Abstract
In heat-shocked human cells, heat shock factor 1 activates transcription of tandem arrays of repetitive Satellite III (SatIII) DNA in pericentromeric heterochromatin. Satellite III RNAs remain associated with sites of transcription in nuclear stress bodies (nSBs). Here we use real-time RT-PCR to study the expression of these genomic regions. Transcription is highly asymmetrical and most of the transcripts contain the G-rich strand of the repeat. A low level of G-rich RNAs is detectable in unstressed cells and a 104-fold induction occurs after heat shock. G-rich RNAs are induced by a wide range of stress treatments including heavy metals, UV-C, oxidative and hyper-osmotic stress. Differences exist among stressing agents both for the kinetics and the extent of induction (>100- to 80.000-fold). In all cases, G-rich transcripts are associated with nSBs. On the contrary, C-rich transcripts are almost undetectable in unstressed cells and modestly increase after stress. Production of SatIII RNAs after hyper-osmotic stress depends on the Tonicity Element Binding Protein indicating that activation of the arrays is triggered by different transcription factors. This is the first example of a non-coding RNA whose transcription is controlled by different transcription factors under different growth conditions.
INTRODUCTION
Cells are adapted to the microenvironment in which they grow and any perturbing agent can be considered as a source of stress. Stressing factors include various environmental (i.e. heat, cold, UV-light, oxygen, ion balance, heavy metals), and pathological factors (infections, inflammation, fever and ischemia). Moreover, the cells also experience the challenge of various physiologically relevant changes occurring during the cell cycle, cellular differentiation and in response to growth stimuli (1).
The type of cell response to stress depends on the nature and the intensity of the stressing condition but also on the cell identity and, in fact, is cell type-, tissue- and organism-dependent (1,2). Some stressing agents directly challenge the integrity of the genome (genotoxic stress) by generating different types of DNA damage or perturbing the DNA replication process. Other agents cause the denaturation of proteins, lipid peroxidation or disturbance in the cellular redox state. The intensity of stress is also important for the type of response mounted by the cells and severe stresses are cytotoxic and may cause permanent growth arrest or cell death either by apoptosis or necrosis (3).
The best characterized stress-defense mechanism, which is triggered by a variety of stressing conditions, involves the transcriptional activation of a set of genes encoding for molecular chaperones and that, for historical reasons, are called heat shock genes. In vertebrate cells, heat shock genes are under the control of a family of transcription factors, the so-called heat shock factors, HSF1 to HSF4 (4,5). Among them, only HSF1 is critical for the activation of heat shock genes after thermal stress. HSF1 is maintained in an inactive form in the cytoplasm. Activation upon heat shock requires a number of events that ultimately lead to HSF1 trimerization and import to the nucleus where this factor binds to the heat shock element (HSE) in the promoters of heat inducible genes (6).
In addition to the heat shock response, other defense mechanisms are activated in a stress-specific manner. For example, the oxidative defense mechanism encompasses both enzymatic (superoxide dismutase, peroxidases, catalases) and non-enzymatic (glutathione, thioredoxin) detoxification mechanisms that destroy ROS (reactive oxygen species) or restore the redox balance (7,8). The cellular defense against cadmium and heavy metals in general entails the synthesis of protective molecules such as metallothioneins and glutathione (9). DNA damaging agents, such as UV-irradiation, cause the activation of the p53 and DNA checkpoint pathways (10). Finally, the cellular response to increased osmolarity can be divided in immediate and delayed phases. The immediate response takes place within seconds and involves an increase in the intracellular concentration of charged ions such as potassium, sodium and chloride that are mediated by pre-existing ion transport systems (11). The delayed or adaptive response, on the other hand, is a slow process, occurring over a period of hours and requires the activation of genes that allow ionic osmolytes to be replaced with non-ionic ones. From this brief summing up, it is clear that several stress response pathways co-exist in human cells.
In recent years, we and others have shown that, in addition to the classical heat shock response, thermal stress affects the distribution of various nuclear factors, which accumulate in the so-called nuclear stress bodies (nSBs) (12–14). The formation of nSBs starts soon after the onset of thermal stress with the association of HSF1 with specific pericentromeric heterochromatic domains of the human genome composed of long arrays of Satellite III (SatIII) DNA sequences (15,16). HSF1 binding triggers a change of the epigenetic status of these regions and results in the production of non-coding RNA molecules of various length composed of SatIII sequences (15–17). SatIII RNAs remain associated with sites of transcription (17) and are bound by several RNA-binding factors thus leading to the formation of nSBs. So far no clear role for SatIII RNAs and nSBs has been demonstrated. The identification of the different conditions eliciting the production of SatIII transcripts can provide some hints to understanding the physiological relevance of these molecules.
We have previously shown that, in addition to heat shock, prolonged exposure to cadmium sulfate, but not other stressing factors, can trigger the formation of nSBs as revealed by immunofluorescence analysis of the distribution of hnRNP HAP/Saf-B, a marker of nSBs (18). By using a more sensitive assay, i.e. quantitative Real-Time PCR, we now show that transcription of SatIII DNA is induced by a wide range of stress treatments. Moreover, transcriptional activation of SatIII DNA can be triggered not only by HSF1 but also by the tonicity enhancer-binding protein (TonEBP) which controls the activity of genes involved in the response to hyper-osmotic stress (19).
MATERIALS AND METHODS
Cell culture and treatment
Human HeLa (adenocarcinoma) cells were grown in DMEM medium (Sigma), 10% fetal calf serum (Sigma), 50 µg/ml gentamicin and 2 mM l-glutamine. B14-150, Chinese hamster ovary cells and hamster > human somatic cell hybrids, previously described (20), were grown in RPMI-1640 (Sigma), 10% fetal bovine serum, 50 µg/ml gentamicin and 2 mM l-glutamine. The HeLa-HSF1i clone expressing siRNA against HSF1 shows a 95% reduction in HSF1 protein (21). For heat shock experiments, cell monolayers were incubated for 1 h at 42°C in complete medium made with 10 mM HEPES pH 7.0 and allowed to recover for different times at 37°C as indicated in the text. For heavy metal stress, cells were grown in the presence of cadmium sulfate (at the indicated concentrations), either for one hour and then allowed to recover for different length of time, or the treatment was continued for increasing times as indicated in the text. For UV stress, a single dose of UV-C at 254 nm was applied to cells in PBS, using the Hoefer UV-C 500 Crosslinker (Amersham Biosciences); fresh medium was then added and cells were allowed to recover for the indicated times. Hyper-osmotic stress was obtained by growing cells in 0.8 M D-sorbitol (Sigma), or 0.8 M urea (Sigma), or 0.1 M NaCl. Hypo-osmotic stress was obtained by growing cells in 30% DMEM medium and 70% water. For oxidative stress, cells were treated with 200 µM H2O2 in PBS for 20 min, cells were then washed and allowed to recover for different times in fresh medium. The following chemicals were used to damage DNA or to affect cell proliferation: 2 mM thymidine (Sigma), 2 µg/ml aphidicolin (Sigma), 3.54 mM methyl methanesulfonate (MMS) (Aldrich) and 100 µM etoposide. Hypoxia was mimicked with Hypoxia was obtained by growing cells in 3% O2 in a Heraeus BB 6220 oxygen electrode incubator (Heraeus Hanau Germany) or in 21% O2 plus 100 µM COCl2 (Meck. Germany) (0–24 h) (22).
