Abstract
The performance of tongue muscles in various feeding behaviours is not well defined. This study was undertaken to examine the role of the intrinsic and extrinsic tongue muscles during natural drinking, food ingestion and chewing.
Ten 12-week-old Yucatan miniature pigs (5 in each gender) were used. Under anesthesia, fine-wire electrodes were inserted into three intrinsic (verticalis and transversus [V/T]; superior and inferior longitudinalis [SL and IL]) and two extrinsic (genioglossus [GG] and styloglossus [SG]) tongue muscles and two jaw muscles (masseter [MA] and anterior digastricus [DI]). Electromyogram (EMG) and jaw movement were recorded and synchronized when pigs were drinking water, ingesting and chewing food freely. Chewing frequency (CF), onset of activation, burst duration and integrated activity (IEMG) were assessed quantitatively, and EMG activities during drinking and ingestion were examined qualitatively.
Results indicate that during chewing, the V/T and GG had one phase of activity starting at early jaw opening, and the V/T activity lasted through late of jaw closing. The SL, IL and SG had double phases with the first starting at jaw opening and the second at late jaw closing phases. The three intrinsic tongue muscles and the SG were active during 35-48% of the chewing cycle. IEMG values of the SL, IL and SG of both sides were significantly greater compared to the other muscles (p < 0.05-0.01). Both the SL and the IL showed significantly higher activities in the contralateral than ipsilateral sides (p < 0.05). The timing sequences of both extrinsic and intrinsic muscles were similar between ingestion and chewing, but amplitudes of the GG and IL were greatly enhanced and those of the MA and SL were reduced during ingestion. The simultaneous activation of the MA, GG and V/T were seen during drinking, along with major activity in the GG and V/T.
These results suggested that the majority of activity in the intrinsic and extrinsic tongue muscles occurred during jaw opening and the occlusal phases of chewing. The activity of the GG and IL played a major role during ingestion, whereas simultaneous activation of jaw, extrinsic and intrinsic tongue muscles and major activity in the GG and V/T occurred during drinking.
Keywords: Tongue muscles, EMG, Mastication, Ingestion, Drinking
1.. Introduction
The mammalian tongue plays an important role in various oral functions such as respiration, speech, deglutition and mastication. As a muscular organ comprised of both intrinsic and extrinsic muscles, the tongue possesses the hydrostat properties which allow localized shape changes while maintaining constant volume and incompressibility.1,2
The intrinsic tongue muscles, which by definition have their origin and insertion within the tongue, include the verticalis, transversus, superior and inferior longitudinalis. However, palatoglossus, styloglossus, genioglossus and hyoglossus have one end attached to bones and the other inserting into the base of the tongue, and are recognized as extrinsic tongue muscles.3
Commonly, the intrinsic muscles are described as merely controlling the shape of the tongue surface, while the extrinsic muscles are believed to move the tongue per se in the orofacial space.4 However, this approach is no longer tenable. It is now known that both muscle groups interdigitate and act in concert with each other during most functional activities, although each muscle may have some specific and independent function, i.e. some tongue regions are controlled by intrinsic muscles independently in mammals.3,5-7
It is known that the extrinsic tongue muscles serve to protrude and retract the tongue. Also, these muscles can elevate (styloglossus) and depress (genioglossus) the base of the tongue.8 With the bilateral activation of the styloglossus and hypoglossus, the tongue is retracted. This movement is accomplished by decreasing the length and thickening the body of the tongue through contraction of intrinsic muscles (see below). The genioglossus is a notably larger muscle in a fan-like projection from below. This muscle, co-activating with the intrinsic muscles, cause the tongue to protrude by increasing the length and decreasing the thickness.8 Unilateral activation of the extrinsic muscles can alter tongue direction in the horizontal plane while retracting or protruding the tongue. Furthermore, these muscles have some other specific functions: the genioglossus may influence tongue shape, e.g. midline grooving, and the styloglossus controls the lateral margins of the tongue.9
The intrinsic muscles are considered specifically to control the position of the tongue tip and tongue shape.10 Along with activation of the genioglossus and the styloglossus, these muscles participate in tongue protrusion and retraction, respectively.8 Shortening of the tongue body can be accomplished by the superior and inferior longitudinalis in concert bilaterally. Moreover, intrinsic muscles are responsible for bending of the tongue. In this deformation, the longitudinal muscle fibres act together with their antagonistic muscle (transversus) fibres.2 It has also been said that most intrinsic and extrinsic muscle fibres are not separate in terms of their mechanical effects.3,11
In the literature, studies on tongue function during feeding have focused on the extrinsic tongue and jaw muscles, and less attention has been paid to the intrinsic tongue muscles.3,5,12 Also, most of the studies on the intrinsic tongue muscles focus on morphology and do not include their motor function which is usually evaluated by electromyography (EMG).6,13 This lack of information stems from the technical challenges associated with recording EMG from the intrinsic muscles under natural conditions, and the lack of an appropriate animal model. With investigations of the architecture, location, fibre organization and contractile properties of the longitudinal muscles in the rat and human, Sokoloff et al. suggested that the localization of these muscle fibres has a role in the regional control, and in particular the superior longitudinalis is organized to control both tongue displacement and regional shape.6,13 Stal et al. examined the fibre composition of the three human intrinsic tongue muscles, and suggested that the existence of type II fibres in all of them allows for generally fast and flexible actions in shaping and positioning the tongue during function.14
In the present study, we used the pig model to examine the functional interactions or mutual activations of the intrinsic and extrinsic tongue muscles during natural feeding. Because the tongue must undergo many small regional deformations associated with placing and controlling the bolus between the teeth during jaw closing,6,8,11 and because most loads on surrounding hard tissues occur during this phase,15,16 we hypothesized that (1) both the intrinsic and extrinsic tongue muscles would exhibit greater activity in jaw closing than opening phases in feeding and (2) the intrinsic tongue muscles would show more activity than do the extrinsic tongue and jaw muscles during rhythmic jaw movements in feeding.
2.. Materials and methods
1.1. Animal care
Ten 12-week-old Yucatan miniature pigs (5 in each gender) were used for the current study. Pigs were acclimated to the laboratory through daily training and handling for 5 days and food deprived for 24 h prior to EMG recordings. The housing, care and experimental protocol were approved by the Institutional Animal Care and Use Committee, University of Washington.
