complicated by the need to identify not only the primary receptor and overt action, but also complimentary players such as couplers (that is, G-proteins) and/or signalling pathways. In the case of allosteric ligands, co-ligand names may be required to describe succinctly effects although it could be argued that only the effects on endogenous agonist need be considered. However, in areas such as the chemokine system where there is redundancy in ligands for receptors (Wells et al., 2006), this may still pose a practical problem even for endogenous ligands. For example, the allosteric HIV-1 entry inhibitor aplaviroc blocks the binding of the natural chemokine MIP-1α but does not block the binding of RANTES, another natural chemokine agonist for this receptor (Watson et al., 2005). Probe dependence may be quite pronounced as in the case of the complete resistance of CXCR4-mediated chemotaxis response to the stromal-derived factor type 1α peptide fragments [Ala-Ser-Leu-Trp] and [Arg-Ser-Val-Met] to antagonism by the CXCR4 antagonist AMD-3100 (Sachpatzidis et al., 2003).
Conclusions
The accurate determination of nuances in ligand efficacy may yield important patterns that can be characterized in vitro in experimental systems and then associated with useful therapeutic profiles in more complex in vivo systems. The binning of molecules into categories (that is, as in the case of ERK1/2 agonist—β-blockers, see Figure 3) is the result of testing of biologically active molecules in numerous pharmacological assay systems; the thermodynamic association of affinity and efficacy would advocate rationale for such extensive testing. This suggests that an important part of drug taxonomy is to define ‘completely' the efficacies of a given biologically active molecule with as many eyes to see activity as possible. This also suggests that the classification of drugs is an ongoing conditional process either with respect to the signalling pathway connected to the receptor and/or the orthosteric molecule co-binding to that receptor. The potential bonus of the identification of collateral or pluridimensional efficacy furnishes the justification. A concomitant system of nomenclature would assist in this process as appropriate labels for drugs would bias how the molecule is used.
Abbreviations
- Aplaviroc (GW873140)
4-{[4-({(3R)-1-butyl-3-[(R)-cyclohexyl(hydroxy)methyl]-2,5-dioxo-1,4,9-triazaspiro[5.5]undec-9-yl}methyl)phenyl]oxy}benzoic acid hydrochloride
- BRET
bioluminescent resonance energy transfer
- DADLE
[D-Ala2-D-Leu5]-enkephalin
- ERK
extracellular signal-related kinase
- FRET
fluorescent resonance energy transfer
- ICI118551
[±]-1-[2,3-(dihydro-7-methyl-1H-inden-4-yl)oxy]-3-[(1-methylethyl)amino-2-butanol]
- MDL 11,939
[α-phenyl-2-(2-phenylmethyl)-4-piperidinemethanol]
- SDF-1α
stromal-derived factor type 1α
- SpD
[D-Arg1,D-Phe5,D-Trp7,9,Leu11]Substance P
Conflict of interest
The author states no conflict of interest.
References
- Aloyo VJ, Dave KD, Rahman T, Harvey JA. Selective and divergent regulation of cortical 5-HT2A receptors in rabbit. J Pharmacol Exp Ther. 2001;299:1066–1072. [PubMed] [Google Scholar]
- Azzi M, Charest PG, Angers S, Rousseau G, Kohout TA. arrestin-mediated activation of MAPK by inverse agonists reveals distinct active conformations for G-protein-coupled receptors. Proc Natl Acad Sci USA. 2003;100:11406–11411. doi: 10.1073/pnas.1936664100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker JG, Hall IP, Hill SJ. Agonist and inverse agonist actions of β-blockers at the human β2-adrenoceptor provide evidence for agonist-directed signaling. Mol Pharmacol. 2003;64:1357–1369. doi: 10.1124/mol.64.6.1357. [DOI] [PubMed] [Google Scholar]
