Abstract
Psychopathy is a personality disorder with interpersonal–emotional and antisocial deviance facets. This study investigated these facets of psychopathy prospectively using normal-range personality traits in a community sample of young adult men who completed a picture-viewing task that included startle blink and skin conductance measures, like tasks used to study psychopathy in incarcerated men. Consistent with prior research, scores on the interpersonal–emotional facet of psychopathy (“fearless dominance”) were associated with deficient fear-potentiated startle. Conversely, scores on the social deviance facet of psychopathy (“impulsive antisociality”) were associated with smaller overall skin conductance magnitudes. Participants high in fearless dominance also exhibited deficient skin conductance magnitudes specifically to aversive pictures. Findings encourage further investigation of psychopathy and its etiology in community samples.
Descriptors: Psychopathy, Startle, Skin conductance, Psychopathic Personality Inventory, Multidimensional Personality Questionnaire, Community sample
Psychopathy is a personality disorder with interpersonal–emotional and antisocial deviance features. In incarcerated samples, the interpersonal–emotional features have been linked to deficits in fear-potentiated startle (e.g., Patrick, Bradley, & Lang, 1993), whereas the antisocial deviance features have been associated instead with lower electrodermal reactivity to stimuli (e.g., Patrick, Cuthbert, & Lang, 1994). However, the relations of these two facets of psychopathy with psychophysiological measures have not been explored as extensively in community samples, largely because screening measures suitable for assessing the two facets of psychopathy in epidemiological samples have not been available. Recently, the Psychopathic Personality Inventory (PPI; Lilienfeld & Andrews, 1996) has been shown to have two distinct factors (Benning, Patrick, Hicks, Blonigen, & Krueger, 2003); furthermore, both factors could be predicted well from the primary trait scales of the Multidimensional Personality Questionnaire (Benning et al., 2003), an omnibus measure of normal personality dimensions (MPQ; Tellegen, in press). These two factors have divergent external correlates that parallel those reported for the two factors of psychopathy in incarcerated samples (Benning, Patrick, Blonigen, Hicks, & Iacono, 2005). In this study, we investigated the relationships between these two factors of psychopathy and psychophysiological reactivity in a picture-viewing paradigm to examine whether associations previously reported in male prisoners would extend to a nonincarcerated community sample.
The Assessment of Psychopathy and Its Facets
Psychopathy has been a focus of clinical concern for decades. Criteria for the disorder were first enumerated in Hervey Cleckley’s (1941/1988) monograph, The Mask of Sanity. Cleckley’s work established 16 descriptive criteria that could be used to identify individuals with the disorder. These criteria included descriptions of interpersonal–emotional features (e.g., superficial charm, absence of nervousness, egocentricity) and antisocial tendencies (e.g., inadequately motivated antisociality, unreliability, failure to follow any life plan). However, Cleckley’s notion of the psychopath was not precisely operationalized until the advent of the Psychopathy Checklist (Hare, 1980). Items on this instrument were chosen to have maximum power in discriminating prisoners who were rated as being high versus low in global ratings of Cleckley psychopathy (Hare, 1980).
However, the Psychopathy Checklist and its successor, the Psychopathy Checklist–Revised (PCL-R) were designed to assess psychopathy in forensic samples through a lengthy scoring procedure, which includes a face-to-face interview and a review of prison file information. The relatively high base rate of psychopathy in prison samples has allowed researchers to conduct studies using extreme-groups designs, based on clinically derived cutoff scores for psychopathy (i.e., a person obtaining PCL-R score of 30 or more may be classified as a psychopath, and a person with a score or 20 or less may be classified as a nonpsychopath; Hare, 2003). Historically, the assessment of psychopathy through self-report, which would allow screening for the disorder in populations in which the base rate of psychopathy is substantially lower than that in prisons, has met with more limited success. Early inventories developed to measure psychopathy through self-report, such as the Socialization scale of the California Personality Inventory (Gough, 1957, 1960) and the Psychopathic Deviate scale of the Minnesota Multiphasic Personality Inventory (Hathaway & McKinley, 1942; McKinley & Hathaway, 1944), appear to index the antisocial deviance component primarily, with limited coverage of the interpersonal–emotional features of psychopathy (Hare, 1985; Harpur, Hare, & Hakstian, 1989). Authors of more recent self-report measures of psychopathy have sought to remedy this shortcoming by including scales designed to explicitly index both the antisocial deviance and the interpersonal–emotional personality features in psychopathy (Levenson, Kiehl, & Fitzpatrick, 1995; Lilienfeld & Andrews, 1996; Lynam, Whiteside, & Jones, 1999).
