Abstract
Cholinergic neurons respond to the administration of nerve growth factor (NGF) in vivo with a prominent and selective increase of choline acetyl transferase activity. This suggests the possible involvement of endogenous NGF, acting through its receptor TrkA, in the maintenance of central nervous system cholinergic synapses in the adult rat brain. To test this hypothesis, a small peptide, C(92-96), that blocks NGF-TrkA interactions was delivered stereotactically into the rat cortex over a 2-week period, and its effect and potency were compared with those of an anti-NGF monoclonal antibody (mAb NGF30). Two presynaptic antigenic sites were studied by immunoreactivity, and the number of presynaptic sites was counted by using an image analysis system. Synaptophysin was used as a marker for overall cortical synapses, and the vesicular acetylcholine transporter was used as a marker for cortical cholinergic presynaptic sites. No significant variations in the number of synaptophysin-immunoreactive sites were observed. However, both mAb NGF30 and the TrkA antagonist C(92-96) provoked a significant decrease in the number and size of vesicular acetylcholine transporter–IR sites, with the losses being more marked in the C(92-96) treated rats. These observations support the notion that endogenously produced NGF acting through TrkA receptors is involved in the maintenance of the cholinergic phenotype in the normal, adult rat brain and supports the idea that NGF normally plays a role in the continual remodeling of neural circuits during adulthood. The development of neurotrophin mimetics with antagonistic and eventually agonist action may contribute to therapeutic strategies for central nervous system degeneration and trauma.
Nerve growth factor (NGF) is the first well characterized member of a family of neurotrophic factors (NTFs) (1) that includes brain-derived neurotrophic factor, neurotrophin 3, and neurotrophin 4 (2, 3). These neurotrophins are known to regulate the survival, differentiation, and phenotypic maintenance of specific neuronal populations, but their role in neuronal plasticity is not fully understood. Investigations in newborn and adult rats have shown that cholinergic neurons in the corpus striatum and those in the basal forebrain projecting to the hippocampus and cortex respond to exogenous NGF with a selective and prominent increase of choline acetyl transferase (ChAT) activity (4–8). These areas are the major targets of ascending projections from cholinergic basal forebrain neurons that retrogradely transport NGF from these areas to the cholinergic cell bodies of the basal forebrain (9, 10). The intracerebral application of NGF prevents the down-regulation of cholinergic markers in septal cholinergic neurons after axotomy (11) and ameliorates both cholinergic and behavioral deficits after basalocortical lesions (12, 13).
Another cholinergic phenotype-specific protein is the vesicular acetylcholine transporter (VAChT) (14). This molecule mobilizes cytosolic acetylcholine (ACh) into the synaptic vesicle compartment. The rat VAChT gene is regulated in a coordinated fashion with ChAT (15, 16). As observed with ChAT activity, exogenous NGF injected in the brain increased VAChT expression in the septum (17).
NGF is expressed in the adult central nervous system (CNS), with the highest levels being present in the hippocampus and in the cerebral cortex and with the lowest levels being in the olfactory bulb (18–20). Its distribution suggests a regulating role for NGF of forebrain cholinergic neurons. Indeed, the application of anti-NGF antibodies inhibits cholinergic phenotype differentiation (21) and blocks the sprouting of acetylcholinesterase-positive branches in the deafferentiated hippocampus (22, 23).
Two receptors for NGF have been identified, namely a low affinity neurotrophin receptor, p75(LNTR), that binds all neurotrophins and a high affinity tyrosine kinase receptor, TrkA, that binds both NGF and neurotrophin 3 (24, 25). Although the receptor-binding domains of the neurotrophin molecules have yet to be fully elucidated, it is most likely that their β-turn (variable regions) are implicated (26). In previous studies, it was shown that a small, cyclic, conformationally constrained peptide, C(92-96), derived from the C-D β-turn region of NGF binds TrkA with an apparent Kd of 10−7 M and behaves as a competitive antagonist of NGF/TrkA interactions (27, 28).
In other studies, it also has been shown that exogenous NGF provokes the generation of new cholinergic synapses in the cerebral cortex (29). In the present study, therefore, we address the question of whether cholinergic synapses in the cerebral cortex of adult animals depend on endogenous NGF for their maintenance in the fully mature CNS. To investigate this, we blocked NGF function by using two different approaches: (i) the immunoneutralization of NGF and (ii) the blockade of TrkA receptors. For the first approach, we applied an anti-NGF monoclonal antibody, coded mAb NGF30, which previously has been shown to block NGF-induced effects in vitro (30). For the second approach, we used a putative TrkA antagonist, the cyclic peptide C(92-96). To validate this approach, we first assessed its effects on cholinergic phenotype in vitro on dissociated embryonic septal cells. These two compounds were infused into the cortex over a 2-week period, and their effects on the number of presynaptic elements (cholinergic and noncholinergic) 2 weeks after cessation of treatments were analyzed.