- Beyermann M, Heinrich N, Fechner K, Ferkurt J, Zhang W, Kraetke O, et al. Ago-antagonists for G protein-coupled peptide hormone receptor by modifying the agonist's signaling domain. Br J Pharmacol. 2007;151:851–859. doi: 10.1038/sj.bjp.0707293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatnagar A, Willins DL, Gray JA, Woods J, Benovic JL, Roth BL. The dynamin-dependent, arrestin-independnet internalization of 5-hydroxytryptamine 2A (5-HT2A) serotonin receptors reveals differential sorting of arrestins and 5-HT2A receptors during endocytosis. J Biol Chem. 2001;276:8269–8277. doi: 10.1074/jbc.M006968200. [DOI] [PubMed] [Google Scholar]
- Bouvier M. Oligomerization of G-protein-coupled transmitter receptors. Nat Rev Neurosci. 2001;2:274–286. doi: 10.1038/35067575. [DOI] [PubMed] [Google Scholar]
- Chidiac P, Nouet P, Bouvier M. Agonist-induced modulation of inverse agonist efficacy at the beta 2-adrenergic receptor. Mol Pharmacol. 1996;50:662–666. [PubMed] [Google Scholar]
- Costa T, Herz A. Antagonists with negative intrinsic activity at δ-opioid receptors coupled to GTP-binding proteins. Proc Natl Acad Sci. 1989;86:7321–7325. doi: 10.1073/pnas.86.19.7321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa T, Klinz FJ, Vachon L, Herz A. Opioid receptors are coupled tightly to G proteins but loosely to adenylate cyclase in NG108–15 cell membranes. Mol Pharmacol. 1988;34:744–754. [PubMed] [Google Scholar]
- Galandrin S, Bouvier M. Distinct signaling profiles of β1 and β2 adrenergic receptor ligands toward adenylyl cyclase and mitogen-activated protein kinase reveals the pluridimensionality of efficacy. Mol Pharmacol. 2006;70:1575–1584. doi: 10.1124/mol.106.026716. [DOI] [PubMed] [Google Scholar]
- Gesty-Palmer D, Chen M, Reiter E, Ahn S, Nelson CD, Wang S, et al. Distinct β-arrestin- and G protein-dependent pathways for parathyroid hormone receptor-stimulated ERK1/2 activation. J Biol Chem. 2006;281:10856–10864. doi: 10.1074/jbc.M513380200. [DOI] [PubMed] [Google Scholar]
- Gether U, Lin S, Kobilka BK. Fluorescent labeling of purified β2-adrenergic receptor: evidence for ligand specific conformational changes. J Biol Chem. 1995;270:28268–28275. doi: 10.1074/jbc.270.47.28268. [DOI] [PubMed] [Google Scholar]
- Ghanouni P, Gryczynski Z, Steenhuis JJ, Lee TW, Farrens DL, Lakowicz JR, et al. Functionally different agonists produce distinct conformations in G-protein coupling domains of the β2-adrenergic receptor. J Biol Chem. 2001;276:24433–24436. doi: 10.1074/jbc.C100162200. [DOI] [PubMed] [Google Scholar]
- Giepmans BNG, Adams SR, Ellisman MH, Tsien RY. Review—The fluorescent toolbox for assessing protein location and function. Science. 2006;312:217–224. doi: 10.1126/science.1124618. [DOI] [PubMed] [Google Scholar]
- Goldstein JM. The new generation of antipsychotic drugs: how atypical are they. Int J Neuropsychopharmacol. 2000;3:339–349. doi: 10.1017/S1461145700002042. [DOI] [PubMed] [Google Scholar]
- Gray JA, Roth BL. Paradoxical trafficking and regulation of 5-HT2A receptors by agonists and antagonists. Brain Res Bull. 2001;56:441–451. doi: 10.1016/s0361-9230(01)00623-2. [DOI] [PubMed] [Google Scholar]
- Groer CE, Tidgewell K, Moyer RA, Harding WW, Rothman RB, Prisinzano TE, et al. An opioid agonist that does not induce m-opioid receptor-arrestin interactions or receptor internalization. Mol Pharmacol. 2007;71:549–557. doi: 10.1124/mol.106.028258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakubic J, Bacakova L, Lisa V, El-Fakahany EE, Tucek S. Positive cooperativity of acetylcholine and other agonists with allosteric ligands on muscarinic acetylcholine receptors. Mol Pharmacol. 1997;52:172–179. doi: 10.1124/mol.52.1.172. [DOI] [PubMed] [Google Scholar]
- Kenakin TP. Pharmacological proteus. Trends Pharmacol Sci. 1995b;16:256–258. doi: 10.1016/s0165-6147(00)89037-9. [DOI] [PubMed] [Google Scholar]
- Kenakin TP, Onaran O. The ligand paradox between affinity and efficacy: can you be there and not make a difference. Trends Pharmacol Sci. 2002;23:275–280. doi: 10.1016/s0165-6147(02)02036-9. [DOI] [PubMed] [Google Scholar]