One such measure is the Psychopathic Personality Inventory (PPI; Lilienfeld & Andrews, 1996), a self-report inventory constructed to provide comprehensive assessment of the personality characteristics in psychopathy. When the subscales of the PPI were factor analyzed, two uncorrelated factors emerged (Benning et al., 2003). One factor, termed “fearless dominance” (Benning et al., 2005), was marked by the Social Potency, Stress Immunity, and Fearlessness subscales of the PPI. The other factor, labeled “impulsive antisociality” (Benning et al., 2005), was marked by the Carefree Nonplanfulness, Blame Externalization, Impulsive Nonconformity, and Machiavellian Egocentricity subscales —the latter reflecting interpersonal aggressiveness. Fearless dominance related positively to such criterion measures as clinically assessed adult symptoms of antisocial personality disorder, verbal intelligence, and socioeconomic status. In contrast, impulsive antisociality related positively to clinically assessed child and adult symptoms of antisocial personality disorder as well as substance use, and negatively with verbal intelligence, socioeconomic status, and socialization. Additionally, fearless dominance was related to Factor 1 of Hare’s (1991) Self-Report Psychopathy–II scale, whereas impulsive antisociality was related to Factor 2 of the Self-Report Psychopathy–II scale, as well as to the factors of Frick and Hare’s (2001) Antisocial Process Screening Device (Benning, Patrick, Salekin, & Leistico, 2005). Furthermore, both the fearless dominance and impulsive antisociality factors of the PPI showed substantial relations with Miller, Lynam, Widiger, and Leukefeld’s (2001) facet-level Five-Factor Model prototype of psychopathy (Ross, Benning, Patrick, Thompson, & Thurston, 2005).
The two factors of the PPI also showed differential relations with normal-range personality traits indexed by the MPQ. The MPQ contains 11 primary trait scales, reflecting three higher-order dimensions of personality: Positive Emotionality, Negative Emotionality, and Behavioral Constraint. In regression analyses using the MPQ primary trait scales, the fearless dominance factor of the PPI was significantly predicted by high Social Potency and low Stress Reaction and Harm Avoidance, whereas the impulsive antisociality factor was significantly predicted by high Alienation and Aggression and low Control, Traditionalism, and Social Closeness (Benning et al., 2003). Notably, though the MPQ and PPI were administered between 4 and 6 years apart, the two factors of the PPI were predicted well in these regressions (multiple Rs ≥ .67; Benning et al., 2003).