Our results show that both C(92-96) and mAb NGF30 are capable of modulating the number of cerebral cortex cholinergic presynaptic sites. The results would indicate that endogenous NTFs might play a role via TrkA receptors on the maintenance of the steady state number of synaptic sites in the adult, fully differentiated CNS.
MATERIALS AND METHODS
Animals.
Adult male Wistar rats, 340–360 g, were used in this study. All procedures followed the guidelines of the Canadian Council on Animal Care and were approved by the McGill University Animal Care Committee.
Materials.
In these experiments, we used a cyclic conformationally constrained peptide, C(92-96) [YCTDEKQCY, (27)], a control cyclic peptide (YCTNYGVCY), and an NGF monoclonal antibody (mAb NGF30) directed against the C termini of NGF that inhibits NGF-induced neurite outgrowth on PC12 cells and ChAT activity in primary septal cell cultures (30).
In Vitro
Septal Neuronal Cultures.
Cell cultures were established from the septal area of 17-day-old rat embryos by using procedures described by Debeir et al. (31). In brief, tissue was incubated in PBS containing trypsin and DNase. Tissue pieces then were mechanically dissociated. After centrifugation, the pellet was suspended in Leibovitz’s L-15 medium containing the chemical components described by Hefti et al. (32). Cells were plated onto 96-multiwell NUNC dishes (105 cells/well) coated with poly-d-lysine (5 μg/ml), and cells were grown for 5 days (days in vitro) at 37°C in a water-saturated air environment containing 5% CO2.
Cell Treatment.
Pure cultures of septal neurons were treated 1 day after plating. Drugs, prepared in medium, were added directly to the cells without changing the initial medium. The incubation was continued until 4, 6, and 8 DIV, at which time ChAT activity was evaluated.
Measurement of ChAT Activity.
At 4, 6, and 8 DIV, the medium was aspirated, and ice-cold lysis buffer (10 mM sodium phosphate, pH 7.4/0.1% Triton X-100) was added. ChAT activity assays were performed directly in the wells by using Fonnum’s method (33). In brief, after incubation at 37°C for 1 h with a solution containing 25 mM EDTA, 25 mM choline chloride, 500 μM acetyl-CoA, 125 mM sodium phosphate buffer (pH 7.4), 750 mM sodium chloride, [3H]acetyl-CoA, and 250 μM eserine or 0.125 units/μl acetylcholine esterase, ChAT activity was stopped by the addition of an excess of acetylcholine at 4°C. Then, [3H]acetylcholine was extracted with 20 mg/ml sodium tetraphenylboron in 3-heptanone.
In Vivo
Drug Treatment.
Animals were anaesthetized with 2.5 ml/kg Equithesin (sodium pentobarbital, 10 mg/ml; chloral hydrate, 40 mg/ml; magnesium sulfate) given i.p. Rats were implanted with cannula according to the following coordinates from Bregma (34): anterior/posterior, 1.3 mm; lateral 3 mm, vertical 2.2 mm (Hindlimb area). The cannula was connected to tubing filled with the drugs diluted in PBS (0.01 M). The tubing was connected to an Alzet (Palo Alto, CA) 2004 osmotic pump (0.25 μl/h) filled with dye (0.1% methylene blue) (35). The control peptide and the C(92-96) peptide were diluted to achieve a delivery rate of 30 and 28 μg, respectively, in 24 h. For mAb NGF30, this rate was 32 ng/day. Two weeks after implantation, the pumps and tubing were removed from anaesthetized rats. Rats were killed 2 weeks after treatments ended (4 weeks in total). To investigate the effects of C(92-96) and mAb NGF30, a total of 15 animals was used, divided into three groups of 5 animals each.
Perfusion and Fixation.
Rats were anaesthetized with Equithesin (2.5 ml/kg, i.p.) and were injected with heparin (4 USP/kg, i.p.) before perfusion. They were perfused briefly through the heart with perfusion buffer (for composition, see ref. 36) and then with 4% paraformaldehyde in 0.1 M phosphate buffer (pH 7.4) for 30 min. Subsequently, the brains were removed from their skulls and were postfixed in the same fixative for 2 h at 4°C, followed by 20% sucrose in phosphate buffer at 4°C for 3 days. The brains then were cut into 50-μm coronal sections with a sledge-freezing microtome (Leitz) at −20°C [between +0.2 mm and −2.8 mm to Bregma (34)]. Alternate sections from the same brain were used for immunohistochemical staining.
Synaptophysin and VAChT Immunostaining.
