Abstract
Reduced P300 amplitude is reliably found in individuals with a personal or family history of alcohol problems. However, alcoholism is part of a broader externalizing spectrum that includes other substance use and antisocial disorders. We hypothesized that reduced P300 is an indicator of the common factor that underlies disorders within this spectrum. Community males (N=969) were assessed at age 17 in a visual oddball task. Externalizing was defined as the common factor underlying symptoms of alcohol dependence, drug dependence, nicotine dependence, conduct disorder, and adult antisocial behavior. A robust association was found between reduced P300 amplitude and the externalizing factor, and this relation accounted for links between specific externalizing disorders and P300. Our findings indicate that reduced P300 amplitude is an indicator of the broad neurobiological vulnerability that underlies disorders within the externalizing spectrum.
Descriptors: P300, externalizing, psychopathology
Diagnostic comorbidity studies and behavioral genetic investigations over the past decade have converged on the idea that alcoholism, drug dependence, and antisocial deviance in childhood and later life comprise a spectrum of related disorders. Coincident with these developments, evidence has accumulated that reduced amplitude of the P300 brain potential response, long known to be an indicator of risk for alcohol problems, is associated with other disorders in this spectrum. The current study addressed the following basic question, arising from these existing lines of evidence: Is reduced P300 amplitude an indicator of the general factor that these disorders have in common, rather than of specific disorders within this spectrum?
It is well established that reduced amplitude of the P300 component of the event-related potential, a positive brainwave deflection evoked by infrequent, task-relevant events in a stimulus sequence, is associated with alcohol problems and alcoholism risk.1 This link was first noted in work comparing abstinent alcoholics with controls (Porjesz, Begleiter, & Garozzo, 1980). Subsequent studies revealed that reduced P300 amplitude was associated not just with active symptoms, but also with risk for the development of alcohol problems. For example, children and adolescents with a paternal history of alcoholism show reliably reduced P300 compared with family-negative controls (Begleiter, Porjesz, Bihari, & Kissin, 1984; Elmasian, Neville, Woods, Schuckit, & Bloom, 1982; Hill & Shen, 2002; for review, see Polich, Pollock, & Bloom, 1994). Additionally, smaller P300 amplitude prospectively predicts the later emergence of alcohol problems (Berman, Whipple, Fitch, & Noble, 1993; Hill, Steinhauer, Lowers, & Locke, 1995; Iacono, Carlson, Malone, & McGue, 2002). These results have led theorists to postulate that reduced P300 response is an indicator of brain-based impairments in cognitive-executive function that confer a risk for alcohol dependence (e.g., Begleiter & Porjesz, 1999; Giancola & Tarter, 1999).
However, alcohol-related problems do not typically occur in isolation. They routinely co-occur with symptoms of other disorders such as drug dependence and antisocial personality (Kessler et al., 1997; Robins & Regier, 1991; Sher & Trull, 1994). Moreover, this comorbidity is systematic rather than random–that is, the presence of alcohol problems reliably predicts symptoms of these other disorders and vice versa (Krueger, 1999; Krueger, Caspi, Moffitt, & Silva, 1998). One interpretation of this systematic co-occurrence is that substance abuse and antisocial behavior disorders are connected at a fundamental etiologic level–that is, they are expressions of a common underlying vulnerability (Iacono, Carlson, Taylor, Elkins, & McGue, 1999; Krueger et al., 1998; Tarter, 1988). Family and twin studies provide support for this position. Antisocial behavior problems are more common among the offspring of substance abusers compared with controls (Clark et al., 1997; Luthar, Merikangas, & Rounsaville, 1993; Malone, Iacono, & McGue, 2002; Sher, Walitzer, Wood, & Brent, 1991) and rates of substance abuse are elevated in offspring of antisocial individuals (Cadoret, Yates, Troughton, Woodworth, & Stewart, 1995). Twin research studies have yielded evidence of common genetic factors underlying pairs of disorders within this spectrum (Grove et al., 1990; Pickens, Svikis, McGue, & LaBuda, 1995; Slutske et al., 1998). Moreover, recent large-scale epidemiological studies with twins have shown that the broad externalizing factor, reflecting the shared variance among disorders of this type, is substantially (>80%) heritable (Kendler, Prescott, Myers, & Neale, 2003; Krueger et al., 2002; Young, Stallings, Corley, Krauter, & Hewitt, 2000).
Of particular relevance to the current study is growing evidence that reduced P300 response is associated with other disorders in the externalizing spectrum besides alcohol dependence, including drug dependence (Attou, Figiel, & Timsit-Berthier, 2001; Biggins, MacKay, Clark, & Fein, 1997; Branchey, Buydens-Branchey, & Horvath, 1993), nicotine dependence (Anokhin et al., 2000; Iacono et al., 2002), child conduct disorder (Bauer & Hesselbrock, 1999a, 1999b; Kim, Kim, & Kwon, 2001), and adult antisocial personality (Bauer, O’Connor, & Hesselbrock, 1994; Costa et al., 2000). Furthermore, reduced P300 is associated with risk for these other disorders as well as with active symptoms. For example, Iacono et al. (2002) reported smaller P300 amplitude among adolescent males whose fathers met criteria for alcohol dependence, drug dependence, or antisocial personality in comparison to controls with no paternal history of these disorders. This was true whether or not the offspring had manifested symptoms of these disorders themselves by the age at which brain potential response was assessed (see also Brigham, Herning, & Moss, 1995). Additionally, P300 amplitude tended to be smallest among participants whose fathers met criteria for more than one externalizing disorder. Taken together, these findings suggest that reduced P300 amplitude reflects an underlying vulnerability not just to alcohol problems, but to all disorders within the externalizing spectrum.
Following from this, the current study addressed the following three interrelated questions:
-
Is There an Association between Externalizing and P300 Amplitude?
Various lines of evidence converge on the hypothesis of a link between reduced P300 and the common factor that underlies disorders within the externalizing spectrum, but this possibility has not been evaluated directly. We tested this hypothesis by performing an analysis in which P300 comprised the dependent variable, and the common factor underlying alcohol dependence, drug dependence, nicotine dependence, conduct disorder, and adult antisocial behavior was included as the primary independent variable.
-
Could the Connection between Externalizing and P300 Account for the Alcohol–P300 Relationship?
A second question was whether the association between the externalizing factor and P300 amplitude might account for the well-documented relationship between alcohol problems and reduced P300–as well as for relations between P300 and other externalizing syndromes (cf. Iacono et al., 1999). To address this question, we performed hierarchical regression analyses for each diagnostic symptom variable, examining whether its association with P300 would be substantially attenuated after controlling for scores on the externalizing factor.
-
Can P300 Amplitude Be Considered an Indicator of Externalizing Vulnerability?
A third question that we addressed was whether reduced P300 amplitude represents an indicator of externalizing vulnerability. If so, when included in a factor analysis with disorder symptom scores, P300 amplitude should show a robust association with a single common externalizing factor rather than defining a separate factor. However, because P300 is assessed in a distinct measurement domain (i.e., physiological response), we predicted that its association with this underlying externalizing factor would be lower than the associations of the diagnostic variables (cf. Campbell & Fiske, 1959).
