Abstract
Background
Research has documented high levels of co-morbidity among childhood externalizing disorders, but its etiology remains in dispute. Specifically, although all behavior genetic studies of the etiology of the co-occurrence of attention deficit-hyperactivity disorder (ADHD), oppositional defiant disorder (ODD), and conduct disorder (CD) agree that genetic factors are important, differences exist across studies in the relative weight assigned to genetic, shared environmental factors (i.e. factors that increase similarity among family members), and non-shared environmental factors (i.e. factors that decrease similarity among family members). Because heritability estimates can vary across informants, we used a biometric informant-effects model to determine whether these discrepancies were a function of systematic differences in maternal and child informant reports of ADHD, CD, and ODD.
Method
We studied 1782 11-year-old twins from the Minnesota Twin Family Study. Symptom counts for each disorder were obtained from interviews administered to twins and their mothers. We fit a model that allowed us to examine, both across and within informants, the genetic and environmental contributions to the co-occurrence among ADHD, CD, and ODD.
Results
The results revealed that the co-occurrence among the disorders common to maternal and child informant reports was influenced largely by shared environmental forces. Genetic factors also contributed, though their impact was only marginally significant. In contrast, the co-occurrence unique to each informant was influenced exclusively by either genetic or non-shared environmental factors.
Conclusions
Such findings offer additional evidence that shared environmental factors are important to the co-morbidity among ADHD, CD, and ODD, and highlight the necessity of considering informant effects when drawing conclusions about the origins of co-morbidity from analyses of genetically informative data.
INTRODUCTION
There is extensive evidence of ‘co-morbidity ’, or co-occurrence at greater than chance levels (ranging between 29 and 71%), among attention deficit-hyperactivity disorder (ADHD), oppositional defiant disorder (ODD), and conduct disorder (CD; Simono. et al. 1997; Waschbusch, 2002). Moreover, these high rates of co-morbidity have important ramifications, as those with multiple disorders have more serious clinical courses with poorer outcomes, more peer difficulties and behavioral aggression, more negative parent-child relationships, more severe psychopathology, and in adulthood, higher rates of psychiatric in-patient admissions than do individuals with single disorders (Dalsgaard et al. 2002; Waschbusch, 2002).
Understanding the origins of this co-morbidity is therefore critical. One useful tool for such an exploration is that of twin studies, which enables us to identify etiological contributions to co-morbidity. Using this approach, a handful of studies have found that the co-morbidity among the child-externalizing disorders is primarily genetically mediated (Silberg et al. 1996; Nadder et al. 1998; Young et al. 2000; Thapar et al. 2001). However, these studies have used only a single informant to assess disorder or symptom presence, rather than a combined informant approach. In contrast, analyses of combined mother- and child-reported symptom counts of ADHD, CD, and ODD revealed that genetic factors contributed only a moderate amount (roughly 30%) to disorder covariation, while a single shared environmental factor (i.e. those environmental forces that increase similarity among family members) accounted for the majority (53%) of the covariation (Burt et al. 2001).
Importantly, these different conclusions have distinct ramifications. In one case, future research would seek only to identify specific genes that predispose one to co-morbid disorders. In the other, future research would also explore phenomena within the child’s familial and contextual environmental (i.e. negative parent-child relationships, etc.) and their contributions to co-morbidity.
Accordingly, there is a need to examine whether informant effects could account for the disparate twin study results noted above. Recently, investigators have argued that the use of a single informant for studies of childhood psychiatric disorders may bias estimates of genetic and environmental contributions (Eaves et al. 1997; Sherman et al. 1997; Simono. et al. 1998; Eaves et al. 2000). Specifically, maternal informant reports appear to consistently yield substantially larger genetic effects than do child reports across multiple childhood disorders (Eaves et al. 1997). For ADHD in particular, such findings may be the result of rater contrast effects (Simono. et al. 1998; Eaves et al. 2000), in which mothers tend to overemphasize the hyperactivity differences in their twins. As this contrast effect appears to be particularly pronounced in the case of dizygotic (DZ) twins (Simono. et al. 1998), acting to suppress DZ correlations, heritability estimates of ADHD are correspondingly inflated. For CD and ODD, however, it may be that mothers over-report the similarities between their monozygotic (MZ) twins, thereby increasing their correlation and similarly acting to artifactually inflate heritability estimates. In both cases, however, the central issue is one of shared method variance, in that the same informant (i.e. the mother) is reporting on symptom presence in both twins, which may act to distort heritability estimates.
Child self-reports, however, may also be troublesome owing to the limited capacity of children to comprehend the questions and give insightful answers. In behavioral genetic studies, such increased unreliability would be expected to manifest as substantially higher estimates of the non-shared environment (Eaves et al. 1997) which includes measurement error. These issues notwithstanding, however, children have firsthand knowledge of particular clinically meaningful acts that their parents do not. For example, adolescent males report twice as many symptoms of CD as do their parents (Hewitt et al. 1997). Consistent with this, subsequent empirical examinations of informant-effects, particularly for CD, have supported the use of both parent and child informant reports (Bird et al. 1992; Hart et al. 1994; Jensen et al. 1999).
However, though there is now extensive evidence that individual disorder variance estimates vary by informant (Simono. et al. 1995; Eaves et al. 1997; Sherman et al. 1997; Simono. et al. 1998), very few studies have evaluated the impact of different informants on disorder co-occurrence. The two exceptions (Eaves et al. 2000; Nadder et al. 2002) both found that while there was evidence of extensive genetic overlap within informant-reports for CD and ODD, and for ADHD and conduct problems, respectively, these genetic factors were largely unique to each informant. However, because these studies explored only these particular disorder pairings, the impact of informant effects on co-morbidity among all three disorders has yet to be examined. Furthermore, there is a need to better understand the impact of single-informant ratings versus composite ratings of multiple informants, given that researchers typically adopt one approach or the other. ‘Rater bias’ or informant-effects models, which examine the genetic and environmental etiology of both the variance common to all informants and the variance unique to each informant (Neale & Stevenson, 1989; Hewitt et al. 1992) allowed us to address both of these concerns.