All immunohistochemical reactions were performed by using free-floating sections. PBS with Triton [0.01 M with 0.2% Triton X-100 (PBS+T)] was used for washing and diluting antibodies. To minimize background staining, all sections were preincubated in 5% normal goat serum (Sigma) (37°C, 30 min), and normal goat serum (2.5%) was added to all of the solutions containing antibodies. The sections were incubated with a mouse monoclonal antibody against synaptophysin (1:40, overnight) (Boehringer Mannheim) or with a rabbit polyclonal antibody against VAChT (1:10,000, 4°C, 72 h) (a gift of R. Edwards, University of California at San Francisco). After washing, the tissue was immersed in a goat anti-mouse IgG (1:160; 1 h, room temperature) (American Qualex, La Mirada, CA), followed by a monoclonal mouse antiperoxidase antibody (1:60) (Medicorp, Montreal) (37) with 5 μg/ml horseradish peroxidase (Sigma, type IV) (1h, room temperature) for synaptophysin staining. For VAChT staining, the tissue was incubated in a biotinylated goat anti-rabbit antibody (1:800, 2 h, room temperature) (Vector Laboratories) followed by an ABC reaction (1:400, 2 h) (Vector Laboratories). After several washes, the tissue was incubated in 0.6% diaminobenzidine (Sigma) in PBS+T (15 min, room temperature). Subsequently, H2O2 was added to the diaminobenzidine solution. After washing, the sections were mounted on gelatin-coated glass slides and were dehydrated and cover-slipped with Entellan (Merck). Omission of primary antibody served as control.
Morphometric Analysis
Quantification of the VAChT and Synaptophysin-Stained Presynaptic Boutons.
To count the immunoreactive punctae, a BH-2 Olympus (New Hyde Park, NY) microscope, equipped with a 100× oil immersion plan achromatic objective and a 10× projection lens, was used. The microscope was connected to an MCID-M4 image-analysis system (Imaging Research, St. Catharines, ON, Canada). The immunopositive punctae (presynaptic boutons and varicosities) were detected by the image analysis system by using software devised for silver grain counting. Measurements were performed on a single focal plane.
For synaptophysin-immunoreactive (IR) counting, segmentation values were selected, following a trial and error method, on the basis of those that would provide the most accurate measurements when compared with the direct visual counting of punctae on the computer screen. The number of presynaptic elements was counted in individual sections. All cell bodies, blood vessels, and cortical tissue that were not in focus were excluded. All measurements were done in the hindlimb and in parietal I and II areas of the cortex. These areas represent, respectively, cortical tissue proximal, intermediate, and distal to the cannula implantation. Twelve 8,500-μm2 sections were digitized from five consecutive sections in each cortical area of each rat (five animals per group). From these values, the number of detectable presynaptic boutons per 1,000 μm2 was calculated in each area. The same approach, without segmentation, was used to count VAChT-IR.
Measurement of VAChT-Positive Cell Size in the Nucleus Basalis.
The VAChT immunoreactive neurons of the nucleus basalis magnocellularis (NBM) were visualized and analyzed with the same system that was used for the presynaptic boutons, with a 20× objective. For analysis of the NBM, a total of six sections from the midbasalis were taken from each animal as described (12). Three random fields for each level were quantified. Results were expressed as mean cross sectional area of the cholinergic cell bodies ± SEM.
Statistical Analysis.
Results are given as mean ± SEM. Statistical analyses were performed by using Duncan’s multiple range test for the in vitro studies. To compare the effects of drugs on the number of boutons, two way ANOVA was used. For cell bodies, statistical significance was obtained by using ANOVA and Newman-Keuls post hoc analysis.
RESULTS
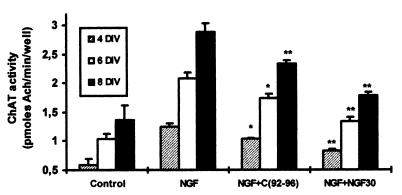
Substantial ChAT activity could be detected in septal neurons maintained 4, 6, and 8 days in a defined culture medium. This activity was increased ≈2-fold when cells were cultured with NGF (1 ng/ml). The TrkA antagonist C(92-96) and mAb NGF30, per se, did not provoke any effect on either ChAT activity or on cell density (data not shown). However, both C(92-96) and mAb NGF30 prevented the up-regulation of ChAT activity induced by 1 ng/ml of NGF (Fig. 1). Molar concentrations 106-fold higher than those of NGF were required for the optimal blockade of neurotrophin activity by C(92-96). These high concentrations are in line with the previous observations on NGF-induced PC12 cells (27) and with other trophic responses applying analogues (31). After 4, 6, and 8 DIV, C(92-96) significantly decreased the NGF-induced ChAT activity (respectively, -33, -33, and -37%).
Figure 1.
Comparative effects of C(92-96) and mAb NGF30 on NGF-increased cholinergic activity of septal neurons. NGF (1 ng/ml), C(92-96) (100 μg/ml), and/or mAb NGF 30 (10 ng/ml) were added to the culture medium 1 day after plating. ChAT activity was measured on the fourth, sixth and eighth days after plating (4, 6, and 8 DIV). Results are means ± SEM of four different experiments, ∗, P < 0.05 vs. NGF alone; ∗∗, P < 0.01 vs. NGF alone.
