Abstract
6-Hydroxydopamine (6-OHDA) is widely used to selectively lesion dopaminergic neurons of the substantia nigra (SN) in the creation of animal models of Parkinson’s disease. In vitro, the death of PC-12 cells caused by exposure to 6-OHDA occurs with characteristics consistent with an apoptotic mechanism of cell death. To test the hypothesis that apoptotic pathways are involved in the death of dopaminergic neurons of the SN caused by 6-OHDA, we created a replication-defective genomic herpes simplex virus-based vector containing the coding sequence for the antiapoptotic peptide Bcl-2 under the transcriptional control of the simian cytomegalovirus immediate early promoter. Transfection of primary cortical neurons in culture with the Bcl-2-producing vector protected those cells from naturally occurring cell death over 3 weeks. Injection of the Bcl-2-expressing vector into SN of rats 1 week before injection of 6-OHDA into the ipsilateral striatum increased the survival of neurons in the SN, detected either by retrograde labeling of those cells with fluorogold or by tyrosine hydroxylase immunocytochemistry, by 50%. These results, demonstrating that death of nigral neurons induced by 6-OHDA lesioning may be blocked by the expression of Bcl-2, are consistent with the notion that cell death in this model system is at least in part apoptotic in nature and suggest that a Bcl-2-expressing vector may have therapeutic potential in the treatment of Parkinson’s disease.
6-Hydroxydopamine (6-OHDA) is a neurotoxin taken up into dopaminergic neurons through the dopamine transporter, where the compound is autooxidized to form semiquinone and superoxide anions that subsequently are converted to hydroxyl radicals through interaction with H2O2 (1, 2). Injection of the toxin into striatonigral projections results in selective damage to dopaminergic neurons and has been used to create widely used models of Parkinson’s disease in rats (3, 4). Neuronal injury after direct injection of the toxin into the region of the substantia nigra is acute (5), but a subacute lesion that progresses over weeks can be created by injection of 6-OHDA into the proximity of the dopaminergic terminal in the striatum. Morphologic (6, 7) behavioral and biochemical effects (8, 9) have been demonstrated with this model.
6-OHDA lesioning of dopaminergic terminals in the striatum of neonatal rats in vivo results in the appearance of apoptotic cell death in phenotypically defined dopaminergic neurons over the course of 10 days postlesioning (10). In older animals the appearance of dying cells suggests a combination of necrotic and apoptotic cell death (10). PC-12 cells induced to die by exposure to 6-OHDA in vitro demonstrate cell shrinkage, chromatin condensation, membrane blebbing, and DNA fragmentation characteristic of apoptosis (11). Additional evidence that cell death in this model proceeds through apoptotic mechanisms is suggested by the up-regulation of p53 and Bax protein expression after 6-OHDA exposure (12) and the observation that cell death can be blocked by addition of a caspase inhibitor (13). Cell death with characteristics of apoptosis also has been demonstrated after exposure to l-dopamine in PC-12 cells (14) and sympathetic neurons (15), suggesting that endogenous oxidative stressors may produce similar toxicity to these cells.
Bcl-2, the prototypical member of a family of at least 12 related proteins that are structural and functional homologues of the nematode protein CED-9 (16), is capable of protecting cells against apoptotic cell death resulting from a variety of cytotoxic insults (17). Bcl-2 is located primarily on the cytoplasmic face of the mitochondrial outer membrane, endoplasmic reticulum, and nuclear envelope. Bcl-2 blocks activation of the caspase cascade and also protects mitochondria, preventing the opening of permeability transition complexes and the release of cytochrome c (18). Dopamine-induced apoptosis of PC-12 cells is inhibited by the overexpression of Bcl-2 (19), as is metamphetamine-induced cell death in an immortalized neuronal cell line (20). Cultured striatal neurons from transgenic mice overexpressing Bcl-2 similarly are resistant to 6-OHDA toxicity in vitro (21).
To determine the contribution of apoptotic cell death to the loss of tyrosine hydroxylase (TH)-expressing neurons of the substantia nigra (SN) after 6-OHDA lesioning of the striatum in vivo, we constructed a multiply deleted replication-incompetent genomic herpes simplex virus (HSV) vector carrying the coding sequence for Bcl-2 under the transcriptional control of the simian cytomegalovirus immediate early promoter (SCMV IEp). Injection of the vector into the striatum 1 week before lesioning with 6-OHDA resulted in a 50% increase in the number of surviving TH-immunoreactive and retrogradely labeled fluorogold (FG) neurons in the substantia nigra 2 weeks after lesioning.
MATERIALS AND METHODS
Generation of a Bcl-2 Expressing Vector.