METHOD
Participants
The sample was drawn from participants in the ongoing Minnesota Twin Family Study (MTFS). Detailed information regarding the design, recruitment procedures, participation rates, and zygosity determination of the MTFS has been provided in Iacono et al. (1999). The participants in the current research ranged in age from 10 to 12, averaging age 11, at the time of their intake visit. The original sample consisted of 753 same-sex, reared-together twin pairs: 373 male (nMZ=253, nDZ=120) and 380 female (nMZ=233, nDZ=147) twin pairs. In addition, we have recently initiated efforts to increase our sample size. To date, we have added 138 additional twin pairs, bringing the total sample to: 415 male (nMZ=281, nDZ=134) and 476 female (nMZ=295, nDZ=181) pairs of twins.
Measures
During their intake visit, all participants and their parents were assessed in-person by separate bachelor- and masters-level interviewers for lifetime DSM-III-R mental disorders (the current manual at the study’s onset). ADHD, CD, and ODD were assessed using the Diagnostic Interview for Children and Adolescents–Revised (DICA-R; Reich & Welner, 1988). The MTFS version of this instrument contained supplementary probes and questions, which were added after consultation with one of the DICA-R’s authors to ensure complete coverage of each symptom. Mothers reported on symptom presence first in one twin and then in the other, while twins reported only on themselves.
Prior to the assignment of mental disorder symptoms, a clinical case conference was completed in which the evidence for every symptom was discussed by at least two advanced clinical psychology graduate students. Only symptoms that were judged to be clinically significant in both severity and frequency were considered present. After symptoms were assigned, computer algorithms were used to create symptom counts corresponding to DSM-III-R criteria. Criteria were included in the symptom count only if they referred to symptoms of the disorders (i.e. symptom duration and hierarchical exclusionary rules were not included), although age of onset information was included. These variables corresponded to (1) the nine criterion-A symptoms of ODD, (2) 12 of the 13 criterion-A symptoms of CD (the exception, ‘has forced someone into sexual activity with him or her ’, was not assessed to avoid potential mandated reporting), and (3) the 14 criterion-A symptoms of ADHD listed in the DSM-III-R†
Symptom counts, rather than diagnoses, were chosen primarily to increase power. Considerable power and information are lost when sub-and supra-threshold variation in diagnostic status is collapsed into a dichotomous variable (Krueger & Finger, 2001). In addition, the relatively low prevalence of diagnosed disorders in a non-clinical sample such as this one would considerably lower our statistical power and potentially obscure relationships among the disorders.
Data analyses
Twin methodology uses the difference in the proportion of genes shared between MZ twin pairs, who share 100%of their genetic material, and dizygotic DZ twin pairs, who share an average of 50% of their segregating genetic material, to estimate the relative genetic, shared environmental, and non-shared environmental contributions to variance within and covariance among observed behaviors or characteristics (phenotypes). The additive genetic component is the independent effect of individual genes summed over loci, and when acting alone, creates MZ correlations double those of DZ correlations. The shared environment indexes environmental effects (e.g. familial and contextual experiences) common to both members of a twin pair that act to increase their similarity. Non-shared environment encompasses environmental factors unique to each twin within a pair (as well as measurement error) that differentiates the siblings, making them less similar. (Readers interested in twin study methodology are referred to Plomin et al. 2001, for more detail.)
Mx, a structural-equation modeling program (Neale, 1997), uses maximum-likelihood model-fitting techniques to fit models to observed variance-covariance matrices. The χ2 test statistic provides a goodness-of-fit index of the fit of the model, which is then converted to the Bayesian information criterion (BIC; BIC= χ2−df (ln N) ; N=891 pairs). BIC measures model fit relative to parsimony to determine the best-fitting model among nested models. Better-fitting models have more negative values. Importantly, Markon & Krueger (in press) found that BIC is generally more robust to distributional misspecification than the traditional behavioral genetic index-of-fit, Akaike’s information criterion (AIC; Akaike, 1987) (AIC=χ2−2 df). In addition, in larger samples and when comparing complex models (such as in the present study), BIC was better able to identify the true model relative to AIC.
Fitting an informant-effects model (see Fig. 1) with Mx allowed us to investigate the co-occurrence among externalizing disorders and their relationship with informant effects via three sets of analyses. First, we examined genetic and environmental contributions to a cross-informant index of co-morbidity, the general Ext factor. This primary factor comprises the variance in ADHD, CD, and ODD that is common across disorders and across informants, or the portion of co-occurrence among the disorders upon which mothers and twins agree. Secondly, we examined genetic and environmental contributions to mother and child factors, which are composed of the variance in ADHD, CD, and ODD that is common across disorders but unique to each informant. Accordingly, evaluation of the mother and child factors revealed whether heritability estimates for co-occurrence within informants differed systematically from each other and from co-occurrence across informants. Of note, these factors are secondary to the general Ext factor, as they are in effect estimated after accounting for disorder co-occurrence common to both informants. Thirdly, the variance remaining for each measured variable, once the effects of the general Ext factor and the relevant informant-specific factor (i.e. either mother or child) have been accounted for, was also decomposed into its genetic and environmental components. These variable-specific residuals index the variance unique to each variable and can be thought of as variance that is both informant- and disorder-specific. Of note, measurement error is contained in the non-shared environmental portion of these variable-specific residuals. Thus, by examining informant-reports of the disorders in these three ways, the informant-effects model allowed us to make inferences regarding the sources of co-occurrence among ADHD, CD, and ODD and the impact of different informants on these sources.
Fig. 1.