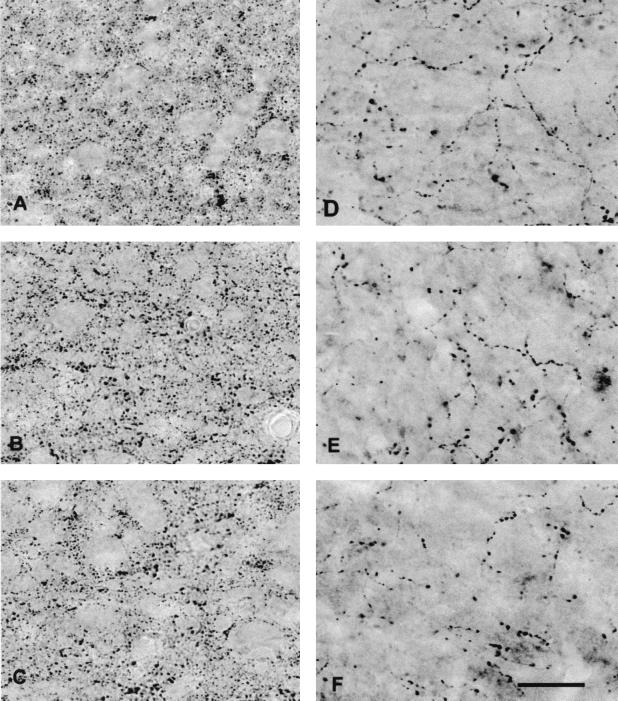
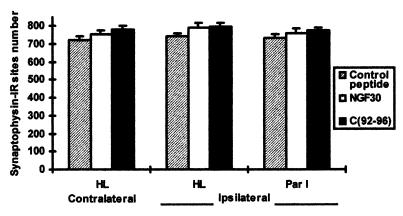
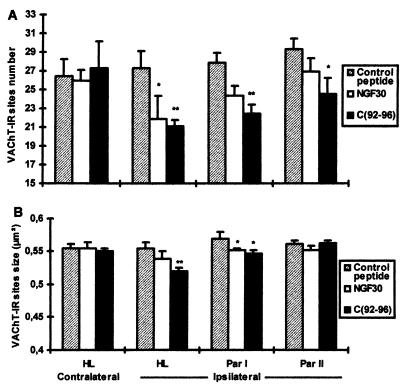
The investigation of the in vivo paradigm revealed that synaptophysin immunoreactivity was restricted to small punctae (Fig. 2) representing the overall population of presynaptic boutons independent of their transmitter content. The blockade of NGF function by the application of either the NGF mAb or the TrkA antagonist did not provoke any significant variations in the number of synaptophysin-IR sites (Figs. 2 and 3). In contrast, the application of mAb NGF30 and C(92-96) provoked a significant ipsilateral depletion in the number of VAChT-IR sites (Figs. 2 and 4A). Furthermore, a significant decrease of the size of the cholinergic boutons (VAChT-IR) was observed in the ipsilateral cortex of the treated rats receiving either mAb NGF30 or C(92-96) as compared with control peptide (Fig. 4B). The decrease in the number of VAChT-IR sites and the shrinkage of bouton size were rather similar for both treatments (antagonist and antibody) proximal to the cannula implantation (hindlimb) (see Fig. 4 A and B). However, the effects of C(92-96) remained evident in cortical areas far away from the cannula implantation. This result is consistent with our observation that 125I-labeled C(92-96) migrates from the site of injection more than the antibody in the rat brain after 2 days postdelivery intracerebroventricular (data not shown). We hypothesize that small (≈1 kDa) peptides penetrate the brain parenchyma better than the larger antibodies (≈150 kDa). The application of the control cyclic peptide did not produce any noticeable effect on boutons or on synaptophysin-IR or VAChT-IR in the ipsilateral cortex (Figs. 3 and 4 A and B). This result would indicate that the microlesion induced by the cannula and the delivery of the compound per se did not provoke any unspecific damage or modification of the cholinergic phenotype in the treated cortices.
Figure 2.
Synaptophysin (A, B, and C) and VAChT (D, E, and F) immunoreactivity in the ipsilateral hindlimb cortex of control peptide (A and D)-, mAb NGF30 (B and E)-, and C(92-96) (C and F)-infused animals. Note the apparent reduction in the number of VAChT-IR in E and F compared with D. (Bar = 20 μm.)
Figure 3.
Effect of cortical injections of C(92-96) and mAb NGF30 on the number of synaptophysin-IR sites in the hindlimb and in the parietal I cortex. The drugs were injected into the hindlimb cortex as indicated in Materials and Methods. Twelve 8,500-μm2 sections were digitized from five consecutive sections in each cortical area of each rat (five animals per group). The number of synaptophysin-IR sites in 1,000 μm2 is expressed as the mean ± SEM. HL, hindlimb; Par I, parietal I.
Figure 4.