- Kenakin TP. Agonist-receptor efficacy II: agonist trafficking of receptor signals. Trends Pharmacol Sci. 1995a;16:232–238. doi: 10.1016/s0165-6147(00)89032-x. [DOI] [PubMed] [Google Scholar]
- Kenakin TP. Inverse, protean, and ligand-selective agonism: matters of receptor conformation. FASEB J. 2001;15:598–611. doi: 10.1096/fj.00-0438rev. [DOI] [PubMed] [Google Scholar]
- Kenakin TP. Efficacy at G protein coupled receptors. Annu Rev Pharmacol Toxicol. 2002;42:349–379. doi: 10.1146/annurev.pharmtox.42.091401.113012. [DOI] [PubMed] [Google Scholar]
- Kenakin TP. MiniReview: efficacy as a vector: the prevalence and paucity of inverse agonism. Mol Pharmacol. 2004;65:2–11. doi: 10.1124/mol.65.1.2. [DOI] [PubMed] [Google Scholar]
- Kenakin TP. Collateral efficacy as pharmacological problem applied to new drug discovery. Expert Opin Drug Disc. 2006;1:635–652. doi: 10.1517/17460441.1.7.635. [DOI] [PubMed] [Google Scholar]
- Lefkowitz RJ. Historical review: a brief history and personal retrospective of seven-transmembrane receptors. Trends Pharmacol Sci. 2004;25:413–422. doi: 10.1016/j.tips.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Lefkowitz RJ, Rajagopal K, Whalen EJ. New roles for beta-arrestins in cell signaling: not just seven-transmembrane receptors [Review] Mol Cell. 2006;24:643–652. doi: 10.1016/j.molcel.2006.11.007. [DOI] [PubMed] [Google Scholar]
- Lefkowitz RJ, Shenoy SK. Transduction of receptor signals by β-arrestins. Science. 2005;308:512–517. doi: 10.1126/science.1109237. [DOI] [PubMed] [Google Scholar]
- Legros J-L. Inhibitory effect of oxytocin on corticotrope function in humans: are vasopressin and oxytocin ying–yang neurohormones. Psychoneuroendocrinology. 2001;26:649–655. doi: 10.1016/s0306-4530(01)00018-x. [DOI] [PubMed] [Google Scholar]
- Li IT, Pham E, Truong K. Protein biosensors based on the principle of fluorescence resonance energy transfer for monitoring cellular dynamics. Biotechnol Lett. 2006;28:1971–1982. doi: 10.1007/s10529-006-9193-5. [DOI] [PubMed] [Google Scholar]
- Littman BH, De Mario L, Plebani M, Marincolas FM. What's next in translational medicine. Clin Sci. 2007;112:217–227. doi: 10.1042/CS20060108. [DOI] [PubMed] [Google Scholar]
- Luttrell LM. Composition and function of G protein-coupled receptor signalsomes controlling mitogen-activated protein kinase activity. J Mol Neurosci. 2005;26:253–263. doi: 10.1385/JMN:26:2-3:253. [DOI] [PubMed] [Google Scholar]
- Mackinnon AC, Waters C, Jodrell D, Haslett C, Sethi T. Bombesin and substance P analogues differentially regulate G-protein coupling to the bombesin receptor. J Biol Chem. 2001;276:28083–28091. doi: 10.1074/jbc.M009772200. [DOI] [PubMed] [Google Scholar]
- Marie N, Aguila B, Allouche S. Tracking the opioid receptors on the way of desensitization. Cell Signal. 2006;18:1815–1833. doi: 10.1016/j.cellsig.2006.03.015. [DOI] [PubMed] [Google Scholar]
- Milligan G, Bond RA. Inverse agonism and the regulation of receptor number. Trends Pharmacol Sci. 1997;18:468–474. doi: 10.1016/s0165-6147(97)01139-5. [DOI] [PubMed] [Google Scholar]
- Mukhopadhyay S, Howlett AC. Chemically distinct ligands promote differential CB1 cannabinoid receptor-Gi protein interactions. Mol Pharmacol. 2005;67:2016–2024. doi: 10.1124/mol.104.003558. [DOI] [PubMed] [Google Scholar]
- Onaran HO, Costa T. Agonist efficacy and allosteric models of receptor action. Ann NY Acad Sci. 1997;812:98–115. doi: 10.1111/j.1749-6632.1997.tb48150.x. [DOI] [PubMed] [Google Scholar]
- Onaran HO, Scheer A, Cotecchia S, Costa T.A look at receptor efficacy. From the signaling network of the cell to the intramolecular motion of the receptor The Pharmacology of Functional, Biochemical, and Recombinant Systems Handbook of Experimental Pharmacology 2000Springer, Heidelberg: Germany; 217–280.In: Kenakin TP, Angus JA (eds).vol 148. [Google Scholar]