Methods and Materials
Participants
The sample consisted of twin participants in the Minnesota Twin Family Study (MTFS), a longitudinal and epidemiological investigation of the origins and development of substance use disorders and related psychopathology. The MTFS employs a population-based ascertainment method in which all twins born in the state of Minnesota within specified birth years are identified through public birth records. (For a comprehensive description of the MTFS sample and study design, including ascertainment and recruitment procedures, see Iacono et al., 1999.) The current sample consisted of all male twin participants from the MTFS for whom diagnostic indicators of externalizing (see below) were available. Specifically, the study sample consisted of 969 adolescent males from 520 families, most assessed around the age of 17 (M=17.66; SD=0.53; range=16.66 to 20.01). This sample combined subjects from the two age cohorts of the MTFS: subjects in one cohort were 17 years old at intake whereas subjects in the other were approximately 11 years old at intake, with data for the latter coming from their second three-year follow-up assessment (i.e., data for the current study were obtained when participants in this cohort were around age 17). Consistent with the demographics of the state of Minnesota at the time the study participants were born, nearly all were Caucasian.
Clinical Assessment
Diagnostic ratings
Subjects who were still legal minors gave written assent to participate and their parents consented to their participation. Subjects who were 18 years old gave written informed consent to participate. Trained interviewers with undergraduate or Master’s degrees administered structured interviews to assess clinical disorder symptoms according to criteria listed in the Diagnostic and Statistical Manual of Mental Disorders, Third Edition-Revised (DSM-III-R; American Psychiatric Association, 1987), the diagnostic standard in place at the time data collection began.
Symptoms of alcohol, drug, and nicotine dependence were assessed using an expanded version of the Substance Abuse Module (SAM; Robins, Babor, & Cottler, 1987) from the Composite International Diagnostic Interview (Robins et al., 1988). Subjects were also interviewed using an instrument developed by MTFS staff to assess symptoms of DSM-III-R conduct disorder occurring before age 15 and adult antisocial behavior symptoms occurring after age 15 (cf. Iacono et al., 1999). Mothers of the twins reported on the substance use and childhood antisocial behaviors of each twin through interviews using the parent version of the Diagnostic Interview for Children and Adolescents–Revised (DICS-R; Reich, 2000; Welner, Reich, Herjanic, Jung, & Amado, 1987). Advanced graduate students trained in descriptive psychopathology determined the presence or absence of DSM-III-R symptoms working in teams, using a consensus, “best-estimate” approach (Kosten, Rounsaville, Kosten, & Merikangas, 1992; Leckman, Sholomskas, Thompson, Belanger, & Weissman, 1982) that combined reports from both the individual and his mother. Because youths were sole informants about their antisocial behavior occurring after age 15, these symptoms were assigned on the basis of self-report only.
Symptom counts (i.e., number of diagnostic criteria met) for each of the following disorders were used: alcohol dependence; illicit drug dependence, representing the number of symptoms of dependence on the illicit substance used most heavily, whether cannabis, amphetamines, cocaine, hallucinogens, opiates, psychedelics, or sedatives; nicotine dependence; conduct disorder (12 of the 13 criterion A symptoms; item 5, concerning coercive sexual activity, was excluded); and adult antisocial behavior (9 of the 10 criterion C symptoms of ASPD; owing to subjects’ young age, the item concerning inability to remain in a monogamous relationship was omitted). Reliability coefficients for the various disorders examined in the present study all achieved acceptable levels and are summarized in Iacono et al. (1999). Kappa coefficients were greater than or equal to .81 for the disorders considered in the present investigation. The frequencies of the various disorders among participants in the sample (where presence of the disorder was defined as probable or definite, meaning at threshold or within one symptom of threshold for a diagnosis) were as follows: alcohol dependence, 16.6%; drug dependence, 10.8%; nicotine dependence, 27.6%; conduct disorder, 38.6%; and adult antisocial behavior, 11.4%.
Externalizing variable
Scores on the externalizing factor were derived from a principal components analysis (PCA) of symptom counts for the syndromes described in the preceding section, that is, alcohol dependence, drug dependence, nicotine dependence, conduct disorder, and adult antisocial behavior. Correlations among the various symptom variables were all significant, and ranged from .33 to .59 (median=.42). For the principal components analysis, scores on each symptom variable were Blom-transformed and rank normalized in order to correct for skewness (cf. Krueger et al., 2002). This involved replacing raw scores with ranks (the mean rank was assigned in cases involving ties) and then expressing these ranks in z-score units. By standard eigenvalue and scree plot criteria, the principal components analysis yielded a single dominant component, accounting for 60% of the variance in the five disorders. Loadings of the individual symptom variables on this common externalizing factor were all robust and comparable in magnitude to those observed by Krueger et al. (2002): alcohol dependence, .81; drug dependence, .78; nicotine dependence, .78; conduct disorder, .66; and adult antisocial behavior, .85. Scores on the externalizing factor were computed for each participant using the regression method (SPSS, 2001), and these scores served as the externalizing variable in the primary analyses reported below.
Psychophysiological Assessment
We used the rotated-heads visual oddball task of Begleiter et al. (1984; see Figure 1), a procedure that yielded robust P300 effects in this and other subsequent alcohol risk studies (e.g., Hill & Steinhauer, 1993; Hill, Shen, et al., 1999; O’Connor, Bauer, Tasman, & Hesselbrock, 1994). Each of the 240 stimuli comprising this task was presented on a computer screen for 98 ms, with the intertrial interval (ITI) varying randomly between 1 and 2 s. A small dot, upon which subjects were instructed to fixate, appeared in the center of the screen during the ITI. On two-thirds of the trials, participants saw a plain oval to which they were instructed not to respond. On the remaining third of the trials, participants saw a superior view of a stylized head, depicting the nose and one ear. These stylized heads served as “target” stimuli.
Figure 1.
Schematic depiction of stimuli used in the rotated-heads visual oddball task. Nontargets (bottom) occurred on 160 trials and required no response. Each of the four target stimuli was presented on 20 trials; for these stimuli, the participant pressed a button with either the left or right hand to indicate the side of the head on which the ear was positioned. For easy head targets (top), the nose was pointed up and thus the correct button response (“left” or “right”) corresponded directly to the side of the screen on which the ear appeared. For hard targets (middle), the nose was pointed down and thus the correct button response was opposite to the side of the screen on which the ear appeared.
Participants were instructed to press one of two response buttons attached to each arm of their chair to indicate whether the ear was on the left side of the head or the right. Half of these target trials consisted of heads with the nose pointed up, such that the left ear would be on the left side of the head as it appeared to the subject (easy discrimination). Half consisted of heads rotated 180° so that the nose pointed down, such that the left ear would appear on the right side of the screen and the right ear would appear on the left side of the screen (hard discrimination).
Recording procedure
All participants completed the assessment at approximately the same time in the morning. They sat in a comfortable high-backed chair while electroencephalographic (EEG) data were recorded from three parietal scalp locations, one on the midline (Pz) and one over each hemisphere (P3 and P4). Linked earlobes served as reference and an electrode on the right shin as ground. Blinks and eye movements were recorded with a pair of biopotential electrodes arranged in a transverse montage, one electrode superior to the eye and the other over the outer canthus. A Grass Model 12A Neurodata acquisition system was used to collect EEG data, with each signal passed through an amplifier with a bandpass of 0.01 to 30 Hz (half-amplitude) and a roll-off of 6 dB per octave. For each trial, 2 s of EEG, including a 500-ms prestimulus baseline, were digitized to 12 bits resolution at a rate of 256 Hz. If participants failed to respond to a given target, or if any EEG signal exceeded the range of the A–D converter, the trial was repeated. Trials repeated more than twice were excluded from averaging.