Effect of cortical injection of C(92-96) and mAb NGF30 on the number (A) and on the size (B) of VAChT-IR sites in the hindlimb and in the parietal I and II cortices. Note in A the loss of cholinergic boutons (VAChT-IR), which is more prominent with the TrkA antagonist C(92-96) and is related to the distance from the injection site; B illustrates the shrinkage of the cholinergic boutons after the administration of both compounds. The drugs were injected into the hindlimb cortex as indicated in Materials and Methods. Twelve 8,500-μm2 sections were digitized from five consecutive sections in each cortical area of each rat (five animals per group). The number of VAChT-IR sites (A) in 1,000 μm2 is expressed as the mean ± SEM. The size of VAChT-IR sites (B) (in square micrometers) is expressed as the mean ± SEM. HL, hindlimb; Par II, parietal II. ∗, P < 0.05 vs. each control peptide area; ∗∗, P < 0.01 vs. each control peptide area.
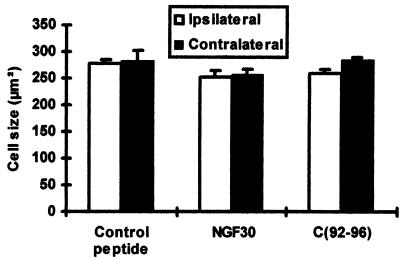
Besides the steady state level of the cholinergic cortical network, we investigated the effects of C(92-96) and of mAb NGF30 on the cell size of the cholinergic NBM neurons (Fig. 5). Under the present experimental conditions, we found no change in the mean cross-section area of NBM cholinergic neurons when compared with the control peptide-treated animals.
Figure 5.
Cortical injections of C(92-96) and mAb NGF30 did not affect the size of VAChT positive neurons of the nucleus basalis. The drugs were injected into the hindlimb cortex as indicated in Materials and Methods. Six sections from the midbasalis were taken from each animal (five animals in each group). Three random fields from each level were quantified. The size of VAChT neurons (in square micrometers) is expressed as the mean ± SEM.
DISCUSSION
The analysis of mice lacking NGF (38) or its signaling TrkA receptors (39, 40) has confirmed that certain PNS neurons require neurotrophin signaling in vivo for their embryonic survival. It also has been shown that TrkA signaling is important for the maturation and for the survival of CNS cholinergic neurons during development (41, 42). The present paper shows that, in the already developed, adult brain, both the immunoneutralization of NGF and the blockade of TrkA receptors cause a selective loss of preexisting cholinergic presynaptic sites in the cerebral cortex of adult animals. The fact that synaptophysin sites were not significantly affected by the “mopping out” of endogenous NGF or by the occupancy of its high affinity receptor sites stresses the specificity of this neurotrophin in the maintenance of the terminal synaptic network in the cerebral cortex.
The results observed support the hypothesis that neurotrophic factors continue modulating the neural phenotype during adulthood, including the regulation of size, location and number of synapses. The results observed in the basalocortical systems are consistent with those of Vantini et al. (21), who showed that the administration of an anti-NGF antibody in the ventricle decreased ChAT activity in the cerebral cortex and in the hippocampus.
It is well established that neurotrophins are responsible for inducing synaptogenesis during development (43) and that exogenously applied neurotrophins in the mature and fully differentiated CNS are capable of generating new synapses in the cerebral cortex of adult animals (29, 44). Furthermore, NGF has been shown to produce a broad increase in synaptophysin in aged rats (45) and in cortically lesioned primates (46) and an increase in VAChT mRNA expression in neonatal rats (17). Our results would indicate that, in normal situations, the endogenous NGF levels are sufficient to regulate only cholinergic synapses without affecting other neurotransmitter systems. Therefore, a distinction has to be made between the physiological and pharmacological effects of this neurotrophin.
The target-derived actions of NGF in the CNS are unquestionable. For instance, there is robust evidence for a retrograde transport mechanism for NGF by NBM neurons projecting to the neocortex (47). Furthermore, the granular immunoreactivity to NGF observed in NBM neurons substantially decreases after the blockade of axonal transport by colchicine (48). The fact that no reduction in NBM cholinergic neuron size was observed after the occupation of TrkA receptors sites or the immunoneutralization of NGF in the cortex can be explained on general grounds: It is likely that a more sustained and drastic reduction of the neurotrophic signal is required to observe noticeable changes in the cell somata of NGF-dependent neurons. This has been well illustrated for the septohippocampal cholinergic system, where massive destruction of the hippocampal neurons has been achieved by the application of N-methyl-d-aspartate with a reduction in size but not the number of septal ChAT-IR neurons (49).
Furthermore, little is known about the autocrine and paracrine effects of neurotrophins in the CNS, although some evidence has been advanced for brain-derived neurotrophic factor (50). In the basalocortical cholinergic system, it has been shown that NGF levels increase in the NBM region after cortical lesions (51) and that NGF synthesis can occur in the basal forebrain (52). Furthermore, we have shown that, in the rat brain, forebrain cholinergic neurons are responsive to a local administration of NGF (53). This indicates that TrkA receptors present in the somatodendritic region of NBM cholinergic neurons are functional and capable of modulating neuronal phenotype in the adult CNS.