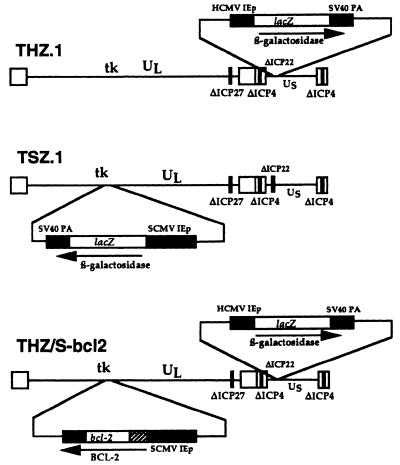
A multiply deleted replication-incompetent genomic HSV vector expressing the human Bcl-2 gene product was constructed from a recombination plasmid containing a Bcl-2 expression cassette flanked by sequences from the HSV genome. The human bcl-2 cDNA was obtained from the ATTC clone number 79804 as an EcoRI-NsII fragment. The cDNA coding for the 5′ untranslated sequence from the rabbit β-globin gene, including the second intron, was added upstream of the translation start site. The cDNA was modified further by the addition of the β-globin 3′ untranslated region and simian virus 40 polyadenylation sequences downstream of the stop codon, and this modified cDNA was placed under the control of the SMCV IEp (22) in a plasmid containing sequences from the HSV thymidine kinase (TK) locus. The expression cassette was flanked by HSV sequences 48634–47978 upstream and 47416–46098 downstream. (All HSV genome numbers refer to the published sequence for HSV strain 17.) The cloning of the expression cassette into this locus resulted in a deletion of the tk gene from 47416–47978, which inactivates the gene. Recombination of the plasmid into the virus genome yields recombinants that are tk− and therefore resistant to treatment with ganciclovir. The vector THZ/S-bcl2 (Fig. 1) was created by homologous recombination of the plasmid into the TK locus of the ICP4-deleted HSV vector d120 (23) using standard calcium phosphate precipitation (24). Recombinants were identified by selection of progeny virus with ganciclovir (5 μg/ml), isolated, and tested for the presence of the bcl-2 gene by Southern blot analysis. Isolates that contained the bcl-2 cassette (d120/S-bcl2) were purified by three rounds of limiting dilution. The d120/S-bcl2 vector was crossed genetically with the ICP4-, ICP22-, and ICP27-deficient vector THZ.1 (25) by infecting complementing 7B cells with both vectors at a multiplicity of infection (moi) of 5, and ganciclovir-resistant plaques that expressed lacZ were selected. The deletion in ICP27 was confirmed by the inability of the isolates to grow on ICP4-complementing cell lines and Southern blot. The final vector was grown to high titer, purified on a Nicodenz gradient, and used in all the experiments described. Two control vectors, similarly deleted for ICP4, ICP22, and ICP27, were used. THZ.1 had the lacZ reporter gene under the control of the HCMV IEp in the ICP22 locus, whereas TSZ.1 was constructed with an SCMV IEp:lacZ reporter cassette in the tk locus of the recombinant HSV vector genome (Fig. 1).
Figure 1.
Schematic representation of genomic HSV vectors. Both the control vectors THZ.1 and TSZ.1 and the Bcl2-expressing vector THZ/S-bcl2 are deleted for the immediate early genes ICP4 (both copies), ICP27, and ICP22, indicated by solid bars in the genome.
Cell Culture and in Vitro Transfections.
Hig-82 cells were cultured in MEM at 37°. Neuronal cultures were prepared from cortex from 17-day-old rat embryos, which were dissociated with 0.25% trypsin-1 mM EDTA (30 min at 37°) and plated on poly-d-lysine (molecular weight, 70,000–150,000; Sigma)-coated coverslips at 2 × 105 cells per well (24-well plate; Falcon) in defined medium [NeuroBasal Medium supplemented with B-27 (1× concentration), Glutamax I (0.5 ml/50 ml) and Albumax II (50 μl/50 ml), all purchased from GIBCO/BRL], and incubated at 37° in humidified 95% air, 5% CO2. These cultures contain greater than 95% neurons as determined by immunostaining with antibodies directed against neurofilament and glial fibrillary acidic protein (data not shown). Seven days after plating, cultures were infected at an moi of 1 in 250 μl of medium, for 1 hr at 37°, with gentle agitation every 10 min. Bcl-2 protein was detected by Western analysis. Forty-eight hours after infection cells were lysed in Laemmli buffer, the protein was separated by 12% SDS/PAGE and transferred to nitrocellulose membrane, which was probed with an anti-Bcl-2 antibody (1:100, Boehringer Mannheim) and detected with an alkaline phosphatase-conjugated secondary antibody (1:2,000; Sigma). Similar numbers of cells were harvested for mock, control, and vector-infected samples, and Ponceau-S staining confirmed the transfer of equivalent amounts of protein per lane. The amount of Bcl-2 in the cell lysate was also determined by ELISA by using a commercially available kit (Endogen, Cambridge, MA) following the manufacturer’s instructions. The number of viable cells was determined by Alamar blue fluorescence. Alamar blue (2% final concentration; Alamar Biosciences, Westlake, OH) was added to the cultures, and after 4-hr incubation at 37°, the viable cells were determined by quantitative fluorescence (530/590, excitation/emission) as described. For immunohistochemistry, cells were fixed in cold 4% paraformaldehyde, incubated overnight at 4° with the primary antibody (anti-Bcl-2 1:100, Boehringer Mannheim; anti-MAP2 1:1,000, Sigma), followed by a CY3-conjugated secondary antibody (1:3,000, Sigma) for 1 hr at room temperature. Apoptotic nuclei in uninfected cultures were identified by terminal deoxynucleotidyltransferase-mediated UTP end labeling (TUNEL). Uninfected cells were fixed in 4% paraformaldehyde, rinsed once with PBS, and permeabilized with 0.17% Triton X-100/0.1% sodium citrate for 2 min on ice, followed by incubation with the TUNEL reaction mixture of the fluorescein in situ cell death detection kit (Boehringer Mannheim) following the manufacturer’s instructions. Negative controls, incubated without enzyme, and positive controls, treated with DNase I, produced appropriate results.