EEG data processing and reduction
The procedure of Gratton, Coles, and Donchin (1983) was used to correct for blinks and other ocular artifacts in the EEG. Signals were digitally filtered using a third-order Butterworth highpass filter at 0.5 Hz to attenuate low frequency artifact present in some of the data due to amplifier drift. Averaged waveforms were constructed for both easy and hard discrimination conditions for the parietal electrode Pz. (Differences in activity between the lateral and central electrodes did not produce significant interactions with scores on the externalizing factor in a preliminary analysis, and thus only data from the Pz electrode are reported.) The P300 was defined as the point between 280 and 600 ms at which amplitude of the average waveform was maximal.2 P300 amplitude scores were obtained for the target conditions (easy discrimination, hard discrimination) at the Pz electrode site.
Statistical Analyses
Performance on the visual discrimination task was assessed using analysis of variance procedures. Behavioral response data were unavailable for 12 subjects, leaving 957 for these specific analyses. A mixed-model analysis of variance (ANOVA) was first conducted in which continuous externalizing factor scores and categorical target difficulty (easy vs. hard) were included as between-subjects and within-subject factors, respectively, and response accuracy (i.e., number of correct button presses following target stimuli) was the dependent variable. A second Externalizing × Target Difficulty mixed-model ANOVA was conducted with response latency (in milliseconds) for correct response trials as the dependent variable. Additionally, the relationship between externalizing scores and number of false alarms (i.e., erroneous button presses to nontarget stimuli) was examined via Pearson correlation.
For the brain potential data, two analyses were conducted. First, a mixed-model ANOVA was constructed in which continuous scores on the externalizing factor served as the between-subjects factor, target difficulty the within-subjects factor, and P300 amplitude the dependent variable. Our main hypothesis was that externalizing scores would be significantly related to P300 amplitude. Second, hierarchical regression analyses were performed separately for each DSM disorder to assess whether externalizing scores accounted for observed relations between specific disorder symptoms and P300 amplitude. The first step in each analysis was to regress P300 amplitude on the symptom count score for a given disorder. In the second step, externalizing score was added to the model. If externalizing accounts for the association between the disorder symptoms and P300 amplitude, then the effect of the disorder should be significant in the absence of the externalizing variable (i.e., in step 1) but become nonsignificant in the presence of the externalizing variable (i.e., in step 2). Our hypothesis was that the externalizing factor would account for all significant relations between individual disorders and P300 amplitude.
A final analysis was conducted to quantify the loading of P300 amplitude on the externalizing factor. This analysis was a principal components analysis incorporating P300 amplitude as a variate in addition to the four DSM-III-R symptom scores.
In what follows, only effects significant beyond p<.01 are reported and discussed as being statistically significant. This more stringent criterion was adopted to adjust for the effect of correlated observations arising from the use of data from twins, who were treated as individual cases in the analyses.3
Results
Behavioral Performance
A total of 240 stimuli were presented: 40 easy targets, 40 hard targets, and 160 non-targets. Overall, the number of correct responses to targets was very high (M=78.81 out of 80, SD=1.70) and the number of erroneous button presses to nontargets was extremely low (M=0.11, SD=0.54; only 60 subjects registered any false alarms). Response accuracy was higher for easy than hard targets, Ms=39.54 and 39.27, respectively, SDs=.90 and 1.19, F(1,955)=45.59, p<.001, and on correct response trials, response latency was faster for easy targets than hard, Ms=833.33 and 1048.16, respectively, SDs=162.16 and 221.04, F(1,955)=2608.54, p<.001. There was no significant effect of externalizing score on either target response accuracy or latency, and no Externalizing × Target Difficulty interaction was evident for either variable. The correlation between externalizing scores and false alarms was not significant, r=.061.
Brain Response
Assessing the relation between P300 amplitude and externalizing
In the analysis of P300 response amplitude to target stimuli, significant main effects were observed for both independent variables: externalizing score, F(1,967)=28.26, p<.001, and target difficulty (easy, hard), F(1,967)=35.35, p<.001. Higher externalizing was associated with significantly smaller P300. Figure 2 illustrates this effect by comparing the average ERP waveform for participants in the lowest quartile of the distribution of scores on the externalizing factor with average waveforms for individuals who scored (a) above the median, (b) in the highest quartile, and (c) in the highest decile (10%) of the distribution. Effects of target difficulty paralleled those for externalizing: Hard targets evoked smaller P300 responses than easy targets, Ms=17.29 and 17.89, respectively, SDs=5.50 and 5.51. The interaction between externalizing and target difficulty was not significant, F(1,967)=.67, n.s.
Figure 2.
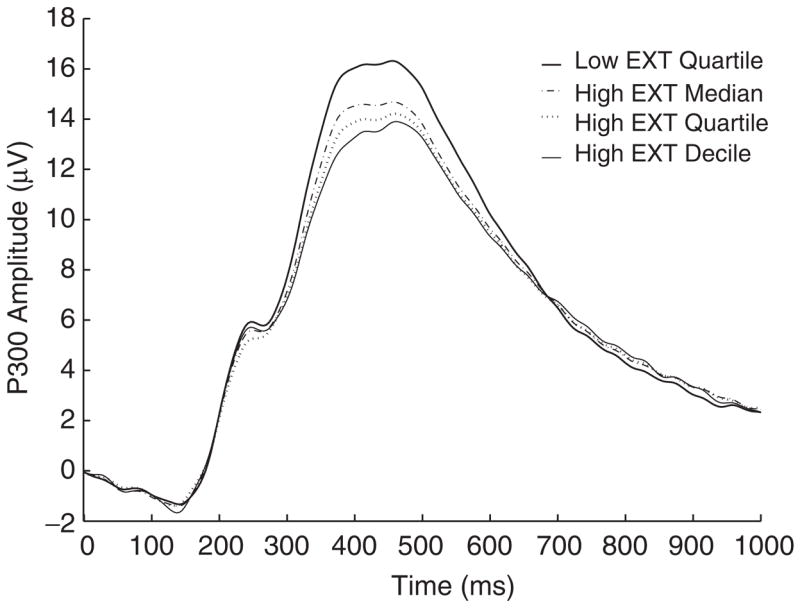
Average ERP waveforms for participants in the lowest quartile of the distribution of scores on the externalizing (EXT) factor, and for individuals who scored (a) above the median of the distribution, (b) in the highest quartile of the distribution, and (c) in the highest decile (10%) of the distribution. The externalizing factor is defined as the first principal component derived from a PCA of DSM-III-R symptoms of alcohol dependence, drug dependence, nicotine dependence, conduct disorder, and adult antisocial behavior. A score on this factor was computed for each individual participant using the regression method.