In situ hybridization to RNA and immunofluorescence
The in situ hybridization to RNA was done as described (15,17). Briefly, cells were grown on coverslips and heat-shocked or treated with other stressing agents as described above. Cells were fixed in 4% paraformaldehyde in PBS for 15 min, washed thoroughly and permeabilized in 0.5% Triton X-100 on ice for 5 min. Hybridization was carried out overnight at 42°C with 5 ng/ml 5′ biotinylated oligonucleotide probe (see Supplementary Table 1). After washing in 2× SSC, the biotinylated probe was detected with FITC-avidin (Vector Laboratories). The signal was amplified by incubation with biotinylated anti-avidin D antibody (Vector Laboratories) followed by FITC-avidin. The oligonucleotide probes used were Direct and Reverse (see Table 1 in Supplementary Data) (15). For immunofluorescence, cells were fixed and treated as described for the in situ hybridization. The following primary antibodies were used: affinity purified rabbit polyclonal antibody against hnRNP HAP/Saf-B (23), anti-heat shock factor 1 (HSF1) rat monoclonal antibody (mAb) 10H8 (Neomarkers, Fremont CA), anti-tonicity enhancer element binding protein (TonEBP) rabbit antibody (generous gift of Dr. Moo Kwon, University of Maryland). Secondary antibodies were rhodamine-conjugated anti-rabbit or anti-mouse IgG goat antibodies (Jackson ImmunoResearch Laboratories, West Grove, PA, USA). Samples were analyzed either with a confocal microscopy as described previously (17) or with an Olympus IX71 microscope, in which case, images were acquired with a Roper scientific Photometrixm digital camera using the Metamorph 6.2r2 software and exported to Adobe Photoshop (Adobe Systems, Mountain View, CA, USA).
Western blotting analysis
Equal amounts of total proteins (35 µg/sample) from whole cell extracts were analyzed in Western blotting according to standard procedures. Antibodies used were rabbit anti-HSF1 (Abcam) and mouse anti-α-tubulin (Sigma). Secondary antibodies were from Jackson Lab. Western were developed with the Super Signal detection kit (Pierce).
RNA extraction and quantitative RT-PCR (qRT-PCR)
Total RNA was purified from HeLa cells using TriReagent (Sigma), following the manufacturer's protocol. Subsequently, the RNA was treated with Turbo DNase (Ambion) for 30 min at 37°C, to remove any contaminating DNA. RNA (0.5 µg) was reverse transcribed with MuLV reverse transcriptase (Applied Biosystems) using an oligo (dT) primer or gene-specific primers. An aliquot (1/10th) of the reaction was then used in a quantitative PCR with DyNAmo SYBR green qPCR kit (Finnzymes). PCR was carried out on Opticon™ 2 Real-Time PCR detection system MJResearch/BioRad. and analysed with Opticon MonitorTM software (MJResearch/BioRad).
Reverse transcription of the G-rich and C-rich SatIII RNAs was carried out with RSM13 and FSM13, respectively. For all the other transcripts an oligo (dT) primer was used.
We first assessed in all samples the level of transcripts encoded from Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (GenBank Accession number NM_002046) and ribosomal hP0 (GenBank Accession number M17885) genes that were used for subsequent normalization of SatIII RNAs levels. Primers used were hGP_F and hGP_R for GAPDH and hP0_F and hP0_R for hP0 (see Supplementary Table 1). We never observed any significant variation in the expression of the two housekeeping genes in response to different stresses (see Supplementary Figure 1 for an example). Finally we measured in the same samples the level of SatIII transcripts. G-rich SatIII cDNAs were amplified with Hur98-R and M13. C-rich molecules were amplified with Hur98-F and M13 (see Supplementary Table 1). In both cases the amplification profile was: 94°C for 30 sec, 58°C for 30 sec and 72°C for 1 min. After normalization, values were then expressed as a function of the SatIII level in unstressed cells taken as a reference. For each stressing condition, the expression of SatIII RNAs was measured in at least three independent experiments. The analysis of each sample was performed in triplicate to reduce as much as possible experimental errors.
Amplification of Hsp70-A1A (GeneBank Accession Number NM_005345) was carried out with Hsp70-F and Hsp70-R.
siRNA directed knockdown of TONEBP and HSF1
siRNA duplexes (400 nM) were administered to cells using oligofectamine reagent (Invitrogen) following the manufacturer's instructions. siRNAs were: siHSF1 (24) (MWG); siTonEBP-569 (25) (MWG). As a negative control we used siLUC (Dharmacon) directed against the firefly's luciferase gene (see Supplementary Table 1 for primer sequences).
RESULTS
Quantitative analysis of SatIII RNAs induction after heat shock
SatIII repeats have a modular structure composed of runs of the GGAAT pentamer separated by the CAAC(C/A)CGAGT ‘terminator’ sequence and can be represented by the general formula (GGAAT)n CAAC(C/A)CGAGT with n > 1 (17). Variations in the n value and in the pentamer and terminator sequences account for the complexity of this repetitive DNA.
We have previously shown that heat shock induces the activation of arrays of SatIII DNA repeats and leads to the production of SatIII RNAs that are undetectable in unstressed cells (15). In northern blotting, these molecules appear as a continuum of transcripts ranging in size from >5 kb to <2 kb. It is still unclear whether both strands of the repeat are transcribed. However, a number of evidences suggest that thermal stress mainly induces transcripts containing the G-rich strand of SatIII DNAs (17). In order to investigate in a quantitative manner, the expression of SatIII sequences and to also understand whether C-rich transcripts are produced we have developed a real-time quantitative RT-PCR (qRT-PCR) assay. The critical elements of this assay are the 40-mer Reverse Transcription primers (RSM13 and FSM13) used to distinguish G-rich from C-rich RNAs. The two oligos share a 20 nt region at the 5′-end corresponding to the M13 sequencing primer (used in the successive PCR) and differ for the 20 nt sequence at the 3′-end that targets either the G-rich (RSM13) or the C-rich (FSM13) strand of the SatIII repeat. These oligos are designed to avoid the PCR amplification of background SatIII cDNAs that are generated during the RT reaction independently of exogenously added primer and that hamper the possibility to independently amplify G-rich and C-rich transcripts.
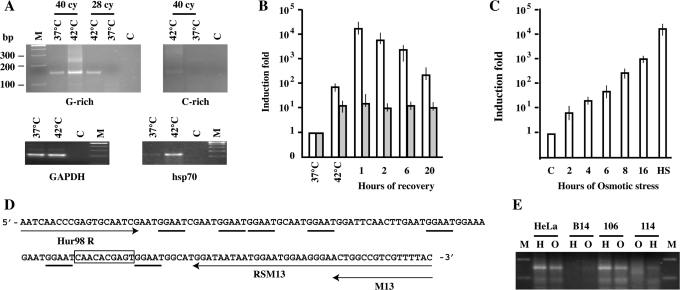
We initially tested these oligos in conventional RT-PCR. When the G-rich RNAs are investigated an amplification band of ∼150 nt is detectable after 25 cycles only in heat-shocked cells. The same band is visible in unstressed cells after additional 15 cycles, suggesting that in HeLa cells SatIII sequences are constitutively transcribed, although to a very low level (Figure 1A). Notably, other primer sets failed to generate a specific SatIII band and produced a continuous smear of PCR products (data not shown). The specificity observed in Figure 1A is probably due to the fact that primers were drawn on the basis of a computer search for the less represented 20-mers in the pool of SatIII RNA sequences previously described (17). However, after 40 cycles of amplification some larger, less abundant, PCR products are detectable in heat-shocked cells even with this set. As shown in Figure 1B, qRT-PCR indicates that the level of G-rich RNAs peaks after 1 h of recovery from stress reaching a maximum of 1–2 × 104-fold over the value observed in unstressed cells. The expression of these RNAs remains relatively constant during the first 6 h of recovery and is still 5 × 102 fold higher than in unstressed cells at 20 h. Notably, qRT-PCR reveals a certain increase in the level of SatIII RNAs already at the end of the 1 h heat shock, a result that escaped previous northern blotting analysis, probably because of the size heterogeneity of these molecules.
Figure 1.