Animal Experiments.
6-OHDA lesioning was carried out according to the protocol of Sauer and Oertel (6). Female Sprague–Dawley rats (200–225 g) were injected into the striatum bilaterally (coordinates anterior–posterior +1.0, medial–lateral 3.0, dorsal–ventral −5.0 relative to the bregma) with the retrograde tracer fluorogold (0.2 μl of 2% FG in 0.9% saline; Fluorochrome, Denver, CO), and at the same time injected unilaterally into the region of the SN (AP −5.3, ML 1.9, DV −7.4) with either vector or control (4 μl at 1 μl/min). At 1 week after inoculation, 6-OHDA (16 μg of free base, 0.2 μg/μl in 0.9% saline) was injected unilaterally into striatum using the same coordinates as the fluorogold, ipsilateral to the vector injection into SN. The experiment was performed with nine animals in each group, and the entire experiment was repeated once with identical results.
Reverse Transcription–PCR (RT-PCR).
Transgene expression of bcl-2 RNA was examined by RT-PCR. Total RNA was isolated from one glass slide-mounted 40-μm section from three vector-injected and two control animals, using TRIZOL Reagent (GIBCO/BRL) as per the manufacturer’s instructions. Approximately 1 μg of RNA was treated with DNase I (Boehringer Mannheim) for 45 min at 42° and then inactivated by incubation at 100° for 5 min. First-strand cDNA was made by using a GeneAmp kit (Perkin–Elmer) as per the manufacturer’s instructions and approximately 0.2 μg of DNase-treated total RNA. Two 40-cycle PCR amplifications, using nested primers to the human Bcl-2 sequence, were performed by using a Perkin–Elmer GeneAmp 2400 thermocycler and Perkin–Elmer Amplitaq Gold enzyme (20 μl total reaction volume, consisting of 0.2 mM dNTP, 10 pM primers, and 0.5 unit of Taq polymerase). Four microliters of cDNA reaction product was used as template for the first round of amplification (forward primer, 5′-gaattccactgtcaagaaagagcagt-3′; reverse primer, 5′-atgctgtggttgatatttcgaaagc-3′; GIBCO/BRL), and 4 μl of the first amplification product was used as template for the second round of amplification (forward primer, 5′-acagaggccctgggccttcctat-3′; reverse primer, 5′-agttccaggtgtggaatatggg-3′; GIBCO/BRL). Cycle conditions were 94° for 1 min, 58° for 1 min, and 72° for 2 min.
Histology and Cell Counting.
Two weeks after 6-OHDA lesioning the animals were sacrificed by intracardiac perfusion with 4% paraformaldehyde in PBS, the brains were cryoprotected with 30% sucrose, and 40-μm sections were mounted directly for examination of FG-labeled cells or processed for immunocytochemistry. For TH-immunocytochemistry, floating sections were incubated with an anti-TH antibody (1:500, overnight at room temperature; Chemicon), followed by a secondary antibody conjugated to biotin (1:200 for 2 hr; Vector Laboratories), and detected with diaminobenzidine by using a commercial kit (Vector Elite; Vector Laboratories). The number of surviving FG-labeled and TH-immunoreactive neurons was determined by counting labeled or immunoreactive cells through the SN (four to seven sections per animal for each technique), and the total number was expressed as a percentage of the unlesioned side. The statistical significance of the difference between the two sides was determined by t test (Systat, Evanston, IL).
RESULTS
THZ/S-bcl2 Vector Expresses Bcl-2.