1.2. Placement of electrodes and markers
After the pig was anaesthetized by isoflurane through a nostril mask, the cheek and submandibular area were shaved and cleaned with 99% alcohol swabs. Eight pairs of fine-wire electrode (0.05 mm nickel-chromium wire, 1 mm bared tip and 2 mm separation between wire tips) were inserted into the following muscles by a 25G needle. Each pair of electrodes was inserted along the fibre direction of the target muscle.
The electrode placements were modified from published approaches3 and from those in our previous studies.12,17 No effort was made to record separately from the verticalis and transverses muscles, as they are interwoven with each other and therefore not spacially distinct. The right verticalis/transversalis was approached from the lateral side ventral to the circumvallate papilla and the electrodes were inserted into the central region of the anterior tongue. Electrodes were inserted into the right superior longitudinalis through the dorsal surface of the tongue in the midline just anterior to the right premolars. The right inferior longitudinalis was approached from the ventral surface and the electrodes were inserted ventrally 1 mm right to the commissure of the lingual frenulum. Additionally, electrodes were inserted into two extrinsic tongue muscles (genioglossus and styloglossus) and two jaw muscles (masseter and digastric). The right genioglossus was hooked at a depth of 10-15 mm after the needle had passed through the mylohyoid and the geniohyoid 5 mm right to the midline in the sub-mandibular region. In order to place electrodes into the syloglossus, the needle was inserted through the skin near the junction of the mandibular body and the ramus where it followed the inner surface of the right mandibular to a depth of 25-35 mm. The right and left masseters and right digastricus were hooked through direct skin penetrations. The palataglossus and hyoglossus were not included in the present study because (1) the difficulty of access and the problem of leading a wire from the palataglossus; (2) lack of a landmark for identifying the hyoglossus; (3) limitation in the number of available EMG channels.
All electrodes from the intrinsic muscles were led out of the mouth from the space between the mandibular canine and first deciduous molar, taped onto the cheek with other electrodes, and hooked up into EMG leads. The leads were then connected to EMG amplifier probes at a collar. Four fluorescent markers were glued on the skin of the upper and lower lips, 2 of each, for digital videotaping of jaw movements. These markers were aligned parallel to the occlusal plane. Finally, anesthesia was ceased, and the pigs awoke and were fed their usual diet (pig pellet chow) and water. The EMG signals were sampled at the rate of 500 Hz by a computer running Acqknowledge III software (MP 100, Biopac System, Inc, CA) for 20-30 min when pigs were ingesting and chewing food or drinking water from a transparent container. Jaw movement was captured using a digital video camera (60 frame/s, Sony Co., Tokyo, Japan) and synchronized with bilateral masseter EMG signals through an Event and Video Control and an Analog to Digital Interface (Peak Performance Technologies Inc.). The behaviours in feeding, including ingestion (cycles in which food is brought into the mouth), chewing and water drinking cycles were identified by direct visualization. Real-time markers were placed on the on-line recordings during masticatory data acquisition in order to identify ingestion from chewing cycles for off-line analyses. After these recordings, feeding was ceased and electrodes were gently removed. A short session of jaw movement in feeding was captured again to examine if there was any functional interferences caused by placements of EMG electrodes.
The accuracy of electrode locations for each muscle was validated through visual observations with back stimulations, i.e. jaw closing and opening for masseter and digastricus, tongue protraction and retraction for genioglossus and styloglossus, ventral and dorsal bending of tongue surface for superior and inferior longitudinalis, and central tongue grooving or narrowing for verticalis/transverses. Additionally, two fresh pig cadaver heads of the same age were used to verify accuracy of electrode placements. Each pair of electrodes was inserted as they were for living animals. The lead wires were then cut, leaving the bared tip ends in place. Dissections were done to identify the sites of each pair of electrode.
1.3. Data processing
After digital filtering (bandpass of 60-250 Hz), EMG signals from 15-20 consecutive chewing or drinking cycles, and 8-15 ingestion cycles from each pig were selected for analysis. Cycles of ingesting and drinking were only analysed qualitatively because of the limited data. Chewing side was identified by looking at the timing and amplitude differences between bilateral masseters, i.e. the higher amplitude and/or delayed onset were considered as an ipsilateral side.16,18 Attention was paid to the rhythm of jaw movements and regularity of muscle activity bursts (constant pattern) in order to exclude any possible transport and swallowing cycles. Only consecutive and rhythmic jaw movement cycles (>5) with stereotyped EMG activity of jaw muscles were selected for the study. The following EMG parameters were quantitatively assessed for each chewing cycle: (1) onset of activation; (2) burst duration measured from the onset to offset of activity burst; (3) integrated activity (IEMG) calculated by rectifying the EMG signal, integrating the signal over a specified interval of time and subsequently forming a time series of the integrated values. Due to variation of chewing frenquency, both onset and duration were standardized to the relative values (%) corresponding to chewing cycle length (chewing frequency) obtained from jaw movement analysis (see below).
Jaw movement images were analysed using Motus software (Peak Performance Technologies Inc. CO). In order to calculate jaw-opening degrees (gape), a template was set which linked each two upper and lower markers to form two segments. The angulations of separation of these two segments were incorporated into each digitized frame. Furthermore, a custom-made add-in macro was integrated into the program which marked a specific frame as an event. Three events were defined by looking at motions of lower markers relating to upper markers from successive frame replaying and were superimposed on each channel of synchronized EMG signals: (1) event 1—the beginning of jaw opening: this event was determined when the lower markers showed the first downward moving, which also indicated the ending of occlusal phase (power stroke); (2) event 2—the beginning of the jaw closing: this event was determined when the lower markers showed the first upward moving, which also indicated the maximal opening; (3) event 3—the beginning of occlusal phase: this event was determined when no visible vertical moving (neither down nor upward) was identified, which also indicated the end of jaw closing. The event frame were decided by forwarding and rewinding 3-5 frames around the event frame to make sure that this was the one right before the transition. For instance, the frame for event 1 was the last frame showing no vertical moving, and a downward moving was identified in the following frame. The periods between event, 1-2, 2-3 and 1-3 were represented as jaw opening, closing and occlusal (power stroke) phases, respectively (Fig. 1). Similar to EMG timing, the durations between these events were also converted to the relative values (% of corresponding chewing cycle length, i.e. time length between two adjacent events). Because only bilateral masseter EMGs were synchronized with jaw movements, the timing of other muscles (onset and duration) were calculated by taking the masseter as the references.
