Abstract
Neuronal connections are arranged topographically such that the spatial organization of neurons is preserved by their termini in the targets. During the development of topographic projections, axons initially explore areas much wider than the final targets, and mistargeted axons are pruned later. The molecules regulating these processes are not known. We report here that the ligands of the Eph family tyrosine kinase receptors may regulate both the initial outgrowth and the subsequent pruning of axons. In the presence of ephrins, the outgrowth and branching of the receptor-positive hippocampal axons are enhanced. However, these axons are induced later to degenerate. These observations suggest that the ephrins and their receptors may regulate topographic map formation by stimulating axonal arborization and by pruning mistargeted axons.
Topographic neuronal projections are regulated, at least in part, by complementary gradients of the Eph family tyrosine kinase receptors and ligands expressed in the pre- and postsynaptic neurons (1–14). During the development of hippocamposeptal topographic projections, the Eph family receptor EphA5 is expressed in a lateral (low) to medial (high) gradient in the hippocampus (13), whereas three ligands of the receptor, ephrin-A2, A3, and A5, are transcribed in the major subcortical hippocampal target, the lateral septum, in a dorsomedial-to-ventrolateral gradient (13, 14). In the mature hippocamposeptal topographic map, medial hippocampal neurons, which express high levels of EphA5, project to the ligand-poor dorsomedial target. In contrast, the lateral hippocampal neurons, which express low levels of the Eph receptor, send axons to the ligand-rich ventrolateral target (13–16). The opposing gradients of the receptor and ligand expression suggest that the receptor–ligand interaction contributes to the development of the hippocamposeptal topographic map by negatively regulating axonal growth of receptor-positive medial hippocampal neurons. Similar ligand–receptor gradients also exist in retinotectal system and are proposed to map retinal ganglion axons along the rostrocaudal and dorsoventral axes of the tectum (1–12, 17–20).
In vitro studies using coculture assays in which hippocampal neurons are exposed to the ephrin-expressing cells showed that ephrin-A2 selectively inhibits neurite outgrowth of receptor-rich medial hippocampal neurons but has little effects on the growth of the lateral neurites (13). In addition, temporal retina axons, which express high levels of EphA3, a different member of the Eph family, are repelled by ephrin-A2- and A5-containing membrane extracts in stripe assays, in which the extracts with or without the ephrins are laid side by side in narrow stripes (8, 9). In contrast, the ephrins have a much weaker repulsive activity against the nasal retina axons, which do not express high levels of the receptor. These in vitro analyses, together with in vivo expression patterns, indicate that the ephrins negatively regulate growth of receptor-positive axons. It has been proposed that such negative interaction between the receptors and ligands expressed in gradients in the pre- and postsynaptic fields helps to establish topographic maps (1–14, 17–20). Axons with certain concentrations of receptors may terminate in targets with matching concentrations of ligands. Axon termini that grow to areas with inappropriately high ligand concentrations may be repelled.
However, in vivo tracing studies in both chicken and rat showed that the initial outgrowth of most axons goes far beyond the proper target zones (21–23). Retinal axons mistarget widely along both the rostrocaudal and dorsoventral axes, even in the presence of appropriate ephrin and Eph receptor gradients in the retina and tectum (8, 9, 21–23). (In lower vertebrates, the targeting appears to be more accurate.) Topographic order is achieved only later during development by elimination of mistargeted axons and branches and by an increase in branching and arborization at proper target zones (21–23). The discrepancy between axonal behavior in vivo and in vitro suggests that ephrins may not function as simple axonal repellents during map development. To elucidate further how ephrins affect axonal behavior, we examined the dynamics of growth and branching of hippocampal axons after exposure to the ligands.
MATERIALS AND METHODS
Expression of Ephrin-A2, A3, and A5.