What is the significance of growth factor dependency for the maintenance of synaptic contacts in the fully mature CNS? For a long time, it has been suspected that CNS synaptic connections do not remain immutable. Hebb postulated that the strength of synaptic connections was conditional to use and that a growth process takes place with improved synaptic efficacy (54). These ideas can be revisited today in light of neurotrophic factor theory. There is strong experimental evidence for an activity-dependent synthesis and release of neurotrophins in the CNS (3, 55, 56). Such a process would imply that activated neurons could release NTFs acting on NTF-sensitive nerve terminals impinging on such neurons. The results of such neuron-to-neuron trophic communication would be the reinforcement of the synaptic connection, whereby the specific NTF would determine location, size, and number of presynaptic contacts terminated on the NTF releasing neuron. From this, the idea that disuse—or, in the present experiments, the neutralization of endogenous NGF or TrkA blockade—would result in the withdrawal or loss of preexisting synaptic contacts may be derived. Such a situation would be consistent with the evidence that sensory stimulation results in an increased number of cortical synapses (57) and that higher levels of education are a protective factor against Alzheimer’s disease (58). Our experiment thus supports the notion that receptor-mediated mechanisms, such as those studied here, are ultimately responsible for the day-to-day maintenance and remodeling of cortical synaptic contacts. The manipulation of these endogenous mechanisms could have a bearing on the rate of decline of higher CNS functions observed in aging and neurodegenerative diseases.
Acknowledgments
This work was supported by grants from the Medical Research Council (Canada) to A.C.C. and H.U.S. T.D. was the recipient of a Lavoisier fellowship from the Government of France.
ABBREVIATIONS
- NGF
nerve growth factor
- NTF
neurotrophic factor
- ChAT
choline acetyl transferase
- ACh
acetylcholine
- VAChT
vesicular acetylcholine transporter
- CNS
central nervous system
- IR
immunoreactive
- NBM
nucleus basalis magnocellularis
References
- 1.Levi-Montalcini R. Science. 1987;237:1154–1162. doi: 10.1126/science.3306916. [DOI] [PubMed] [Google Scholar]
- 2.Lindsay R M, Wiegand S J, Altar C A, DiStefano P S. Trends Neurosci. 1994;17:182–190. doi: 10.1016/0166-2236(94)90099-x. [DOI] [PubMed] [Google Scholar]
- 3.Thoenen H. Science. 1995;270:593–598. doi: 10.1126/science.270.5236.593. [DOI] [PubMed] [Google Scholar]
- 4.Fusco M, Oderfeld-Nowak B, Vantini G, Schiavo N, Gradkowska M, Zaremba M, Leon A. Neuroscience. 1989;33:47–52. doi: 10.1016/0306-4522(89)90309-6. [DOI] [PubMed] [Google Scholar]
- 5.Gnahn H, Hefti F, Heumann R, Schwab M E, Thoenen H. Dev Brain Res. 1983;9:45–52. doi: 10.1016/0165-3806(83)90107-4. [DOI] [PubMed] [Google Scholar]
- 6.Johnston H V, Rutkowski J L, Wainer B H, Long J B, Mobley W C. Neurochem Res. 1987;12:985–994. doi: 10.1007/BF00970927. [DOI] [PubMed] [Google Scholar]
- 7.Mobley W C, Rutkowski J L, Tennekoon G I, Buchanana K, Johnson M W. Science. 1985;229:284–287. doi: 10.1126/science.2861660. [DOI] [PubMed] [Google Scholar]
- 8.Mobley W C, Rutkowski J L, Tennekoon G, Gemski J, Buchanan K, Johnston H V. Mol Brain Res. 1986;1:53–62. doi: 10.1016/0169-328x(86)90020-3. [DOI] [PubMed] [Google Scholar]
- 9.DiStefano P S, Friedman B, Radziejewski C, Alexander C, Boland P, Schick C M, Lindsay R M, Wiegand S J. Neuron. 1992;8:983–993. doi: 10.1016/0896-6273(92)90213-w. [DOI] [PubMed] [Google Scholar]
- 10.Seiler M, Schwab M E. Brain Res. 1984;300:34–39. doi: 10.1016/0006-8993(84)91338-6. [DOI] [PubMed] [Google Scholar]