Expression of Satellite III RNAs. (A) RT-PCR analysis of SatIII transcripts in total RNA prepared from unstressed (37°C) and heat-shocked (1 h at 42°C followed by 2 h at 37°C) (42°C) HeLa cells. G-rich transcripts are reverse transcribed with the RSM13 primer and PCR amplified with Hur98R and M13 primers for 25 or 40 cycles. C-rich RNAs are reverse transcribed with FSM13 and amplified for 40 cycles with Hur98F and M13. The same amount of RNA (1 µg) was reverse transcribed with an oligo dT and amplified with oligos specific for GAPDH and hsp70.1 transcripts (see Supplementary Table 1). M: GeneRuler 100 bp DNA ladder plus (Fermentas) (B) qRT-PCR analysis of G-rich (white columns) and C-rich (gray columns) transcripts in total RNAs prepared from unstressed HeLa cells, from heat-shocked cells and from cells allowed to recover for the indicated times at 37°C. Primers used are the same described in panel A. Columns: mean of three independent experiments. Error bars are indicated. (C) qRT-PCR analysis of G-rich transcripts in total RNA prepared from HeLa cells subjected to Osmotic stress (0.8 M sorbitol) for the indicated times. Results were normalized against the housekeeping hP0 mRNA level and expressed as a function of the SatIII RNA level observed in unstressed control (C) cells. (HS): RNAs from HeLa cells kept 1 h at 42°C and allowed to recover 1 h at 37°C. Columns: mean of three independent experiments. Error bars are indicated. (D) Sequence of the amplified G-rich RNA in panel A (25 cy). Arrows: primers used in RT-PCR. GGAAT pentamers are underlined. The terminator sequence is boxed. (E) RT-PCR analysis of SatIII transcripts in total RNA prepared from HeLa, B14-150, GM-106 and GM-114 cell lines. For each cell lines we have analyzed RNA extracted from cells subjected to heat shock (H; 1h at 42°C followed by 1 h at 37°C) or to osmotic stress (8 h in 0.8 M sorbitol). G-rich transcripts were PCR amplified for 40 cycles.
Concerning the C-rich transcripts, their level is significantly lower than that of the G-rich RNAs. In conventional RT-PCR an amplification band is barely detectable in stressed cells after 40 PCR cycles while no band is visible in unstressed cells (Figure 1A). In qRT-PCR the level of C-rich transcripts in unstressed cells is only slightly over the background and increases of about 10-fold after heat shock. We calculated that even in stressed cells the level of C-rich RNAs is lower than that of G-rich molecules in unstressed cells.
The 150 bp fragment deriving from the amplification of G-rich transcripts was cloned and sequenced. The analysis of ten randomly selected clones indicates that fragments share a sequence identity higher than 95%. As shown in Figure 1D, the amplified fragment has a complex structure with a non-monotonous arrangement of GGAAT repeats and terminator sequences. This sequence is not present in SatIII RNA molecules previously described (17) suggesting that primers recognize a specific set of SatIII transcripts.
SatIII transcription is induced by a wide range of stress treatments
The results in Figure 1B indicate that the level of SatIII RNAs is a function of the time of recovery from stress. Another parameter that is critically linked to the level of SatIII RNAs is the temperature applied to the cells (Supplementary Figure 2). Indeed, the peak of SatIII RNAs is observed when cells are stressed at 42–43°C. Heat shock at 41°C or 44°C results in ∼10% of the maximal transcription, whereas lower temperatures (39–40°C) or severe stressing conditions (45°C) have little or no effect on the expression of SatIII DNA repeats.
As reviewed in the Introduction section, in addition to heat shock, cells can be stressed by wide range of environmental and physical factors. In a previous study, we identified cadmium sulfate as the only other stressing agent able to induce the formation of nSBs (18). In that study, we used hnRNP HAP/Saf-B to label nSBs and we considered only treatments able to trigger nSBs as efficiently as heat shock. Others have observed induction of nSBs using HSF1 (13) or HSF2 as markers (37). However, in all these cases the approach used was indirect and not particularly sensitive. We decided to investigate more directly the effect of different stress treatments by measuring the level of SatIII RNA by qRT-PCR.
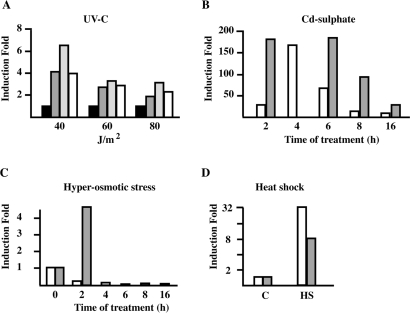
As shown in Table 1, we can distinguish three levels of G-rich SatIII RNA induction: low (<100-fold), medium (from 100- to 1000-fold) and high (>1000-fold). Consistently with our previous analysis (18), only prolonged (>6 h) cadmium treatment (50 µM) gives an induction comparable (or even higher) to that observed in heat-shocked cells. Other stressing agents, namely hyper-osmotic stress (0.8 M sorbitol), UV-C (80 J/m2) and recovery from a short (1 h) exposure to cadmium sulfate (50 µM), cause medium induction. A low expression of SatIII RNAs is observed with a number of additional treatments, while some stresses have no effect, at least under the considered conditions (Table 1). Collectively this analysis suggests that transcription of SatIII DNA repeats is a rather general phenomenon triggered by a wide range of stressing conditions.
Table 1.
Effect of different stress treatments on the production of SatIII RNAs. Classes of induction are defined in the text
| No induction | Low induction | Medium induction | High induction | |
|---|---|---|---|---|
| Hypoxia Cobalt Thymidine Hypo-osmotic | ||||
| Early | MMS | Cadmium (Recovery) | Heat shock | |
| Medium | Etoposide Aphidicolin | Cadmium (constant) | ||
| Late | Oxidation | UV-C Hyper-osmotic |
As observed with heat shock, the extent of SatIII transcription depends on the severity of the treatment. The maximal response to cadmium sulfate is obtained after 8 h of continuous growth at 50 µM but a 50% induction is observed at 5 µM. No increase of SatIII RNAs is observed when cells are grown in 0.5 µM cadmium sulfate. A similar dose–response is observed after UV irradiation. The minimal UV-C dose that triggers SatIII induction (about 300-fold after 16 h of recovery) is 40 J/m2 that has no appreciable effect on cell survival during the period considered for the experiment. A higher induction (about 600-fold) occurs at 60 and 80 J/m2. However, a certain level of cytotoxicity is observed at 60 J/m2 and approximately half of the cells detach from the plate at 80 J/m2. Stressing treatments differed not only for the extent but also for the time required to achieve maximal SatIII transcription. Also for this parameter we can define three classes of response: ‘early’ when the peak of transcription occurs within 2–4 h (heat shock and recovery from cadmium sulfate), ‘medium’ from 6 to 8 h (constant cadmium sulfate stress), after which the induction is defined as ‘late’ (UV-C and hyper-osmotic stress). The origin of these differences in intensity and timing of induction is still unknown. In the case of hyper-osmotic stress, we observed a progressive increase in the level of SatIII transcripts throughout the experiment (Figure 1C) from about 10-fold after 2 h of recovery to about 1000-fold at 16 h. It is unclear whether this induction is part of the delayed or adaptive osmotic response, which involves the transcriptional regulation of several genes. Finally, induction of C-rich SatIII RNAs is observed only in cells grown for 8 h in 50 µM cadmium sulfate (200-fold) and we never detected an increased level of these transcripts in cells subjected to hyper-osmotic stress or UV-C irradiation.
Collectively, these differences in the kinetics and level of SatIII expression may reflect the existence of distinct pathways activated in a stress-specific manner.