Role of externalizing in accounting for relations between individual DSM disorders and P300
Table 1 summarizes the results of hierarchical regression analyses that were conducted to determine whether observed relations between individual disorder symptoms and P300 amplitude were attributable to the shared variance among disorders represented by externalizing factor scores. Because the ANOVA that included data for all task trials revealed only a main effect of externalizing, with no moderation as a function of task difficulty (easy, hard), average P300 amplitude across all target stimuli was used as the dependent variable in these regression analyses. A significant negative association between symptom count and P300 amplitude was apparent in step 1 of the analysis for all symptom variables. However, for all disorders this relationship became negligible when externalizing score was entered in step 2. This indicates that relations between individual DSM syndromes and P300 amplitude are due to the shared variance among these disorders (i.e., externalizing). In no case did the unique variance associated with a particular disorder contribute significantly to its relation with P300 independently of externalizing.
Table 1.
Hierarchical Regression Analyses Demonstrating that Externalizing Vulnerability Accounts for Relations between Individual DSM-III-R Disorders and P300 Amplitude
| Unadjusted (step 1)
|
Adjusted (step 2)
|
|||||
|---|---|---|---|---|---|---|
| Disorder | B | t | p | B | t | p |
| Alcohol dependence | −0.70 | −4.20 | <.001 | 0.02 | 0.08 | .935 |
| Drug dependence | −0.54 | −3.18 | .002 | 0.38 | 1.42 | .156 |
| Nicotine dependence | −0.82 | −4.92 | <.001 | −0.37 | −1.39 | .164 |
| Conduct disorder | −0.60 | −3.59 | <.001 | −0.04 | −0.17 | .869 |
| Adult Antisocial behavior | −0.75 | −4.46 | <.001 | 0.02 | 0.07 | .947 |
Note. B is the raw regression coefficient obtained by regressing P300 amplitude, averaged across easy and hard target stimuli, on normalized symptom counts of each individual DSM disorder. It therefore reflects the decrease in P300 amplitude (in microvolts) associated with a standard deviation increase in symptoms of the relevant disorder. t is the test statistic for each B coefficient, and p its associated probability. The unadjusted coefficients were derived in the first step, in which disorder symptom count for each disorder was the sole predictor in the model. Adjusted coefficients were derived in the second step of each analysis, in which externalizing vulnerability score was added to the model as a second predictor, and therefore are adjusted for scores on this vulnerability factor.
P300 as an indicator of externalizing vulnerability
To evaluate P300 amplitude as an indicator of externalizing and to quantify its relation with the externalizing factor, we conducted a principal components analysis including overall P300 amplitude scores for each participant (i.e., across easy and hard targets) along with symptom scores for the four DSM-III-R disorders as variates. The analysis yielded evidence of a single dominant component: Only one eigenvalue exceeded a magnitude of 1 (i.e., 3.07), and the scree plot revealed a marked break after the first component (i.e., remaining eigenvalues=0.96, 0.71, 0.47, 0.45, and 0.35, respectively). The first component accounted for 51.10% of the total variance in scores. The loadings of the five symptom variables on this component were: alcohol dependence, .81; drug dependence, .77; nicotine dependence, .78; conduct disorder, .66; and adult antisocial behavior, .85. The loading for P300 amplitude was −.25. Figure 3 depicts the association between P300 amplitude and scores on the broad externalizing factor across individuals in this analysis.
Figure 3.
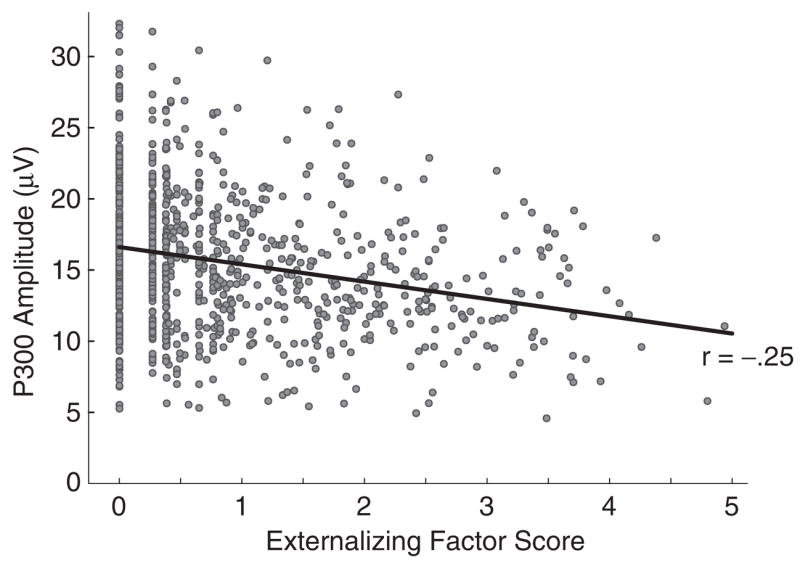
Scatterplot of the association between mean P300 amplitude and continuous scores on the externalizing factor across all study participants (N=969); solid line is best-fitting regression line. A standard geometric transformation, using the rotation matrix
where θ represents the desired rotation angle, was applied to the raw factor score data in order to vertically align low scores on externalizing; the data were normalized to unit-length axes prior to rotation and rescaled to the original units afterward. For purposes of plotting, the minimum score on the externalizing factor was subtracted from each resultant value, such that 0 now represents the minimum score, rather than the mean.
We evaluated the significance of the loading of each variable on the first principal component by computing 99% bootstrap confidence intervals for the values of these loadings. Each bootstrap confidence interval was bias-corrected and accelerated, based on 10,000 bootstrap samples. Bias-corrected and accelerated bootstrap confidence intervals have been shown to approximate actual coverage well when exact values are known (Efron & Tibishirani, 1998). In no case did the 99% confidence interval include zero–that is, loadings for all variables, including P300 amplitude, were significant at p<.01.
Discussion
Based on recent research findings, we predicted a relationship between P300 brain potential amplitude and the broad externalizing factor linking alcohol dependence, drug dependence, nicotine dependence, conduct disorder, and adult antisocial deviance. In a multivariate analysis in which continuous externalizing scores, defined as scores on the first component extracted from a PCA of symptom scores for these disorders, were included as a factor along with task difficulty (easy vs. hard), a robust main effect of externalizing was found: Higher scores on the externalizing factor, reflecting greater severity and breadth of externalizing symptoms, were associated with smaller P300 amplitude.
With regard to the second major question of the study, a hierarchical regression analysis revealed that scores on the broad externalizing factor accounted for the association between alcohol dependence and P300 amplitude in the current study sample. When externalizing was controlled statistically, the significant alcohol–P300 correlation was reduced to nonsignificance. Parallel analyses revealed that relations between P300 and each of the other symptom variables (drug dependence, nicotine dependence, conduct disorder, and adult antisocial behavior) were also attributable entirely to this general externalizing factor. This pattern of results has important implications. It encourages a shift away from the perspective that reduced P300 is associated specifically with alcohol problems. It also provides an alternative to the idea that the association between P300 and alcohol problems is mediated by its relationship with antisocial deviance (cf. Bauer & Hesselbrock, 1999a, 1999b). Instead, the current data suggest that it is what these disorders have in common, rather than what is unique to any one of them, that is primarily associated with reduced P300 response.
Finally, when mean P300 amplitude was included with the five diagnostic variables in a principal components analysis, a single dominant factor was evident on which all variables loaded significantly. Because it reflects a separate measurement domain with its own unique method variance (cf. Campbell & Fiske, 1959), the loading of P300 on the common externalizing factor was modest (−.25) in comparison to the very high loadings for the symptom variables (M=.77)–but it was nonetheless statistically robust. Moreover, the fact that P300 amplitude loaded with the symptom variables on a common factor rather than defining a separate method component indicates that it is tapping the same underlying construct as the symptom variables.