Full-length mouse ephrin-A2 and human ephrin-A3 and A5 were cloned into a retroviral vector pLIG, which contains a β-galactosidase gene fused to an aminoglycoside phosphotransferase for G418 resistance (24). The constructs then were transfected into NIH 3T3 cells. G418-resistant colonies were selected and screened for receptor binding by using an alkaline phosphatase-tagged EphA5 extracellular domain fusion protein (EphA5-AP) (13). Positive EphA5-AP binding was found in the ligand-transfected cells. In contrast, no significant binding was observed in parental or vector-transfected control NIH 3T3 cells.
Neurite Outgrowth Assay.
For assaying the effects of ephrins on the growth of neurites, medial or lateral hippocampal neurons were dissected from embryonic day (E) 18 Sprague–Dawley rat fetuses. The hippocampus was separated from the cerebral cortex and brain stem by using a no. 11 surgical blade (Becton Dickinson) under a microscope. The medial hippocampal neurons used in this study were derived from the medial most one-third of the hippocampus. The lateral hippocampal neurons were from the lateral most one-fourth of the hippocampus. Dissected tissues were dissociated to single neurons by gentle trituration. Dissociated neurons were plated in 12-well dishes (1 × 105 cells per well) preseeded with a confluent monolayer of ephrin-expressing or control NIH 3T3 cells in DMEM supplemented with fetal bovine serum (10%), penicillin (50 μg/ml), and streptomycin (50 μg/ml). Cells usually were grown for 48 hr unless indicated otherwise. After incubation, cultures were fixed in 4% paraformaldehyde in PBS and stained with anti-neuron-specific enolase (NSE; 1:500 dilution; Chemicon) or anti-tau-1 (1:500 dilution; Boehringer Mannheim) antibodies with Vectastain ABC kit (Vector Laboratories).
RESULTS
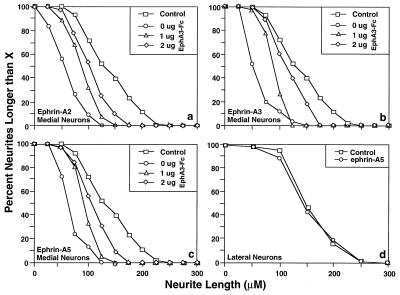
To examine the biological response of the hippocampal neurons to the ligands expressed in the septal target, ephrin-A2, A3, and A5 were expressed in NIH 3T3 cells. Medial and lateral hippocampal neurons were cocultured with cells expressing each of the three ligands. The growth of neurites was examined by immunocytochemical staining with NSE antibody for 48–96 hr. The medial hippocampal neurons had significantly shorter neurites when cocultured with each of the ligand-expressing cells than when cocultured with control cells transfected with only the expression vector (Figs. 1 and 2 a and b). This inhibitory effect on medial hippocampal neurite outgrowth specifically was a result of ligands expressed on cell surfaces because a competitive inhibitor, the ligand-binding domain of EphA3 fused to the IgG Fc domain (EphA3-Fc), which competes for ligand-binding, effectively reduced the inhibitory effects of the ligand-expressing cells (Fig. 1). In contrast, neurite outgrowth of lateral hippocampal neurons was comparable to control when cocultured with the ligand-expressing cells (Fig. 1d). These observations indicate that the ephrins can discriminate between hippocampal neurons of different spatial origin and specifically reduce the length of topographically inappropriate medial neurites.
Figure 1.
Ephrins inhibit the growth of medial hippocampal neurites. Medial or lateral hippocampal neurons were cocultured with a confluent monolayer of NIH 3T3 cells expressing each of the three ligands ephrin-A2, A3, and A5, or with vector-transfected NIH 3T3 cells (controls). Neurons were detected with anti-NSE. All three ligands reduce the length of the neurites of the medial hippocampal neurons. The inhibitory effects were reversed partially by inclusion of the competitive inhibitor EphA3-Fc in the tissue culture medium. The concentrations of EphA3-Fc used are indicated in the Insets. EphA3-Fc had no significant effects on the control cells.