Induction of SatIII transcription is accompanied by the formation of nSBs
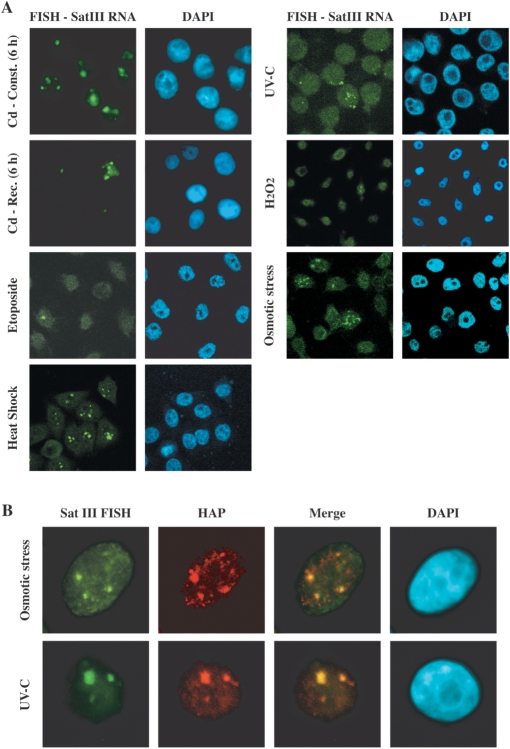
SatIII transcripts are integral components of nSBs induced by heat shock (15). We decided to investigate whether other stress treatments able to induce SatIII RNAs can trigger the formation of nSBs. We selected for each treatment the best conditions to induce SatIII transcription, as revealed by the qRT-PCR analysis, and then analyzed stressed cells by in situ hybridization with a biotinylated probe complementary to the G-rich strand of the SatIII repeat. In accord with the qRT-PCR results, prolonged treatment with 5 µM cadmium sulfate is comparable to heat shock both for the fraction of cells with nSBs (>90%) and the size of nSBs per cell (Figure 2A). The fraction of cells with nSBs is drastically reduced when cadmium is added for a short interval (1 h) followed by recovery in fresh medium. A lower fraction of cells with nSBs (30–40%) is observed also after hyper-osmotic stress (0.8 M sorbitol) or UV-C irradiation (40 J/m2) and the size of these structures is usually smaller (Figure 2A). Finally, treatments that modestly increase the expression of SatIII RNAs trigger the formation of nSBs only in a small fraction of cells. Thus, after oxidative stress (200 µM H2O2 for 20 min followed by 16 h of recovery) or treatment with the anticancer drug etoposide (100 µM) less than 5% of cells form only 1 or 2 tiny nSBs (Figure 2A). Collectively these results indicate a relationship between the level of SatIII transcripts and the appearance of nSBs and suggest that, as in heat-shocked cells, SatIII RNAs remain associated with the sites of transcription. Consistently with our previous analysis (15), we failed to observe specific staining of specific nuclear districts with a biotinylated oligo complementary to the C-rich SatIII sequence (data not shown). This could be due to the extremely low abundance of these molecules.
Figure 2.
In situ hybridization analysis of HeLa cells subjected to different stress treatments. Stressed cells were hybridized to a biotinylated probe complementary to the G-rich strand of the SatIII repeat (A) HeLa cells were subjected to the indicated stress treatments (details in Materials and Methods section). Cd-Const (6 h): treatment with 5 µM Cadmium-sulfate for 6 h. Cd-Rec (6 h): cadmium for 1 h and then fresh medium for 6 h. Etoposide: 100 µM for 2 h. Heat shock: 1 h at 42°C followed by 1 h at 37°C. UV-C: 40 J/m2 followed by 8 h of recovery. Osmotic stress: HeLa cells grown 8 h in 0.8 M sorbitol. Oxidative stress: HeLa cells grown 20 min in 200 µM H2O2 followed by 16 h of recovery. (B) UV-C and hyper-osmotic stress induce nSBs. HeLa cells were subjected to hyper-osmotic stress (8 h in 0.8 M sorbitol) or UV-C irradiated (40 J/m2) and allowed to recover for 16 h. Cells were analyzed by in situ hybridization with a biotinylated probe complementary to the G-rich strand of the SatIII repeat and co-stained with an antibody against hnRNP HAP/Saf-B. Confocal images of the same cells were taken and merged.
In addition to SatIII RNAs, several proteins have been shown to localize to the nSBs during heat shock (26,27) and after treatment with cadmium sulfate (18). Among these there are pre-mRNA processing factors, such as splicing factor SF2/ASF, which binds to SatIII transcripts (28), and hnRNP HAP/Saf-B, which is recruited to nSBs via an interaction with SF2/ASF (26). As shown in Figure 2B, hnRNP HAP/Saf-B co-localizes with SatIII RNAs in cells irradiated with UV-C or subjected to hyper-osmotic stress indicating that these stress treatments trigger the formation of structures identical to nSBs in heat-shocked cells.
Relationship between nSBs induced by heat shock and osmotic stress
The analysis in the previous section indicates that hyper-osmotic stress and heat shock differ not only for the extent of SatIII RNA induction and the fraction of cells displaying nSBs but also for the number of these structures per cell (Figure 2A). This difference is quantified in the histogram in Figure 3A. After heat shock (1 h at 24°C followed by 2 h of recovery) most (∼60%) of the cells contain a number of bodies ranging from 1 to 5. On the contrary, after 8 h in 0.8 M sorbitol two major groups of cells are detectable: one containing 1–5 bodies (∼36%) and the other 10–15 nSBs (34%). One plausible explanation for this difference is that distinct arrays of SatIII DNA are activated by the two types of stress. However, the fact that the same amplification product, both in size and in sequence, is observed after heat shock and osmotic stress (Figure 1E and Supplementary Figure 3) indicates that at least a common pool of sequences is activated under both conditions. In order to address this aspect in more detail, we studied whether the same chromosomal domains are activated by the two stress treatments. For this analysis we took advantage of our previous observation that the formation of nSBs in heat shocked hamster>human cells hybrids depends on specific human chromosomes among which HSA 9 and 15 (20). Therefore, we studied by RNA FISH the formation of nSBs in parental B14-150 hamster cells and in 3 hamster>human somatic cells hybrids: YXY-95S (containing HSA2. 3, 13, 21 and X), GM-106 (HSA9) and GM-114 (HSA15). As shown in Figure 3B, RNA hybridization with the oligonucleotide specific for the G-rich transcripts indicates that both heat shock and osmotic stress induce the formation of nSBs only in cells containing HSA 9 or 15. Notably, GM-104 and GM-106 cells are indistinguishable in their ability to respond to heat shock (∼90% of cells with nSBs) and osmotic stress (∼30%). No nSBs are, instead, detectable in YXY-95S and in parental B14-150 cells. A different result was obtained when SatIII RNAs were measured by qRT-PCR (Figure 3C). Consistently with the in situ hybridization analysis no signal over the background is detectable in B14-150 and YXY-95S cells. A robust transcriptional activation of SatIII sequences, comparable with that measured in HeLa cells, is obtained in GM-106 (HSA9) both after heat shock and osmotic stress. Surprisingly, a very small induction (about 100-fold) is observed in GM-114 cells after heat shock and only a 20- to 30-fold induction is detectable in response to osmotic stress. This is confirmed by the conventional RT-PCR in Figure 4C. The discrepancy with the results of the RNA-FISH analysis indicates that the primer set used in qRT-PCR is specific for sequences mainly present on HSA9.
Figure 3.
Different SatIII chromosomal domains are activated by osmotic stress. (A) The number of nSBs per cell has been measured. Cells have been grouped in four classes containing the indicated number of nSBs. Percentages have been calculated on 300 cells with nSBs in three different experiments. White columns: Cells grown for 8 h in 0.8 M Sorbitol. Gray columns: 1 h at 42°C followed by 1 h at 37°C. (B) Parental B14-150 cells, and somatic human>hamster cell hybrids GM-106, GM-114 and YXY-95S grown 8 h in 0.8 M sorbitol, heat shocked (1 h at 42°C followed by 1 h at 37°C) or untreated (NT). Cells were analyzed by RNA in situ hybridization (FISH) and co-stained with DAPI. Confocal images of the same cells are shown. (C) qRT-PCR analysis of G-rich SatIII RNAs extracted from the indicated cells lines untreated (C), heat shocked (HS) or grown for the indicated hours in 0.8 M sorbitol. RNAs were first standardized by measuring by qRT-PCR the level of the GAPDH transcripts. The induction fold has been calculated using the level of SatIII RNAs in unstressed cells as reference. Dots represent the results of a second experiment. ND: non detectable.
Figure 4.