What is the nature of the common factor on which P300 amplitude loads along with symptoms of these various externalizing disorders? The findings of recent behavior genetic investigations suggest that this factor reflects a broad underlying neurobiological vulnerability to disorders of this kind. For example, Krueger et al. (2002) assessed genetic and environmental contributions to this factor (defined as the shared variance among symptoms of alcohol dependence, drug dependence, conduct disorder, adult antisocial behavior, and disinhibitory personality) in a large twin cohort. The externalizing factor was found to be strongly (81%) genetic (see also Kendler et al., 2003; Young et al., 2000). In contrast, environmental factors contributed most to the unique part of each disorder not attributable to this common factor. These findings support a hierarchical model of the externalizing spectrum in which varying disorders are viewed as alternative manifestations of a broad, heritable vulnerability. This broad vulnerability confers a risk for a range of different disorders. However, its expression as one disorder or another is determined by specific causal influences.
The present results establish that reduced P300 is associated with higher externalizing vulnerability as defined by current symptomatology. However, P300 response amplitude, like externalizing vulnerability, is highly heritable (Katsanis, Iacono, McGue, & Carlson, 1997; O’Connor, Morzorati, Christian, & Li, 1994; van Beijsterveldt, Molenaar, de Geus, & Boomsma, 1998). This raises the possibility that reduced P300 amplitude may represent a quantitative endophenotype of externalizing vulnerability. An endophenotype is a biological characteristic that arises from, and thus directly reflects, an underlying genotypic predisposition (Gottesman & Shields, 1972; Iacono, 1998; John & Lewis, 1966).4
If reduced P300 is an endophenotype for externalizing vulnerability, it should also occur at higher rates among asymptomatic individuals who are at risk for developing externalizing problems by virtue of a positive parental history of such problems (cf. Elmasian et al., 1982). Consistent with this, Iacono et al. (2002) reported reduced P300 in the adolescent sons of fathers who met criteria for alcohol dependence, drug abuse/dependence, or antisocial personality, whether or not the offspring themselves met criteria for a diagnosis. This study also found that reduced P300 at age 17 predicted the development of externalizing problems of various kinds at age 20, even among individuals who were free from disorder at the time of P300 assessment. From these findings, it seems reasonable to expect that higher levels of externalizing vulnerability in fathers would predict smaller P300 amplitude in their offspring, and that individuals with reduced P300 amplitude early in life would show higher levels of externalizing symptomatology later in life. These hypotheses will be interesting to test in future research.
Concerning the status of P300 as an indicator of externalizing vulnerability, an important question concerns its specificity, because P300 amplitude reduction has also been found in disorders outside the externalizing spectrum–most notably major depression and schizophrenia (e.g., Blackwood et al., 1987; Bruder et al., 1995; Roth & Cannon, 1972). With regard to the former, comorbid depression has been found to account for reduced P300 amplitude in some alcoholic samples (e.g., Hill, Locke, & Steinhauer, 1999). However, in the current study, lifetime reports of symptoms of major depression, assessed via the Structured Clinical Interview for DSM-III-R Axis I (SCID-I; Spitzer, Williams, Gibbon, & First, 1992), showed only a marginal association with P300 amplitude, r= −.05, p>.14, and the relation between externalizing and P300 remained highly significant after controlling for depressive symptoms. Moreover, in contrast with alcoholism, P300 amplitude shows a return to normal levels when depressive symptoms remit (Yanai, Fujikawa, Osada, Yamawaki, & Touhouda, 1997), and reduced P300 has not been shown to predict the emergence of depression in persons at risk for mood disorder. This suggests that reduced P300 may act as a state (vs. trait) indicator of depression. On the other hand, evidence does exist for P300 as a trait marker of schizophrenia (cf. Ford, 1999). However, the association between schizophrenia and P300 is more reliable for auditory than visual tasks (Jeon & Polich, 2003), whereas the reverse is true for alcohol problems (Polich et al., 1994)–suggesting that the mechanisms underlying P300 reduction in schizophrenia and externalizing psychopathology are not identical. Nevertheless, more research would be needed to establish the specificity of visual P300 amplitude reduction as a vulnerability marker for externalizing problems.
A further issue concerns the generality of our findings. The current sample consisted of community males assessed around age 17. Some recent research suggests that there may be a developmental transition in the association between alcohol risk and P300: In a large-scale longitudinal study comparing individuals from families with a high density of alcohol dependence with control participants, Hill, Shen, et al. (1999) reported a marked reduction of P300 amplitude in high-risk individuals during childhood and adolescence relative to age-matched controls, but this group difference became less reliable in early adulthood. There is also evidence that reduced P300 amplitude may be less indicative of risk for alcohol problems in women than in men, especially when using visual tasks (Hill, Muka, Steinhauer, & Locke, 1995). Relations between P300 and other externalizing syndromes may also be less reliable among women than men (Iacono et al., 1999; Justus, Finn, & Steinmetz, 2001). In future studies of the association between externalizing vulnerability and P300, it will be important to examine moderating effects of age and gender.
A final question concerns the neurobiological underpinnings of the externalizing vulnerability factor, and the basis of its association with P300 brain potential response. Begleiter and Porjesz (1999) postulated that the inherited predisposition to alcohol dependence entails a hyperexcitability of the central nervous system that enhances risk for a variety of dishinhibitory syndromes. In this model, reduced P300 amplitude is believed to reflect a diminished capacity for neuronal inhibition. Relatedly, Iacono, Carlson, and Malone (2000) proposed that vulnerability to substance abuse and antisocial deviance reflects a dysfunction in inhibitory control associated with frontal brain regions (see also Giancola & Tarter, 1999), and that reduced P300 response is one indicator of this inhibitory deficit.
A notable feature of the current findings was that reduced P300 amplitude among high externalizing individuals was not accompanied by reduced performance on the visual discrimination task. This lack of behavioral differences helps to rule out the possibility that brain response differences were due to differential engagement in the task. On the other hand, it might be argued that the lack of behavioral differences calls into question the functional significance of brain response differences. However, we would assert that the presence of P300 brain response differences in the context of normal behavioral performance implies a meaningful difference in cognitive processing associated with the task–for example, reduced cognitive-evaluative processing of the task stimuli, or a difference in “cognitive set” or strategy associated with performance of the task. Although the precise nature of this difference in cognitive processing remains to be elucidated, the current findings are nonetheless important because they establish reduced P300 in the context of this task as an indicator of general externalizing vulnerabilty.
A key challenge for future research will be to precisely identify the neuro-cognitive processing impairments that underlie the vulnerability to these types of problems, the brain systems that are involved, and the genes that contribute to these brain-based differences. We argue on the basis of the current findings that the primary target phenotype in this effort should be the externalizing vulnerability that these disorders share, definable as the covariance among disorders within this spectrum (Krueger et al., 2002). This vulnerability is highly heritable and, as we have shown, it accounts for relations between P300 and specific externalizing disorders. As such, it is a logical referent for genetic and neurobiological studies. At the same time, we advocate complementary research aimed at elucidating unique etiologic factors that shape the expression of this general vulnerability in specific ways (i.e., as alcohol dependence, drug dependence, or antisocial behavior) and identifying other problem behaviors and syndromes that arise from this broad trait disposition.