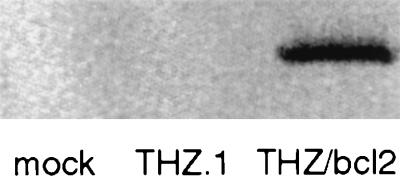
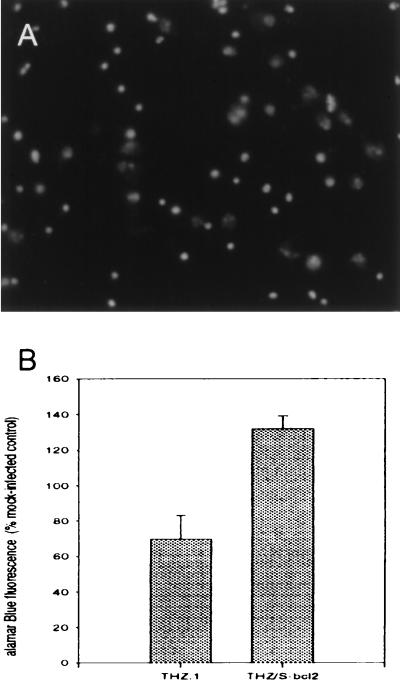
The ability of the THZ/S-bcl2 vector to express biologically active Bcl-2 first was determined in vitro. Hig-82 cells, infected at an moi of 1, were harvested 48 hr postinfection. A 26-kDa bcl-2 immunoreactive band was found in the lysate of THZ/S-bcl2-infected cells, but not in uninfected or control vector infected cells (Fig. 2). The amount of Bcl-2 in the lysate of infected cells was approximately 400 pg/well as determined by ELISA. Control vector (THZ.1) infection produced essentially no detectable Bcl-2 (<25 pg/well). The biological activity of vector-mediated Bcl-2 was assessed after infection of primary cortical neurons in culture. These cells, grown in defined medium in the absence of trophic factors, undergo naturally occurring cell death between 2 and 4 weeks in culture. The apoptotic nature of cell death in this model system was confirmed by TUNEL staining of the remaining nuclei at 3 weeks after plating (Fig. 3A). Cultures were infected with the control vector (THZ.1) or the bcl-2-expressing vector (THZ/S-bcl2), and 3 weeks postinfection the number of viable cells were determined by Alamar blue fluorescence. Cultures infected with the Bcl-2-producing vector had twice as many surviving cells as control virus-infected cultures and 130% of uninfected cultures (Fig. 3B). The identity of surviving cells as neurons was confirmed by microtubule-associated protein 2 (MAP2) immunoreactivity (data not shown). These cells were larger and had longer and larger processes than the cells in uninfected or control-infected cultures.
Figure 2.
Bcl-2 expression in vitro. Western blot of lysates of infected Hig-82 cells shows a 26-kDa BCl-2 immunoreactive band in THZ/S-bcl2 but not mock- or THZ.1-infected cells.
Figure 3.
Expression of Bcl-2 in cortical neurons in vitro inhibits naturally occurring cell death. (A) TUNEL staining of uninfected primary cortical neuron cultures at 3 weeks. The majority of residual shrunken nuclei in uninfected cultures was TUNEL-positive, whereas viable neurons were unlabeled. Similar patterns were seen in cultures at 2 and 4 weeks after plating. (B) Three weeks after infection, cultures infected with THZ/S-bcl2 demonstrated increased cell survival compared with uninfected cultures or cultures infected with THZ.1. Cell viability was determined by Alamar blue fluorescence, and the data are presented as a percentage of mock-infected cells. The data presented were obtained from three independent repetitions of the same experiment, with four wells in each group for each experiment. (P < .05 for the comparison of THZ.1 with THZ/S-bcl2 by t test.)
THZ/S-bcl2 Vector Protects SN Neurons from 6-OHDA Toxicity.