Quantitative RT-PCR analysis of hsp70.1 mRNA level in HeLa cells subjected to different stress treatments. (A) Total RNA (1 µg) from HeLa cells irradiated with UV-C at the indicated doses was reverse transcribed with oligo dT. An aliquot (1/10th) was tested in qPCR to assess the level of hsp70 A1A mRNA. Black bars: no recovery. Dark gray bars: 4 h of recovery. Light gray bars: 8 h of recovery. White bars: 15 h of recovery after irradiation. (B) HeLa cells were treated with 5 µM cadmium sulfate for 1 h and allowed to recover for the indicated times (white bars). Gray bars: cell treated for the indicated times with 5 µM cadmium sulfate. (C) HeLa cells were grown for the indicated time periods in 0.8 M sorbitol. White columns: hsp70.1. Gray columns: hsp70.2. (D) HeLa cells heat shocked 1 h at 42°C and the allowed to recover 1 h at 37°C (HS). White columns: hsp70.1. Gray columns: hsp70.2. C represents unstressed cells.
Induction of SatIII transcription during hyper-osmotic stress does not depend on HSF1
HSF1 controls the expression of heat shock genes, among which the hsp70.1 and hsp70.2 genes, whose induction is considered the paradigm for the transcriptional response to environmental stresses (29). We observed co-expression of SatIII and hsp70.1 upon UV-C irradiation and treatment with cadmium sulfate (Figure 4). On the contrary, the level of hsp70.1 mRNA decreases upon hyper-osmotic stress. Intriguingly, in the same cells we observed activation of hsp70.2. This differential expression of the two heat shock genes is in line with a recent report by Heo et al. (30) showing that in human cells only the expression of hsp70. 2 and not that of hsp70.1 is up-regulated by hypertonic stress in a HSF1-independent manner. On the basis of this result we decided to investigate whether HSF1 is required for transcription of SatIII DNA after hyper-osmotic stress.
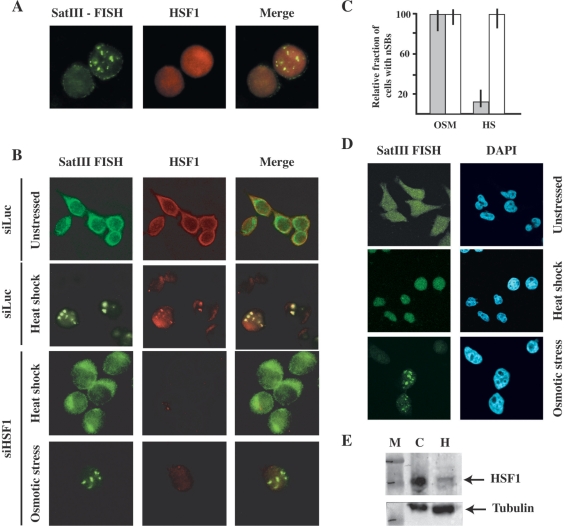
Upon heat shock HSF1 colocalizes with SatIII RNA in nSBs (16). As shown in Figure 5B, osmotic stress causes the progressive redistribution of HSF1 from the cytoplasm to the nucleus. Although we failed to observe HSF1 hyper-phosphorylation (Figure 5A), which is a marker of the activation, the redistribution clearly indicates that some level of HSF1 activation is actually occurring in these cells. However, we never detected accumulation of HSF1 in specific sub-nuclear compartments nor colocalization with SatIII RNAs in cells subjected to osmotic cells (Figure 6A). A plausible interpretation of this result is that HSF1 is not involved in the transcriptional activation of SatIII repeats after hyper-osmotic stress. To verify this hypothesis, we transfected HeLa cells with an siRNA directed against HSF1 (siHSF1) or with a control oligo against the Luciferase gene (siLuc). After 2 rounds of transfections, cells were either heat-shocked at 42°C for 45 min or grown for 8 h in 0.8 M Sorbitol. Cells were then fixed and stained with an anti-HSF1 antibody and with a biotinylated oligo against G-rich SatIII RNAs. As expected, down-regulation of HSF1 abrogates the production of SatIII transcripts in heat-shocked cells while siLuc has no effect. Notably, down-regulation of HSF1 does not prevent the production of SatIII RNAs and the formation of nSBs in sorbitol-treated cells (Figure 6B) arguing against the involvement of this transcription factor in the activation of SatIII arrays in response to hyper-osmotic stress. As a further indication that this is indeed the case we studied the response to heat shock and osmotic stress in HeLa cells (21) in which HSF1 is down-regulated by the stable expression of a specific siRNA (Figure 6E). This reduction drastically hampers the formation of nSBs triggered by heat shock (Figure 6D). Only 5% of these cells show nSBs (compared to 90% of control cells) and in positive cells only one small body is detectable. In contrast, stable down-regulation of HSF1 does not affect the fraction of cells with nSBs after osmotic stress (∼40%) nor the number of nSBs per cell.
Figure 5.
Effect of osmotic stress on HSF1. (A) Western blot analysis of total cell extracts prepared from unstressed HeLa cells (C), from heat-shocked cells and from cells subjected to osmotic stress (0.8 M sorbitol) for the indicated time periods. Extracts were analyzed with an antibody to HSF1 and normalized for the level of α-tubulin. The phosphorylated and un-phosphorylated forms of HSF1 are indicated. (B) HeLa cells either unstressed (C) or grown for the indicated time periods in 0.8 M sorbitol were co-stained with DAPI, with an oligo specific for G-rich SatIII RNAs and with an antibody against HSF1. Confocal images of the same cells are shown.
Figure 6.
HSF1 is dispensable for expression of SatIII RNAs after hyper-osmotic stress. (A) HSF1 does not colocalize with SatIII RNAs in HeLa cells after hyper-osmotic stress. HeLa cells were treated for 8 h with 0.8 M sorbitol and then co-stained with an anti-HSF1 antibody and with a biotinylated probe complementary to the G-rich strand of the SatIII repeat. Confocal images of the same cell are shown. (B) HeLa cells were transfected with an siRNA against HSF1. After two rounds of transfection, the cell population was split in two pools, one subjected to heat shock (45 min at 42°C) the other to hyper-osmotic stress (8 h in 0.8 M sorbitol). Cells were then co-stained with an anti HSF1 antibody and with a biotinylated probe complementary to the G-rich strand of the SatIII repeat. Confocal images of the same cells are shown. As a control cells were transfected with an anti-luciferase siRNA and analyzed either before or after heat shock. (C) The histogram represents the fraction of cells with SatIII RNAs in nSBs after heat shock (HS) or hyper-osmotic stress (OSM). Gray bars: cells transfected with the anti-HSF1 siRNA. White bars: cells transfected with the anti-luciferase siRNAs. Values are calculated on three independent experiments and are expressed as percentages of the fraction of control non-transfected cells displaying nSBs. Error bars are indicated. (D) Analysis of HeLa-HSF1i cells in which HSF1 level is reduced through stable expression of a specific siRNA. Cells were costained with DAPI and with a biotinylated oligo specific for G-rich SatIII molecules. Confocal images of the same cells are shown. (E) Western blot analysis of total cell extract prepared for HeLa cells (C) and from HeLa-HSF1i cells that stably express the siRNA against HSF1 (H). Western blots were analyzed with antibodies directed against HSF1 and α-tubulin. M: Biorad MW markers.
Transcription factor TonEBP is required for induction of SatIII RNAs during hyper-osmotic stress
Transcription of genes required for the delayed or adaptive response to hyper-osmotic stress, among which hsp70.2 [(30) and Figure 4], depends on the activity of the tonicity enhancer-binding protein (TonEBP), also known as osmotic response element-binding protein (OREBP) or nuclear factor of activated T cell 5 (NFAT5) (19). We decided to investigate whether this factor was also involved in SatIII transcription in cells grown in sorbitol containing medium. Immunofluorescence of cells subjected to hyper-osmotic stress for 8 h reveals a punctate nuclear distribution of TonEBP that colocalizes with the nSBs stained by the biotinylated oligo against SatIII RNAs (Figure 7).
Figure 7.
