Abstract
Fragile X mental retardation syndrome is a repeat expansion disease caused by expansion of a CGG·CCG-repeat tract in the 5′ UTR of the FMR1 gene. In humans, small expansions occur more frequently on paternal transmission while large expansions are exclusively maternal in origin. It has been suggested that expansion is the result of aberrant DNA replication, repair or recombination. To distinguish amongst these possibilities we crossed mice containing 120 CGG·CCG-repeats in the 5′ UTR of the mouse Fmr1 gene to mice with mutations in ATR, a protein important in the cellular response to stalled replication forks and bulky DNA lesions. We show here that ATR heterozygosity results in increased expansion rates of maternally, but not paternally, transmitted alleles. In addition, age-related somatic expansions occurred in mice of both genders that were not seen in ATR wild-type animals. Some ATR-sensitive expansion occurs in postmitotic cells including haploid gametes suggesting that aberrant DNA repair is responsible. Our data suggest that two mechanisms of repeat expansion exist that may explain the small and large expansions seen in humans. In addition, our data provide an explanation for the maternal bias of large expansions in humans and the lower incidence of these expansions in mice.
INTRODUCTION
Expansion of a tandem repeat array is responsible for disease pathology in the repeat expansion diseases, a group of genetic disorders that includes Fragile X mental retardation syndrome (FXS) (1,2). In FXS, the repeat unit is the triplet CGG·CCG. The mechanism responsible for expansion is unknown. However, it differs from the genome-wide microsatellite instability seen in diseases like hereditary non-polyposis colorectal carcinoma (HNPCC) in showing an expansion bias (more expansions than contractions) and occurring at a single genetic locus. In addition, at least in the case of mouse models for the disorders resulting from CAG·CTG-repeat expansion, mutations in DNA mismatch repair genes, like MSH2, MSH3 and PMS2, actually decrease the frequency of repeat expansion while the opposite is true for HNPCC (3–9).
Different diseases in this group involve repeats with different sequences and repeat unit sizes (10). These repeats have the potential to form secondary structures that are thought to play a role in the expansion process [see (11) for a recent review]. However, since not all of the repeats have the same properties, it is unclear whether all repeats expand via the same mechanism. Studies in bacteria and yeast have shown that variety of mechanisms can cause repeat instability in these organisms including DNA slippage during replication, errors in Okazaki fragment processing as well as aberrant DNA repair or recombination (12–42).
However, much is currently unknown about the events responsible for expansion in humans. For example, some expansion diseases only show small changes in repeat number on intergenerational transfer, while others result in alleles many times larger than the parental allele from which they are derived. The small expansions are typically seen when the repeats fall within an open reading frame, as in the case of the CAG·CTG-repeat responsible for Huntington disease (HD). These expansions show a paternal transmission bias. Large expansions, like those that cause FXS, are characteristically seen in regions outside of the open reading frame and occur almost exclusively on maternal transmission. Whether large and small expansions share the same mechanism is not known.
In addition, some diseases involve significant somatic instability while others do not and it is not known if the same mechanism is responsible for both germline and somatic expansion. The timing of intergenerational expansion is also unclear. Most studies have focussed on CAG·CTG-repeats. Some studies in transgenic mice have suggested that small expansions occur premeiotically in spermatogonia (9), whilst others suggest that expansions occur in haploid gametes (8,43). Some studies also suggest a second event occurs in the early female embryo. In some transgenic mouse models this event is an expansion (44) and in others it is a contraction (45,46). The differences in these two models has been attributed to the different genomic context of the repeats (44). This would be consistent with work in bacteria, yeast and tissue culture models that have implicated orientation, proximity to origins of replication and transcription as cis-acting factors affecting expansion (15,21,24,27,31,36,38,39,47,48).
In order to study the Fragile X repeat in as close to normal a chromosomal context as possible, we generated a FXS premutation knock-in (KI) mouse containing 120 CGG·CCG-repeats in the murine Fmr1 gene (49). Since the murine Fmr1 gene is located in a region of the X chromosome that is syntenic with the corresponding region of the human X chromosome, differences in cis-acting signals involved in expansion may be small. As was seen in other transgenic and Knockin mouse models of CGG·CCG-repeat expansion (50–53), instability in these animals resembles what is seen in humans in that the frequency is high and shows an expansion bias (49). In our mouse model large expansions that generate alleles in the full mutation range (>200 repeats) were seen, but at a much lower frequency than in humans (49). In fact most expansions in these mice are small, involving fewer than five repeats per generation and occurring more commonly in males than in females. In this respect these expansions resemble what is seen in human carriers of FMR1 common or intermediate sized alleles (grey-zone alleles) (54) or those diseases such as HD that involve relatively small increases in repeat number.
Expansion occurring during the perigametic interval—between the last premeiotic mitosis and the first post-meiotic one—could explain the differences between mice and humans and the maternal CGG·CCG-repeat expansion bias in the human FMR1 gene. This interval can last decades in human females creating a large window of opportunity for expansion to occur. In contrast, this interval lasts only months in female mice and in weeks in males of either species. Depletion of the DNA repair capacity for much of spermatogenesis could also exacerbate the differences in maternal and paternal expansion rates (55). It may also be that some of the differences between humans and mice reflect differences in the efficacy of DNA damage repair or checkpoint proteins.