A follow-up study demonstrated that the MPQ-estimated PPI factors exhibited similar meaningful patterns of associations with criterion variables in samples of community twins, undergraduates, and incarcerated men (Benning et al., 2005). In all three samples, MPQ-estimated fearless dominance correlated with fewer self-reported and clinically assessed symptoms of social and specific phobias, reduced temperamental fearfulness and subjective distress, higher thrill and adventure seeking as indexed by Zuckerman’s (1979) sensation seeking scale, and higher self-reported narcissistic personality features (Benning et al., 2005). In contrast, MPQ-estimated impulsive antisociality correlated with self-reported and clinically assessed symptoms of externalizing disorders (childhood and adult antisocial personality disorder and alcohol and drug dependence; Krueger et al., 2002) and symptoms of depression, temperamental negative emotionality, the boredom susceptibility and disinhibition components of sensation seeking, and lower socialization (Benning et al., 2005). Furthermore, in prisoners, fearless dominance was related selectively to the interpersonal-affective Factor 1 of the PCL-R (particularly its interpersonal features), whereas impulsive antisociality was related preferentially to the antisocial lifestyle Factor 2 of the PCL-R (Benning et al., 2005). In a behavioral genetic study, the genetic factors underpinning fearless dominance were negatively related to those underpinning internalizing disorders, whereas the genetic factors underlying impulsive antisociality were correlated with those underlying externalizing disorders (Blonigen, Hicks, Krueger, Patrick, & Iacono, 2005).
These results suggest that both fearless dominance and impulsive antisociality can be assessed validly through weighted composites of normal-range personality dimensions. Constructs are defined by their lawful relations with observable variables and other constructs (Cronbach & Meehl, 1955), and thus scales that share the same patterns of relationships with criterion variables (as do PPI- and MPQ-estimated fearless dominance and impulsive antisociality; Benning et al., 2005) can be considered indices of similar constructs. Considering the aforementioned evidence for the criterion-related validity of the two PPI factors (Benning et al., 2003, 2005), firm grounds exist for concluding that MPQ-estimated fearless dominance and impulsive antisociality index distinctive facets of the psychopathy construct in their own right. In summary, although the current study employed constructs operationalized using scores on a normal-range personality inventory, we maintain that these constructs nevertheless provide a valid basis for evaluating the psychophysiological correlates of psychopathy in a nonincarcerated, community participant sample.
The Psychophysiology of Psychopathy
Psychophysiological measures have been used in many studies to test the hypothesis that emotional reactivity is deficient in individuals with psychopathy. Skin conductance response magnitude has been used in psychopathy research as a measure of physiological arousal. Compared to controls, individuals with psychopathy identified through global ratings of Cleckley psychopathy exhibit deficient skin conductance responses to conditioned stimuli in aversive conditioning tasks (Hare, 1965a; Hare & Quinn, 1971, Lykken, 1957) and to avoidable aversive tones in vigilance tasks (Hare, 1982), during shock anticipation (Hare, 1965b; Hare & Craigen, 1974) and aggression tasks (Dengerink & Bertilson, 1975), and in response to adrenalin infusions (Hare, 1972). Moreover, Lorber’s (2004) meta-analysis found that psychopathy was consistently inversely related to a variety of skin conductance measures (cf. Raine, 1997). The findings of these studies indicate that individuals with psychopathy are less electrodermically aroused by aversive stimuli, consistent with the notion that such individuals are relatively fearless (Lykken, 1957, 1995).
However, increases in skin conductance magnitude are not specific to aversive stimuli. Skin conductance also increases with exposure to pleasant arousing stimuli (Bradley, Codispoti, Cuthbert,& Lang, 2001; Lang, Greenwald, Bradley, & Hamm, 1993), indicating that it acts as a nonspecific measure of sympathetic activation. In fact, there is evidence that individuals with psychopathy show global deficits in skin conductance magnitudes to all emotional pictures (Herpertz et al., 2001; Pastor, Molto, Vila, & Lang, 2003). A psychophysiological measure with greater specificity to aversive emotional states is the facilitation, or potentiation, of the defensive startle blink reflex. Within the first second after picture onset, startle blink magnitudes during aversive pictures increase such that they are potentiated relative to blink reflex magnitudes during neutral pictures (Bradley, Cuthbert, & Lang, 1993; Hawk & Cook, 2000; Vrana, Spence, & Lang, 1988). The potentiation of the startle blink appears to be driven by activity from the amygdala feeding into the neural circuitry of the startle reflex (Hitchcock & Davis, 1986), which suggests that startle blink potentiation can be interpreted as an index of defensive fear processing.