Acknowledgments
This research was supported by grants MH 65137, DA 05147, and AA 09367 from the National Institutes of Health, and by funds from the Hathaway endowment at the University of Minnesota.
Footnotes
The term P3b is sometimes used for this frequency-sensitive component, to distinguish it from the “P3a” or “novelty P3,” maximal at fronto-central sites, which follows the occurrence of an unexpected rare nontarget stimulus (Coles & Rugg, 1995). Unless otherwise specified, “P300” here refers to the P3b component, which has been studied most extensively in relation to substance abuse and other externalizing disorders.
In addition to analyses employing these peak amplitude scores, we performed a principal components analysis on the ERP waveform to isolate the P300 component, and reran the main analysis assessing the relation between P300 and externalizing using the scores for this component in place of the peak amplitude scores. This analysis yielded essentially the same results. Because the peak amplitude parameter is more straightforward and more commonly used in psychopathology studies, we report this as the primary measure in the current analyses.
We also conducted supplementary analyses in which we directly controlled for the correlation between twins with respect to P300 amplitude. These analyses employed multilevel models with random intercepts to account for characteristics shared by twins that relate to P300 amplitude (cf. Goldstein, 1995). The results of these more complex supplemental analyses–consisting of multivariate analyses with target difficulty and externalizing as fixed effects as well as multilevel versions of the mediational analyses–corroborated those of our primary analyses. Because the primary analyses are more readily interpretable, we focus on the findings of these in the main text.
It is conceivable that reduced P300 might reflect the toxic effects of long-term alcohol consumption, rather than an underlying, genetically mediated vulnerability. The youth of subjects in this study argues against viewing P300 amplitude reductions associated with externalizing as a consequence of their substance use, rather than a constitutional liability. However, a number of subjects had considerable experience with alcohol, even to the point of meeting diagnostic criteria for dependence. A measure of estimated alcohol consumption in the preceding year was, in fact, correlated with both P300 amplitude and externalizing. Nonetheless, partial correlations between externalizing and P300 amplitude measures, holding estimated consumption constant, remained highly significant, whereas correlations between estimated consumption and P300, holding externalizing constant, were nonsignificant. These results support an interpretation of P300 amplitude reduction as related to an underlying vulnerability to externalizing problems, rather than to neurotoxic effects of excessive alcohol consumption.
References
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 3. Washington, DC: Author; 1987. [Google Scholar]
- Anokhin AP, Vedeniapin AB, Sirevaag EJ, Bauer LO, O’Connor SJ, Kuperman S, et al. The P300 brain potential is reduced in smokers. Psychopharmacology. 2000;149:409–413. doi: 10.1007/s002130000387. [DOI] [PubMed] [Google Scholar]
- Attou A, Figiel C, Timsit-Berthier M. ERP assessment of heroin detoxification and methadone treatment in chronic heroin users. Clinical Neurophysiology. 2001;31:171–180. doi: 10.1016/s0987-7053(01)00253-2. [DOI] [PubMed] [Google Scholar]
- Bauer LO, Hesselbrock VM. P300 decrements in teenagers with conduct problems: Implications for substance abuse risk and brain development. Biological Psychiatry. 1999a;46:263–272. doi: 10.1016/s0006-3223(98)00335-7. [DOI] [PubMed] [Google Scholar]
- Bauer LO, Hesselbrock VM. Subtypes of family history and conduct disorder: Effects on P300 during the Stroop test. Neuropsychopharmacology. 1999b;21:51–62. doi: 10.1016/S0893-133X(98)00139-0. [DOI] [PubMed] [Google Scholar]
- Bauer LO, O’Connor S, Hesselbrock VM. Frontal P300 decrements in antisocial personality disorder. Alcoholism: Clinical and Experimental Research. 1994;18:1300–1305. doi: 10.1111/j.1530-0277.1994.tb01427.x. [DOI] [PubMed] [Google Scholar]
- Begleiter H, Porjesz B. What is inherited in the predisposition toward Alcoholism? A proposed model. Alcholism: Clinical and Experimental Research. 1999;23:1125–1135. doi: 10.1111/j.1530-0277.1999.tb04269.x. [DOI] [PubMed] [Google Scholar]
- Begleiter H, Porjesz B, Bihari B, Kissin B. Event-related brain potentials in boys at risk for alcoholism. Science. 1984;225:1493–1496. doi: 10.1126/science.6474187. [DOI] [PubMed] [Google Scholar]
- Berman SM, Whipple SC, Fitch RJ, Noble EP. P3 in young boys as a predictor of adolescent substance use. Alcohol. 1993;10:69–76. doi: 10.1016/0741-8329(93)90055-s. [DOI] [PubMed] [Google Scholar]
- Biggins CA, MacKay S, Clark W, Fein G. Event-related potential evidence for frontal cortex effects of chronic cocaine dependence. Biological Psychiatry. 1997;42:472–485. doi: 10.1016/S0006-3223(96)00425-8. [DOI] [PubMed] [Google Scholar]
- Blackwood DH, Whalley LJ, Christie JE, Blackburn IM, St Clair DM, McInnes A. Changes in auditory P3 event-related potential in schizophrenia and depression. British Journal of Psychiatry. 1987;150:154–160. doi: 10.1192/bjp.150.2.154. [DOI] [PubMed] [Google Scholar]
- Branchey MH, Buydens-Branchey L, Horvath TB. Event-related potentials in substance-abusing individuals after long-term abstinence. American Journal of Addictions. 1993;2:141–148. [Google Scholar]
- Brigham J, Herning RI, Moss HB. Event-related potentials and alpha synchronization in preadolescent boys at risk for psychoactive substance use. Biological Psychiatry. 1995;37:834–846. doi: 10.1016/0006-3223(94)00218-R. [DOI] [PubMed] [Google Scholar]
- Bruder GE, Tenke CE, Stewart JW, Towey JP, Leite P, Voglmaier M, et al. Brain event-related potentials to complex tones in depressed patients: Relations to perceptual asymmetry and clinical features. Psychophysiology. 1995;32:373–381. doi: 10.1111/j.1469-8986.1995.tb01220.x. [DOI] [PubMed] [Google Scholar]
- Cadoret RI, Yates WR, Troughton E, Woodworth G, Stewart MA. Adoption study demonstrating two genetic pathways to drug abuse. Archives of General Psychiatry. 1995;52:42–52. doi: 10.1001/archpsyc.1995.03950130042005. [DOI] [PubMed] [Google Scholar]
- Campbell DT, Fiske DW. Convergent and discriminant validity by the multitrait-multimethod matrix. Psychological Bulletin. 1959;56:81–105. [PubMed] [Google Scholar]