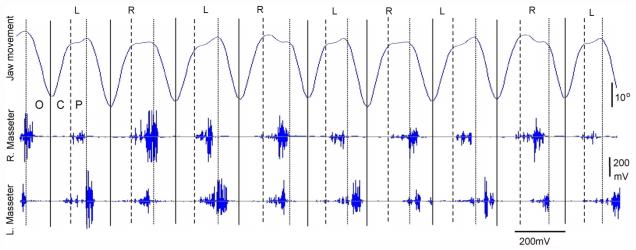
Fig. 1.
A typical jaw movement tracing (the top) with synchronized masseter EMG activities (bottom two) during chewing. Lines indicate marked events during jaw movement digitization. Solid line: maximal opening; Dashed line: maximal closing; Dotted line: end of power stroke (occlusal) O: jaw opening phase, C: jaw closing phase, P: occlusal (power stroke) phase. The letters (L and R) above the figure indicate chewing sides.
2. Statistical analysis
SPSS (version 11.0) for Windows was used for the statistical analysis. Since the distribution of the data was asymmetric, Mann-Whitney U test was used to determine the side differences of each muscle and to compare phase I and II in the muscles which showed 2-phased activity. Kruskall-Wallis H test was performed to detect the differences in the onset, duration and IEMG across the recorded muscles, followed by Tukey test for pair-wise comparisons with Bonferroni correction. p < 0.05 was adopted as the significance level for Mann-Whitney U and Kruskall-Wallis H tests.
3. Results
3.1. Chewing, ingestion and drinking
Generally, pigs awoke from anesthesia and began to eat in less than 20 min. After the first 5-10 min, animals usually stood and ate food as usual. Ingestion and chewing cycles were consecutive with a few visible transitional cycles. Ingestion was much shorter than chewing, and lasted less than 15 cycles. Drinking was recorded separately from those of food eating, and rhythmic jaw movement was slight. Stereotyped activity patterns were found in both intrinsic and extrinsic tongue muscles during chewing, ingestion and drinking. Jaw movement analyses indicated that placement of EMG electrodes did not cause functional impairment during these three feeding behaviours, but this verification might not exclude possible alteration in tongue function.
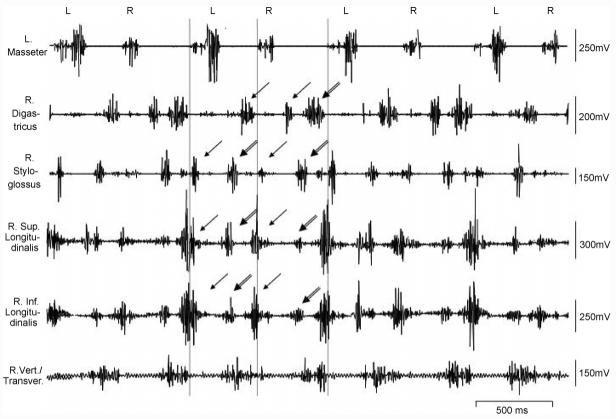
The superior and inferior longitudinalis and styloglossus showed a 2-phased activity pattern during chewing, with the late part of the second phase overlapping with the early part of masseter activation. The 2-phase pattern was also sometimes identified in the ipsilateral digatricus (2 out of 10) (Fig. 2).
Fig. 2.
Raw EMGs of jaw, tongue extrinsic and intrinsic muscles during chewing. Lines indicate the onset of masseter activation. Arrows show double-phased activity. Single- and double-headed arrows indicate the first and second phases, respectively. Note that of 10 animals, only 2 showed double-phased activity in the ipsilateral digastricus. The letters above EMGs indicate chewing side.
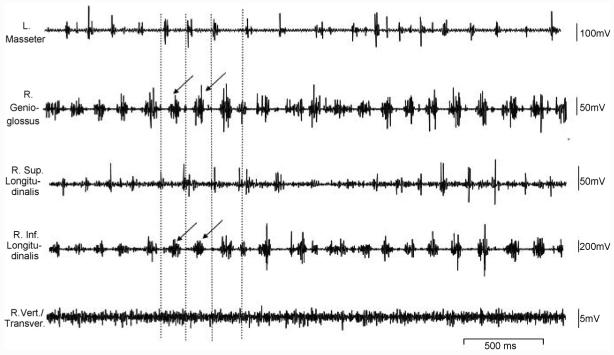
The frequency of ingestion cycles was almost 2 times higher than that of chewing. The timing sequence (relationship of onsets) during ingestion and chewing were similar for both intrinsic and extrinsic muscles, but the burst durations and activity amplitudes were different. During ingestion, the burst duration of the masseter decreased three times and those of the superior and inferior longitudinalis and genioglossus were also greatly reduced compared to chewing. The activity of the genioglossus and inferior longitudinalis was comparable to that seen in chewing, whereas masseter activity decreased as much 5 times compared to chewing. Furthermore, qualitative observations suggested that the activity of the superior longitudinalis and verticalis/transversus were also diminished compared to that during chewing, and the periodic activation burst of the latter was not as clear as it was during chewing (Fig. 3).
Fig. 3.
Raw EMGs of jaw, tongue extrinsic and intrinsic muscles during food ingestion. Arrows indicate major activity in the genioglossus and inferior logitudinalis.
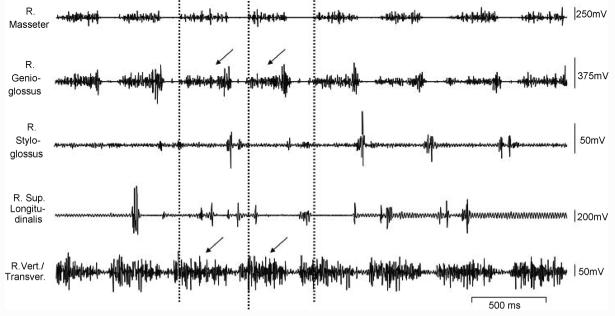
The frequency of drinking was similar to that of chewing. The striking differences between drinking and chewing involved in both time sequences and activity amplitudes. Instead of activation during jaw opening, the genioglossus and the verticalis/transversus activated almost in line with that of masseter (i.e. jaw closing). The styloglossus showed a single phase in jaw opening and did not overlap with masseter activation as it did in chewing. Compared to chewing, the durations of the verticalis/transversus and genioglossus were considerably extended, and the activity amplitude of the genioglossus was enhanced. Interestingly, the duration of the masseter was also extended up to 150% of that of chewing with moderate reduction of activity amplitude, while the burst duration of styloglossus was diminished (Fig. 4).