This resultant linear pattern of startle reflex modulation by emotion (i.e., diminished during pleasant vs. enhanced during unpleasant) has been interpreted as a measure of the degree to which the underlying motivational state elicited by each picture primes or inhibits the protective action of the startle reflex (Lang, Bradley, & Cuthbert, 1990). In contrast to normal participants, psychopathic prisoners show inhibition of the startle blink reflex during both pleasant and unpleasant pictures, yielding a quadratic pattern of valence modulation (Levenston, Patrick, Bradley,& Lang, 2000; Pastor et al., 2003; Patrick et al., 1993; Sutton, Vitale, & Newman, 2002) that has been interpreted as reflecting foreground attentional engagement as opposed to motivational priming (Levenston et al., 2000). Psychopathic prisoners also exhibit deficient startle reflex potentiation during cues to an impending aversive noise compared to nonpsychopathic prisoners (Patrick, 1994).
The Current Study
There have been few studies of the psychophysiological correlates of psychopathy using community samples. Ishikawa, Raine, Lencz, Bihrle, and LaCasse (2001) used adult men from temporary employment agencies to study successful and unsuccessful psychopaths in the community. Successful psychopaths, who had never been convicted of a crime, had lower scores on the antisocial deviance factor of the PCL-R (Factor 2) than unsuccessful psychopaths, who had been convicted of a crime (though the two groups did not differ in their scores on the interpersonal–emotional factor of the PCL-R [Factor 1] or in rates of self-reported crime), and they exhibited significantly more heart rate reactivity to a social stressor. Furthermore, startle modulation scores in this sample during a picture viewing paradigm were negatively related to scores on the interpersonal–emotional factor of the PCL-R and positively related to scores on the antisocial deviance factor (Vanman, Mejia, Dawson, Schell, & Raine, 2003). However, even the control participants in this sample, who scored in the bottom third of the sample on the PCL-R and had never been convicted of a crime, had a mean PCL-R score that was substantially greater than those reported in Hare (2003) for other community samples, and they reported having committed significantly more crimes than the average citizen. Therefore, a study employing an epidemiologically designed community sample that is more representative of the general population would be valuable.
The current study used young adult male twins from a large-scale community-epidemiological sample who completed the MPQ at age 17 and participated in an affective picture-viewing assessment at age 20 (see Carlson, Katsanis, Iacono, & McGue, 1997, for details). There were two primary hypotheses. The first was that diminished fear-potentiated startle would be selectively associated with fearless dominance—that is, participants high in fearless dominance would exhibit deficient fear-potentiated startle, whereas those high on impulsive antisociality would show normal fear-potentiated startle. This hypothesis derives from the finding that people high in the interpersonal–emotional features of psychopathy show deficient fear-potentiated startle (Patrick, 1994; Patrick et al., 1993; Vanman et al., 2003). The second was that impulsive antisociality would be associated with deviant skin conductance modulation. However, based on previous findings, the direction of this prediction was less clear. One alternative is that participants high on impulsive antisociality would show selective deficits in skin conductance to aversive pictures. This hypothesis is based on the lack of reactivity that persistently antisocial people show during aversive stimuli. Consistent with the results of Herpertz et al. (2001), an alternative hypothesis is that individuals high in impulsive antisociality would show reduced skin conductance to all pictures in this study. This prediction derives from a study in which impulsive antisociality was inversely related to overall skin conductance responses during a concealed information test (Verschuere, Crombez, De Clercq, & Koster, 2005). However, neither fearless dominance nor impulsive antisociality was expected to show relationships with affective ratings of the pictures used in the experiment.