One potential DNA damage checkpoint protein that may affect expansion frequency is the Ataxia-telangiectasia and rad3-related (ATR) kinase. ATR primarily responds to stalled replication forks and bulky DNA adducts like those arising from UV irradiation (56) or S(N)1-type alkylating agents (57). Here we show that ATR heterozygosity leads to increased maternally transmitted expansions and somatic expansions in mice of both genders that appear to involve aberrant DNA repair.
MATERIAL AND METHODS
Mice breeding and maintenance
The generation of the Fragile X premutation mice was described previously (49). The ATR+/− mice were a kind gift of Dr Eric Brown (Caltech, Pasadena, CA). Mice were maintained in accordance with the guidelines of the NIDDK Animal Care and Use Committee and with the Guide for the Care and Use of Laboratory Animals (NIH publication no. 85-23, revised 1996).
Data analysis
Genomic DNA is prepared from mouse tail DNA or homogenized mouse tissue as previously described (49). Genotyping to detect the presence or absence of the disrupted ATR gene was carried out as described elsewhere (58). The primer pair, frax-c and frax-f (1), was used to detect both wildtype (WT) Fmr1 and FXS premutation alleles. The size of the CGG·CCG-repeat tract was monitored by Polymerase chain reaction (PCR) using the primers frax-m4 (5′-CTTGAGGCCCAGCCGCCGTCGGCC-3′) and frax-m5 (5′-CGGGGGGCGTGCGGTAACGGCCCAA-3′). The binding sites for these primers are located immediately adjacent to the repeat tract and their 3′ ends are unique to the KI allele. The PCR reaction was done in one of two ways. Radiolabelled PCR products were generated by inclusion of α-P32-dCTP in the reaction mix as previously described (49). The resultant PCR products were analysed on a 5% polyacrylamide sequencing gels. PCR reactions were also carried out using one 6-carboxyfluorescein (FAM)-labelled primer. The reaction products were then run on a 3130XL Genetic Analyzer and analysed using GeneMapper® 3.7 (Applied Biosystems, Foster City, CA). Instability seen using the Genetic Analyzer was also clearly visible by conventional gel electrophoresis. Results were confirmed where necessary by Southern blotting. PCR across long repeats typically produces multiple bands. In young mice these bands show a Gaussian distribution about the mean that is very similar for alleles in the same size range. The mean size of each allele was calculated based on the mobility of the central band in the cluster. Comparison of the parental mean and the offspring's; mean determined from samples run on the same gel, allows the changes in the offspring's; allele to be reproducibly determined. Statistical analysis of instability was carried out using the Chi square and Student's t-tests.
RESULTS
The checkpoint protein ATR is responsible for activating pathways that lead to the repair of stalled DNA replication forks and bulky lesions in DNA. Thus the effect of ATR mutations on the expansions seen in Fragile X premutation mice may shed light on the mechanism of repeat expansion. ATR null mice die during early embryogenesis (58). However, since ATR heterozygous mice show a small increase in tumour incidence and a small decrease in overall survival it is apparent that some effects of ATR deficiency can be seen even in the heterozygous state (58). We thus analysed the transmission of a FXS premutation allele with 120 CGG·CCG-repeats in mice heterozygous for a disrupted ATR gene. The results obtained for the repeat length changes in the offspring of these mice are summarized in Table 1.
Table 1.
Expansions of a premutation allele in WT and ATR+/− mice on paternal and maternal transmission
| Offspring | |||||||
|---|---|---|---|---|---|---|---|
| Cross | % mice with expansions | Mean no. repeats added | |||||
| Male × Female | (1) Total | (2) Males | (3) Females | (4) ATR+/− | (5) WT | ||
| 1 | *Fmr1w, ATR+/+ × Fmr1w/p, ATR+/+ | 37≠ | 39‡ | 33¶ | – | 37 Ø | 2.2• |
| 2 | Fmr1w, ATR+/+ × Fmr1w/p, ATR+/− | 86≠,□ | 89‡ | 83¶ | 96# | 63Ø,# | 5.0• |
| 3 | *Fmr1p, ATR+/+ × Fmr1w/w, ATR+/+ | 61 | – | 61 | – | 61 | 3.1§ |
| 4 | Fmr1p, ATR+/− × Fmr1w/w, ATR+/+ | 68□ | – | 68 | 69 | 67 | 5.2§ |
| 5 | Fmr1p, ATR+/+ × Fmr1w/w, ATR+/− | 63 | – | 63 | 63 | 63 | 2.2 |
W: wildtype Fmr1 allele; P: Fmr1 premutation allele; -: indicates offspring from crosses that should not have either the premutation or a mutated ATR allele; *: Data source: Entezam et al. (47); ≠,□,‡,¶,ØNumbers sharing one of these symbols were compared using the Chi-squared test and shown to be significantly different with a P-value <0.005. •,§Numbers sharing these symbols were compared using the Student's t-test and shown to be significantly different with a P-value <0.005.