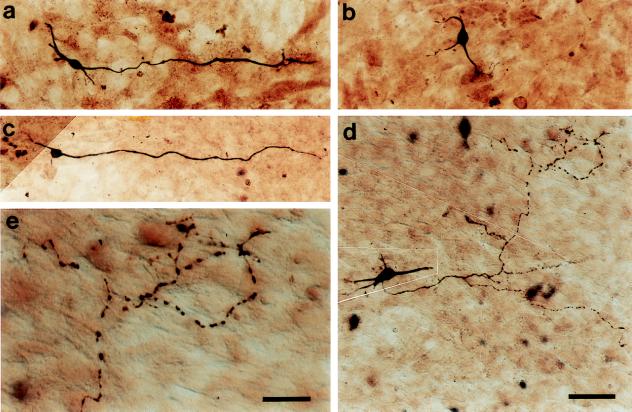
Figure 2.
Ephrins promote growth and branching and induce fragmentation of the medial hippocampal axons. Medial hippocampal neurons from E18 rat embryos were dissected and cocultured for 96 hr. (a and b) Medial hippocampal neurons cocultured with control or ephrin-A5-expressing cells. Neurons were detected with anti-NSE antibody. The neurons grew long and thick axons on the control cells (a), but only very short neurites on the ephrin-A5-expressing cells (b). (c and d) Medial hippocampal neurons cultured on control and ephrin-A5 cells, respectively, and stained with anti-tau antibody. Long, fragmented axons with numerous branches were observed on the ephrin-A5 cells with anti-tau antibody (d). Note that anti-NSE and anti-tau revealed similar neurons with long axons on the control cells. However, on ephrin-A5 cells, anti-NSE detected neurons with only much shorter neurites, in contrast to anti-tau antibody. (e) A higher magnification of a degenerated axon on the ligand-expressing cells. Numerous gaps were observed along the axons. [Bars = 37.5 μm (a–d) and 18.75 μm (e).]
To determine the effects of ephrins specifically on axons, cocultured neurons were stained with a mAb against tau-1, an axon-specific marker (25–28). The medial hippocampal neurons grew long and thick axons when cultured on the control cells (Fig. 2c). Surprisingly, the neuritic length on the ligand-expressing cells appeared significantly longer than on the control cells in contrast to that revealed by anti-NSE staining. Furthermore, the number of axonal branches was also significantly larger than the control (Figs. 2 d and e and 3). However, unlike axons on the control cells, which were straight and continuous, axons on the ligand-expressing cells became fragmentary, suggesting that degeneration had occurred (Figs. 2 d and e and 3). Loading of neurons with a vital dye 5(6)-carboxyfluorescein diacetate (CFDA), which labeled both axons and dendrites (29), revealed only short neurites, similar to that observed with anti-NSE antibody staining (data not shown), indicating that the tau-positive fragments were not physically connected to the cell soma.
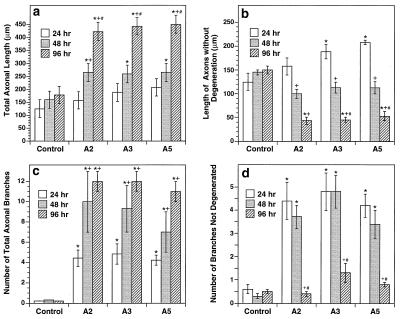
Figure 3.
Quantitative analyses of ephrin-dependent axonal growth, branching, and degeneration. Neurons were cocultured with ligand-expressing or control cells for 24, 48, and 96 hr and then fixed and stained with anti-tau antibody. Eight to 10 randomly selected neurons were quantitated for each parameter. (a) Total length of each axon. The measurements include both unfragmented and fragmented stretches of axons and are the sum of the length of axons and all branches. The total axonal length increased over time, primarily because of the increase in the number of branches. (b) Length of the region of each axon without fragmentation. Axonal fragmentation usually starts at the growth cones and progresses toward the cell somas. The segments of axons closest to the somas and without fragmentation were measured. (c) Number of total branches of each axon. (d) Number of branches without fragmentation. Data analyzed with two-factor ANOVA (substrate condition and time in culture). Bars = SEM. ∗, +, and #, Significant differences compared with the control, 24-hr, or 48-hr time points, respectively (P < 0.05; Scheffe’s test).