Fig. 4.
Raw EMGs of jaw, tongue extrinsic and intrinsic muscles during food ingestion. Arrows indicate major activity in the genioglossus and verticalis/transversus.
3.2. Time sequences and durations during chewing
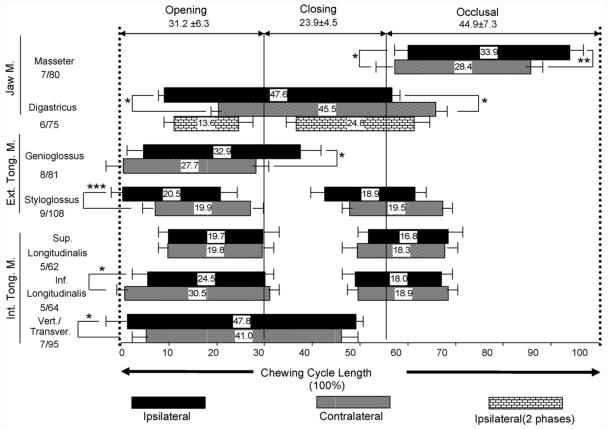
Jaw opening, closing and occlusal phases took up 31.2% ± 6.3%, 23.9 ± 4.5% and 44.9% ± 7.3% of chewing cycle length, respectively. The beginning of the occlusal phase preceded the onset of the masseter, and the opening phase was close to its offset (Fig. 5). The transition from jaw opening to closing was in the middle of the cessation period of masseter activity.
Fig. 5.
The comparison of activation onsets and burst durations in relation to jaw gape in chewing. Two dotted lines indicate one chewing cycle length, and two solid lines indicate maximal jaw opening and closing. Bar lengths indicate the mean percentages of burst duration and the distances of the bars to left dotted line indicate activation onsets in relation to the start of jaw opening in chewing. Error bars left and right to each histogram bars indicate 1 standard deviation (S.D.) of onset and duration, respectively. Asterisks indicate significant difference between ipsilateral and contralateral sides in the onset (left) and duration (right). Numbers below each muscle name indicate sample size (animal numbers/chewing cycle numbers). *p < 0.05; **p < 0.01; ***p < 0.001.
The time sequence of all muscles in relation to jaw movements is illustrated in Fig. 5. The onsets of the ipsilateral diagistricus, styloglossus and verticalis/transversus significantly preceded those of contralateral ones (p < 0.05-0.001), whereas the onsets of the contralateral genioglossus, superior and inferior longitudinalis preceded those of ipisilateral ones with statistical significance only for the inferior longitudinalis (p < 0.05). The duration of activity of the intrinsic tongue muscles and the styloglossus was about 35-48% of the chewing cycle length. The shortest and longest durations were seen in the genioglossus (28-33%) and the digastricus (46-48%), respectively. Furthermore, the durations of these two muscles were significantly longer on the ipsilateral than the contralateral sides (p < 0.05) (Fig. 5 and Table 1). If there was two phases in ipsilateral digastricus, the late phase was generally longer than the early phase (Fig. 5).
Table 1. Comparisons of EMG burst durations.
| Masseter |
Digastricus |
Genioglossus |
Styloglossus |
Sup. Longitudinalis |
Inf. Longitudinalis |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| I | C | I | C | I | C | I | C | I | C | I | C | |
| Digastricus | ||||||||||||
| I | ns | - | ∼ | ∼ | ** | - | ns | - | ns | - | ns | - |
| C | - | ** | ∼ | ∼ | - | ** | - | *** | - | * | - | *** |
| Genioglossus | ||||||||||||
| I | ns | - | ** | - | ∼ | ∼ | ns | - | ns | - | ns | - |
| C | - | ns | - | ** | ∼ | ∼ | - | *** | - | ** | - | *** |
| Styloglossus | ||||||||||||
| I | ns | - | ns | - | ns | - | ∼ | ∼ | ns | - | ns | - |
| C | - | ns | - | *** | - | *** | ∼ | ∼ | - | - | - | ns |
| Sup. Longitudinalis | ||||||||||||
| I | ns | - | ns | - | ns | - | ns | - | ∼ | ∼ | ns | - |
| C | - | ns | - | * | - | ** | - | - | ∼ | ∼ | - | ns |
| Inf. Longitudinalis | ||||||||||||
| I | ns | - | ns | - | ns | - | ns | - | ns | - | ∼ | ∼ |
| C | - | * | - | *** | - | *** | - | ns | - | ns | ∼ | ∼ |
| Vert./Transver. | ||||||||||||
| I | *** | - | *** | - | *** | - | *** | - | ** | - | ** | - |
| C | - | * | - | *** | - | *** | - | ns | - | ns | - | ns |
ipsilateral side
contralateral side
not avaialble
not applicable
p < 0.05
p < 0.01
p < 0.001
not significant
3.3. Muscle activities during chewing
For those muscles showing 2-phase activity, IEMGs were calculated separately. However, statistical tests (nonparametric, 2-related sample) showed no significant differences between the first and second phases for both ipsilateral and contralateral sides. Thus these values were combined.
The IEMG values of the ipsilateral and contralateral styloglossus, and superior and inferior longitudinalis were significant greater compared to those of the verticalis/transversus, genioglossus and the two jaw muscles (p < 0.05-0.01). Both superior and infereior longitudinalis showed significantly higher IEMG in the contralateral than the ipsilateral sides (p < 0.05). A similar trend was also found in the styloglossus and digastricus. On the contrary, the masseter showed significant higher activity in the ipsilateral side (p < 0.05) (Fig. 6).
Fig. 6.