Intergenerational instability
ATR heterozygosity had no effect on the deletion frequency and no effect of ATR heterozygosity was seen on the stability of the normal mouse Fmr1 repeat (data not shown). In contrast, a significant increase in the expansion frequency was seen when the premutation allele was maternally transmitted in ATR+/− mice compared to WT mice (86% versus 37%; cross 2 versus cross 1 in Table 1). This suggests that ATR is normally involved in protecting the genome against intergenerational expansions in female mice carrying FXS premutation alleles. In contrast, the expansion frequency on paternal transmission by ATR+/− mice was not statistically different from mice WT with respect to ATR (68% versus 61%, cross 4 versus cross 3 in Table 1). There was an apparent increase in the average number of repeats added per expansion on both paternal and maternal transmission in ATR heterozygotes despite the fact that no increase in expansion frequency was seen on paternal transfer.
There was no significant gender bias in the expansion frequency in the offspring of ATR+/− mothers. This is similar to what is seen in with Fragile X repeats in humans (59) and differs from the male expansion bias seen in a transgenic mouse model of CAG·CTG-expansions (45). ATR WT males showed no increase in the transmission of an expanded CGG·CCG-allele when crossed to ATR+/− females (Cross 5 in Table 1).
An expansion frequency of 63% was seen in the WT progeny of females carrying the premutation who are heterozygous for ATR (Cross 2). This is significantly higher than the 37% seen in offspring of females WT for ATR. The expansion frequency was even higher in the ATR+/− offspring of ATR+/− mothers (96%, cross 2 in Table 1).
Somatic instability
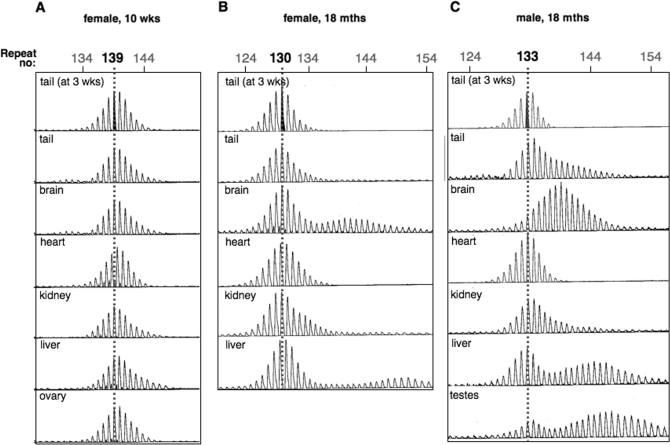
To study the role of ATR in somatic instability we examined the size of the repeat in different organs of young (10 weeks old) and old (18 months old) ATR+/− mice. As can be seen from Figure 1A, very limited somatic instability was seen in the liver of young mice as evidenced by the very slightly skewed distribution of repeat sizes. No instability was detected in other tissues. Small pool PCR was also negative for instability (data not shown). Old mice showed much more significant changes in organs like brain, testes and liver. In some instances little remained of the original allele size (see brain sample in Figure 1C). These changes showed a clear expansion bias. We have previously shown that no such somatic instability was seen in mice of a similar age that were WT for ATR (49). In some organs, like the male brain, the expansions resulted in a shift of the average allele size without changing the basic monophasic distribution of allele sizes (Figure 1C). In organs like liver and testes a biphasic distribution of allele sizes was apparent (e.g. Figure 1C). Whether the biphasic distribution reflects a predisposition of certain cells within the organ to expand is currently under investigation. A biphasic distribution of somatic expansion products has been reported in the liver in a mouse model knockin mouse model for myotonic dystrophy, a CAG·CTG-expansion disorder, where it was attributed to changes in ploidy in a subset of liver cells (60).
Figure 1.
Somatic instability in ATR+/− mice carrying an FXS premutation allele. Genomic DNA was isolated from various organs of young (10 weeks old) and old (18 months old) FXS premutation mice and the repeat tract analysed by PCR using one FAM-labelled primer and an ABI GeneAnalyzer as described in the Materials and Methods. The number repeats in the modal allele in the tail DNA at 3 weeks of age is shown in black font and is indicated in the other organ samples by the grey dotted line. Similar results were obtained using PCR using 32P-α-dCTP and denaturing gel electrophoresis as described previously (49).
DISCUSSION
We have shown that a maternal ATR insufficiency leads to an increase in the frequency of intergenerational expansions. ATR heterozygosity also causes the appearance of age-related expansion products in certain organs of older males and females.
A number of lines of evidence support a prezygotic origin for the ATR-sensitive intergenerational expansions seen in females. The elevated expansion frequency in ATR WT offspring of ATR heterozygous mothers (Cross 2, column 5 in Table 1 compared to Cross 1, column 5) demonstrates that expansion can occur prior to fertilization of the oocyte. This is supported by the fact that the paternal expansion frequency in mice WT for ATR is the same whether the dam is ATR+/− or WT for ATR (Cross 5 in Table 1).
Despite the maternal expansion bias, there is no gender bias in the likelihood of inheriting an expansion. This together with the fact that female mice do not show more somatic instability in adult tissues than males argues against somatic expansions that occur specifically either in the early female embryo or in the female germline prior to meiosis.