- 11.Hefti F. J Neurosci. 1986;6:2155–2162. doi: 10.1523/JNEUROSCI.06-08-02155.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garofalo L, Cuello A C. Exp Neurol. 1994;125:195–217. doi: 10.1006/exnr.1994.1024. [DOI] [PubMed] [Google Scholar]
- 13.Cuello A C, Garofalo L, Kenigsberg R L, Maysinger D. Proc Natl Acad Sci USA. 1989;86:2056–2060. doi: 10.1073/pnas.86.6.2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Usdin T B, Eiden L E, Bonner T I, Erickson J D. Trends Neurosci. 1995;18:218–224. doi: 10.1016/0166-2236(95)93906-e. [DOI] [PubMed] [Google Scholar]
- 15.Berrard S, Varoqui H, Cervini R, Israel M, Mallet J, Diebler M F. J Neurochem. 1995;65:939–942. doi: 10.1046/j.1471-4159.1995.65020939.x. [DOI] [PubMed] [Google Scholar]
- 16.Erickson J D, Varoqui H, Schafer M K, Modi W, Diebler M F, Weihe E, Rand, Eiden L E, Bonner T I, Usdin T B. J Biol Chem. 1994;269:21929–21932. [PubMed] [Google Scholar]
- 17.Tian X T, Sun X Y, Suszkiw J B. Neurosci Lett. 1996;209:134–136. doi: 10.1016/0304-3940(96)12629-x. [DOI] [PubMed] [Google Scholar]
- 18.Conner J M, Muir D, Varon S, Hagg T, Manthorpe M. J Comp Neurol. 1992;319:454–462. doi: 10.1002/cne.903190310. [DOI] [PubMed] [Google Scholar]
- 19.Korsching S, Auburger G, Heumann R, Scott J, Thoenen H. EMBO J. 1985;4:1389–1393. doi: 10.1002/j.1460-2075.1985.tb03791.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Whittemore S R, Ebendal T, Lärkfors L, Olson L, Seiger Å, Strömberg I, Persson H. Proc Natl Acad Sci USA. 1986;83:817–821. doi: 10.1073/pnas.83.3.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vantini G, Schiavo N, DiMartino A, Polate P, Triban C, Callegro L, Toffano G, Leon A. Neuron. 1989;3:267–273. doi: 10.1016/0896-6273(89)90251-1. [DOI] [PubMed] [Google Scholar]
- 22.Van der Zee C E, Rashid K, Le K, Moore K A, Stanisz J, Diamond J, Racine R J, Fahnestock M. J Neurosci. 1995;15:5316–5323. doi: 10.1523/JNEUROSCI.15-07-05316.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Van der Zee C E E M, Fawcett J, Diamond J. J Comp Neurol. 1992;326:91–100. doi: 10.1002/cne.903260108. [DOI] [PubMed] [Google Scholar]
- 24.Chao M V. J Neurobiol. 1994;25:1373–1385. doi: 10.1002/neu.480251106. [DOI] [PubMed] [Google Scholar]
- 25.Kaplan D R, Stephens R M. J Neurobiol. 1994;25:1404–1417. doi: 10.1002/neu.480251108. [DOI] [PubMed] [Google Scholar]
- 26.Nanduri J, Vroegop S M, Buxser S E, Neet K E. J Neurosci Res. 1994;37:433–444. doi: 10.1002/jnr.490370402. [DOI] [PubMed] [Google Scholar]
- 27.LeSauteur L, Wei L, Gibbs B F, Saragovi H U. J Biol Chem. 1995;270:6564–6569. doi: 10.1074/jbc.270.12.6564. [DOI] [PubMed] [Google Scholar]
- 28.LeSauteur L, Cheung N K V, Lisbona R, Saragovi H U. Nat Biotechnol. 1996;14:1120–1122. doi: 10.1038/nbt0996-1120. [DOI] [PubMed] [Google Scholar]
- 29.Garofalo L, Ribeiro-da-Silva A, Cuello A C. Proc Natl Acad Sci USA. 1992;89:2639–2643. doi: 10.1073/pnas.89.7.2639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kenigsberg R L, Elliott P J, Cuello A C. J Immunol Methods. 1991;136:247–257. doi: 10.1016/0022-1759(91)90011-4. [DOI] [PubMed] [Google Scholar]
- 31.Debeir T, Benavides J, Vige X. Brain Res. 1996;708:159–166. doi: 10.1016/0006-8993(95)01237-0. [DOI] [PubMed] [Google Scholar]
- 32.Hefti F, Hartikka J J, Eckenstien F, Gnahn H, Heumann R, Schwab M. Neuroscience. 1985;14:55–68. doi: 10.1016/0306-4522(85)90163-0. [DOI] [PubMed] [Google Scholar]
- 33.Fonnum F. J Neurochem. 1975;24:407–409. doi: 10.1111/j.1471-4159.1975.tb11895.x. [DOI] [PubMed] [Google Scholar]
- 34.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Sydney: Academic; 1986. [DOI] [PubMed] [Google Scholar]
- 35.Vahlsing H L, Varon S, Hagg T, Fass-Holmes B, Dekker A, Manley M, Manthorpe M. Exp Neurol. 1989;150:233–243. doi: 10.1016/0014-4886(89)90125-8. [DOI] [PubMed] [Google Scholar]