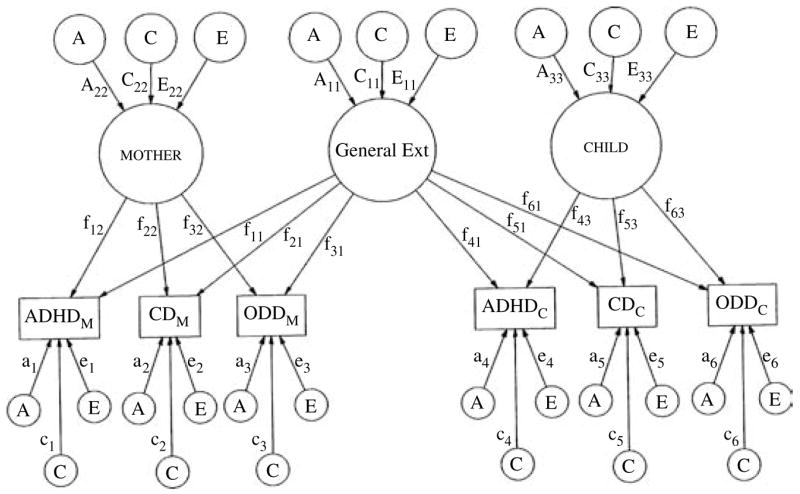
Path diagram of a full ACE informant-effects model for maternal and child informant-reports of attention deficit-hyperactivity disorder (ADHD), conduct disorder (CD), and oppositional defiant disorder (ODD). To promote ease of presentation, this path diagram represents only one twin in a pair (though the results are identical for the co-twin). General Ext represents the primary general factor loading onto both mother and child informant-reports. mother and child represent the secondary relevant informant-specific factors. The variance in each factor is parsed into that which is due to additive genetic effects (A), shared environmental effects (C), and non-shared environmental effects (E). Paths are represented by uppercase letters followed by two subscript numerals (e.g. A11, A22, and A33). Factor loading paths are represented by a lowercase ‘ f ’, followed by two subscript numerals. The first numeral corresponds to variable ‘number’ and the second to the factor that is loading on the variable. The variable-specific residual paths load directly onto each disorder, and are indicated by a lowercase letter followed by a single subscript numeral (e.g. a1).
Prior to interpretation of the informant-effects model, however, we evaluated its overall appropriateness for our data. That is, we fit a Cholesky model, in which minimal structure is imposed on the data and heritability estimates are freely estimated (i.e. variables are not constrained to covary within higher order factors). Importantly, the informant-effects model is nested within the Cholesky model, allowing a direct comparison of model fit statistics, thereby enabling us statistically to evaluate the fit of the general Ext and informant-specific factors. Accordingly, we were able to determine whether there was meaningful covariation among the disorders within and across informants. Finally, the moderating effects of gender were also examined in these analyses, as it is well known that male children generally display a higher frequency of externalizing behaviors than do female children (Hewitt et al. 1997).
RESULTS
Descriptive statistics
In order to index the severity of externalizing symptomatology, mean maternal and child symptom counts were computed separately by sex (see Table 1). Independent-samples t tests indicated that the mean symptom counts differed significantly by sex (p<0.001, two-tailed), with males having higher symptom counts than females for all six disorder informant-reports, collectively indicating that the boys in this sample are more symptomatic than are the girls. Because exclusionary rules and duration requirements were omitted from our symptom count variables, these rates are upper estimates of disorder prevalences in our sample. Consistent with other studies of this age range (Achenbach et al. 1987), maternal and child reports were correlated 0.24, 0.26, and 0.25 for ADHD, CD, and ODD, respectively. Given these correlations, it appears that while maternal and child informant-reports do overlap, much of the variance is unique to each informant.
Table 1.
Number of attention deficit hyperactivity disorder (ADHD), conduct disorder (CD), and oppositional defiant disorder (ODD) symptoms by informant-report and gender
| Mean | s.d. | Max | Min | % past diagnostic cut-off | n | |
|---|---|---|---|---|---|---|
| Male–mother reports | ||||||
| ADHD | 1.08* | 2.23 | 12 | 0 | 3.0 | 838 |
| CD | 0.40* | 0.84 | 6 | 0 | 3.0 | 838 |
| ODD | 1.21* | 1.54 | 9 | 0 | 5.3 | 838 |
| Male–child reports | ||||||
| ADHD | 0.47* | 1.36 | 12 | 0 | 0.5 | 839 |
| CD | 0.41* | 0.89 | 8 | 0 | 3.3 | 839 |
| ODD | 0.70* | 1.17 | 8 | 0 | 1.7 | 838 |
| Female–mother reports | ||||||
| ADHD | 0.71* | 1.66 | 13 | 0 | 1.8 | 971 |
| CD | 0.14* | 0.47 | 5 | 0 | 0.5 | 967 |
| ODD | 0.83* | 1.26 | 9 | 0 | 2.3 | 971 |
| Female–child reports | ||||||
| ADHD | 0.33* | 1.09 | 11 | 0 | 0.5 | 971 |
| CD | 0.09* | 0.36 | 4 | 0 | 0.2 | 970 |
| ODD | 0.40* | 0.79 | 5 | 0 | 0.3 | 970 |
The number of symptoms required for a definite diagnosis of ADHD, CD, and ODD in DSM-III-R are 8, 3, and 5, respectively, which can be used to compute the percentage of individuals who are past the diagnostic cut-off. Because duration requirements were omitted from our symptom count variables, these rates are upper estimates of the possible prevalences of these disorders in our sample.
Male symptom count significantly greater than female symptom count, p<0.001.
The symptom count distributions were positively skewed for each disorder. In order to better approximate normality, the symptom count variables were individually Blom-transformed and rank-normalized prior to model-fitting, a procedure which was recently found to optimize model selection (van den Oord et al. 2000). This procedure involved replacing raw symptom counts with their rank values. Ties were assigned the mean rank of the tied values. The adjustments were conducted separately by sex, but without regard to zygosity. However, due to the level of positive skew in the data prior to transformation, particularly for child informant-reports, the data remained somewhat skewed even after normalization.
Correlations
Prior to multivariate model-fitting analyses, intra-class and cross-twin, cross-trait correlations were calculated for each sex-zygosity cohort, as well as pooled across sex (see Table 2). These correlations were calculated using the double-entry method, which removes the variance associated with the arbitrary ordering of the twins within each pair. Maternal reports and child self-reports are presented separately. Intra-class correlations were computed for each zygosity between, for example, the ADHD symptom counts of Twin A and Twin B. The MZ and DZ intra-class correlations were then compared to obtain a preliminary indication of the extent to which genetic and environmental factors influenced a specific informant-report of a disorder. In contrast, the cross-twin, cross-trait correlations offer a preliminary indication of the sources of co-occurrence among the disorders for both maternal and child informants. They were computed separately for MZ and DZ twins using, for example, the CD symptom count of one twin and the ODD symptom count of his or her co-twin. For both intra-class and cross-twin, cross-trait correlations, MZ correlations that are double those of DZ correlations implicate genetic effects, whereas MZ correlations that are not double, but are still greater than those of DZ correlations implicate both genetic and shared environmental effects. However, if the correlation among MZ twin pairs is equivalent to that of the DZ twin pairs, primarily shared environmental mediation is indicated.