To examine the dynamics of axonal growth and degeneration, the medial hippocampal neurons were cocultured with ephrin-expressing or control cells for various times and subsequently fixed and stained with anti-tau antibody. Although the total length of axons, which include fragmented regions, increased over time (Fig. 3a), the length of axonal regions without fragmentation was longest at 24 hr of culture and decreased with time thereafter (Fig. 3b). Similarly, the number of total branches increased over time, but the number of branches that were not fragmented decreased after 24 hr (Fig. 3 c and d). These observations suggest that the ligands promoted the growth and branching of the axons initially and degeneration occurred later. In the first 24 hr, growth and branching were more robust than degeneration. After 24 hr, fragmentation surpassed growth promotion. The fragmentation of axons provided an explanation of why neurites on the ligand-expressing cells revealed by anti-NSE staining were very short, because NSE is a cytoplasmic enzyme and likely is lost in fragmented axons. These observations suggest that the ligands of the Eph family exhibit dual effects on the medial hippocampal neurons: initially promoting growth and branching and later inducing fragmentation of axons.
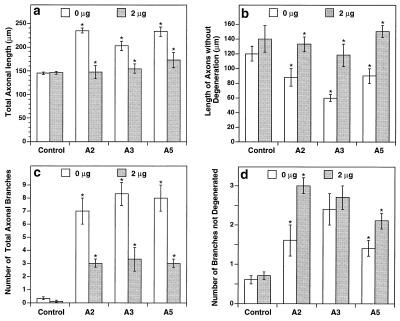
To determine whether the seemingly opposite effects were both a result of ephrins, we cocultured the hippocampal neurons with ligand-expressing cells in the presence of the competitive inhibitor EphA3-Fc. If the ligands cause both promotion of axonal growth and branching and induction of fragmentation, EphA3-Fc should reduce the total length of axons as well as the extent of fragmentation. Indeed, in the presence of 2 μg/ml EphA3-Fc in the culture medium, the average total axonal length was reduced to the level of control (Fig. 4a), whereas the length of axons without degeneration was increased to that of control (Fig. 4b). Similarly, the total number of axonal branches extended by neurons cultured on the ligand-expressing cells was reduced significantly by EphA3-Fc, whereas the number of unfragmented branches was increased (Fig. 4 c and d). The inhibition of the dual effects by Eph-A3-Fc indicates that the Eph ligands specifically promote neurite outgrowth and branching and induce axonal fragmentation.
Figure 4.
Inhibition of the effects of the Eph ligands by the competitive inhibitor EphA3-Fc. Medial hippocampal neurons were cocultured with the ligand-expressing or control cells in the presence of the soluble extracellular domain of EphA3, EphA3-Fc. The cells were incubated for 48 hr before analysis. Fifteen to 20 neurons were quantitated for each parameter. (a) Inhibition of ephrin-induced growth promotion by EphA3-Fc. In the presence of EphA3-Fc, the total axonal length of medial hippocampal neurons cocultured with ligand-expressing cells was comparable to that elicited by control cells. (b) Inhibition of axonal fragmentation by EphA3-Fc. (c) Reduction of the total number of axonal branches by EphA3-Fc. (d) Inhibition of fragmentation of axonal branches by EphA3-Fc. Asterisks indicate significant differences (P < 0.05; t test) between cultures with and without the inhibitor.
DISCUSSION
Using a coculture assay, we have shown that three different ephrins, which are expressed in the hippocampal target, the lateral septum in a dorsomedial-to-ventrolateral gradient, have dual effects on the growth and branching of the medial hippocampal axons. All three ligands cause a very extensive initial growth and branching of these axons. However, the axons and branches degenerate over time, leaving neurons with only very short processes. The degeneration effect primarily is limited to the receptor-positive medial hippocampal neurons, because the ligands did not inhibit significantly the growth of the lateral axons. These observations suggest that the receptor and ligand gradients in the hippocampus and the septal target interact to restrict the projection of the receptor-positive medial axons from terminating at the topographically inappropriate, ligand-rich ventral septum probably by eliminating mistargeted axons.