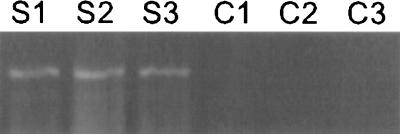
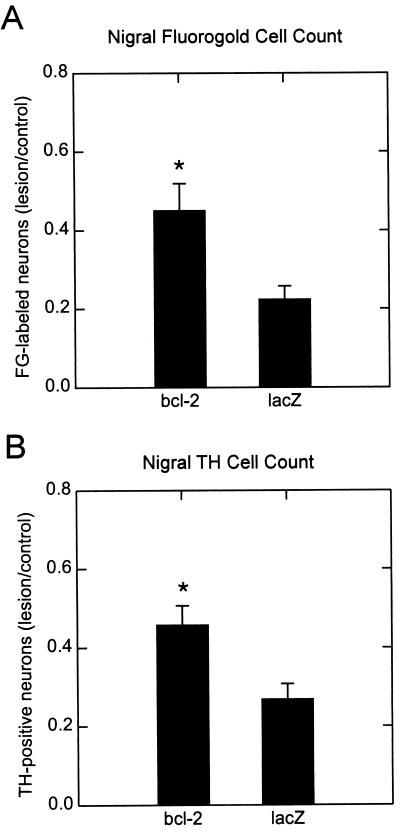
The effect of transgene expression of Bcl-2 in vivo was assessed by striatal 6-OHDA lesioning after injection of the vector into the region of the SN. Expression of the human bcl-2 RNA in vector-injected rat SN was confirmed by RT-PCR (Fig. 4). Bcl-2 produced from the THZ/S-bcl2 vector prevented 6-OHDA-induced degeneration of retrogradely labeled FG-positive neurons (Figs. 5A and 6) as well as the degeneration of TH+ dopaminergic cells of the SN (Figs. 5B and 7). Approximately twice as many cells of the SN, labeled retrogradely by FG or identified by the TH immunoreactivity, survived 2 weeks postlesioning in the bcl-2 vector-treated animals compared with control vector-injected animals. Injection of the bcl-2 vector or the control vector alone, without 6-OHDA lesioning, resulted in approximately 40% reduction in the number of TH+ or FG-labeled cells on the injected side at 3 weeks postinjection, although inspection of hematoxylin/eosin-stained sections revealed no evidence of an inflammatory infiltrate in animals sacrificed 3 weeks after vector injection (data not shown).
Figure 4.
Transgene-driven expression of human Bcl-2 RNA in rat SN. RT-PCR from a single 40-μm section of rat SN demonstrates bcl-2 in vector-injected (S1–S3) but not control (C1–C2) brains. C3 is a water blank.
Figure 5.
Expression of Bcl-2 in SN protects neurons from 6-OHDA toxicity. (A) Cell survival as estimated from cell counts of nigral FG+ cells 2 weeks after 6-OHDA lesion. THZ/S-bcl2 transfection resulted in a significantly greater number of FG+ cells surviving the lesion as compared with THZ.1 control transfected SN (n = 9 animals in each group, P < .05). (B) Cell survival as estimated from counts of TH-immunoreactive cells 2 weeks after 6-OHDA lesion. THZ/S-bcl2 transfection resulted in a significantly greater number of TH-immunoreactive cells surviving the lesion as compared with TSZ.1 control transfected SN (n = 9 animals in each group, P < .05). The experiment was repeated twice with the same results.
Figure 6.
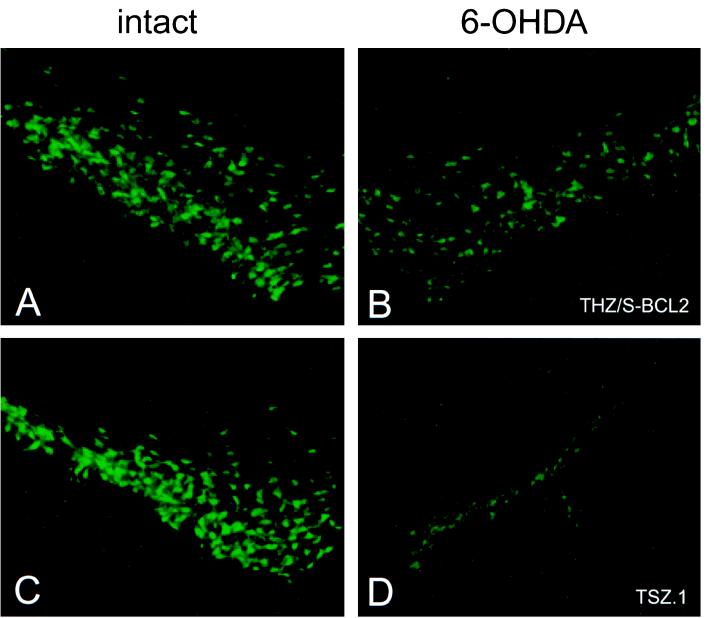
Photomicrographs of intact and lesioned/vector-injected SN 2 weeks after 6-OHDA lesion (FG+ cells). Representative photomicrographs of FG+ cells in contralateral intact SN (A and C) and ipsilateral SN injected with THZ/S-bcl2 (B) and TSZ.1 (D) are shown.
Figure 7.