Table 2.
Intra-class and cross-twin, cross-trait correlations for maternal and child self-reports of attention deficit hyperactivity disorder (ADHD), conduct disorder (CD), and oppositional defiant disorder (ODD) for each sex-zygosity cohort
| Participants | MZ male (n=562) | DZ male (n=268) | MZ female (n=590) | DZ female (n=362) | MZ all (n=1152) | DZ all (n=630) |
|---|---|---|---|---|---|---|
| Mother reports | ||||||
| Intra-class correlations | ||||||
| AD1 & AD2 | 0.805** | 0.252 | 0.795** | 0.351 | 0.800** | 0.309 |
| CD1 & CD2 | 0.868** | 0.682 | 0.930** | 0.361 | 0.901** | 0.518 |
| OD1 & OD2 | 0.818** | 0.469 | 0.857** | 0.554 | 0.838** | 0.518 |
| Cross-twin, cross-trait correlations | ||||||
| AD1 & CD2 | 0.313* | 0.176 | 0.163* | 0.051 | 0.235** | 0.109 |
| AD1 & OD2 | 0.280* | 0.157 | 0.153 | 0.308 | 0.215 | 0.242 |
| CD1 & OD2 | 0.381 | 0.353 | 0.258 | 0.193 | 0.317 | 0.267 |
| Child reports | ||||||
| Intra-class correlations | ||||||
| AD1 & AD2 | 0.163 | 0.092 | 0.212** | 0.034 | 0.190** | 0.063 |
| CD1 & CD2 | 0.280 | 0.229 | 0.244** | −0.074 | 0.261** | 0.068 |
| OD1 & OD2 | 0.296* | 0.168 | 0.320* | 0.182 | 0.309** | 0.177 |
| Cross-twin, cross-trait correlations | ||||||
| AD1 & CD2 | 0.171 | 0.085 | 0.104 | 0.017 | 0.136* | 0.050 |
| AD1 & OD2 | 0.143 | 0.130 | 0.115 | 0.168 | 0.129 | 0.153 |
| CD1 & OD2 | 0.198 | 0.138 | 0.198** | 0.028 | 0.199** | 0.079 |
AD1, CD1, and OD1 indicate attention deficit hyperactivity disorder, conduct disorder, and oppositional defiant disorder, respectively, for Twin A, while AD2, CD2, and OD2 indicate attention deficit-hyperactivity disorder, conduct disorder, and oppositional defiant disorder, respectively, for Twin B. Maternal and child informant-reports are presented separately. MZ correlations that are statistically larger than the corresponding DZ correlations are indicated above.
p<0.01, one-tailed;
p<0.05, one-tailed.
Intra-class
For maternal reports of both males and females, the MZ correlations for ADHD, CD, and ODD were significantly greater than the DZ correlations (p≤0.01), suggesting that genetic influences make important contributions to the individual variance in maternal reports of each disorder. Moreover, for ADHD in males and CD in females, MZ correlations are more than double DZ correlations, suggesting the presence of either non-additive genetic variance or contrast effects. In contrast, for ODD in females and CD in males, the MZ twin correlations were less than double those of the DZ twins, suggesting that shared environmental components are also contributing to maternal reports of these disorders. When males and females are pooled, the results were quite similar. Specifically, genetic effects are implicated for all three disorders, shared environmental effects are suggested for CD and ODD, and non-additive genetic variance or contrast effects are again suggested for ADHD.
In contrast, child self-reports of the disorders did not suggest such strong genetic effects. Specifically, only the reports of ADHD and CD in females and ODD in both males and females revealed evidence of genetic mediation. Genetic effects did appear somewhat stronger in the pooled correlations, likely because pooling across sex increases power to detect effects as significant. Of note, MZ twin correlations obtained using child self-reports did not replicate the very high correlations obtained with the maternal reports, suggesting that maternal reports may be hampered by an inability to discriminate between their MZ twins.
Cross-twin, cross-trait
For maternal reports, there was evidence of genetic influence on the relationship between ADHD and CD for both males and females, and between ADHD and ODD for males. However, MZ/DZ differences for maternal reports of CD/ODD were uniformly non-significant, implicating shared environmental factors. In contrast, for child self-reports, only the difference between MZ and DZ correlations for CD/ODD in females was significant. All other MZ/DZ differences were non-significant, highlighting the role of shared environmental factors. When pooled across sex, the correlations continued to suggest genetic influence on the co-occurrence of ADHD and CD, irrespective of informant, as well as on the co-occurrence among CD and ODD.
Multivariate modeling
We tested an ACE Cholesky and an ACE informant-effects model, in which the variance attributable to genetic (A), shared environmental (C), and unique environmental plus measurement error (E) factors were all estimated. It should be noted that, although the models were fit to variance-covariance matrices, standardized estimates are being reported. The Cholesky and the informant-effects models were fit both allowing for sex differences in variance parameter estimates (Cholesky: χ2=322.90 on 186 df, BIC=−940.416; informant-effects: χ2= 399.505 on 240 df, BIC=−1230.575), and constraining variance parameter estimates to be equal across sex (Cholesky: χ2=431.83 on 249 df, BIC=−1259.38; informant-effects : χ2= 490.208 on 276 df, BIC=−1384.384). The best-fitting model (i.e. that which resulted in the most negative BIC value), was the informant-effects no-sex-differences model (presented in Fig. 2), indicating that there is meaningful co-occurrence across the disorders, both within and across informants. The improved fit of the no-sex-differences model is consistent with other studies (Burt et al. 2001; Rhee & Waldman, 2002), and suggests that although boys are more symptomatic than girls, the genetic and environmental architecture underlying the disorders does not vary across sex.