We have previously shown that male mice show no bias against the transmission of large repeats (49). Thus the female expansion bias in an ATR+/− background is not likely to be due to somatic expansions that are selected against in males. Furthermore, the excess of expansions in ATR+/− offspring (96% compared to 63% in ATR WT offspring) is not consistent with a somatic origin in the mother either since all her somatic cells would be ATR+/−. Thus the most likely origin of the additional expansions in the ATR+/− offspring is in the haploid oocyte. Given that scheduled DNA replication does not occur in haploid gametes, DNA damage is thus likely to be responsible. The haploid gamete that gives rise to ATR WT offspring would not be ATR deficient. Thus if expansion were confined to this stage, we would not expect the expansion frequency to be higher than that seen in offspring of a mother WT for ATR. Therefore, some ATR-sensitive expansions in the WT offspring of ATR+/− mothers could have occurred in the oogonia or diploid oocyte. Female gametes remain diploid until just prior to ovulation. Thus the window of opportunity for expansions in these cells is much larger than it is for haploid gametes although the rate of expansion may in fact be lower.
It may be that the ATR heterozygosity in these mice reveals the existence of two different intergenerational expansion mechanisms, the first showing a higher expansion frequency in males that is less sensitive to ATR haploinsufficiency and the second, occurring predominantly in females, that is sensitive. This is not to say that ATR mutations are necessary for maternal expansions in humans, but rather that an ATR insufficiency in mice allows events that would have years to accumulate in humans, to be visible within the rodent lifespan.
The relatively ATR-insensitive mechanism may account for the paternal transmission bias seen with intermediate and grey-zone FMR1 alleles in humans (54). A higher mutation frequency in males is usually attributed to errors occurring during DNA replication since mature sperm are the product of more rounds of cell division than ova (61). While it is possible that the increase in the average expansion size seen on paternal transmission reflects measurement errors, it may be that in males the ATR-deficiency simply delays the resolution of the replication problem. For example, in a strand-slippage scenario, such a delay could result in the incorporation of additional bases into the expanded allele without affecting the frequency with the initiation of strand-slippage occurs.
ATR-sensitive expansions may be more common in female mice since our data suggest that the mechanism responsible is related to the repair of DNA damage and not genomic replication. Expansions can thus occur at any point during gametogenesis, a process that lasts significantly longer in females. It is appealing to think of this mechanism as the basis for the strong maternal bias in the transmission of Fragile X full mutation alleles in humans. Since gametogenesis takes so much longer in human females, this may provide a much larger window of opportunity for such expansions to occur even in the presence of normal amounts of ATR.
Somatic expansion is also sensitive to ATR heterozygosity. One organ that showed evidence of significant somatic expansion is the brain. In some cases very little of the original allele was seen (see adult male brain in Figure 1C). Since a significant fraction of cells in the adult brain are post-mitotic, somatic expansion is also probably not limited to dividing cells. Thus these expansions may also arise from an aberrant DNA repair process rather than a problem with scheduled DNA replication. Expansion limited to some organs could be explained by differences in either the frequency with which the DNA damage that initiates expansion occurs in these organs or the frequency with which such mutations are repaired or eliminated. Organs such as liver and brain may be predisposed to expansion since ATR is expressed at a lower level in cells with a low proliferative capacity (62) and shows a lower affinity for chromatin in such cells (63).
In a transgenic mouse model of CAG·CTG-repeat expansion a deficiency of OGG1, an enzyme involved in the repair of the oxidation product of guanine, 7,8-dihydro-8-oxoguanine, reduces somatic expansion frequency (64). Oxidative DNA damage-induced expansion is likely to be high in organs like brain that in the Fragile X premutation mice show elevated levels of ATR-sensitive somatic mutations. However, since an OGG1 deficiency did not affect germline instability in the CAG–CAG-mouse model, its significance for intergenerational instability in the CAG·CTG-expansion diseases and in the etiology of Fragile X syndrome is unclear. In Fragile X premutation mice, an ATR insufficiency may prevent error-free DNA repair pathways from being activated to repair DNA damage, forcing the cell to use a secondary repair pathway that results in expansions. Potential pathways could be non-homologous end-joining or some sort of homologous recombination.
The effect of ATR mutations on the CGG·CCG-expansion frequency in female mice raises the possibility that DNA damage checkpoint proteins or proteins involved in DNA repair have the potential to affect expansion risk in humans. Such transacting factors may explain why the risk of expansion in premutation carriers is lower in those carriers identified by general prenatal screening than in carriers from known fragile X families (65). It could also explain why some intergenerational instability is apparent in some families with alleles in the ‘grey zone’ and not others (66) and the transition from an allele in the normal size range to a full mutation in two generations reported in one family (67). Our data also raise the possibility that there may be tissue variation in repeat lengths in older human premutation carriers that may be relevant for diagnosis and the severity of disease symptoms.
ACKNOWLEDGEMENTS
The authors wish to thank the people in the NIDDK animal care facility who take such good care of our mice, thus making this work possible. The authors also wish to thank Maria Jose Lopez Barragan and Jianbing Mu, NIAID, NIH for their help with the genotyping assay. This work was carried out with funding from the Intramural Program of the NIDDK (NIH). Funding to pay the Open Access publication charges for this article was provided by the Intramural Program of the NIDDK (NIH).