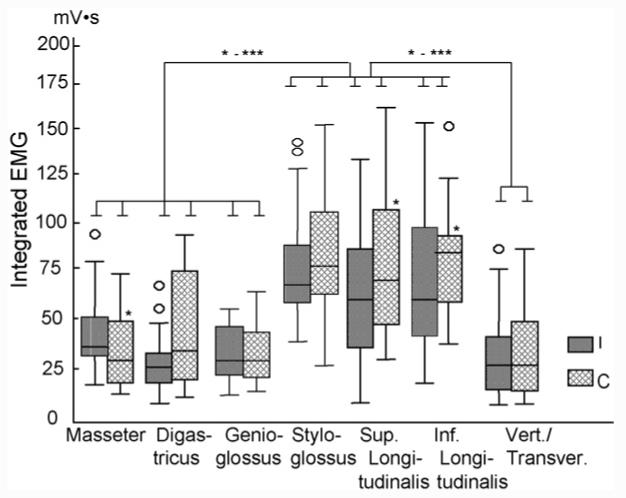
Boxplots of integrated activity values (IEMG) among the recorded muscles during chewing. Upper and lower limits of box represent 75th and 25th percentile, respectively. Horizontal line in each box represents the median. Small circles outside box represent outliers (1.3-3 of box length). The asterisks above the solid lines indicate significant differences between muscles, and asterisks above the boxes indicate the significant differences between ipsilateral and contralateral sides. I: ipsilateral side; C: contralateral side. See Fig. 5 for other symbols.
4. Discussion
4.1. Design and limitations
Pigs were chosen for the present study because their masticatory apparatus (jaw muscle locations and joint structures, tooth and dentition forms, jaw movement pattern) most closely resembles that of higher primates among any other species of non-primate mammals.19,20 However, it must be mentioned that the anatomical differences between humans and pigs in the craniofacial region are obvious, and are mostly associated with the significantly long snout, long oral cavity, long anterior portion of the tongue and long post-canine occlusal table. These may contribute to functional differences in mastication, food acquisition and swallowing.
Because the experiments presented here were not part of terminal procedures, the actual recording sites in the muscle were not confirmed by direct visualization. Although back stimulations were used to verify the accuracy of electrode sites, it has been generally known that back stimulation is insufficient to verify which muscle the electrode is in, as the receptive field may not represent the stimulus. To overcome this limitation, a confirmatory study was carried out on two cadavers to examine the electrode placement sites in the muscles when electrodes were inserted by the described manner. These experiments confirmed that electrode placements were accurate. However, again, cadaver experiments might still not reflect which muscle was being recorded due to the possibilities of anatomic (muscle size and location), physiologic (functional performance) and experimental (methodological error) variations, and therefore, the source of EMG signals might be ambiguous in the present study due to these limitations.
The present study also has limited capacity to describe function at the muscle level, particularly for the intrinsic tongue muscles because there are compartments within these muscles and each compartment may function differently.21 Thus, the recording presented here might only represent the function of one compartment, not the overall function of the muscle. Secondly, the intrinsic muscles are often interwoven, particularly the verticalis with transversus, and the genioglossus with the intrinsic muscles. Most of the muscles from which we recorded are spacially distinct, and the probability was low that signals from different electrode pairs might have recorded from the same muscle. However, we were unable to distinguished two intrinsic muscles (verticalis and transverses) with the current electrodes approaches, although the EMG patterns were highly consistent across animals. Therefore, the signals from this pair of electrodes could be from either one or both. Finally, because jaw and tongue movements are broadly correlated,22 it is likely that altered tongue function would have resulted in changes in jaw movement. While it cannot be confirmed, the fact that electrode placement did not interfere with jaw movement suggests tongue function was normal.
In the present study, chewing, ingestion and drinking were visually discriminated, but other two important feeding behaviours, food transport and swallowing, were not identified due to the restriction of the methods (no visualization of intra-oral and pharyngeal behaviours). Thus, these two behaviours could be included in the chewing sequence in the present study. Based on the studies in humans and animals,23-27 it has been suggested food transport and swallowing are generally not featured with stereotyped and consecutive cycles as seen in chewing, ingestion and drinking. Therefore, considering the selection criteria for the chewing cycles described above, it is most unlikely that the data analysed were mixed with either or both behaviours.
4.2. EMG timing during chewing
In the present study, all extrinsic and intrinsic muscles started activation during the cessation period of masseter activity (e.g. early jaw opening). The burst durations of the genioglossus (ipsilateral side) and verticalis/transversus (both sides) lasted through most of the jaw closing phase, but in the styloglossus, and superior and inferior longitudinalis, their first-phase activation ended right before or at the early jaw closing phase. In the occlusal phase, the onset of the second-phase in these three muscles preceded that of the masseter and ceased at the middle half of the masseter activity burst, resulting in a multiple overlapping (co-activation) of EMG activity in the early stage of the occlusal phase. On the contrary, no co-activation of tongue muscles was found in the late half of masseter activation. This striking difference in the early and late stages of jaw occlusal phase (power stroke) may suggest that: (1) co-activation of jaw, extrinsic and intrinsic tongue muscles for the bolus placement and grinding during early power stroke; (2) little tongue movement and/or shape change during late power stroke while the jaw moves to the opposite side.
Tongue movement and shape change are featured by co-activation of the intrinsic and extrinsic muscles.11,28 According to our preliminary observation on the jaw and tongue movement from chewing sequences using a high-speed video camera (250 frame/s), tongue retraction started before jaw closing and continued until early jaw closing (unpublished data). Thus, it could be speculated that tongue retraction is initiated by co-activation of the styloglossus, and the superior and inferior longitudinalis in late jaw opening and this movement lasts through early jaw closing (first phase of EMG activity). The tongue retraction movement may slow down or cease in late jaw closing while the genioglossus and verticalis/tranversus activate, and resume in the early power stroke (Figs. 2 and 5). The secondary retraction of the tongue just before the activation of the masseter, presented as the second phase of activity in the above three muscles, may relate to bolus transfer and placement in the molar region.
Since the genioglossus is the major tongue protrusor, it is reasonable to expect the activity of this muscle during jaw opening. Some animal studies have identified the close neuronal and functional links between the genioglossus and jaw opening muscles in various animal models.12,29 However, human studies indicated that the activity of the genioglossus occurs during both jaw opening and closing phases.1,30 These findings are in accordance with the current study since the genioglossus was active through jaw opening and closing phases without overlapping the occlusal phase of jaw movement and masseter activity during chewing (Figs. 2 and 5).