Technical Considerations.
The repulsive axonal guidance activity that regulates retinotectal topographic map development has been studied primarily by using the stripe assay developed by Bonhoeffer and colleagues (30). In this assay, membrane extracts from the anterior and posterior tectum are laid down side by side in narrow stripes and temporal axons appear to grow preferentially on the anterior extracts. This preference is a result of the presence of repulsive activities in the posterior extracts. Although this assay is instrumental in the identification and characterization of repulsive axonal guidance activity in the tectum, it does not reproduce all aspects of axonal behavior in vivo: the strong repulsive effect of posterior tectum on temporal axons was not observed during retinotectal development. Temporal axons widely overshoot their target zones both in the chicken and in rodents (21–23). One potential difference between the stripe assay and in vivo guidance is that the in vitro assay generates an artificially sharp boundary between the anterior and posterior membrane extracts, which is lacking in the tectum. Instead, ephrin-A2 and A5, which account, at least in part, for the repulsive activity in the posterior tectum, are expressed in a continuous gradient (8, 9, 14). Axon behavior in a repulsive gradient field may be different from that encountering a sharp boundary, and the elegant guidance effect of the repulsive cues in the stripe assay may be a manifestation of this particular assay. In vivo tracing studies indicate that retinal axons can grow up a repulsive gradient for considerable distance and overshoot beyond proper target zones (22). This raises the issue of how the Eph family positional cues confer topographic specificity.
The coculture assay used in this study resembles in vivo guidance in two aspects: first, axons are exposed to repulsive molecules over a long stretch; second, axons interact with ligands expressed in live cell surface, rather than molecules isolated through biochemical fractionation, which may alter the native conformation of these ligands. The ligand conformation has been shown to be important in mediating their biological effects (31). Consistent with these similarities, axon behavior in the cocultures recapitulates several events in vivo, including the initial outgrowth and branching in the presence of ephrin molecules and later degeneration of axons and branches. However, we must emphasize that the coculture assay is not completely physiological either, because the ligands are distributed uniformly, rather than in gradient as found in vivo, and, therefore, the results observed here may reflect merely axon behavior when they encounter a particular concentration of ephrins. The distinct effects on axons revealed by these two methods argue strongly that multiple assays must be used in analyzing the biological function of these mapping molecules.
Dual Effects of Ephrins on Medial Hippocampal Axons.
In this study, we observed dual effects of ephrins on the growth and branching of medial hippocampal axons: the initial stimulation and later degeneration. This process appears to be extremely dynamic. At no point in the experiment do we observe extensive unfragmented axons (Figs. 1 and 3b), although axonal remnants can be two times as long as the control (Fig. 3a). This suggests that axons and branches are degraded rapidly immediately after they are generated. The total axon length continued to increase even after the degeneration had started, indicating that axons continued to grow throughout the period of experiment, possibly through the addition of new branches. The observation that the ephrins promote axonal growth and branching is consistent with a recent study showing that ephrin-A5 can induce branching of cortical layer 6 neurons (32). The ephrins are not unique in having multiple activities. Netrin, a diffusable axon guidance molecule, functions as either a chemoattractant or a chemorepellant, depending on the source of axons (33, 34). Even for the same type of axons, netrin can function as both attractant and repellent, depending on the level of cyclic AMP in the environment (35).
The stimulation effect of ephrins was not noted in previous studies using stripe assays or uniform carpets containing endogenous ephrins (8, 11, 36). In these studies, axons were marked by carbocyanine dyes that label cytoplasmic membranes. Because degenerated axons lost their membranes, the transient stimulation effects revealed by axon debris in our study were unlikely to be observed. Consistent with this, no degeneration was noted in these studies as well. Another possibility for the lack of observable stimulation effect in previous studies is that, in the stripe assay, axons were repelled from ligand-containing stripes because of the presence of sharp ligand boundary and, therefore, no persistent stimulation could be achieved. Future studies examining whether tau-positive axon debris are present in the stripe assay are needed to resolve this discrepancy.