- Clark DB, Moss HB, Kirisci L, Mezzich AG, Miles R, Ott P. Psychopathology in preadolescent sons of fathers with substance use disorders. Journal of the American Academy of Child and Adolescent Psychiatry. 1997;36:495–505. doi: 10.1097/00004583-199704000-00012. [DOI] [PubMed] [Google Scholar]
- Coles MGH, Rugg MD. Event-related potentials: An introduction. In: Rugg MD, Coles MGH, editors. Electrophysiology of mind: Event-related potentials and cognition. New York: Oxford University Press; 1995. pp. 1–26. [Google Scholar]
- Costa L, Bauer L, Kuperman S, Porjesz B, O’Connor S, Hesselbrock V, et al. Frontal P300 decrements, alcohol dependence, and antisocial personality disorder. Biological Psychiatry. 2000;47:1064–1071. doi: 10.1016/s0006-3223(99)00317-0. [DOI] [PubMed] [Google Scholar]
- Efron B, Tibishirani RJ. An introduction to the bootstrap. Boca Raton, FL: Chapman & Hall; 1998. [Google Scholar]
- Elmasian R, Neville H, Woods D, Schuckit M, Bloom F. Event-related brain potentials are different in individuals at high and low-risk for developing alcoholism. Proceedings of the National Academy of Sciences. 1982;79:7900–7903. doi: 10.1073/pnas.79.24.7900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford JM. Schizophrenia: The broken P300 and beyond. Psychophysiology. 1999;36:667–682. [PubMed] [Google Scholar]
- Giancola PR, Tarter RE. Executive cognitive functioning and risk for substance abuse. Psychological Science. 1999;10:203–205. [Google Scholar]
- Goldstein H. Multilevel statistical models. 2. London: Edward Arnold; 1995. [Google Scholar]
- Gottesman II, Shields J. Schizophrenia and genetics: A twin study vantage point. New York: Academic Press; 1972. [Google Scholar]
- Gratton G, Coles MG, Donchin E. A new method for off-line removal of ocular artifact. Electroencephalography and Clinical Neurophysiology. 1983;55:468–484. doi: 10.1016/0013-4694(83)90135-9. [DOI] [PubMed] [Google Scholar]
- Grove WM, Eckert ED, Heston L, Bouchard TJ, Jr, Segal N, Lykken DT. Heritability of substance abuse and antisocial behavior: A study of monozygotic twins reared apart. Biological Psychiatry. 1990;27:1293–1304. doi: 10.1016/0006-3223(90)90500-2. [DOI] [PubMed] [Google Scholar]
- Hill SY, Locke J, Steinhauer SR. Absence of visual and auditory P300 reduction in non-depressed male and female alcoholics. Biological Psychiatry. 1999;46:982–989. doi: 10.1016/s0006-3223(99)00054-2. [DOI] [PubMed] [Google Scholar]
- Hill SY, Muka D, Steinhauer S, Locke J. P300 amplitude decrements in children from families of alcoholic female probands. Biological Psychiatry. 1995;37:622–632. doi: 10.1016/0006-3223(94)00384-7. [DOI] [PubMed] [Google Scholar]
- Hill SY, Shen S. Neurodevelopmental patterns of visual P3b in association with familial risk for alcohol dependence and childhood diagnosis. Biological Psychiatry. 2002;51:621–631. doi: 10.1016/s0006-3223(01)01301-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill SY, Shen S, Locke J, Steinhauer SR, Konicky C, Lowers L, Connolly J. Developmental delay in P300 production in children at high risk for developing alcohol-related disorders. Biological Psychiatry. 1999;46:970–981. doi: 10.1016/s0006-3223(99)00032-3. [DOI] [PubMed] [Google Scholar]
- Hill SY, Steinhauer SR. Assessment of prepubertal and postpubertal boys and girls at risk for developing alcoholism with P300 from a visual discrimination task. Journal of Studies on Alcohol. 1993;54:350–358. doi: 10.15288/jsa.1993.54.350. [DOI] [PubMed] [Google Scholar]
- Hill SY, Steinhauer S, Lowers L, Locke J. Eight-year longitudinal follow-up of P300 and clinical outcome in children from high-risk for alcoholism families. Biological Psychiatry. 1995;37:823–827. doi: 10.1016/0006-3223(95)00041-E. [DOI] [PubMed] [Google Scholar]
- Iacono WG. Identifying psychophysiological risk for psychopathology: Examples from substance abuse and schizophrenia research. Psychophysiology. 1998;35:621–637. [PubMed] [Google Scholar]
- Iacono WG, Carlson SR, Malone SM. Identifying a multivariate endophenotype for substance use disorders using psychophysiological measures. International Journal of Psychophysiology. 2000;38:81–96. doi: 10.1016/s0167-8760(00)00132-x. [DOI] [PubMed] [Google Scholar]
- Iacono WG, Carlson SR, Malone SM, McGue M. P3 event-related potential amplitude and risk for disinhibitory disorders in adolescent boys. Archives of General Psychiatry. 2002;59:750–757. doi: 10.1001/archpsyc.59.8.750. [DOI] [PubMed] [Google Scholar]
- Iacono WG, Carlson SR, Taylor J, Elkins IJ, McGue M. Behavioral disinhibition and the development of substance use disorders: Findings from the Minnesota Twin Family Study. Development and Psychopathology. 1999;11:869–900. doi: 10.1017/s0954579499002369. [DOI] [PubMed] [Google Scholar]
- Jeon YW, Polich J. Meta-analysis of P300 and schizophrenia: Patients, paradigms, and practical implications. Psychophysiology. 2003;40:684–701. doi: 10.1111/1469-8986.00070. [DOI] [PubMed] [Google Scholar]
- John B, Lewis K. Chromosome variability and geographic distribution in insects. Science. 1966;152:711–721. doi: 10.1126/science.152.3723.711. [DOI] [PubMed] [Google Scholar]
- Justus AN, Finn PR, Steinmetz JE. P300, disinhibited personality, and early alcohol problems. Alcoholism: Clinical and Experimental Research. 2001;25:1457–1466. doi: 10.1097/00000374-200110000-00008. [DOI] [PubMed] [Google Scholar]
- Katsanis J, Iacono WG, McGue MK, Carlson SR. P300 event-related potential heritability inmonozygotic and dizygotic twins. Psychophysiology. 1997;34:47–58. doi: 10.1111/j.1469-8986.1997.tb02415.x. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Prescott CA, Myers J, Neale MC. The structure of genetic and environmental risk factors for common psychiatric and substance use disorders in men and women. Archives of General Psychiatry. 2003;60:929–937. doi: 10.1001/archpsyc.60.9.929. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Crum RM, Warner LA, Nelson CB, Schulenberg J, Anthony JC. Lifetime co-occurrence of DSM-III-R alcohol abuse and dependence with other psychiatric disorders in the National Comorbidity Survey. Archives of General Psychiatry. 1997;54:313–321. doi: 10.1001/archpsyc.1997.01830160031005. [DOI] [PubMed] [Google Scholar]
- Kim MS, Kim JJ, Kwon JS. Frontal P300 decrement and executive dysfunction in adolescents with conduct problems. Child Psychiatry and Human Development. 2001;32:93–106. doi: 10.1023/a:1012299822274. [DOI] [PubMed] [Google Scholar]