Method
Participants
Participants were 355 male twins who participated in the Minnesota Twin Family Study (Iacono, Carlson, Taylor, Elkins, & McGue, 1999). This study is an ongoing epidemiological-longitudinal study investigating the genetic and environmental factors that contribute to the development of substance abuse and related psychopathology in reared-together, same-sex twins and their parents. Twin families were identified through public birth records and recruited to participate the year the twins turned 17 years old. Male twins born in the years 1972–1977 were identified. For any given year, the study was able to locate over 90% of twin families. Of the families located, 17% declined participation. Families were excluded from participation if they lived further than a day’s drive from the Minneapolis laboratories or if either twin had a serious mental or physical handicap that would preclude him or her from completing the in-person intake assessment. Individuals who did not participate were largely similar to participants in the study in terms of their diagnostic and socioeconomic status (Iacono et al., 1999). The twins included in this study were 20 years old and completed a day-long assessment at the Minneapolis laboratories during the first follow-up assessment of the study, which included a battery of psychophysiological tasks. These twins had previously completed the MPQ as part of their initial contact at age 17.
Data were available for varying numbers of participants for each measure. All participants (n = 355) had scoreable skin conductance data. Seven participants failed to complete the ratings procedure, leaving a final n = 348 for this measure. Participants (n = 26) with more than 50% invalid startle blink trials (i.e., trials identified as having an unstable pre-noise-probe baseline or for which the onset of the startle blink response occurred<20 ms after noise probe presentation) were excluded from the analyses, as were those who failed to exhibit a startle response on at least 50% of all trials (n = 22), leaving a final n of 307 for the startle blink measure.
Stimuli
The picture-viewing paradigm used 27 pictures from the International Affective Picture System. Pictures were assigned an affective valence based on normative valence and arousal ratings for these pictures. There were nine pleasant pictures, nine neutral pictures, and nine aversive pictures. Pleasant pictures consisted mainly of erotic women and adventure scenes and were chosen to be maximally pleasant and highly arousing for male participants; neutral pictures consisted of household objects and were chosen so that they were rated neutrally in valence and low in arousal; aversive pictures consisted mainly of scenes of mutilated bodies and disease that were chosen to be maximally unpleasant and highly arousing.1 Pleasant and aversive pictures were chosen to be equidistant from neutral pictures in terms of their valence and arousal ratings.
Pleasant, neutral, and aversive pictures were presented in the following order: N, P, N, A, P, A, P, N, A, P, N, N, A, N, P, A, A, P, A, N, P, P, N, A, A, P, N, with pictures from each valence category presented in the sequence listed in the footnote; acoustic startle probes were delivered during pictures marked with an asterisk (six per valence category). The acoustic startle probes were 90-dB, 50-ms noise bursts with nearly instantaneous rise time produced by a Coulbourn white noise generator (S81-02). Startle probes were presented binaurally through Sennheiser HD 540 reference II headphones. Because startle responses to the first noise probe were significantly greater than those to all subsequent probes, suggesting that it acted as a habituation stimulus (Carlson et al., 1997), startle responses for the initial picture trial were excluded from the analyses (cf. Patrick & Berthot, 1995).
Measures
Psychopathy factors
Standardized scores on the 11 primary trait scales of the MPQ were used to compute scores on the fearless dominance and impulsive antisociality facets of psychopathy embodied in the PPI, using the regression equations in Benning et al. (2003). Fearless dominance is significantly predicted by high Social Potency, low Stress Reaction, and low Harm Avoidance scales; impulsive antisociality is significantly predicted by high Aggression and Alienation, low Control and Traditionalism, and low Social Closeness. As noted earlier, prior research has demonstrated high correspondence between MPQ-estimated PPI factor scores and actual factor scores, even when the two instruments were administered several years apart (Benning et al., 2005).
Affective ratings
Self-report reactions to the pictures were rated with a paper and pencil version of the Self-Assessment Manikin (Bradley & Lang, 1994). Separate ratings of subjective valence and arousal were completed for each picture, allowing these dimensions of emotional experience (Russell, 1979) to be assessed independently.