Previous studies also showed that ephrin-A5 causes growth cone collapse (8, 37, 38). This appears to contradict our results that there is initial stimulation of axonal growth and branching. However, it has been difficult to correlate growth cone collapse activity with long-term effects on axonal growth. For example, brain-derived neurotrophic factor (BDNF), a well characterized neurotrophic factor that supports survival and neurite outgrowth of a variety of neurons, also induces growth cone collapse and neurite retraction when applied acutely, and the effect is clearly dependent on the activation of its receptor, trkB tyrosine kinase (39). The collapsing activity of BDNF appears also to be regulated by intracellular cAMP levels (39). Thus, it is conceivable that the complex effects of the ephrins on axons are regulated by different concentrations of intracellular-signaling molecules activated by the binding of ephrins to the receptors. There may be a threshold below which axons are promoted to grow and above which they are induced to degenerate. In early cultures (<24 hr), the concentration of the signaling molecules is relatively low and ephrins show positive effects on axonal growth. With prolonged exposure, the signaling molecules may accumulate to higher concentrations, which may result in axonal degeneration. Consistent with this proposal, reducing the levels of ligands in vivo by homologous recombination in ephrin-A5 knock-out mice causes increased overshooting of retinal ganglion axons (40). In these mice, only ephrin-A2 is expressed in the posterior tectum, in contrast to the wild type in which both ephrin-A2 and A5 are expressed. The reduction of ligand levels may reduce the degeneration effects but still allow the stimulation effects on incoming axons because this may require only low levels of signals. Indeed, less degeneration of the hippocampal neurites was observed on fibroblast cell lines with lower levels of ephrin expression, although the stimulation effects were comparable to that observed on fibroblast cells expressing high levels of the ligands (data not shown). Another possibility for the switch in response to ephrins is that receptors or receptor compositions in neurons are altered during development in vivo or during culture in vitro. Further work clearly is needed to differentiate these possibilities.
Correlation with Axon Behavior in Vivo.
During the development of retinotectal or retinocollicular projections in higher vertebrates (chicken and rat), retinal axons initially mistarget widely both in rostrocaudal and dorsoventral axes (21–23). Axons also form branches in many topographically inappropriate positions. Topographic specificity is achieved only later by massive remodeling, which involves elimination of large segments of mistargeted axons and branches, and a dramatic increase in branching and arborization at topographically appropriate sites (21–23). Our coculture assays appear to recapitulate this process. The initial stimulation of axonal growth and branching by the ephrins may ensure that at least some axonal branches reach proper target zones. There may be no topographic specificity in the initial outgrowth. However, axons reaching topographically incorrect areas may be pruned in later developmental stages by process fragmentation induced by the ligands expressed in these regions. Our observations provide evidence that the ephrins may regulate topographic projection by specific axonal pruning. The ability to stimulate axonal growth and branching may suggest that in the target zone with proper levels of expression, ephrins may stimulate branching and arborization, a process also observed in vivo during topographic map development. Thus, it is tempting to speculate that the ephrins may contribute to the development of topographic maps by stimulating axonal growth and arborization at the proper target zones and pruning mistargeted axons, although this does not exclude involvement of other positive and negative guidance cues.
Acknowledgments
We thank L. Chong, I. Daar, and E. Dicicco-Bloom for critical reading of the manuscript. Research was supported in part by grants to R.Z. from the National Institutes of Health (1RO1NS36788-01 and PO1HD23315) and the Alzheimer’s Foundation.