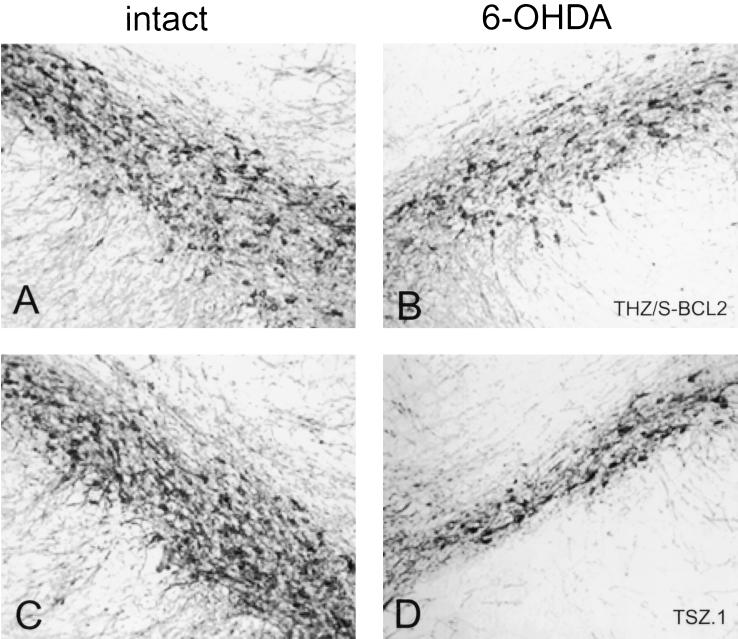
Photomicrographs of intact and lesioned/vector-injected SN 2 weeks after 6 OHDA lesion (TH-immunoreactive cells). Representative photomicrographs of TH-immunoreactive cells in contralateral intact SN (A and C) and from ipsilateral SN injected with THZ/S-bcl2 (B) and TSZ.1 (D).
DISCUSSION
The data presented in this study demonstrate that expression of bcl-2 in the SN protects dopaminergic neurons of the SN from degeneration induced by 6-OHDA. We have used the well characterized model described by Sauer and Oertel (6) in which nigrostriatal dopaminergic neurons degenerate over a period of weeks, with most of the cell loss completed by 2 weeks postlesioning. Retrograde labeling with FG demonstrates that the cell loss does not merely represent the loss of the dopaminergic phenotype of surviving cells; the amount of protection afforded was similar using either of the measures employed.
Evidence in cell culture model systems by others has demonstrated that exposure to 6-OHDA may result in cell death with features suggestive of apoptosis (11–13), and in vivo histologic and biochemical markers of apoptotic cell death can be demonstrated in the SN after 6-OHDA lesioning (10). Two recent reports have shown that transgenic mice overexpressing bcl-2 are resistant to 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) toxicity (21, 26). Surprisingly, in the latter study, protection against the acute model of MPTP toxicity, which is characterized histologically by necrotic cell death, was more robust than protection against the “chronic” model of MPTP toxicity, in which cell death is more clearly apoptotic in nature (26).
It has been demonstrated previously that delivery of the glial-derived neurotrophic factor (GDNF) directly to the region of the SN (27) protects nigrostriatal DA cells from degeneration in the same model of 6-OHDA toxicity and that production of GDNF mediated by either an E1–E3-deleted recombinant adenoviral vector (28, 29) or a recombinant adeno-asssociated viral vector (30) is also capable of protecting these neurons from degeneration and loss of TH immunoreactivity. The magnitude of protection demonstrated in our study, comparing control vector with the experimental vector, is similar to the magnitude of protection provided by the GDNF vectors. There is no direct evidence that GDNF effects are mediated through prevention of apoptosis. In cells of hematopoietic lineage, Bcl-2 delays ATP depletion induced by cytokine withdrawal (31). Whether the protective effect we see with Bcl-2 would be additive to the protective effects of GDNF remains to be determined. In addition to the effects of Bcl-2 on the caspase cascade in apoptosis, there is evidence that Bcl-2 may function directly to protect cells from injury because of oxidative stress (32–34). Bcl-2 shifts the redox potential to a more reduced state (35) and enhances mitochondrial calcium uptake (36). Some of these effects may occur upstream of caspase activation or independent of apoptotic mechanisms.
The HSV-based vector employed for transgene delivery and expression in this study has several potential advantages. Recombinant genomic HSV-based vectors have a large capacity (37–39), and during latency the viral genome is entirely, transcriptionally silent with the exception of the latency-associated transcript and transgene expression. The multiply deleted vector employed in this study is incapable of replicating in the absence of complementation of the essential immediate early genes ICP4 and ICP27 and, therefore, does not replicate in vivo, and the relative absence of an immune response is a distinct advantage in the use of these vectors.
The 6-OHDA model is an excellent model of cell death restricted to dopaminergic neurons of the SN, although the relevance of the cell death in this model to that which occurs in human Parkinson’s disease has not been established fully. Several investigators have reported detection of cells with morphologic characteristics of apoptosis in the SN of brains from Parkinson’s patients examined at autopsy (40–43). The short time frame during which cells undergoing apoptotic cell death may be detected compared with the prolonged duration of the disease process makes large-scale detection of apoptotic cell bodies in the late stage of the disease unlikely. Nonetheless, if Bcl-2 expression can prevent such cell death in the human disease, long-term expression from a defective HSV vector may have therapeutic potential.
Acknowledgments
This work was supported by grants from the National Institutes of Health (J.C.G. and D.J.F.), the Department of Veterans Affairs (M.M. and D.J.F.), the Department of Defense (M.Y., J.C.G., and D.J.F.) and the GenVec Corporation (J.C.G. and D.J.F.).