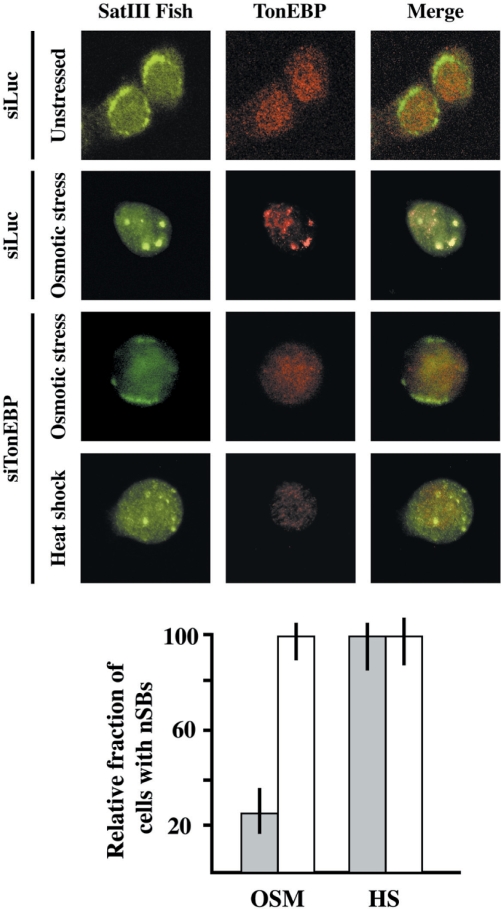
TonEBP is required for expression of SatIII RNAs after hyper-osmotic stress. HeLa cells were transfected with an siRNA against TonEBP. After two rounds of transfection the cell population was split in two pools, one subjected to heat shock (45 min at 42°C) the other to hyper-osmotic stress (8 h in 0.8 M sorbitol). Cells were then co-stained with an anti TonEBP antibody and with a biotinylated probe complementary to the G-rich strand of the SatIII repeat. Confocal images of the same cells are shown. As a control cells transfected with an anti-luciferase siRNA were subjected to hyper-osmotic stress. The histogram represents the fraction of cells with SatIII RNAs in nSBs after heat shock (HS) or hyper-osmotic stress (OSM). Gray bars: cells transfected with the anti-TonEBP siRNA. White bars: cells transfected with the anti-luciferase siRNAs. Values are calculated on three independent experiments and are expressed as percentages of the fraction of control non-transfected cells displaying nSBs.
In order to confirm the involvement of TonEBP, we down-regulated the expression of this factor by the siRNA technology. After 2 rounds of transfections cells were heat-shocked for 45 min or treated for 8 h with 0.8 M sorbitol and then stained for TonEBP and SatIII transcripts. As shown in Figure 7, siRNA-mediated down-regulation of TonEBP does not prevent formation of nSBs after heat shock but drastically reduces the fraction of cells displaying nSBs upon osmotic stress (8% versus 34% in control cells transfected with siRNA against the luciferase gene). This is consistent with the fact that siRNA reduces the level of TonEBP of ∼75% (data not shown). Collectively this analysis demonstrates that TonEBP and not HSF1 is the transcription factor involved in the induction of SatIII RNA in cells subjected to hyper-osmotic stress.
DISCUSSION
In this article, we have investigated the expression of SatIII RNAs in unstressed cells and in response to different stressing conditions. For these studies, we developed a qRT-PCR assay designed to independently amplify transcripts encoded by the G-rich and C-rich strands of the SatIII repeat. Some aspects should be considered for a correct interpretation of the results. First of all, due to the repetitive nature of SatIII transcripts, qRT-PCR could provide an over-estimation of the actual number of SatIII RNA molecules (more targets sites for PCR primers within a single transcript). On the other hand, because of the sequence divergence of SatIII units, it is possible that the primer set recognizes only a subset of transcripts. This is suggested also by the observation that SatIII RNAs encoded by HSA15 and detectable by in situ hybridization, are not recognized by the PCR primers (Figures 1E and 3C). Thus, our analysis is likely to provide a relative rather than an absolute measurement of the amount of SatIII RNAs in the cells. Keeping this in mind, however, our data supports several conclusions about the expression of SatIII DNA.
Transcription of the two strands of Satellite III DNA is asymmetrical
As with protein coding genes, transcription of SatIII DNA arrays is highly asymmetrical and almost the totality of transcripts corresponds to the G-rich strand of the repeat. A basal level of G-rich SatIII RNAs is detectable in unstressed HeLa cells. This is not completely unexpected since, as shown by Gilbert et al. (31), a fraction of SatIII arrays normally exists in an open chromatin conformation that is permissive for transcription. The low expression of SatIII RNAs observed under normal growth conditions could indicate that a subset of SatIII arrays is activated only in a fraction of cells. However, we did not observe any significant variation in the level of SatIII molecules during the cell cycle (data not shown). It is also possible that the low abundance of these non-coding RNAs is controlled at the post-transcriptional level through efficient processing or degradation.
In agreement with previous data (15,16), we have found that heat shock triggers a dramatic increase in the level of SatIII RNAs. Again similarly to what observed with protein-coding genes, transcriptional activation is specific for one strand of the repeat, i.e. the one expressed at a basal level in unstressed cells. Upon heat shock the abundance of target sites of primers specific for G-rich transcripts, in fact, augments of approximately 104-fold, while a modest increase (about 10-fold) is observed for the complementary C-rich RNAs. It is plausible that only transcription of G-rich RNAs is directly controlled by HSF1 whereas induction of C-rich RNAs may reflect a change in the epigenetic status of the SatIII arrays. Indeed, although low, the expression of C-rich RNAs parallels that of G-rich molecules and is maximal (about 200-fold induction) after prolonged treatment with cadmium-sulfate, i.e. a condition under which we observed the highest induction of G-rich molecules (up to 8 × 104-fold). Thanks to the high sensitivity of the qRT-PCR assay we can appreciate a certain level of SatIII RNAs induction (100-fold) after 1 h incubation at 42°C, a result that escaped previous northern blot analysis (15). This apparent discrepancy can be at least partially explained by the fact that even after a 100-fold induction the level of SatIII RNAs is still very low. Moreover, the different sensitivity northern blotting and qRT-PCR is further exacerbated by the heterogeneity in size of SatIII RNAs.
Different stress treatments trigger production of SatIII RNAs
An important result of our analysis is the observation that G-rich SatIII RNAs are induced, although to different extents and with different kinetics, by a wide range of stresses including DNA damaging agents (MMS and etoposide), oxidative stress (H2O2), hypoxia (Cobalt chloride and low O2), hyper-osmotic stress (sorbitol) and heavy metals (cadmium) (Table 1). These treatments are known to elicit distinct signal transduction cascades, which in turn affect different gene expression programs. It is surprising, therefore, that all of them trigger transcription of SatIII arrays. It is worth considering that some of these treatments, such as Cd-sulfate, UV light and oxidative stress directly induce the heat shock response and activate HSF1 (32–34). In other cases, such as etoposide, the effect on the heat shock response is less clear. However, it is conceivable that perturbations of the cell metabolism and cell structure may ultimately activate HSF1.
Finally we would like to underline that the level of SatIII RNAs does not simply reflect the harshness of the considered stress and cannot be considered as a marker of cell death pathways. Indeed, in the case of heat shock maximal induction is achieved at 42–43°C (mild heat shock) whereas higher temperatures, which lead to cytotoxicity and cell death, have very limited effects (Supplementary Figure 2).
TonEBP is involved in the transcriptional activation of SatIII arrays
As stated above SatIII transcription is a rather general phenomenon elicited by a wide range of stressing conditions. It is unlikely, therefore, that HSF1 is the only factor involved in its induction. This prediction is supported by our analysis of cells subjected to hyper-osmotic stress, which differs form other stressing treatments for its ability to depress the hsp70.1 mRNA level (Figure 4) (30). One of the crucial parts of the adaptive response to hyper-osmotic stress is the expression of osmo-protective proteins that control the intracellular level of compatible osmolytes such as sorbitol. In the absence of this response the cells accumulate an excess of double-stranded DNA breaks and eventually undergo apoptosis (35). The expression program activated to cope with hyper-osmosis is controlled through the interaction between the Tonicity responsive Elements (TonE), found in the regulatory region of genes activated by hyper-tonicity, and TonEBP (TonE Binding Protein), the only known osmo-sensitive mammalian transcription factor (36). We have shown that TonEBP but not HSF1 is critical for the expression of SatIII RNAs in response to high concentrations of osmolytes such as sorbitol (Figure 7).