- Kosten TR, Rounsaville BJ, Kosten TA, Merikangas KR. Gender differences in the specificity of alcoholism transmission among the relatives of opioid addicts. Journal of Nervous and Mental Disease. 1992;179:392–400. doi: 10.1097/00005053-199107000-00002. [DOI] [PubMed] [Google Scholar]
- Krueger RF. The structure of common mental disorders. Archives of General Psychiatry. 1999;56:921–926. doi: 10.1001/archpsyc.56.10.921. [DOI] [PubMed] [Google Scholar]
- Krueger RF, Caspi A, Moffitt TE, Silva PA. The structure and stability of common mental disorders (DSM-III-R): A longitudinal-epidemiological study. Journal of Abnormal Psychology. 1998;107:216–227. doi: 10.1037//0021-843x.107.2.216. [DOI] [PubMed] [Google Scholar]
- Krueger RF, Hicks B, Patrick CJ, Carlson S, Iacono WG, McGue M. Etiologic connections among substance dependence, antisocial behavior, and personality: Modeling the externalizing spectrum. Journal of Abnormal Psychology. 2002;111:411–424. [PubMed] [Google Scholar]
- Leckman JF, Sholomskas D, Thompson WD, Belanger A, Weissman MM. Best estimate of lifetime psychiatric diagnosis: A methodological study. Archives of General Psychiatry. 1982;39:879–883. doi: 10.1001/archpsyc.1982.04290080001001. [DOI] [PubMed] [Google Scholar]
- Luthar SS, Merikangas KR, Rounsaville BJ. Parental psychopathology and disorders in offspring: A study of relatives of drug abusers. Journal of Nervous and Mental Disease. 1993;181:351–357. doi: 10.1097/00005053-199306000-00003. [DOI] [PubMed] [Google Scholar]
- Malone SM, Iacono WG, McGue M. Drinks of the father: Father’s maximum number of drinks consumed predicts externalizing disorders, substance abuse, and substance use disorders in preadolescent and adolescent offspring. Alcoholism: Clinical and Experimental Research. 2002;26:1823–1832. doi: 10.1097/01.ALC.0000042222.59908.F9. [DOI] [PubMed] [Google Scholar]
- O’Connor S, Bauer L, Tasman A, Hesselbrock V. Reduced P3 amplitudes are associated with both a family history of alcoholism and antisocial personality disorder. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 1994;18:1307–1321. doi: 10.1016/0278-5846(94)90095-7. [DOI] [PubMed] [Google Scholar]
- O’Connor S, Morzorati S, Christian JC, Li TK. Heritable features of the auditory oddball event-related potential: Peaks, latencies, morphology and topography. Electroencephalography and Clinical Neurophysiology. 1994;92:115–125. doi: 10.1016/0168-5597(94)90052-3. [DOI] [PubMed] [Google Scholar]
- Pickens RW, Svikis DS, McGue M, LaBuda MC. Common genetic mechanisms in alcohol, drug, and mental disorder comorbidity. Drug and Alcohol Dependence. 1995;39:129–138. doi: 10.1016/0376-8716(95)01151-n. [DOI] [PubMed] [Google Scholar]
- Polich J, Pollock VE, Bloom FE. Meta-analysis of P300 amplitude from males at risk for alcoholism. Psychological Bulletin. 1994;115:55–73. doi: 10.1037/0033-2909.115.1.55. [DOI] [PubMed] [Google Scholar]
- Porjesz B, Begleiter H, Garozzo R. Visual evoked potential correlates of information processing deficits in chronic alcoholics. In: Begleiter H, editor. Biological effects of alcohol. New York: Plenum; 1980. pp. 603–623. [DOI] [PubMed] [Google Scholar]
- Reich W. Diagnostic Interview for Children and Adolescents (DICA) Journal of the American Academy of Child and Adolescent Psychiatry. 2000;39:59–66. doi: 10.1097/00004583-200001000-00017. [DOI] [PubMed] [Google Scholar]
- Robins LN, Babor TF, Cottler LB. Composite international diagnostic interview: Expanded substance abuse module. St. Louis: Authors; 1987. [Google Scholar]
- Robins LN, Regier DA. Psychiatric disorders in America: The epidemiological catchment area study. New York: The Free Press; 1991. [Google Scholar]
- Robins LN, Wing J, Wittchen HU, Helzer JE, Babor TF, Burke J, et al. The composite international diagnostic interview. Archives of General Psychiatry. 1988;45:1069–1077. doi: 10.1001/archpsyc.1988.01800360017003. [DOI] [PubMed] [Google Scholar]
- Roth WT, Cannon EH. Some features of the auditory evoked response in schizophrenics. Archives of General Psychiatry. 1972;27:466–471. doi: 10.1001/archpsyc.1972.01750280034007. [DOI] [PubMed] [Google Scholar]
- Sher KJ, Trull TJ. Personality and disinhibitory psychopathology: Alcoholism and antisocial personality disorder. Journal of Abnormal Psychology. 1994;103:92–102. doi: 10.1037//0021-843x.103.1.92. [DOI] [PubMed] [Google Scholar]
- Sher KJ, Walitzer KS, Wood P, Brent EE. Characteristics of children of alcoholics: Putative risk factors, substance use and abuse, and psychopathology. Journal of Abnormal Psychology. 1991;100:427–448. doi: 10.1037//0021-843x.100.4.427. [DOI] [PubMed] [Google Scholar]
- Slutske WS, Heath AC, Dinwiddie SH, Madden PAF, Bucholz KK, Dunne MP, et al. Common genetic risk factors for conduct disorder and alcohol dependence. Journal of Abnormal Psychology. 1998;107:363–374. doi: 10.1037//0021-843x.107.3.363. [DOI] [PubMed] [Google Scholar]
- Spitzer RL, Williams JBW, Gibbon M, First MB. The structured clinical interview for DSM-III-R (SCID). I: History, rationale, and description. Archives of General Psychiatry. 1992;49:624–636. doi: 10.1001/archpsyc.1992.01820080032005. [DOI] [PubMed] [Google Scholar]
- SPSS. SPSS for Windows (Version 11.0.1) Chicago, IL: Author; 2001. [Google Scholar]
- Tarter RE. Are there inherited behavioral traits that predispose to substance abuse? Journal of Consulting and Clinical Psychology. 1988;56:189–196. doi: 10.1037//0022-006x.56.2.189. [DOI] [PubMed] [Google Scholar]
- van Beijsterveldt CE, Molenaar PC, de Geus EJ, Boomsma DI. Individual differences in P300 amplitude: A genetic study in adolescent twins. Biological Psychology. 1998;47:97–120. doi: 10.1016/s0301-0511(97)00025-2. [DOI] [PubMed] [Google Scholar]
- Welner Z, Reich W, Herjanic B, Jung K, Amado H. Reliability, validity, and parent-child agreement studies of the Diagnostic Interview for Children and Adolescents (DICA) Journal of the American Academy of Child and Adolescent Psychiatry. 1987;26:649–653. doi: 10.1097/00004583-198709000-00007. [DOI] [PubMed] [Google Scholar]
- Yanai I, Fujikawa T, Osada M, Yamawaki S, Touhouda Y. Changes in auditory P300 in patients with major depression and silent cerebral infarction. Journal of Affective Disorders. 1997;46:263–271. doi: 10.1016/s0165-0327(97)00100-6. [DOI] [PubMed] [Google Scholar]
- Young SE, Stallings MC, Corley RP, Krauter KS, Hewitt JK. Genetic and environmental influences on behavioral disinhibition. American Journal of Medical Genetics (Neuropsychiatric Genetics) 2000;96:684–695. [PubMed] [Google Scholar]