Digastricus is one of the muscles that control jaw opening in the vertical and horizontal planes.16 In the present study, the ipsilateral digastric activity preceded and lasted significantly longer than that of the contralateral one. This result is not in accordance with findings in rabbits,12,31 and may relate to the basic differences in anatomic configuration of digastricus and chewing patterns between the two animals. The rabbit has only anterior digsatricus and usually chews unilaterally with a larger component of lateral jaw movement,12 while the pig chews alternately with a moderate lateral shift in jaw movements.32 Our finding of 2-phase ipsilateral digastric is consistent with the study by Thexton and McGarrick.33
Our results also demonstrate that chewing side is related to the timing pattern of tongue muscles, and ipsilateral and contralateral styloglossus activated before and after the genioglossus, respectively (Fig. 5). This implies that the tongue body may protrude and bend toward the working side in chewing. The predominantly overlapping activities of all five tongue muscles during most jaw opening and early closing phases further prove the co-activation nature in either protrusion-retrusion or extrinsic-intrinsic muscular organization schemes in the tongue.11 Therefore, bodily movements of the tongue are accompanied by complex change of shapes including multiple direction changes in different tongue regions. The features of these three dimensional changes (tongue deformation) in relation to EMG and jaw movements during mastication are published elsewhere.34
4.3. EMG activity during chewing
It is interesting that IEMG values of the superior and inferior longitudinalis significantly surpassed those of the extrinsic tongue and jaw muscles during chewing. Furthermore, they were even much higher in the contralateral than ipsilateral sides. These two intrinsic tongue muscles are responsible for tongue curvature changes, i.e. being more concave by the superior and more convex by the inferior longitudinalis.2,3 Thus, the higher IEMG in these muscles may indicate that active tongue curvature changes occur during chewing. The higher IEMG in the contralateral side of these two muscles may reflect their roles of not only bending the tongue sagitally by altering the curvature, but also twisting the tongue transversely in order to transport food to the ipsilateral side where molar grinding occurs. On the other hand, the verticalis/transversus may play the role of tongue grooving without side predominance during chewing.
Similar to the two intrinsic muscles, the styloglossus also showed distinct side differences. The IEMG values of this muscle are comparable to those of the superior and inferior longitudinalis, whereas those of the genioglossus are very close to the verticalis/transverses (Fig. 6). These results may be related to the fact that the styloglossus is a lateral tongue muscle and contributes to the tongue movement and deformation with side predominance. However, the verticalis/transverses and genioglossus are midline tongue muscles and the primary orientation of the fibres of these muscles are perpendicular to the long axis of the tongue from gross anatomical considerations,13 thus they may not be related to the side predominance during chewing.
Previous studies have revealed that the masseter has stronger activity in the ipsilateral than the contralateral sides during chewing in primates and pigs.16,35-37 On the contrary, we found that the activity of digastricus tends to be higher in contralateral than ipsilateral sides (Fig. 6). Because chewing cycles in the present study was defined starting at jaw closing, this stronger digastric activity may be helpful for depressing the jaw to the contralateral side after power stroke during chewing.
It was hypothesized that both the intrinsic and extrinsic tongue muscles have greater activity in jaw closing than opening phases. This hypothesis can not be supported by present chewing EMG findings because both tongue intrinsic and extrinsic muscles showed major activity during both jaw opening and closing, with more activity in jaw opening (masseter cessation) instead of jaw closing phases. However, the second hypothesis that the intrinsic tongue muscles are more active than the extrinsic tongue and jaw muscles can be partly supported because the intrinsic tongue muscles (superior and inferior longtudinalis but not verticalnalis/transversus) showed more activity than those of jaw and extrinsic tongue muscles, i.e. genioglossus, masseter and digastricus.
4.4. EMG patterns during food ingestion and drinking
Few studies have recorded EMG from tongue muscles, particularly the intrinsic tongue muscles during food ingestion and drinking. Because of the short-term (a few consecutive cycles) nature of ingestion and an unwillingness to drink, it was difficult to perform quantitative analysis as was done for chewing. Despite this, we were still able to describe these two important feeding behaviours qualitatively.
It has been reported that EMG activity in the extrinsic tongue muscles are higher in chewing than ingestion cycles in the cat.33 This finding is in accordance with our findings in jaw and the extrinsic tongue muscles. During ingestion, the genioglossus and inferior longitudinalis not only activated simultaneously, but showed notably higher activity compared to other tongue muscles (verticalis/transversus, styloglossus and superior longitudinalis). Another feature of ingestion was a significant delay of the activation in the superior longitudinalis (about 120 ms, 70% of ingestion cycle length). In addition, the simultaneous reduction of activity in the verticalis/transversus and masseter may be recognized as the third EMG feature during ingestion in pigs. In contrast to chewing, EMG patterns in the two longitudinalis were divergent, and the superior one seemed to be less involved in ingestion. Our previous study using the same pigs demonstrated that tongue length change is significantly larger during ventral than dorsal bending by manipulation.38 Therefore, it could be speculated that during food ingestion, the pig elongates the tongue forward with the anterior part bending ventrally to pick up food, as indicated by the co-activation of the genioglossus and inferior longtudinalis right after the masseter burst (Fig. 3). This type of tongue behaviour in the pig is more or less similar to the findings in the cat.29
The previous studies indicated that drinking frequency (cycle length) is significantly higher than that of chewing with decreased jaw opening and increased activity in the genioglossus, digastricus and geniohyoideus in awake rabbits and rats.17,39 No intrinsic tongue muscle activity during natural drinking has been reported in the literature. Unlike the rabbit, the present study indicates that the frequency of drinking was similar to that of chewing in pigs. Compared to chewing, the most significant changes in drinking were observed in the verticalis/transversus and the genioglossus. First, the onsets of these two muscles were very close to that of the masseter. Second, their burst durations were significantly extended, same as seen in the masseter (Fig. 4). Therefore, it can be concluded that with the co-activation of these two extrinsic and intrinsic muscles and masseter, possibly along with activation of the hyoglossus,40 the base of the tongue depresses, and the tongue midline grooving forms in order to transport liquid to esophagus during drinking. Meanwhile, the jaw keeps slightly closing by contraction of the masseter. There were no multi-phase EMG activities and side predominance in drinking as seen in chewing, resulting in more stereotyped pattern of EMG than chewing and ingestion (Fig. 4). The drinking pattern found in the present study is very similar to other minipig studies.41,42 Because of no obvious tongue movement in the antero-posterior direction,42 tongue shape changes through co-activations of the two midline intrinsic (verticalis/transverses) and extrinsic (geniglossus) may be the major behaviour of the tongue during drinking.