Startle blink
Blink responses to noise probes were measured with a pair of Ag-AgCl electrodes placed 5 mm below the lower lid of the right eye. Hewlett Packard Redux Paste was used as the conducting medium. One electrode was placed directly underneath the pupil, and the second was placed 5 mm to the right of the pupil, toward the outer canthus. The right shin was used as ground. Impedances for the blink and ground electrodes were kept below 20 kΩ. EMG signals were recorded with a Grass Model 12A Neurodata acquisition system. Signals were filtered with − 3 dB attenuation low and high frequency settings at 100 and 1000 Hz, respectively, consistent with similar studies of startle blink magnitude published at the time these data were collected (cf. Bradley et al., 1993; Patrick et al., 1993), and were amplified 50,000×. Off-line, EMG signals were rectified and smoothed using a third-order Butterworth filter (Smith, 2001, chap. 21) with an 80-ms time constant using software written by the first author for version 6.0.0.88 of the Matlab environment (MathWorks, 2000).
Startle blink magnitude was scored from the smoothed EMG signal as a baseline-to-peak difference for each noise probe trial. The baseline was calculated as the mean orbicularis EMG activity during the 25 ms prior to the onset of the noise probe, whereas the peak of the startle blink reflex was identified as the maximum EMG level reached within 30–120 ms after the onset of the startle probe. Negative startle blink magnitude values were set to zero. Individual startle blink trials were flagged for rejection if the variability of the pre-probe baseline EMG activity of the trial was at least 0.5 SD above the mean pre-probe variability over all trials for all participants. These flagged trials were visually inspected to determine whether the baseline EMG activity was too noisy to allow for a valid startle magnitude score to be obtained, or whether the startle blink may have occurred during the refractory period of a pre-probe blink. If either of these conditions were met, the trial was rejected.
Skin conductance
Skin conductance was recorded using a constant current (0.5 V) skin conductance signal conditioner. Participants washed their hands with Ivory dish soap before electrodes were attached. A pair of 1.27-cm-diameter TDE-20 Ag-AgCl electrodes were placed on the tips of the index and ring fingers of the participant’s left and right hands; electrode collars created a contact surface of 0.079 cm2 on each fingertip. A paste consisting of 0.5 M NaCl electrolyte mixed with Unibase cream was the conducting medium. The right shin was used as ground. Skin conductance signals were filtered with a − 3 dB attenuation high frequency setting at 3 Hz and amplified 5,000× through Grass DC amplifiers.
Skin conductance magnitude was scored as a baseline-to-peak difference for each trial. The baseline was calculated as the mean of all samples during the 1 s prior to the onset of the picture, whereas the peak of the skin conductance was taken as the maximum skin conductance level reached between 900 and 4000 ms after the onset of the picture (cf. Bradley et al., 2001). Negative skin conductance magnitudes were set to zero. To avoid including skin conductance responses evoked by startle probes in the analyses, trials in which the startle probe occurred earlier than 3 s after picture onset were excluded. Skin conductance magnitudes were averaged across the two hands for all analyses.
Procedure
Participants were seated in a padded lounge chair within a darkened room and watched the sequence of 27 pictures. Pictures were presented in a darkened room with a Kodak Ektagraphic III AMT projector on a screen 205 cm from the participant. Projected slides occupied a maximum visual angle of 28°, and a Vincent Uniblitz model D122 shutter driver and shutter presented each picture for 6 s. Startle probes were delivered during 18 of the 27 pictures, at random points between 2 s and 5 s after the onset of the picture. To reduce the predictability of the startle stimulus, six additional probes were delivered at random points during intertrial intervals, which ranged between 10 and 20 s, with a mean duration of 15 s. However, startle blink responses were recorded only for probes that were delivered during the pictures. After the picture sequence was completed, participants viewed each picture again and completed affective ratings of the picture. Participants were allowed to view each picture for as long as they desired during the ratings period.