- Palanche T, Ilien B, Zoffmann S, Reck MP, Nucher B, Edelstein SJ, et al. The Neurokinin A receptor activates calcium and cAMP responses through distinct conformational states. J Biol Chem. 2001;276:34853–34861. doi: 10.1074/jbc.M104363200. [DOI] [PubMed] [Google Scholar]
- Persani L, Calebiro D, Bonomi M. Technology Insight: modern methods to monitor protein-protein interactions reveal functional TSH receptor oligomerization. Nat Clin Pract Endocrin Metab. 2007;3:180–190. doi: 10.1038/ncpendmet0401. [DOI] [PubMed] [Google Scholar]
- Pfleger KDG, Eidne KA. Illuminating insights into protein-protein interactions using bioluminescence resonance energy transfer (BRET) Nat Methods. 2006;3:165–174. doi: 10.1038/nmeth841. [DOI] [PubMed] [Google Scholar]
- Ribot-Ciscar E, Roll JP. Ago-antagonist muscle spindle inputs contribute together to joint movement coding in man. Brain Res. 1998;791:167–176. doi: 10.1016/s0006-8993(98)00092-4. [DOI] [PubMed] [Google Scholar]
- Sachpatzidis A, Benton BK, Manfredi JP, Wang H, Hamilton A, Dohlman HG, et al. Identification of allosteric peptide agonists. J Biol Chem. 2003;278:896–907. doi: 10.1074/jbc.M204667200. [DOI] [PubMed] [Google Scholar]
- Schwartz TW, Holst B. Ago-allosteric modulation and other types of allostery in dimeric 7TM receptors. J Recept Signal Transduct Res. 2006;26:107–128. doi: 10.1080/10799890600567570. [DOI] [PubMed] [Google Scholar]
- Smit MJ, Leurs R, Alewijnse AE, Blauw J, Amerongen GPV, Vandevrede Y, et al. Inverse agonism of histamine H-2 antagonists accounts for up-regulation of spontaneously active histamine H-2 receptors. Proc Natl Acad Sci USA. 1996;93:6802–6807. doi: 10.1073/pnas.93.13.6802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephenson RP. A modification of receptor theory. Br J Pharmacol. 1956;11:379–393. doi: 10.1111/j.1476-5381.1956.tb00006.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swaminath G, Xiang Y, Lee TW, Steenhuis J, Parnot C, Kobilka BK. Sequential binding of agonists to the β2-adrenoceptor. Kinetic evidence for intermediate conformation states. J Biol Chem. 2004;279:686–691. doi: 10.1074/jbc.M310888200. [DOI] [PubMed] [Google Scholar]
- Terrillon S, Bouvier M. Receptor activity-independent recruitment of β-arrestin reveals specific signaling modes. EMBO J. 2004;23:3950–3961. doi: 10.1038/sj.emboj.7600387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urban JD, Clarke WP, Zastrow M, Nichols DE, Kobilka B, Weinstein H, et al. Functional selectivity and classical concepts of quantitative pharmacology. J Pharmacol Exp Ther. 2007;320:1–13. doi: 10.1124/jpet.106.104463. [DOI] [PubMed] [Google Scholar]
- Watson C, Chen G, Irving PE, Way J, Chen W-J, Kenakin TP. The use of stimulus-biased assay systems to detect agonist-specific receptor active states: implications for the trafficking of receptor stimulus by agonists. Mol Pharmacol. 2000;58:1230–1238. doi: 10.1124/mol.58.6.1230. [DOI] [PubMed] [Google Scholar]
- Watson C, Jenkinson S, Kazmierski W, Kenakin TP. The CCR5 Receptor-based mechanism of action of 873140, a potent allosteric non-competitive HIV entry-inhibitor. Mol Pharmacol. 2005;67:1268–1282. doi: 10.1124/mol.104.008565. [DOI] [PubMed] [Google Scholar]
- Wells TNC, Power CA, Shaw JP, Proudfoot AEI. Chemokine blockers-therapeutics in the making. Trends Pharmacol Sci. 2006;27:42–47. doi: 10.1016/j.tips.2005.11.001. [DOI] [PubMed] [Google Scholar]
- Willins DL, Berry SA, Alsayegh L, Backstrom L, Sander-Bush E, Friedman L, et al. Clozapine and other 5-hydroxytryptamine-2A receptor antagonists alter the subcellular distribution of 5-hydroxytryptamine-2A receptors in vitro and in vivo. Neuroscience. 1999;91:599–606. doi: 10.1016/s0306-4522(98)00653-8. [DOI] [PubMed] [Google Scholar]