Acknowledgments
The authors would like to thank Dr. Sue Herring for her invaluable advice and critical comments, Dr. Katherine Rafferty for her comments, help with experiments and English editing, and Ms. Xian-Qin Bai for her general lab assistance. Thanks also go to two anonymous reviewers and the Associate Editor for their constructive comments and suggestions on earlier versions of the manuscript. This study was supported by the grant R01DE15659 from NIDCR to Dr. Liu.
Footnotes
A portion of this study was presented at the 84th General Session of the International Association for Dental Research (IADR), Brisbane, Australia, June 28-July 1, 2006.
REFERENCES
- 1.Hiyama S, Iwamoto S, Ono T, Ishiwata Y, Kuroda T. Genioglossus muscle activity during rhythmic open-close jaw movements. J Oral Rehabil. 2000;27:664–70. doi: 10.1046/j.1365-2842.2000.00560.x. [DOI] [PubMed] [Google Scholar]
- 2.Kier WM, Smith KK. Tongues, tentacles and trunks: the biomechanics of movement in muscular-hydrostats. Zool J Linn Soc. 1985;83:307–24. [Google Scholar]
- 3.Bailey EF, Fregosi RF. Coordination of intrinsic and extrinsic tongue muscles during spontaneous breathing in the rat. J Appl Physiol. 2004;96:440–9. doi: 10.1152/japplphysiol.00733.2003. [DOI] [PubMed] [Google Scholar]
- 4.Hiiemae KM, Palmer JB. Tongue movements in feeding and speech. Crit Rev Oral Biol Med. 2003;14:413–29. doi: 10.1177/154411130301400604. [DOI] [PubMed] [Google Scholar]
- 5.Sauerland EK, Mitchell SP. Electromyographic activity of intrinsic and extrinsic muscles of the human tongue. Tex Rep Biol Med. 1975;33:444–55. [PubMed] [Google Scholar]
- 6.Sokoloff AJ. Localization and contractile properties of intrinsic longitudinal motor units of the rat tongue. J Neurophysiol. 2000;84:827–35. doi: 10.1152/jn.2000.84.2.827. [DOI] [PubMed] [Google Scholar]
- 7.Hiiemae KM, Hayenga SM, Reese A. Patterns of tongue and jaw movement in a cinefluorographic study of feeding in the macaque. Arch Oral Biol. 1995;40:229–46. doi: 10.1016/0003-9969(95)98812-d. [DOI] [PubMed] [Google Scholar]
- 8.McClung JR, Goldberg SJ. Functional anatomy of the hypoglossal innervated muscles of the rat tongue: a model for elongation and protrusion of the mammalian tongue. Anat Rec. 2000;260:378–86. doi: 10.1002/1097-0185(20001201)260:4<378::AID-AR70>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 9.Stone M. A three-dimensional model of tongue movement based on ultrasound and X-ray microbeam data. J Acoust Soc Am. 1990;87:2207–17. doi: 10.1121/1.399188. [DOI] [PubMed] [Google Scholar]
- 10.Napadow VJ, Chen Q, Wedeen VJ, Gilbert RJ. Intramural mechanics of the human tongue in association with physiological deformations. J Biomech. 1999;32:1–12. doi: 10.1016/s0021-9290(98)00109-2. [DOI] [PubMed] [Google Scholar]
- 11.Sokoloff AJ. Activity of tongue muscles during respiration: it takes a village? J Appl Physiol. 2004;96:438–9. doi: 10.1152/japplphysiol.01079.2003. [DOI] [PubMed] [Google Scholar]
- 12.Liu ZJ, Masuda Y, Inoue T, Fuchihata H, Sumida A, Takada K, et al. Coordination of cortically induced rhythmic jaw and tongue movements in the rabbit. J Neurophysiol. 1993;69:569–84. doi: 10.1152/jn.1993.69.2.569. [DOI] [PubMed] [Google Scholar]
- 13.Slaughter K, Li H, Sokoloff AJ. Neuromuscular organization of the superior longitudinalis muscle in the human tongue. 1: Motor endplate morphology and muscle fiber architecture. Cells Tissues Organs. 2005;181:51–64. doi: 10.1159/000089968. [DOI] [PubMed] [Google Scholar]
- 14.Stal P, Marklund S, Thornell LE, De Paul R, Eriksson PO. Fibre composition of human intrinsic tongue muscles. Cells Tissues Organs. 2003;173:147–61. doi: 10.1159/000069470. [DOI] [PubMed] [Google Scholar]
- 15.Liu ZJ, Green JR, Moore CA, Herring SW. Time series analysis of jaw muscle contraction and tissue deformation during mastication in miniature pigs. J Oral Rehabil. 2004;31:7–17. doi: 10.1111/j.1365-2842.2004.01156.x. [DOI] [PubMed] [Google Scholar]
- 16.Liu ZJ, Herring SW. Masticatory strains on osseous and ligamentous components of the temporomandibular joint in miniature pigs. J Orofac Pain. 2000;14:265–78. [PubMed] [Google Scholar]
- 17.Liu ZJ, Ikeda K, Harada S, Kasahara Y, Ito G. Functional properties of jaw and tongue muscles in rats fed a liquid diet after being weaned. J Dent Res. 1998;77:366–76. doi: 10.1177/00220345980770020501. [DOI] [PubMed] [Google Scholar]
- 18.Huang X, Zhang G, Herring SW. Effects of oral sensory afferents on mastication in the miniature pig. J Dent Res. 1993;72:980–6. doi: 10.1177/00220345930720061401. [DOI] [PubMed] [Google Scholar]
- 19.Herring SW. Mastication and maturity: a longitudinal study in pigs. J Dent Res. 1977;56:1377–82. doi: 10.1177/00220345770560111701. [DOI] [PubMed] [Google Scholar]
- 20.Herring SW. Animal models of temporomandibular disorders: how to choose. In: Sessle BJ, Bryant PS, Dionne RA, editors. Temporomandibular disorders and related pain conditions. IASP Press; Seattle: 1995. pp. 323–8. [Google Scholar]
- 21.Mu L, Sanders I. Neuromuscular specializations of the pharyngeal dilator muscles: I. Compartments of the canine geniohyoid muscle. Anat Rec. 1998;250:146–53. doi: 10.1002/(SICI)1097-0185(199802)250:2<146::AID-AR3>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 22.Palmer JB, Hiiemae KM, Liu J. Tongue-jaw linkages in human feeding: a preliminary videofluorographic study. Arch Oral Biol. 1997;42:429–41. doi: 10.1016/s0003-9969(97)00020-4. [DOI] [PubMed] [Google Scholar]