Conflict of interest statement. None declared.
REFERENCES
- 1.Fu YH, Kuhl DP, Pizzuti A, Pieretti M, Sutcliffe JS, Richards S, Verkerk AJ, Holden JJ, Fenwick RG, Jr, et al. Variation of the CGG repeat at the fragile X site results in genetic instability: resolution of the Sherman paradox. Cell. 1991;67:1047–1058. doi: 10.1016/0092-8674(91)90283-5. [DOI] [PubMed] [Google Scholar]
- 2.Verkerk AJ, Pieretti M, Sutcliffe JS, Fu YH, Kuhl DP, Pizzuti A, Reiner O, Richards S, Victoria MF, et al. Identification of a gene (FMR-1) containing a CGG repeat coincident with a breakpoint cluster region exhibiting length variation in fragile X syndrome. Cell. 1991;65:905–914. doi: 10.1016/0092-8674(91)90397-h. [DOI] [PubMed] [Google Scholar]
- 3.Foiry L, Dong L, Savouret C, Hubert L, te Riele H, Junien C, Gourdon G. Msh3 is a limiting factor in the formation of intergenerational CTG expansions in DM1 transgenic mice. Hum. Genet. 2006;119:520–526. doi: 10.1007/s00439-006-0164-7. [DOI] [PubMed] [Google Scholar]
- 4.Manley K, Shirley TL, Flaherty L, Messer A. Msh2 deficiency prevents in vivo somatic instability of the CAG repeat in Huntington disease transgenic mice. Nat. Genet. 1999;23:471–473. doi: 10.1038/70598. [DOI] [PubMed] [Google Scholar]
- 5.van den Broek WJ, Nelen MR, Wansink DG, Coerwinkel MM, te Riele H, Groenen PJ, Wieringa B. Somatic expansion behaviour of the (CTG)n repeat in myotonic dystrophy knock-in mice is differentially affected by Msh3 and Msh6 mismatch-repair proteins. Hum. Mol. Genet. 2002;11:191–198. doi: 10.1093/hmg/11.2.191. [DOI] [PubMed] [Google Scholar]
- 6.Gomes-Pereira M, Fortune MT, Ingram L, McAbney JP, Monckton DG. Pms2 is a genetic enhancer of trinucleotide CAG.CTG repeat somatic mosaicism: implications for the mechanism of triplet repeat expansion. Hum. Mol. Genet. 2004;13:1815–1825. doi: 10.1093/hmg/ddh186. [DOI] [PubMed] [Google Scholar]
- 7.Owen BA, Yang Z, Lai M, Gajek M, Badger JD, 2nd, Hayes JJ, Edelmann W, Kucherlapati R, Wilson TM, et al. (CAG)(n)-hairpin DNA binds to Msh2-Msh3 and changes properties of mismatch recognition. Nat. Struct. Mol. Biol. 2005;12:663–670. doi: 10.1038/nsmb965. [DOI] [PubMed] [Google Scholar]
- 8.Kovtun IV, McMurray CT. Trinucleotide expansion in haploid germ cells by gap repair. Nat. Genet. 2001;27:407–411. doi: 10.1038/86906. [DOI] [PubMed] [Google Scholar]
- 9.Savouret C, Garcia-Cordier C, Megret J, te Riele H, Junien C, Gourdon G. MSH2-dependent germinal CTG repeat expansions are produced continuously in spermatogonia from DM1 transgenic mice. Mol. Cell. Biol. 2004;24:629–637. doi: 10.1128/MCB.24.2.629-637.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Usdin K, Grabczyk E. DNA repeat expansions and human disease. Cell. Mol. Life Sci. 2000;57:914–931. doi: 10.1007/PL00000734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mirkin SM. DNA structures, repeat expansions and human hereditary disorders. Curr. Opin. Struct. Biol. 2006;16:351–358. doi: 10.1016/j.sbi.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 12.Bowater RP, Jaworski A, Larson JE, Parniewski P, Wells RD. Transcription increases the deletion frequency of long CTG.CAG triplet repeats from plasmids in Escherichia coli. Nucleic Acids Res. 1997;25:2861–2868. doi: 10.1093/nar/25.14.2861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bowater RP, Rosche WA, Jaworski A, Sinden RR, Wells RD. Relationship between Escherichia coli growth and deletions of CTG.CAG triplet repeats in plasmids. J. Mol. Biol. 1996;264:82–96. doi: 10.1006/jmbi.1996.0625. [DOI] [PubMed] [Google Scholar]
- 14.Hashem VI, Rosche WA, Sinden RR. Genetic recombination destabilizes (CTG)n.(CAG)n repeats in E. coli. Mutat. Res. 2004;554:95–109. doi: 10.1016/j.mrfmmm.2004.03.012. [DOI] [PubMed] [Google Scholar]
- 15.Hirst MC, White PJ. Cloned human FMR1 trinucleotide repeats exhibit a length- and orientation-dependent instability suggestive of in vivo lagging strand secondary structure. Nucleic Acids Res. 1998;26:2353–2358. doi: 10.1093/nar/26.10.2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Iyer RR, Wells RD. Expansion and deletion of triplet repeat sequences in Escherichia coli occur on the leading strand of DNA replication. J. Biol. Chem. 1999;274:3865–3877. doi: 10.1074/jbc.274.6.3865. [DOI] [PubMed] [Google Scholar]