- 36.Côté S, Ribeiro-da-Silva A, Cuello A C. In: Current Protocols for Light Microscopy Immunocytochemistry. Cuello A C, editor. New York: Wiley; 1993. pp. 147–168. [Google Scholar]
- 37.Semenenko F M, Bramwell S, Sidebottom E, Cuello A C. Histochemistry. 1985;83:405–408. doi: 10.1007/BF00509200. [DOI] [PubMed] [Google Scholar]
- 38.Crowley C, Spencer S D, Nishimura M C, Chen K S, Pitts M, Armanini M P, Ling L H, MacMahon S B, Shelton D L, Levinson A D. Cell. 1994;76:1001–1011. doi: 10.1016/0092-8674(94)90378-6. [DOI] [PubMed] [Google Scholar]
- 39.Smeyne R J, Klein R, Schnapp A, Long L K, Bryant S, Lewin A, Lira S A, Barbacid M. Nature (London) 1994;368:246–249. doi: 10.1038/368246a0. [DOI] [PubMed] [Google Scholar]
- 40.Fagan A M, Zhang H, Landis S, Smeyne R J, Silos S, Barbacid M. J Neurosci. 1996;16:6208–6218. doi: 10.1523/JNEUROSCI.16-19-06208.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fagan A M, Garber M, Barbacid M, Silos-Santiago I, Holtzman D M. J Neurosci. 1997;17:7644–7654. doi: 10.1523/JNEUROSCI.17-20-07644.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li Y, Holtzman D M, Kromer L F, Kaplan D R, Chua-Couzens J, Clary D O, Knüsel B, Mobley W C. J Neurosci. 1995;15:2888–2905. doi: 10.1523/JNEUROSCI.15-04-02888.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lewin G R, Barde Y A. Annu Rev Neurosci. 1996;19:289–317. doi: 10.1146/annurev.ne.19.030196.001445. [DOI] [PubMed] [Google Scholar]
- 44.Cuello A C, Garofalo L, Liberini P, Maysinger D. Prog Brain Res. 1994;101:337–355. doi: 10.1016/s0079-6123(08)61961-5. [DOI] [PubMed] [Google Scholar]
- 45.Chen K S, Masliah E, Mallory M, Gage F H. Neuroscience. 1995;68:19–27. doi: 10.1016/0306-4522(95)00099-5. [DOI] [PubMed] [Google Scholar]
- 46.Burgos I, Cuello A C, Liberini P, Pioro E P, Masliah E. Brain Res. 1995;692:154–160. doi: 10.1016/0006-8993(95)00696-n. [DOI] [PubMed] [Google Scholar]
- 47.Seiler M, Schwab M E. Brain Res. 1984;300:33–39. doi: 10.1016/0006-8993(84)91338-6. [DOI] [PubMed] [Google Scholar]
- 48.Conner J M, Varon S. J Comp Neurol. 1992;326:347–362. doi: 10.1002/cne.903260304. [DOI] [PubMed] [Google Scholar]
- 49.Sofroniew M V, Galletly N P, Isacson O, Svenden C N. Science. 1990;247:338–342. doi: 10.1126/science.1688664. [DOI] [PubMed] [Google Scholar]
- 50.Acheson A, Conover J C, Fandl J P, DeChiara T M, Russell M, Thadani A, Squinto S P, Yancopoulos G D, Lindsay R M. Nature (London) 1995;374:450–453. doi: 10.1038/374450a0. [DOI] [PubMed] [Google Scholar]
- 51.Lorez H, vonFrankenber M, Weskamp G, Otten U. Brain Res. 1988;454:355–360. doi: 10.1016/0006-8993(88)90837-2. [DOI] [PubMed] [Google Scholar]
- 52.Lauterborn J C, Bizon J L, Tran T M D, Gall C M. J Comp Neurol. 1995;360:454–462. doi: 10.1002/cne.903600307. [DOI] [PubMed] [Google Scholar]
- 53.Hu L, Cote S L, Cuello A C. Exp Neurol. 1997;143:162–171. doi: 10.1006/exnr.1996.6357. [DOI] [PubMed] [Google Scholar]
- 54.Hebb C O. The Organization of Behavior: A Neuropsychological Theory. New York: Wiley; 1949. [Google Scholar]
- 55.Zafra F, Hengerer B, Leibrock J, Thoenen H, Lindholm D. EMBO J. 1990;9:3545–3550. doi: 10.1002/j.1460-2075.1990.tb07564.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lindholm D, Castren E, Berzaghi M, Blochl A, Thoenen H. J Neurobiol. 1994;25:1362–1372. doi: 10.1002/neu.480251105. [DOI] [PubMed] [Google Scholar]
- 57.Kilgard M P, Merzenich M M. Science. 1998;279:1714–1718. doi: 10.1126/science.279.5357.1714. [DOI] [PubMed] [Google Scholar]
- 58.Evans D A, Hebert L E, Beckett L A, Scherr P A, Albert M S, Chown M J, Pilgrim D M, Taylor J O. Arch Neurol (Chicago) 1997;54:1399–1405. doi: 10.1001/archneur.1997.00550230066019. [DOI] [PubMed] [Google Scholar]