ABBREVIATIONS
- 6-OHDA
6-hydroxydopamine
- SN
substantia nigra
- TH
tyrosine hydroxylase
- FG
fluorogold
- HSV
herpes simplex virus
- RT-PCR
reverse transcription–PCR
- TUNEL
terminal deoxynucleotidyltransferase-mediated UTP end labeling
- GDNF
glial-derived neurotrophic factor
Footnotes
This paper was submitted directly (Track II) to the Proceedings office.
References
- 1.Heikkila R E, Hess A, Duvoisin R C. Science. 1984;224:1451–1453. doi: 10.1126/science.6610213. [DOI] [PubMed] [Google Scholar]
- 2.Nicklas W J, Vyas I, Heikkila R E. Life Sci. 1985;36:2503–2508. doi: 10.1016/0024-3205(85)90146-8. [DOI] [PubMed] [Google Scholar]
- 3.Ungerstedt U. Eur J Pharmacol. 1968;5:107–110. doi: 10.1016/0014-2999(68)90164-7. [DOI] [PubMed] [Google Scholar]
- 4.Ungerstedt U, Arbuthnott G W. Brain Res. 1970;24:485–493. doi: 10.1016/0006-8993(70)90187-3. [DOI] [PubMed] [Google Scholar]
- 5.Jeon B S, Jackson-Lewis V, Burke R E. Neurodegeneration. 1995;4:131–137. doi: 10.1006/neur.1995.0016. [DOI] [PubMed] [Google Scholar]
- 6.Sauer H, Oertel W H. Neuroscience. 1994;59:401–415. doi: 10.1016/0306-4522(94)90605-x. [DOI] [PubMed] [Google Scholar]
- 7.Berger K, Przedborski S, Cadet J L. Brain Res Bull. 1991;26:301–307. doi: 10.1016/0361-9230(91)90242-c. [DOI] [PubMed] [Google Scholar]
- 8.Cadet J L, Last R, Kostic V, Przedborski S, Jackson-Lewis V. Brain Res Bull. 1991;26:707–713. doi: 10.1016/0361-9230(91)90164-f. [DOI] [PubMed] [Google Scholar]
- 9.Kirik D, Rosenblad C, Bjorklund A. Exp Neurol. 1998;152:259–277. doi: 10.1006/exnr.1998.6848. [DOI] [PubMed] [Google Scholar]
- 10.Marti M J, James C J, Oo T F, Kelly W J, Burke R E. J Neurosci. 1997;17:2030–2039. doi: 10.1523/JNEUROSCI.17-06-02030.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Walkinshaw G, Waters C M. Neuroscience. 1994;63:975–987. doi: 10.1016/0306-4522(94)90566-5. [DOI] [PubMed] [Google Scholar]
- 12.Blum D, Wu Y, Nissou M F, Arnaud S, Alim Louis B, Verna J M. Brain Res. 1997;751:139–142. doi: 10.1016/s0006-8993(96)01358-3. [DOI] [PubMed] [Google Scholar]
- 13.Ochu E E, Rothwell N J, Waters C M. J Neurochem. 1998;70:2637–2640. doi: 10.1046/j.1471-4159.1998.70062637.x. [DOI] [PubMed] [Google Scholar]
- 14.Walkinshaw G, Waters C M. J Clin Invest. 1995;95:2458–2464. doi: 10.1172/JCI117946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ziv I, Zilkha-Falb R, Offen D, Shirvan A, Barzilai A, Melamed E. Movement Disorders. 1997;12:17–23. doi: 10.1002/mds.870120105. [DOI] [PubMed] [Google Scholar]
- 16.Adams J M, Cory S. Science. 1998;281:1322–1326. doi: 10.1126/science.281.5381.1322. [DOI] [PubMed] [Google Scholar]
- 17.Reed J C. Nature (London) 1997;387:773–776. doi: 10.1038/42867. [DOI] [PubMed] [Google Scholar]
- 18.Kroemer G. Nat Med. 1997;3:614–620. doi: 10.1038/nm0697-614. [DOI] [PubMed] [Google Scholar]
- 19.Offen D, Ziv I, Panet H, Wasserman L, Stein R, Melamed E, Barzilai A. Cell Mol Neurobiol. 1997;17:289–304. doi: 10.1023/A:1026390201168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cadet J L, Ordonez S V, Ordonez J V. Synapse. 1997;25:176–184. doi: 10.1002/(SICI)1098-2396(199702)25:2<176::AID-SYN8>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 21.Offen D, Beart P M, Cheung N S, Pascoe C J, Hochman A, Gorodin S, Melamed E, Bernard R, Bernard O. Proc Natl Acad Sci USA. 1998;95:5789–5794. doi: 10.1073/pnas.95.10.5789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chang Y N, Jeang K T, Chiou C J, Chan Y J, Pizzorno M, Hayward G S. J Virol. 1993;67:516–529. doi: 10.1128/jvi.67.1.516-529.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.DeLuca N A, McCarthy A M, Schaffer P A. J Virol. 1985;56:558–570. doi: 10.1128/jvi.56.2.558-570.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Krisky D, Marconi P, Goins W F, Glorioso J C. Methods in Molecular Medicine, Gene Therapy Protocols. Totowa, NJ: Humana; 1996. pp. 79–102. [DOI] [PubMed] [Google Scholar]