ABBREVIATION
- NSE
neuron-specific enolase
References
- 1.Harris W A, Holt C E. Neuron. 1995;15:241–244. doi: 10.1016/0896-6273(95)90000-4. [DOI] [PubMed] [Google Scholar]
- 2.Tessier-Lavigne M. Cell. 1995;82:345–348. doi: 10.1016/0092-8674(95)90421-2. [DOI] [PubMed] [Google Scholar]
- 3.Friedman G C, O’Leary D D M. Curr Opin Neurobiol. 1996;6:127–133. doi: 10.1016/s0959-4388(96)80018-3. [DOI] [PubMed] [Google Scholar]
- 4.Orioli D, Klein R. Trends Genet. 1997;13:354–359. doi: 10.1016/s0168-9525(97)01220-1. [DOI] [PubMed] [Google Scholar]
- 5.Zisch A H, Pasquale E B. Cell Tissue Res. 1997;290:217–226. doi: 10.1007/s004410050926. [DOI] [PubMed] [Google Scholar]
- 6.Flanagan J G, Vanderhaeghen P. Annu Rev Neurosci. 1998;21:309–345. doi: 10.1146/annurev.neuro.21.1.309. [DOI] [PubMed] [Google Scholar]
- 7.Zhou R. Pharmacol Ther. 1998;77:151–181. doi: 10.1016/s0163-7258(97)00112-5. [DOI] [PubMed] [Google Scholar]
- 8.Drescher U, Kremoser C, Handwerker C, Loschinger J, Masaharu N, Bonhoeffer F. Cell. 1995;82:359–370. doi: 10.1016/0092-8674(95)90425-5. [DOI] [PubMed] [Google Scholar]
- 9.Cheng H-J, Nakamoto M, Bergemann A D, Flanagan J G. Cell. 1995;82:371–381. doi: 10.1016/0092-8674(95)90426-3. [DOI] [PubMed] [Google Scholar]
- 10.Nakamoto M, Cheng H-J, Friedman G C, McLaughlin T, Hansen M J, Yoon C H, O’Leary D D M, Flanagan J G. Cell. 1996;86:755–766. doi: 10.1016/s0092-8674(00)80150-6. [DOI] [PubMed] [Google Scholar]
- 11.Monschau B, Kremoser C, Ohta K, Tanaka H, Kaneko T, Yamada T, Handwerker C, Hornberger M R, Loschinger J, Pasquale E B, et al. EMBO J. 1997;16:1258–1267. doi: 10.1093/emboj/16.6.1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Frisen J, Yates P A, McLaughlin T, Friedman G C, O’Leary D D M, Barbacid M. Neuron. 1998;20:235–243. doi: 10.1016/s0896-6273(00)80452-3. [DOI] [PubMed] [Google Scholar]
- 13.Gao P P, Zhang J H, Racey Y B, Dreyfus C F, Black I B, Zhou R. Proc Natl Acad Sci USA. 1996;93:11161–11166. doi: 10.1073/pnas.93.20.11161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang J H, Cerritti D P, Yu T, Flanagan J G, Zhou R. J Neurosci. 1996;16:7182–7190. doi: 10.1523/JNEUROSCI.16-22-07182.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Swanson L W, Cowan W M. J Comp Neurol. 1977;172:49–84. doi: 10.1002/cne.901720104. [DOI] [PubMed] [Google Scholar]
- 16.Swanson, L. W., Kohler, C. & Bjorklund, A. (1987) The Limbic Region I: The Septohippocampal System, in Handbook of Chemical Neuroanatomy, Vol. 5: Integrated Systems of the CNS, Part I, pp. P124–P278.