Similarly to HSF1 in heat-shocked cells, TonEBP colocalizes with SatIII RNAs in nSBs suggesting its direct interaction with SatIII DNA. Accordingly, computer analysis of a 45 kb cluster of SatIII DNA on chromosome 4 (BAC clone RP11-1281K21 Accession number AC118282) identifies 11 sequences that match the consensus motif TGGAAANN(C/T)N(C/T) for TonEBP (19). Thus, our analysis adds TonEBP to the list of transcription factors that control the activity of SatIII DNA repeats, the others being HSF1 (27) and HSF2 (37). The involvement of TonEBP further supports the idea that induction of SatIII RNAs may have a role in the cell response to stressing conditions. The activity of TonEBP is physiologically essential in the kidney medulla where it plays a major role in protecting renal cells from the deleterious effects of ambient hyper-tonicity associated with urinary concentration (38). In addition to regulate gene expression in response to osmotic stress, TonEBP, which is also called NFAT5 (Nuclear factors of activated T cells) is involved in specific aspects of host defense and is necessary for the development of the thymus (39).
Functional considerations
SatIII RNAs are induced by various types of stress through the activity of transcription factors belonging to different stress-response pathways, which points to a role of these molecules in the ability of human cells to cope with stress. However, the function of these ncRNAs is still a matter of speculations. We have previously suggested that SatIII RNAs, by recruiting specific pre-mRNA processing factors to nSBs, may be part of a regulatory circuit that modulates alternative splicing of pre-mRNAs (12). Another hypothesis, proposed by Caroline Jolly and Claire Vourc'h, suggests the possibility that nSBs may somehow protect the large heterochromatic blocks in 9q12 from chromosomal rearrangements induced by stress (27). Support to this hypothesis has recently come from the observation that a number of genotoxic and non-genotoxic stresses including MMS, UV-C and heat shock, specifically induce pairing of heterochromatin of chromosome 9 (9q12) without affecting the distribution of euchromatic domains (40). It has been speculated that pairing of this heterochromatic region may be relevant for recombinational repair via stalled transcripts as recently shown in yeast (41). However, heterochromatin paring after heat shock occurs only in 10% of the cells (40) while, under the same conditions, transcription of SatIII arrays takes place in at least 90% of the cells. Thus, the relationship between two events is dubious. We would like to propose a further model whereby transcriptional activation of SatIII arrays and the recruitment of several transcription and pre-mRNA processing factors to nSBs may serve to organize the nuclear function during the recovery from stress. In this model, besides affecting alternative splicing in the surrounding nucleoplasm, the assembly of nSBs would create a nuclear district enriched in proteins involved in gene expression regulation, which could affect the activity of genes in close proximity to SatIII arrays, either on the chromosome or in the nuclear volume. Distinct SatIII arrays and sets of genes would be activated by different stress-specific transcription factors. In this hypothesis SatIII arrays on different chromosomes would act as regulatory elements to modulate the expression of specific sets of genes in response to stress treatments. Studies are in progress to verify this hypothesis.
SUPPLEMENTARY DATA
Supplementary Data are available at NAR Online.
ACKNOWLEDGEMENTS
This work was supported by grants from the Associazione Italiana per la Ricerca sul Cancro (AIRC) and the European Union (EURASNET) Network of Excellence on Alternative Splicing (EURASNET) to G.B. Funding to pay the Open Access publication charges for this article was provided by EURASNET, FP6 contract LSH-2004-1.1.5-3.
Conflict of interest statement. None declared.
REFERENCES
- 1.Morimoto RI, Sarge KD, Abravaya K. Transcriptional regulation of heat shock genes. A paradigm for inducible genomic responses. J. Biol. Chem. 1992;267:21987–21990. [PubMed] [Google Scholar]
- 2.Pirkkala L, Nykanen P, Sistonen L. Roles of the heat shock transcription factors in regulation of the heat shock response and beyond. Faseb J. 2001;15:1118–1131. doi: 10.1096/fj00-0294rev. [DOI] [PubMed] [Google Scholar]
- 3.Herr I, Debatin KM. Cellular stress response and apoptosis in cancer therapy. Blood. 2001;98:2603–2614. doi: 10.1182/blood.v98.9.2603. [DOI] [PubMed] [Google Scholar]
- 4.Morimoto RI. Cells in stress: transcriptional activation of heat shock genes. Science. 1993;259:1409–1410. doi: 10.1126/science.8451637. [DOI] [PubMed] [Google Scholar]
- 5.Mathew A, Mathur SK, Jolly C, Fox SG, Kim S, Morimoto RI. Stress-specific activation and repression of heat shock factors 1 and 2. Mol. Cell. Biol. 2001;21:7163–7171. doi: 10.1128/MCB.21.21.7163-7171.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cotto JJ, Morimoto RI. Stress-induced activation of the heat–shock response: cell and molecular biology of heat-shock factors. Biochem. Soc. Symp. 1999;64:105–118. [PubMed] [Google Scholar]
- 7.Mager WH, de Boer AH, Siderius MH, Voss HP. Cellular responses to oxidative and osmotic stress. Cell. Stress Chaperones. 2000;5:73–75. doi: 10.1379/1466-1268(2000)005<0073:crtoao>2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Veal EA, Day AM, Morgan BA. Hydrogen peroxide sensing and signaling. Mol. Cell. 2007;26:1–14. doi: 10.1016/j.molcel.2007.03.016. [DOI] [PubMed] [Google Scholar]
- 9.Wimmer U, Wang Y, Georgiev O, Schaffner W. Two major branches of anti-cadmium defense in the mouse: MTF–1/metallothioneins and glutathione. Nucleic Acids Res. 2005;33:5715–5727. doi: 10.1093/nar/gki881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Latonen L, Laiho M. Cellular UV damage responses – functions of tumor suppressor p53. Biochim. Biophys. Acta. 2005;1755:71–89. doi: 10.1016/j.bbcan.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 11.Ho SN. Intracellular water homeostasis and the mammalian cellular osmotic stress response. J. Cell. Physiol. 2006;206:9–15. doi: 10.1002/jcp.20445. [DOI] [PubMed] [Google Scholar]
- 12.Biamonti G. Nuclear stress bodies: a heterochromatin affair? Nat. Rev. Mol. Cell. Biol. 2004;5:493–498. doi: 10.1038/nrm1405. [DOI] [PubMed] [Google Scholar]
- 13.Cotto J, Fox S, Morimoto R. HSF1 granules: a novel stress-induced nuclear compartment of human cells. J. Cell. Sci. 1997;110(Pt 23):2925–2934. doi: 10.1242/jcs.110.23.2925. [DOI] [PubMed] [Google Scholar]
- 14.Jolly C, Morimoto R, Robert-Nicoud M, Vourc'h C. HSF1 transcription factor concentrates in nuclear foci during heat shock: relationship with transcription sites. J. Cell. Sci. 1997;110(Pt 23):2935–2941. doi: 10.1242/jcs.110.23.2935. [DOI] [PubMed] [Google Scholar]