Fig. 2.
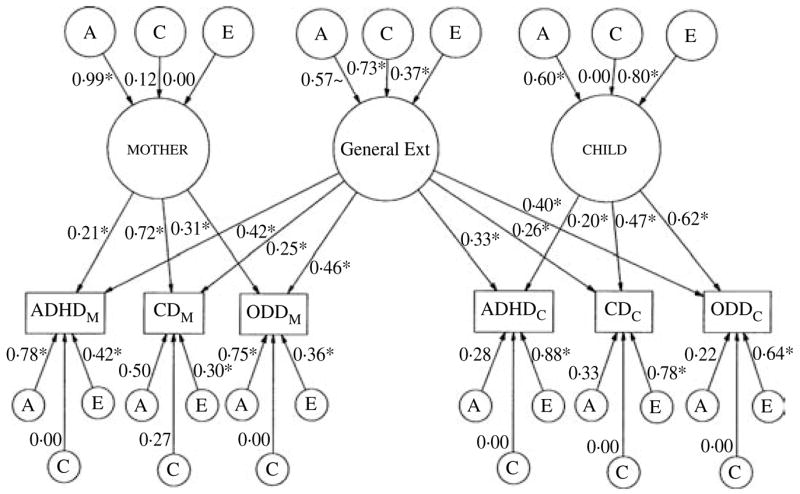
Standardized path diagram of informant-effects model for maternal and child informant-reports of attention defici-thyperactivity disorder (ADHD), conduct disorder (CD), and oppositional defiant disorder (ODD). Standardized path estimates of the genetic and shared environmental contributions to the covariance within each factor and the variance unique to each informant-report of the disorders are illustrated. The paths that are significant at p<0.05 are indicated by an asterisk. ~Indicates path is marginally significant at p<0.10. All paths are squared to estimate the proportion of variance accounted for.
Factor loadings
The general Ext factor is composed of the common variance underlying and uniting the maternal and child informant-reports of ADHD, CD, and ODD. The mother and child factors are composed of the remaining variance common to the disorders, but that is unique to each informant. Factor loadings (as seen in Fig. 2; e.g. f11, f12) are squared to index the percentage of variance in the variable (e.g. ADHDmother) accounted for by that factor. To facilitate interpretation of the results, the percentages of variance accounted for by the factors are presented in Table 3. Though small to moderate in magnitude, the factor loadings are uniformly significant at p<0.05, indicating that they do make a significant contribution. Such findings reveal the presence of significant co-occurrence among ADHD, CD, and ODD, both across and within informants, a finding that serves to highlight the importance of considering informant effects in examinations of co-morbidity. Of note, the relatively small amounts of variance accounted for by the general Ext factor are not unexpected given the modest correlations between maternal and child self-reports (0.24 – 0.26).
Table 3.
Percentages of variance in attention deficit hyperactivity disorder (ADHD), conduct disorder (CD), and oppositional defiant disorder (ODD) accounted for by factor loadings
| General Ext | mother | child | |
|---|---|---|---|
| ADHDmother | 17.6 | 4.4 | |
| CDmother | 6.3 | 51.8 | |
| ODDmother | 21.2 | 9.6 | |
| ADHDchild | 10.9 | — | 4.0 |
| CDchild | 6.8 | — | 22.1 |
| ODDchild | 16.0 | — | 38.4 |
ADHDmother, CDmother, and ODDmother represent maternal reports and ADHDchild, CDchild, and ODDchild represent child self-reports. The general Ext factor is composed of the common variance uniting the maternal and child informant-reports of ADHD, CD, and ODD. The maternal and child factors are composed of the remaining co-occurrence among the disorders that is unique to each informant. Factor loadings have been squared to index the percentage of variance in the variable accounted for by that factor.
All percentages of variance are significant at p<0.05.
Genetic and environmental influences
Genetic (A), shared (C), and non-shared (E) environmental contributions to the factors and unique to each variable (i.e. the variable-specific residuals) can be obtained by squaring their path coefficients, as presented in Fig. 2. To ease facilitation, the proportions of variance obtained via this method are presented in Table 4. These results indicated that the general Ext factor was influenced primarily by shared environmental forces, though the unique environment also contributed. Though moderate in magnitude, the genetic effect was only marginally significant (Δχ2=2.76 on 1 df, p<0.10). Such findings serve to highlight the contribution of shared environmental factors to the co-morbidity among ADHD, CD, and ODD. In contrast, the variance contained in the mother factor was uniformly attributed to genetic factors (i.e. 98%). The variance contained in the child factor was also influenced by genetic forces, though unlike the mother factor, non-shared environmental influences were also quite influential. Of note, because factors do not contain measurement error (error is encapsulated within the residuals), the large non-shared environmental influences on the child factor likely index that part of the child’s experience and perspective that is not shared with his or her co-twin, but that serves to increase covariation among the disorders.
Table 4.
Percentages of variance in attention deficit hyperactivity disorder (ADHD), conduct disorder (CD), and oppositional defiant disorder (ODD) accounted for by genetic (A), shared environmental (C), and non-shared environmental (E) influences
| Genetic (A) | Shared (C)environment | Non-shared (E) environment | |
|---|---|---|---|
| General Ext | 32.5† | 53.3* | 13.7* |
| Maternal factor | 98.0* | 1.4 | 0.0 |
| Child factor | 36.0* | 0.0 | 64.0* |
| Variable-specific residuals | |||
| ADHDmother | 60.8* | 0 | 17.6* |
| CDmother | 25.0 | 7.3 | 9.0* |
| ODDmother | 56.3* | 0 | 13.0* |
| ADHDchild | 7.8 | 0 | 77.4* |
| CDchild | 10.9 | 0 | 60.8* |
| ODDchild | 4.8 | 0 | 41.0* |
The general Ext factor is composed of the common variance uniting the maternal and child informant reports of ADHD, CD, and ODD. The maternal and child factors are composed of the remaining co-occurrence among the disorders that is unique to each informant. Each factor was decomposed into its genetic and environmental components, and therefore each row sums to 100% of the variance within that factor. Within the variable-specific residuals, ADHDmother, CDmother, and ODDmother represent maternal reports of attention deficit-hyperactivity disorder, conduct disorder, and oppositional defiant disorder, respectively. ADHDchild, CDchild, and ODDchild represent child self-reports. Because these estimates index the genetic and environmental contributions to the variance remaining in each variable after that contributed by the factors is accounted for, these rows do not sum to 100%. However, when the variance contributed by the factors (see Table 3) is added, 100% of the variance (allowing for some rounding error) in each variable should be recovered. Thus, for example, the variance in ADHDmother was broken down into that contributed by general Ext (17.6%), that contributed by mother (4.4%), and that contributed by the variable-specific residuals (60.8%, 0%, and 17.6%). Accordingly, ADHDmother=17.6%+4.4%+60.8%+17.6%=100.4%. Factor loadings that account for a significant proportion of the variance are indicated in bold (
significant at p<0.05;
marginally significant at p<0.10).