- 17.Jaworski A, Rosche WA, Gellibolian R, Kang S, Shimizu M, Bowater RP, Sinden RR, Wells RD. Mismatch repair in Escherichia coli enhances instability of (CTG)n triplet repeats from human hereditary diseases. Proc. Natl Acad. Sci. USA. 1995;92:11019–11023. doi: 10.1073/pnas.92.24.11019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kang S, Ohshima K, Jaworski A, Wells RD. CTG triplet repeats from the myotonic dystrophy gene are expanded in Escherichia coli distal to the replication origin as a single large event. J. Mol. Biol. 1996;258:543–547. doi: 10.1006/jmbi.1996.0266. [DOI] [PubMed] [Google Scholar]
- 19.Napierala M, Bacolla A, Wells RD. Increased negative superhelical density in vivo enhances the genetic instability of triplet repeat sequences. J. Biol. Chem. 2005;280:37366–37376. doi: 10.1074/jbc.M508065200. [DOI] [PubMed] [Google Scholar]
- 20.Napierala M, Dere R, Vetcher A, Wells RD. Structure-dependent recombination hot spot activity of GAA.TTC sequences from intron 1 of the Friedreich's ataxia gene. J. Biol. Chem. 2004;279:6444–6454. doi: 10.1074/jbc.M309596200. [DOI] [PubMed] [Google Scholar]
- 21.Parniewski P, Bacolla A, Jaworski A, Wells RD. Nucleotide excision repair affects the stability of long transcribed (CTG*CAG) tracts in an orientation-dependent manner in Escherichia coli. Nucleic Acids Res. 1999;27:616–623. doi: 10.1093/nar/27.2.616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Parniewski P, Jaworski A, Wells RD, Bowater RP. Length of CTG.CAG repeats determines the influence of mismatch repair on genetic instability. J. Mol. Biol. 2000;299:865–874. doi: 10.1006/jmbi.2000.3796. [DOI] [PubMed] [Google Scholar]
- 23.Schmidt KH, Abbott CM, Leach DR. Two opposing effects of mismatch repair on CTG repeat instability in Escherichia coli. Mol. Microbiol. 2000;35:463–471. doi: 10.1046/j.1365-2958.2000.01727.x. [DOI] [PubMed] [Google Scholar]
- 24.Schumacher S, Fuchs RP, Bichara M. Expansion of CTG repeats from human disease genes is dependent upon replication mechanisms in Escherichia coli: the effect of long patch mismatch repair revisited. J. Mol. Biol. 1998;279:1101–1110. doi: 10.1006/jmbi.1998.1827. [DOI] [PubMed] [Google Scholar]
- 25.Schumacher S, Pinet I, Bichara M. Modulation of transcription reveals a new mechanism of triplet repeat instability in Escherichia coli. J. Mol. Biol. 2001;307:39–49. doi: 10.1006/jmbi.2000.4489. [DOI] [PubMed] [Google Scholar]
- 26.Wells RD, Parniewski P, Pluciennik A, Bacolla A, Gellibolian R, Jaworski A. Small slipped register genetic instabilities in Escherichia coli in triplet repeat sequences associated with hereditary neurological diseases. J. Biol. Chem. 1998;273:19532–19541. doi: 10.1074/jbc.273.31.19532. [DOI] [PubMed] [Google Scholar]
- 27.White PJ, Borts RH, Hirst MC. Stability of the human fragile X (CGG)(n) triplet repeat array in Saccharomyces cerevisiae deficient in aspects of DNA metabolism. Mol. Cell. Biol. 1999;19:5675–5684. doi: 10.1128/mcb.19.8.5675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bhattacharyya S, Lahue RS. Saccharomyces cerevisiae Srs2 DNA helicase selectively blocks expansions of trinucleotide repeats. Mol. Cell. Biol. 2004;24:7324–7330. doi: 10.1128/MCB.24.17.7324-7330.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bhattacharyya S, Rolfsmeier ML, Dixon MJ, Wagoner K, Lahue RS. Identification of RTG2 as a modifier gene for CTG*CAG repeat instability in Saccharomyces cerevisiae. Genetics. 2002;162:579–589. doi: 10.1093/genetics/162.2.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dixon MJ, Lahue RS. Examining the potential role of DNA polymerases eta and zeta in triplet repeat instability in yeast. DNA Repair. 2002;1:763–770. doi: 10.1016/s1568-7864(02)00095-2. [DOI] [PubMed] [Google Scholar]
- 31.Freudenreich CH, Stavenhagen JB, Zakian VA. Stability of a CTG/CAG trinucleotide repeat in yeast is dependent on its orientation in the genome. Mol. Cell. Biol. 1997;17:2090–2098. doi: 10.1128/mcb.17.4.2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jankowski C, Nag DK. Most meiotic CAG repeat tract-length alterations in yeast are SPO11 dependent. Mol. Genet. Genom. 2002;267:64–70. doi: 10.1007/s00438-001-0635-4. [DOI] [PubMed] [Google Scholar]