- 25.Marconi P, Krisky D, Oligino T, Poliani P L, Ramakrishnan R, Goins W F, Fink D J, Glorioso J C. Proc Natl Acad Sci USA. 1996;93:11319–11320. doi: 10.1073/pnas.93.21.11319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang L, Matthews R T, Schulz J B, Klockgether T, Liao A W, Martinou J C, Penney J B, Jr, Hyman B T, Beal M F. J Neurosci. 1998;18:8145–8152. doi: 10.1523/JNEUROSCI.18-20-08145.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sauer H, Rosenblad C, Bjorklund A. Proc Natl Acad Sci USA. 1995;92:8935–8939. doi: 10.1073/pnas.92.19.8935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Choi-Lundberg D L, Lin Q, Chang Y-N, Chiang Y L, Hay C M, Mohajeri H, Davidson B L, Bohn M C. Science. 1997;275:838–841. doi: 10.1126/science.275.5301.838. [DOI] [PubMed] [Google Scholar]
- 29.Bilang-Bleuel A, Revah F, Colin P, Locquet I, Robert J J, Mallet J, Horellou P. Proc Natl Acad Sci USA. 1997;94:8818–8823. doi: 10.1073/pnas.94.16.8818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mandel R J, Spratt S K, Snyder R O, Leff S E. Proc Natl Acad Sci USA. 1997;94:14083–14088. doi: 10.1073/pnas.94.25.14083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Garland J M, Halestrap A. J Biol Chem. 1997;272:4680–4688. doi: 10.1074/jbc.272.8.4680. [DOI] [PubMed] [Google Scholar]
- 32.Kane D J, Ord T, Anton R, Bredesen D E. J Neurosci Res. 1995;40:269–275. doi: 10.1002/jnr.490400216. [DOI] [PubMed] [Google Scholar]
- 33.Hockenbery D M, Oltvai Z N, Yin X M, Milliman C L, Korsmeyer S J. Cell. 1993;75:241–251. doi: 10.1016/0092-8674(93)80066-n. [DOI] [PubMed] [Google Scholar]
- 34.Bredesen D E. Ann Neurol. 1995;38:839–851. doi: 10.1002/ana.410380604. [DOI] [PubMed] [Google Scholar]
- 35.Ellerby L M, Ellerby H M, Park S M, Holleran A L, Murphy A N, Fiskum G, Kane D J, Testa M P, Kayalar C, Bredesen D E. J Neurochem. 1996;67:1259–1267. doi: 10.1046/j.1471-4159.1996.67031259.x. [DOI] [PubMed] [Google Scholar]
- 36.Murphy A N, Bredesen D E, Cortopassi G, Wang E, Fiskum G. Proc Natl Acad Sci USA. 1996;93:9893–9898. doi: 10.1073/pnas.93.18.9893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fink D J, Glorioso J C. Nat Med. 1997;3:357–359. doi: 10.1038/nm0397-357. [DOI] [PubMed] [Google Scholar]
- 38.Fink D J, DeLuca N A, Glorioso J C. Annu Rev Neurosci. 1996;19:265–287. doi: 10.1146/annurev.ne.19.030196.001405. [DOI] [PubMed] [Google Scholar]
- 39.Glorioso J C, Fink D J. In: Advances in Pharmacology: Gene Therapy. August J T, Anders M W, Munrad F, Coyle J T, editors. San Diego: Academic; 1996. [Google Scholar]
- 40.Mochizuki H, Goto K, Mori H, Mizuno Y. J Neurol Sci. 1996;137:120–123. doi: 10.1016/0022-510x(95)00336-z. [DOI] [PubMed] [Google Scholar]
- 41.Anglade P, Vyas S, Javoy-Agid F, Herrero M T, Michel P P, Marquez J, Mouatt-Prigent A, Ruberg M, Hirsch E C, Agid Y. Histol Histopathol. 1997;12:25–31. [PubMed] [Google Scholar]
- 42.Anglade P, Vyas S, Hirsch E C, Agid Y. Histol Histopathol. 1997;12:603–610. [PubMed] [Google Scholar]
- 43.Tompkins M M, Basgall E J, Zamrini E, Hill W D. Am J Pathol. 1997;150:119–131. [PMC free article] [PubMed] [Google Scholar]