The variance in the variable-specific residuals was also decomposed into its genetic and environmental components (see Table 4). Of note, the shared environmental residuals was estimated to be zero in five of six cases, and for the only exception, maternal reports of CD, it did not approach statistical significance (Δχ2 = 0.381 on 1 df, p>0.25). These results thus strongly suggest that all shared environmental effects were held in common by the disorders. In contrast, the non-shared environmental-disorder-specific residuals were uniformly significant. Moreover, the non-shared residuals for the child self-reports of the disorders were quite large, accounting for between 41 and 77% of the variance. As measurement error is contained in the non-shared environmental residuals, such results highlight the likelihood of increased measurement error in child self-reports. It should be noted, however, that true disorder-specific unique environmental experiences are confounded with measurement error within the non-shared environmental residuals. As such, we cannot be certain of the magnitude of child measurement error. Finally, the genetic residuals were significant for maternal reports of ADHD and ODD, but were noted to be particularly pronounced in the case of ADHD, a finding which may reflect previously found rater contrast effects on maternal reports of ADHD (Simonoff et al. 1998; Eaves et al. 2000).
DISCUSSION
These results warrant several interrelated conclusions. First, both the mother factor and the child factor were significantly influenced by moderate to strong genetic forces. However, when informant-reports were combined in the general Ext factor, genetically-mediated disorder co-occurrence was only marginally significant. Such findings strongly suggest that, when using only maternal or child informant-reports, the co-occurrence among the disorders will appear more genetic in origin than when using a combined informant approach. In this way, these findings shed light on the different conclusions reached in various studies (Silberg et al. 1996; Nadder et al. 1998; Young et al. 2000; Burt et al. 2001, 2003; Thapar et al. 2001), suggesting that many of the differences in the relative weight assigned to genetic influences may be due to the use of a single (usually maternal) informant.
Of note, however, informants are not the only variable that differs across twin studies. The age range of the current sample and that in Burt et al. (2001) was quite narrow (ages 10–12); however, the samples in Silberg et al. (1996), Nadder et al. (1998), Thapar et al. (2001), and Young et al. (2000) all assessed broader age ranges (ages 10–18, 7–13, 5–17, and 12–18, respectively). Because estimates of genetic influence may increase from childhood to adulthood (McGue et al. 1993; Lyons et al. 1995), age may also contribute to the larger proportion of variance assigned to genetic influences in some studies. Regardless, our results suggest that informant effects are critical to understanding variability in genetic estimates across studies.
Next, when viewed in conjunction with the intra-class correlations, which approached unity solely for maternal MZ reports, the primarily genetic mediation of the maternal reports suggests that mothers of twins may accentuate the similarity of their MZ twins, artifactually inflating maternal heritability estimates relative to child heritability estimates. Such findings suggest that heritability estimates obtained using maternal-only reports should be interpreted cautiously. Similarly, the very large non-shared environmental residuals found for child-only reports also implicate (though not conclusively) increased measurement error in child reports, limiting their usefulness when examined in isolation.
Although the results of this study suggest that conclusions based solely on one informant should be made only tentatively, conclusions obtained solely from the perspective common to informants may also be less than ideal. Specifically, parent–child agreement is notoriously low, with cross-informant correlations hovering near 0.3 (Achenbach et al. 1987), indicating that studies that center on cross-informant agreement will explain relatively little of the variance in a given disorder or across disorders. In the present study, for example, the general Ext factor accounted for only 6–21% of the variance in any given informant-report. Furthermore, evidence indicates that in spite of potential informant biases, each informant also provides unique and incrementally valid information. For example, we reported previously that both mother and child provide predictive information not provided by the other when evaluated against externally-collected teacher reports of behavior problems and grades (Burt et al. 2001), findings that are supported by numerous other studies (Bird et al. 1992; Hart et al. 1994; Angold & Costello, 1996; Jensen et al. 1999). Collectively, these findings suggest that examining only that which is common to informants, without also considering that which is unique to each informant, yields an incomplete picture. Instead, combining multiple informants via the ‘OR’ rule (i.e. symptom is assigned if it is endorsed by either the mother or the child) may provide the most valid indication of the disorders (Bird et al. 1992; Hart et al. 1994; Angold & Costello, 1996), and therefore, their heritability estimates.
Consistent with this conclusion, it may be that shared environmental contributions to ADHD have been prematurely dismissed. Specifically, though our results are consistent with studies of maternal reports of ADHD in suggesting that ADHD is largely (60–80%) genetic in origin (Silberg et al. 1996; Eaves et al. 1997; Sherman et al. 1997; Nadder et al. 1998), the finding of a significant shared environmental influence on ADHD is unusual, perhaps reflecting the combined informant nature of the general Ext factor.
Finally, our findings provide additional evidence that shared environmental effects are a critical source of co-morbidity among the childhood externalizing disorders. Consistent with the findings in Burt et al. (2001), the shared environmental contributions to the disorders were contained solely within the general Ext factor, indicating that they are not informant-specific, and accounted for the majority of its variance. (The path estimates in Fig. 2 are similar in magnitude when based on only the original 753 MTFS twin pairs used in Burt et al. (2001). Thus, adding the recently collected 138 twin pairs acted primarily to increase power.)