- 33.Collins NS, Bhattacharyya S, Lahue RS. Rev1 enhances CAG.CTG repeat stability in Saccharomyces cerevisiae. DNA Repair. 2007;6:38–44. doi: 10.1016/j.dnarep.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 34.Gordenin DA, Resnick MA. Yeast ARMs (DNA at-risk motifs) can reveal sources of genome instability. Mutat. Res. 1998;400:45–58. doi: 10.1016/s0027-5107(98)00047-5. [DOI] [PubMed] [Google Scholar]
- 35.Lahiri M, Gustafson TL, Majors ER, Freudenreich CH. Expanded CAG repeats activate the DNA damage checkpoint pathway. Mol. Cell. 2004;15:287–293. doi: 10.1016/j.molcel.2004.06.034. [DOI] [PubMed] [Google Scholar]
- 36.Miret JJ, Pessoa-Brandao L, Lahue RS. Instability of CAG and CTG trinucleotide repeats in Saccharomyces cerevisiae. Mol. Cell. Biol. 1997;17:3382–3387. doi: 10.1128/mcb.17.6.3382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang J, Freudenreich CH. Haploinsufficiency of yeast FEN1 causes instability of expanded CAG/CTG tracts in a length-dependent manner. Gene. 2007;393:110–115. doi: 10.1016/j.gene.2007.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Claassen DA, Lahue RS. Expansions of CAG{middle dot}CTG repeats in immortalized human astrocytes. Hum. Mol. Genet. 2007;16:3088–3096. doi: 10.1093/hmg/ddm270. [DOI] [PubMed] [Google Scholar]
- 39.Maurer DJ, O'Callaghan BL, Livingston DM. Orientation dependence of trinucleotide CAG repeat instability in Saccharomyces cerevisiae. Mol. Cell. Biol. 1996;16:6617–6622. doi: 10.1128/mcb.16.12.6617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cleary JD, Nichol K, Wang YH, Pearson CE. Evidence of cis-acting factors in replication-mediated trinucleotide repeat instability in primate cells. Nat. Genet. 2002;31:37–46. doi: 10.1038/ng870. [DOI] [PubMed] [Google Scholar]
- 41.Cleary JD, Pearson CE. The contribution of cis-elements to disease-associated repeat instability: clinical and experimental evidence. Cytogenet. Genome Res. 2003;100:25–55. doi: 10.1159/000072837. [DOI] [PubMed] [Google Scholar]
- 42.Freudenreich CH, Lahiri M. Structure-forming CAG/CTG repeat sequences are sensitive to breakage in the absence of Mrc1 checkpoint function and S-phase checkpoint signaling: implications for trinucleotide repeat expansion diseases. Cell Cycle. 2004;3:1370–1374. doi: 10.4161/cc.3.11.1246. [DOI] [PubMed] [Google Scholar]
- 43.Yoon SR, Dubeau L, de Young M, Wexler NS, Arnheim N. Huntington disease expansion mutations in humans can occur before meiosis is completed. Proc. Natl Acad. Sci. USA. 2003;100:8834–8838. doi: 10.1073/pnas.1331390100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Savouret C, Brisson E, Essers J, Kanaar R, Pastink A, te Riele H, Junien C, Gourdon G. CTG repeat instability and size variation timing in DNA repair-deficient mice. Embo J. 2003;22:2264–2273. doi: 10.1093/emboj/cdg202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kovtun IV, Therneau TM, McMurray CT. Gender of the embryo contributes to CAG instability in transgenic mice containing a Huntington's disease gene. Hum. Mol. Genet. 2000;9:2767–2775. doi: 10.1093/hmg/9.18.2767. [DOI] [PubMed] [Google Scholar]
- 46.Kovtun IV, Welch G, Guthrie HD, Hafner KL, McMurray CT. CAG repeat lengths in X- and Y-bearing sperm indicate that gender bias during transmission of Huntington's disease gene is determined in the embryo. J. Biol. Chem. 2004;279:9389–9391. doi: 10.1074/jbc.M313080200. [DOI] [PubMed] [Google Scholar]
- 47.Cleary JD, Pearson CE. Replication fork dynamics and dynamic mutations: the fork-shift model of repeat instability. Trends Genet. 2005;21:272–280. doi: 10.1016/j.tig.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 48.Nichol Edamura K, Leonard MR, Pearson CE. Role of replication and CpG methylation in fragile X syndrome CGG deletions in primate cells. Am. J. Hum. Genet. 2005;76:302–311. doi: 10.1086/427928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Entezam A, Biacsi R, Orrison B, Saha T, Hoffman GE, Grabczyk E, Nussbaum RL, Usdin K. Regional FMRP deficits and large repeat expansions into the full mutation range in a new Fragile X premutation mouse model. Gene. 2007;395:125–134. doi: 10.1016/j.gene.2007.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bontekoe CJ, de Graaff E, Nieuwenhuizen IM, Willemsen R, Oostra BA. FMR1 premutation allele (CGG)81 is stable in mice. Eur. J. Hum. Genet. 1997;5:293–298. [PubMed] [Google Scholar]