- 23.Hiiemae KM, Palmer JB. Food transport and bolus formation during complete feeding sequences on foods of different initial consistency. Dysphagia. 1999;14:31–42. doi: 10.1007/PL00009582. [DOI] [PubMed] [Google Scholar]
- 24.German RZ, Crompton AW, Owerkowicz T, Thexton AJ. Volume and rate of milk delivery as determinants of swallowing in an infant model animal (Sus scrofia) Dysphagia. 2004;19:147–54. doi: 10.1007/s00455-004-0001-x. [DOI] [PubMed] [Google Scholar]
- 25.Liu ZJ, Wang HY, Masuda Y, Morimoto T. A comparative study between cortically induced fictive mastication and actual mastication in acute and chronic rabbits. Zhonghua Kou Qiang Yi Xue Za Zhi. 1994;29:305–8. [PubMed] [Google Scholar]
- 26.Hylander WL, Johnson KR, Crompton AW. Loading patterns and jaw movements during mastication in Macaca fascicularis: a bone-strain, electromyographic, and cineradiographic analysis. Am J Phys Anthropol. 1987;72:287–314. doi: 10.1002/ajpa.1330720304. [DOI] [PubMed] [Google Scholar]
- 27.Thexton A, Hiiemae KM. The effect of food consistency upon jaw movement in the macaque: a cineradiographic study. J Dent Res. 1997;76:552–60. doi: 10.1177/00220345970760010501. [DOI] [PubMed] [Google Scholar]
- 28.Fuller DD, Williams JS, Janssen PL, Fregosi RF. Effect of co-activation of tongue protrudor and retractor muscles on tongue movements and pharyngeal airflow mechanics in the rat. J Physiol. 1999;519(Pt 2):601–13. doi: 10.1111/j.1469-7793.1999.0601m.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thexton AJ, McGarrick JD. Tongue movement of the cat during lapping. Arch Oral Biol. 1988;33:331–9. doi: 10.1016/0003-9969(88)90066-0. [DOI] [PubMed] [Google Scholar]
- 30.Takada K, Yashiro K, Sorihashi Y, Morimoto T, Sakuda M. Tongue, jaw, and lip muscle activity and jaw movement during experimental chewing efforts in man. J Dent Res. 1996;75:1598–606. doi: 10.1177/00220345960750081201. [DOI] [PubMed] [Google Scholar]
- 31.Liu ZJ, Yamagata K, Kasahara Y, Ito G. Electromyographic examination of jaw muscles in relation to symptoms and occlusion of patients with temporomandibular joint disorders. J Oral Rehabil. 1999;26:33–47. doi: 10.1046/j.1365-2842.1999.00356.x. [DOI] [PubMed] [Google Scholar]
- 32.Sun Z, Liu ZJ, Herring SW. Movement of temporomandibular joint tissues during mastication and passive manipulation in miniature pigs. Arch Oral Biol. 2002;47:293–305. doi: 10.1016/s0003-9969(02)00004-3. [DOI] [PubMed] [Google Scholar]
- 33.Thexton AJ, McGarrick JD. The electromyographic activities of jaw and hyoid musculature in different ingestive behaviours in the cat. Arch Oral Biol. 1994;39:599–612. doi: 10.1016/0003-9969(94)90136-8. [DOI] [PubMed] [Google Scholar]
- 34.Liu ZJ, Kayalioglu M, Shcherbatyy V, Seifi A. Tongue deformation, jaw movement and muscle activity during mastication in pigs. Arch Oral Biol. 2007;52:309–12. doi: 10.1016/j.archoralbio.2006.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vinyard CJ, Williams SH, Wall CE, Johnson KR, Hylander WL. Jaw-muscle electromyography during chewing in Belanger’s treeshrews (Tupaia belangeri) Am J Phys Anthropol. 2005;127:26–45. doi: 10.1002/ajpa.20176. [DOI] [PubMed] [Google Scholar]
- 36.Ross CF, Hylander WL. Electromyography of the anterior temporalis and masseter muscles of owl monkeys (Aotus trivirgatus) and the function of the postorbital septum. Am J Phys Anthropol. 2000;112:455–68. doi: 10.1002/1096-8644(200008)112:4<455::AID-AJPA4>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 37.Vinyard CJ, Wall CE, Williams SH, Johnson KR, Hylander WL. Masseter electromyography during chewing in ring-tailed lemurs (Lemur catta) Am J Phys Anthropol. 2006;130:85–95. doi: 10.1002/ajpa.20307. [DOI] [PubMed] [Google Scholar]
- 38.Liu ZJ, Shcherbatyy V, Kayalioglu M, Seifi A. Dimensional changes of the tongue during activation of hypoglossal nerves and contraction of tongue muscles. In: Davidovotch Z, Mah J, Suthanarak S, editors. Biological Mechanisms of Tooth Eruption, Resorption, and Movement. Harvard Society of Advanced Orthodontics, The University of Southern California; 2006. pp. 305–12. [Google Scholar]
- 39.Liu ZJ, Wang HY, Masuda Y, Morimoto T. Coordination of jaw, tongue and hyoid muscles during drinking and mastication in the awake rabbit. In: Morimoto T, Matsuya T, Takatda K, editors. Brain and oral function—oral motor function and dysfunction. Elsevier Science B.V; The Netherlands: 1995. pp. 597–600. [Google Scholar]
- 40.Napadow VJ, Chen Q, Wedeen VJ, Gilbert RJ. Biomechanical basis for lingual muscular deformation during swallowing. Am J Physiol. 1999;277:G695–701. doi: 10.1152/ajpgi.1999.277.3.G695. [DOI] [PubMed] [Google Scholar]
- 41.Thexton AJ, Crompton AW, German RZ. Transition from suckling to drinking at weaning: a kinematic and electromyographic study in miniature pigs. J Exp Zool. 1998;280:327–43. doi: 10.1002/(sici)1097-010x(19980401)280:5<327::aid-jez2>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 42.Herring SW, Scapino RP. Physiology of feeding in miniature pigs. J Morphol. 1973;141:427–60. doi: 10.1002/jmor.1051410405. [DOI] [PubMed] [Google Scholar]