- 17.Braisted J E, McLaughlin T, Wang H U, Friedman G C, Anderson D J, O’Leary D D. Dev Biol. 1997;191:14–28. doi: 10.1006/dbio.1997.8706. [DOI] [PubMed] [Google Scholar]
- 18.Holash J A, Pasquale E B. Dev Biol. 1995;172:683–693. doi: 10.1006/dbio.1995.8039. [DOI] [PubMed] [Google Scholar]
- 19.Marcus R C, Gale N W, Morrison M E, Mason C A, Yancopoulos G D. Dev Biol. 1996;180:786–789. doi: 10.1006/dbio.1996.0347. [DOI] [PubMed] [Google Scholar]
- 20.Sefton M, Araujo M, Nieto M A. Dev Biol. 1997;188:363–368. doi: 10.1006/dbio.1997.8638. [DOI] [PubMed] [Google Scholar]
- 21.Simon D K, O’Leary D D M. Dev Biol. 1990;137:125–134. doi: 10.1016/0012-1606(90)90013-9. [DOI] [PubMed] [Google Scholar]
- 22.Simon D K, O’Leary D D M. J Neurosci. 1992;12:1212–1232. doi: 10.1523/JNEUROSCI.12-04-01212.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nakamura H, O’Leary D D M. J Neurosci. 1989;9:3776–3795. doi: 10.1523/JNEUROSCI.09-11-03776.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lillian L. Nature (London) 1996;377:158–162. [Google Scholar]
- 25.Binder L I, Frankfurter A, Rebhun L I. J Cell Biol. 1985;101:1371–1378. doi: 10.1083/jcb.101.4.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kosik K S, Finch E A. J Neurosci. 1987;7:3142–3153. doi: 10.1523/JNEUROSCI.07-10-03142.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ferreira A, Busciglio J, Caceres A. Brain Res. 1987;431:9–31. doi: 10.1016/0165-3806(87)90191-x. [DOI] [PubMed] [Google Scholar]
- 28.Brion J P, Guilleminot J, Couchie D, Flament-Durand J, Nunez J. Neuroscience. 1988;25:139–146. doi: 10.1016/0306-4522(88)90013-9. [DOI] [PubMed] [Google Scholar]
- 29.Petroski R E, Geller H M. J Neurosci Met. 1994;52:23–32. doi: 10.1016/0165-0270(94)90051-5. [DOI] [PubMed] [Google Scholar]
- 30.Walter J, Henke-Fahle S, Bonhoeffer F. Development. 1987;101:909–913. doi: 10.1242/dev.101.4.909. [DOI] [PubMed] [Google Scholar]
- 31.Stein E, Lane A A, Cerretti D P, Schoecklmann H O, Schroff A D, Van Etten R L, Daniel T O. Genes Dev. 1998;12:667–678. doi: 10.1101/gad.12.5.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Castellani V, Yue Y, Gao P P, Zhou R, Bolz J. J Neurosci. 1998;15:4663–4672. doi: 10.1523/JNEUROSCI.18-12-04663.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Serafini T, Kennedy T E, Galko M J, Mirzayan C, Jessell T M, Tessier-Lavigne M. Cell. 1994;78:409–424. doi: 10.1016/0092-8674(94)90420-0. [DOI] [PubMed] [Google Scholar]
- 34.Colamarino S A, Tessier-Lavigne M. Cell. 1995;81:621–629. doi: 10.1016/0092-8674(95)90083-7. [DOI] [PubMed] [Google Scholar]
- 35.Ming G-l, Song H-j, Berninger B, Holt C E, Tessier-Lavigne M, Poo M-m. Neuron. 1997;19:1225–1235. doi: 10.1016/s0896-6273(00)80414-6. [DOI] [PubMed] [Google Scholar]
- 36.Walter J, Kern-Veits B, Huf J, Stolze B, Bonhoeffer F. Development. 1987;101:685–696. doi: 10.1242/dev.101.4.685. [DOI] [PubMed] [Google Scholar]
- 37.Meima L, Kljavin I J, Moran P, Shih A, Winslow J W, Caras I W. Eur J Neurosci. 1997;9:177–188. doi: 10.1111/j.1460-9568.1997.tb01365.x. [DOI] [PubMed] [Google Scholar]
- 38.Meima L, Moran P, Matthews W, Caras I W. Mol Cell Neurosci. 1997;9:314–328. doi: 10.1006/mcne.1997.0621. [DOI] [PubMed] [Google Scholar]
- 39.Wang Q, Zheng J O. J Neurosci. 1998;18:4973–4984. doi: 10.1523/JNEUROSCI.18-13-04973.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Frisén J, Yates P A, McLaughlin T, Friedman G C, O’Leary D D M, Barbacid M. Neuron. 1998;20:235–243. doi: 10.1016/s0896-6273(00)80452-3. [DOI] [PubMed] [Google Scholar]