- 51.Willemsen R, Hoogeveen-Westerveld M, Reis S, Holstege J, Severijnen LA, Nieuwenhuizen IM, Schrier M, van Unen L, Tassone F, et al. The FMR1 CGG repeat mouse displays ubiquitin-positive intranuclear neuronal inclusions; implications for the cerebellar tremor/ataxia syndrome. Hum. Mol. Genet. 2003;12:949–959. doi: 10.1093/hmg/ddg114. [DOI] [PubMed] [Google Scholar]
- 52.Lavedan C, Grabczyk E, Usdin K, Nussbaum RL. Long uninterrupted CGG repeats within the first exon of the human FMR1 gene are not intrinsically unstable in transgenic mice. Genomics. 1998;50:229–240. doi: 10.1006/geno.1998.5299. [DOI] [PubMed] [Google Scholar]
- 53.Peier AM, Nelson DL. Instability of a premutation-sized CGG repeat in FMR1 YAC transgenic mice. Genomics. 2002;80:423–432. doi: 10.1006/geno.2002.6849. [DOI] [PubMed] [Google Scholar]
- 54.Sullivan AK, Crawford DC, Scott EH, Leslie ML, Sherman SL. Paternally transmitted FMR1 alleles are less stable than maternally transmitted alleles in the common and intermediate size range. Am. J. Hum. Genet. 2002;70:1532–1544. doi: 10.1086/340846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Adler ID. Comparison of the duration of spermatogenesis between male rodents and humans. Mutat. Res. 1996;352:169–172. doi: 10.1016/0027-5107(95)00223-5. [DOI] [PubMed] [Google Scholar]
- 56.Jiang G, Sancar A. Recruitment of DNA damage checkpoint proteins to damage in transcribed and nontranscribed sequences. Mol. Cell. Biol. 2006;26:39–49. doi: 10.1128/MCB.26.1.39-49.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yoshioka K, Yoshioka Y, Hsieh P. ATR kinase activation mediated by MutSalpha and MutLalpha in response to cytotoxic O6-methylguanine adducts. Mol. Cell. 2006;22:501–510. doi: 10.1016/j.molcel.2006.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Brown EJ, Baltimore D. ATR disruption leads to chromosomal fragmentation and early embryonic lethality. Genes Dev. 2000;14:397–402. [PMC free article] [PubMed] [Google Scholar]
- 59.Nolin SL, Brown WT, Glicksman A, Houck GE, Jr, Gargano AD, Sullivan A, Biancalana V, Brondum-Nielsen K, Hjalgrim H, et al. Expansion of the fragile X CGG repeat in females with premutation or intermediate alleles. Am. J. Hum. Genet. 2003;72:454–464. doi: 10.1086/367713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.van den Broek WJ, Wansink DG, Wieringa B. Somatic CTG*CAG repeat instability in a mouse model for myotonic dystrophy type 1 is associated with changes in cell nuclearity and DNA ploidy. BMC Mol. Biol. 2007;8:61. doi: 10.1186/1471-2199-8-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Drost JB, Lee WR. Biological basis of germline mutation: comparisons of spontaneous germline mutation rates among drosophila, mouse, and human. Environ. Mol. Mutagen. 1995;25(Suppl. 26):48–64. doi: 10.1002/em.2850250609. [DOI] [PubMed] [Google Scholar]
- 62.Jones GG, Reaper PM, Pettitt AR, Sherrington PD. The ATR-p53 pathway is suppressed in noncycling normal and malignant lymphocytes. Oncogene. 2004;23:1911–1921. doi: 10.1038/sj.onc.1207318. [DOI] [PubMed] [Google Scholar]
- 63.Dart DA, Adams KE, Akerman I, Lakin ND. Recruitment of the cell cycle checkpoint kinase ATR to chromatin during S-phase. J. Biol. Chem. 2004;279:16433–16440. doi: 10.1074/jbc.M314212200. [DOI] [PubMed] [Google Scholar]
- 64.Kovtun IV, Liu Y, Bjoras M, Klungland A, Wilson SH, McMurray CT. OGG1 initiates age-dependent CAG trinucleotide expansion in somatic cells. Nature. 2007;447:447–452. doi: 10.1038/nature05778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Geva E, Yaron Y, Shomrat R, Ben-Yehuda A, Zabari S, Peretz H, Naiman T, Yeger H, Orr-Urtreger A. The risk of fragile X premutation expansion is lower in carriers detected by general prenatal screening than in carriers from known fragile X families. Genet. Test. 2000;4:289–292. doi: 10.1089/10906570050501524. [DOI] [PubMed] [Google Scholar]
- 66.Abramowicz MJ, Parma J, Cochaux P. Slight instability of a FMR-1 allele over three generations in a family from the general population. Am. J. Med. Genet. 1996;64:268–269. doi: 10.1002/(SICI)1096-8628(19960809)64:2<268::AID-AJMG6>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 67.Terracciano A, Pomponi MG, Marino GM, Chiurazzi P, Rinaldi MM, Dobosz M, Neri G. Expansion to full mutation of a FMR1 intermediate allele over two generations. Eur. J. Hum. Genet. 2004;12:333–336. doi: 10.1038/sj.ejhg.5201154. [DOI] [PubMed] [Google Scholar]