In addition, genetic contributions to comorbidity appeared largely unique to each informant. As noted, however, such informant-specific information is likely to optimize, rather than distort, our understanding of the child’s psychopathology. Together, such findings suggest that, rather than focusing solely on specific genes, we should look to factors within the child’s familial and contextual environment (Burt et al. 2003), as well as to common genes, when attempting to identify the psychopathological processes linking these disorders (Clark et al. 1995; Krueger, 1999, 2002).
Acknowledgments
This research was funded in part by NIH Grants DA05147, AA09367, AA00175, DA13240, and MH65137. The primary author was funded by the Doctoral Dissertation Fellowship at the University of Minnesota.
Footnotes
Criterion-A symptoms for each of these disorders have changed somewhat from DSM-III-R to the current manual, DSM-IV. ADHD has been subtyped into nine possible symptoms of inattentivity and hyperactivity-impulsivity (yielding 18 possible symptoms for the combined subtype). Of these, six symptoms are needed for a diagnosis of either ADHD subtype, and 12 are required for the combined subtype. CD now includes up to 15 symptoms, of which three are needed for a diagnosis. ODD now includes only eight symptoms, of which four are needed for a diagnosis.
DECLARATION OF INTEREST
None.
References
- Achenbach TM, McConaughy SH, Howell CT. Child/adolescent behavioral and emotional problems: implications of cross-informant correlations for situational specificity. Psychological Bulletin. 1987;101:213–232. [PubMed] [Google Scholar]
- Akaike H. Factor analysis and AIC. Psychometrika. 1987;52:317–332. [Google Scholar]
- Angold A, Costello EJ. The relative diagnostic utility of child and parent reports of oppositional defiant behaviors. International Journal of Methods of Psychiatric Research. 1996;6:253–259. [Google Scholar]
- Bird HR, Gould M, Staghezza B. Aggregating data from multiple informants in child psychiatry epidemiological research. Journal of the American Academy of Child & Adolescent Psychiatry. 1992;31:78–85. doi: 10.1097/00004583-199201000-00012. [DOI] [PubMed] [Google Scholar]
- Burt SA, Krueger R, McGue M, Iacono W. Sources of covariation among ADHD, CD, and ODD: the importance of shared environment. Journal of Abnormal Psychology. 2001;110:516–525. doi: 10.1037/0021-843X.110.4.516. [DOI] [PubMed] [Google Scholar]
- Burt SA, Krueger R, McGue M, Iacono W. Parent-child conflict and the co-morbidity among childhood externalizing disorders. Archives of General Psychiatry. 2003;60:505–513. doi: 10.1001/archpsyc.60.5.505. [DOI] [PubMed] [Google Scholar]
- Clark LA, Watson D, Reynolds S. Diagnosis and classification of psychopathology: challenges to the current system and future directions. Annual Review of Psychology. 1995;46:121–153. doi: 10.1146/annurev.ps.46.020195.001005. [DOI] [PubMed] [Google Scholar]
- Dalsgaard S, Mortensen PB, Frydenberg M, Thomsen PH. Conduct problems, gender, and adult psychiatric outcome with children with attention deficit hyperactivity disorder. British Journal of Psychiatry. 2002;181:416–421. doi: 10.1192/bjp.181.5.416. [DOI] [PubMed] [Google Scholar]
- Eaves LJ, Rutter M, Silberg JL, Shillady L, Maes H, Pickles A. Genetic and environmental causes of covariation in interview assessment of disruptive behavior and child and adolescent twins. Behavior Genetics. 2000;30:321–334. doi: 10.1023/a:1026553518272. [DOI] [PubMed] [Google Scholar]
- Eaves LJ, Silberg JL, Meyers JM, Maes HH, Simonoff E, Pickles A, Rutter M, Neale MC, Reynolds CA, Erikson MT, Heath AC, Loeber R, Truett KR, Hewitt JK. Genetics and developmental psychopathology: 2. The main effects of genes and environment on behavioral problems in the Virginia twin study of adolescent development. Journal of Child Psychology and Psychiatry. 1997;38:965–980. doi: 10.1111/j.1469-7610.1997.tb01614.x. [DOI] [PubMed] [Google Scholar]
- Hart EL, Lahey BB, Loeber R, Hanson KS. Criterion validity of informants in the diagnosis of disruptive behavior disorders in children: a preliminary study. Journal of Consulting and Clinical Psychology. 1994;62:410–414. doi: 10.1037/0022-006X.62.2.410. [DOI] [PubMed] [Google Scholar]
- Hewitt JK, Silberg JL, Neale MC, Eaves LJ, Erickson M. The analysis of parental rating of children’s behavior using LISREL. Behavior Genetics. 1992;22:293–317. doi: 10.1007/BF01066663. [DOI] [PubMed] [Google Scholar]
- Hewitt JK, Silberg JL, Rutter M, Simonoff E, Meyer JM, Maes H, Pickles A, Neale MC, Loeber R, Erickson MT, Kendler KS, Heath AC, Truett KR, Reynolds CA, Eaves LJ. Genetics and developmental psychopathology: 1. Phenotypic assessment in the Virginia twin study of adolescent behavioral development. Journal of Child Psychology and Psychiatry. 1997;38:943–963. doi: 10.1111/j.1469-7610.1997.tb01613.x. [DOI] [PubMed] [Google Scholar]
- Iacono WG, Carlson SR, Taylor J, Elkins IJ, McGue M. Behavioral disinhibition and the development of substance use disorders: findings from the Minnesota twin family study. Development and Psychopathology. 1999;11:869–900. doi: 10.1017/s0954579499002369. [DOI] [PubMed] [Google Scholar]