- 15.Rizzi N, Denegri M, Chiodi I, Corioni M, Valgardsdottir R, Cobianchi F, Riva S, Biamonti G. Transcriptional activation of a constitutive heterochromatic domain of the human genome in response to heat shock. Mol. Biol. Cell. 2004;15:543–551. doi: 10.1091/mbc.E03-07-0487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jolly C, Metz A, Govin J, Vigneron M, Turner BM, Khochbin S, Vourc'h C. Stress-induced transcription of satellite III repeats. J. Cell. Biol. 2004;164:25–33. doi: 10.1083/jcb.200306104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Valgardsdottir R, Chiodi I, Giordano M, Cobianchi F, Riva S, Biamonti G. Structural and functional characterization of noncoding repetitive RNAs transcribed in stressed human cells. Mol. Biol. Cell. 2005;16:2597–2604. doi: 10.1091/mbc.E04-12-1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chiodi I, Biggiogera M, Denegri M, Corioni M, Weighardt F, Cobianchi F, Riva S, Biamonti G. Structure and dynamics of hnRNP-labelled nuclear bodies induced by stress treatments. J. Cell. Sci. 2000;113(Pt 22):4043–4053. doi: 10.1242/jcs.113.22.4043. [DOI] [PubMed] [Google Scholar]
- 19.Miyakawa H, Woo SK, Dahl SC, Handler JS, Kwon HM. Tonicity-responsive enhancer binding protein, a rel-like protein that stimulates transcription in response to hypertonicity. Proc. Natl Acad. Sci. USA. 1999;96:2538–2542. doi: 10.1073/pnas.96.5.2538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Denegri M, Moralli D, Rocchi M, Biggiogera M, Raimondi E, Cobianchi F, De Carli L, Riva S, Biamonti G. Human chromosomes 9, 12, and 15 contain the nucleation sites of stress-induced nuclear bodies. Mol. Biol. Cell. 2002;13:2069–2079. doi: 10.1091/mbc.01-12-0569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rossi A, Ciafre S, Balsamo M, Pierimarchi P, Santoro MG. Targeting the heat shock factor 1 by RNA interference: a potent tool to enhance hyperthermochemotherapy efficacy in cervical cancer. Cancer Res. 2006;66:7678–7685. doi: 10.1158/0008-5472.CAN-05-4282. [DOI] [PubMed] [Google Scholar]
- 22.Piret JP, Mottet D, Raes M, Michiels C. CoCl2, a chemical inducer of hypoxia-inducible factor-1, and hypoxia reduce apoptotic cell death in hepatoma cell line HepG2. Ann. N. Y. Acad. Sci. 2002;973:443–447. doi: 10.1111/j.1749-6632.2002.tb04680.x. [DOI] [PubMed] [Google Scholar]
- 23.Weighardt F, Cobianchi F, Cartegni L, Chiodi I, Villa A, Riva S, Biamonti G. A novel hnRNP protein (HAP/SAF-B) enters a subset of hnRNP complexes and relocates in nuclear granules in response to heat shock. J. Cell. Sci. 1999;112(Pt 10):1465–1476. doi: 10.1242/jcs.112.10.1465. [DOI] [PubMed] [Google Scholar]
- 24.Zaarur N, Gabai VL, Porco J.A., Jr, Calderwood S, Sherman MY. Targeting heat shock response to sensitize cancer cells to proteasome and Hsp90 inhibitors. Cancer Res. 2006;66:1783–1791. doi: 10.1158/0008-5472.CAN-05-3692. [DOI] [PubMed] [Google Scholar]
- 25.Na KY, Woo SK, Lee SD, Kwon HM. Silencing of TonEBP/NFAT5 transcriptional activator by RNA interference. J. Am. Soc. Nephrol. 2003;14:283–288. doi: 10.1097/01.asn.0000045050.19544.b2. [DOI] [PubMed] [Google Scholar]
- 26.Denegri M, Chiodi I, Corioni M, Cobianchi F, Riva S, Biamonti G. Stress-induced nuclear bodies are sites of accumulation of pre-mRNA processing factors. Mol. Biol. Cell. 2001;12:3502–3514. doi: 10.1091/mbc.12.11.3502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jolly C, Konecny L, Grady DL, Kutskova YA, Cotto JJ, Morimoto RI, Vourc'h C. In vivo binding of active heat shock transcription factor 1 to human chromosome 9 heterochromatin during stress. J. Cell. Biol. 2002;156:775–781. doi: 10.1083/jcb.200109018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chiodi I, Corioni M, Giordano M, Valgardsdottir R, Ghigna C, Cobianchi F, Xu RM, Riva S, Biamonti G. RNA recognition motif 2 directs the recruitment of SF2/ASF to nuclear stress bodies. Nucleic. Acids. Res. 2004;32:4127–4136. doi: 10.1093/nar/gkh759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee JS, Seo JS. Differential expression of two stress-inducible hsp70 genes by various stressors. Exp. Mol. Med. 2002;34:131–136. doi: 10.1038/emm.2002.19. [DOI] [PubMed] [Google Scholar]
- 30.Heo JI, Lee MS, Kim JH, Lee JS, Kim J, Park JB, Lee JY, Han JA, Kim JI. The role of tonicity responsive enhancer sites in the transcriptional regulation of human hsp70-2 in response to hypertonic stress. Exp. Mol. Med. 2006;38:295–301. doi: 10.1038/emm.2006.35. [DOI] [PubMed] [Google Scholar]
- 31.Gilbert N, Boyle S, Fiegler H, Woodfine K, Carter NP, Bickmore WA. Chromatin architecture of the human genome: gene-rich domains are enriched in open chromatin fibers. Cell. 2004;118:555–566. doi: 10.1016/j.cell.2004.08.011. [DOI] [PubMed] [Google Scholar]
- 32.Muramatsu T, Tada H, Kobayashi N, Yamji M, Shirai T, Ohnishi T. Induction of the 72-kD heat shock protein in organ-cultured normal human skin. J. Invest. Dermatol. 1992;98:786–790. doi: 10.1111/1523-1747.ep12499953. [DOI] [PubMed] [Google Scholar]
- 33.McDuffee AT, Senisterra G, Huntley S, Lepock JR, Sekhar KR, Meredith MJ, Borrelli MJ, Morrow JD, Freeman ML. Proteins containing non-native disulfide bonds generated by oxidative stress can act as signals for the induction of the heat shock response. J. Cell. Physiol. 1997;171:143–151. doi: 10.1002/(SICI)1097-4652(199705)171:2<143::AID-JCP4>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 34.Koizumi S, Gong P, Suzuki K, Murata M. Cadmium-responsive element of the human heme oxygenase-1 gene mediates heat shock factor 1-dependent transcriptional activation. J. Biol. Chem. 2007;282:8715–8723. doi: 10.1074/jbc.M609427200. [DOI] [PubMed] [Google Scholar]
- 35.Kultz D, Chakravarty D. Hyperosmolality in the form of elevated NaCl but not urea causes DNA damage in murine kidney cells. Proc. Natl Acad. Sci. USA. 2001;98:1999–2004. doi: 10.1073/pnas.98.4.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ferraris JD, Williams CK, Persaud P, Zhang Z, Chen Y, Burg MB. Activity of the TonEBP/OREBP transactivation domain varies directly with extracellular NaCl concentration. Proc. Natl Acad. Sci. USA. 2002;99:739–744. doi: 10.1073/pnas.241637298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Alastalo TP, Hellesuo M, Sandqvist A, Hietakangas V, Kallio M, Sistonen L. Formation of nuclear stress granules involves HSF2 and coincides with the nucleolar localization of Hsp70. J. Cell. Sci. 2003;116:3557–3570. doi: 10.1242/jcs.00671. [DOI] [PubMed] [Google Scholar]
- 38.Woo SK, Lee SD, Na KY, Park WK, Kwon HM. TonEBP/NFAT5 stimulates transcription of HSP70 in response to hypertonicity. Mol. Cell. Biol. 2002;22:5753–5760. doi: 10.1128/MCB.22.16.5753-5760.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lopez-Rodriguez C, Aramburu J, Jin L, Rakeman AS, Michino M, Rao A. Bridging the NFAT and NF-kappaB families: NFAT5 dimerization regulates cytokine gene transcription in response to osmotic stress. Immunity. 2001;15:47–58. doi: 10.1016/s1074-7613(01)00165-0. [DOI] [PubMed] [Google Scholar]
- 40.Abdel-Halim HI, Mullenders LH, Boei JJ. Pairing of heterochromatin in response to cellular stress. Exp. Cell. Res. 2006;312:1961–1969. doi: 10.1016/j.yexcr.2006.02.028. [DOI] [PubMed] [Google Scholar]
- 41.Aboussekhra A, Al-Sharif IS. Homologous recombination is involved in transcription-coupled repair of UV damage in Saccharomyces cerevisiae. EMBO J. 2005;24:1999–2010. doi: 10.1038/sj.emboj.7600665. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.