- Jensen PS, Rubio-Stipec M, Canino G, Bird HR, Duncan MK, Schwab-Stone ME, Lahey BB. Parent and child contributions to diagnosis of mental disorder: are both informants always necessary. Journal of the American Academy of Child & Adolescent Psychiatry. 1999;38:1569–1579. doi: 10.1097/00004583-199912000-00019. [DOI] [PubMed] [Google Scholar]
- Krueger RF. The structure of common mental disorders. Archives of General Psychiatry. 1999;56:921–926. doi: 10.1001/archpsyc.56.10.921. [DOI] [PubMed] [Google Scholar]
- Krueger RF. Psychometric perspectives on co-morbidity. In: Helzer JE, Hudziak JJ, editors. Defining Psychopathology in the 21st Century: DSM-V and Beyond. American Psychiatric Publishing; Washington, DC: 2002. pp. 41–54. [Google Scholar]
- Krueger RF, Finger MS. Using item response theory to understand co-morbidity among anxiety and unipolar mood disorders. Psychological Assessment. 2001;13:140–151. [PubMed] [Google Scholar]
- Lyons MJ, True WR, Eisen SA, Goldberg J, Meyer JM, Faraone SV, Eaves LJ, Tsuang MT. Differential heritability of adult and juvenile antisocial traits. Archives of General Psychiatry. 1995;52:906–915. doi: 10.1001/archpsyc.1995.03950230020005. [DOI] [PubMed] [Google Scholar]
- Markon KE, Krueger RF. An empirical comparison of information-theoretic model selection criteria. Behavior Genetics. doi: 10.1007/s10519-004-5587-0. in press. [DOI] [PubMed] [Google Scholar]
- McGue M, Bouchard TJ, Jr, Iacono WG, Lykken DT. Behavior genetics of cognitive ability: a life-span perspective. In: Plomin R, McClearn GE, editors. Nature, Nuture, and Psychology. American Psychological Association; Washington, DC: 1993. [Google Scholar]
- Nadder TS, Rutter M, Silberg JL, Maes HH, Eaves LJ. Genetic effects on the variation and covariation of attention deficit-hyperactivity disorder (ADHD) and oppositional-defiant disorder/conflict disorder (ODD/CD) symptomatologies across informant and occasion of measurement. Psychological Medicine. 2002;32:39–53. doi: 10.1017/s0033291701004792. [DOI] [PubMed] [Google Scholar]
- Nadder TS, Silberg JL, Eaves LJ, Maes HH. Genetic effects on ADHD symptomatology in 7- to 13-year-old twins: results from a telephone survey. Behavior Genetics. 1998;28:83–99. doi: 10.1023/a:1021686906396. [DOI] [PubMed] [Google Scholar]
- Neale MC. Mx: Statistical Modeling. 4. Department of Psychiatry; Box 710 MCV, Richmond, VA 23298: 1997. [Google Scholar]
- Neale MC, Stevenson J. Rater bias in the EASI Temperament Scales: a twin study. Journal of Personality and Social Psychology. 1989;56:446–455. doi: 10.1037//0022-3514.56.3.446. [DOI] [PubMed] [Google Scholar]
- Plomin R, DeFries JC, McClearn GE, McGuffin P. Behavioral Genetics. 4. Worth Publishers and W. H. Freeman & Co; New York: 2001. [Google Scholar]
- Reich W, Welner Z. Diagnostic Interview for Children and Adolescents–Revised: DSM-III-R version (DICA-R) Washington University; St Louis, MO: 1988. [Google Scholar]
- Rhee S, Waldman ID. Genetic and environmental influences on antisocial behavior: a meta-analysis of twin and adoption studies. Psychological Bulletin. 2002;128:490–529. [PubMed] [Google Scholar]
- Sherman DK, McGue MK, Iacono WG. Twin concordance for attention deficit hyperactivity disorder: a comparison of teachers’ and mothers’ reports. American Journal of Psychiatry. 1997;154:532–535. doi: 10.1176/ajp.154.4.532. [DOI] [PubMed] [Google Scholar]
- Silberg J, Rutter M, Meyer J, Maes H, Hewitt J, Simonoff E, Pickles A, Loeber R, Eaves L. Genetic and environmental influences on the covariation between hyperactivity and conduct disturbance in juvenile twins. Journal of Child Psychology and Psychiatry. 1996;37:803–816. doi: 10.1111/j.1469-7610.1996.tb01476.x. [DOI] [PubMed] [Google Scholar]
- Simonoff E, Pickles A, Hervas JL, Silberg J, Rutter M, Eaves L. Genetic influences on childhood hyperactivity: contrast effects imply parental rating bias, not sibling interaction. Psychological Medicine. 1998;28:825–837. doi: 10.1017/s0033291798006886. [DOI] [PubMed] [Google Scholar]
- Simonoff E, Pickles A, Hewitt J, Silberg J, Rutter M, Loeber R, Meyer J, Neale M, Eaves L. Multiple raters of disruptive child behavior: using a genetic strategy to examine shared views and bias. Behavior Genetics. 1995;25:311–326. doi: 10.1007/BF02197280. [DOI] [PubMed] [Google Scholar]
- Simonoff E, Pickles A, Meyer J, Silberg J, Maes H, Loeber R, Rutter M, Hewitt J. The Virginia twin study of adolescent behavioral development. Archives of General Psychiatry. 1997;54:800–808. doi: 10.1001/archpsyc.1997.01830210039004. [DOI] [PubMed] [Google Scholar]
- Thapar A, Harrington R, McGuffin P. Examining the co-morbidity of ADHD-related behaviors and conduct problems using a twin study design. British Journal of Psychiatry. 2001;179:224–229. doi: 10.1192/bjp.179.3.224. [DOI] [PubMed] [Google Scholar]
- van den Oord E, Simonoff E, Eaves L, Pickles A, Silberg J, Maes H. An evaluation of different approaches for behavior genetic analyses with psychiatric symptom scores. Behavior Genetics. 2000;30:1–18. doi: 10.1023/a:1002095608946. [DOI] [PubMed] [Google Scholar]
- Waschbusch DA. A meta-analytic examination of co-morbid hyperactive-impulsive-attention problems and conduct problems. Psychological Bulletin. 2002;128:118–150. doi: 10.1037/0033-2909.128.1.118. [DOI] [PubMed] [Google Scholar]
- Young SE, Stallings M, Corley RP, Krauter KS, Hewitt JK. Genetic and environmental influences on behavioral disinhibition. American Journal of Medical Genetics. 2000;96:684–695. [PubMed] [Google Scholar]