Data Analysis
Score transformation
There was no relationship between overall blink reflex magnitude and scores on either psychopathy factor (rs = − .05 and .00, ps>.3, for fearless dominance and impulsive antisociality, respectively). Furthermore, in comparisons for extreme psychopathy factor groups (see below), raw startle blink responses to neutral pictures did not differ between high and low scoring participants, ts<1.3, ps>.2. However, because individuals can differ greatly in both the absolute magnitude and variability of their startle blink responses, blink magnitudes were standardized within subjects to establish a common metric prior to conducting statistical analyses of blink modulation patterns (Levenston et al., 2000; Patrick et al., 1993; Sutton et al., 2002). Specifically, blink magnitudes were standardized across trials within each participant using a z transformation. To reduce the impact of potential outliers, standardized blink magnitudes greater than 3 or less than − 3 were winsorized to 3 or − 3, respectively (Bradley et al., 2001; Patrick et al., 1993).
Analyses of skin conductance were performed using raw skin conductance scores (in μmhos) because: (a) group differences in absolute skin conductance magnitudes were involved in the hypotheses of the study, (b) standardization, logarithmic, square root, and inverse transformations of the skin conductance data did not normalize the distribution of skin conductance responses (whereas standardization of blink magnitudes normalized the distribution of blink responses), and (c) the results for transformed skin conductance scores were nearly identical to those reported below for raw skin conductance scores.
Manipulation checks
To examine effects of picture valence for each dependent measure in the sample as a whole, repeated-measures multivariate analyses of variance (MANOVAs) were conducted with picture valence as the within-subjects factor. Significant main effects of picture valence were subsequently parsed into orthogonal linear and quadratic contrasts to evaluate the effects of emotional valence and arousal, respectively (Levenston et al., 2000; Patrick et al., 1993). Members of each twin pair had their data weighted by .5 using the weighted least-squares algorithm in SPSS 11.0 to account for their statistical dependence on one another.
Extreme-groups analyses
Because most previous research in psychopathy has used extreme-groups strategies, the primary analyses were conducted using extreme groups on each psychopathy factor. Specifically, we identified participants scoring in the top and bottom 10% on fearless dominance (top 10% M = 1.37, SD = 0.38; bottom 10%M = − 1.05, SD = 0.26) and impulsive antisociality (top 10% M = 1.23, SD = 0.47; bottom 10% M = −1.23, SD = 0.28) to evaluate the effects of psychopathy factor group and picture valence on affective ratings, startle blinks, and skin conductance magnitudes. Effect sizes were computed as partial η2 s (i.e., SSeffect/SSerror in the ANOVA table). This metric is similar to that used in Verschuere et al. (2005), in which they reported that effect sizes of .01, .10, and .25 correspond to small, medium, and large effect sizes, respectively. Participants in the high fearless dominance group did not differ in their levels of impulsive antisociality (M = 0.05, SD = 0.74) from those low in fearless dominance (M = − 0.20, SD = 0.51), t(60) = 1.56, p>.12. Likewise, participants in the high impulsive antisociality group did not differ in their levels of fearless dominance (M = 0.30, SD = 0.72) from those low in impulsive antisociality (M = 0.02, SD = 0.72), t(60) = 1.57, p>.12.
Because individuals with psychopathy show deficits in their physiological responses to aversive stimuli, the contrasts of primary interest involved fear potentiation of startle blink and skin conductance magnitudes to aversive pictures compared against those to neutral pictures. Hence, one set of analyses entailed examining the interaction term in 2 × 2 ANOVAs to compare the difference between startle blink and skin conductance magnitudes to aversive versus neutral pictures in high versus low psychopathy Group participants. We also conducted a second set of analyses, each consisting of a 3 × 2 MANOVA, for all measures to investigate the overall effects of pleasant, neutral, and aversive picture Valence in high versus low psychopathy group participants. Significant multivariate Group × Valence interactions were parsed into orthogonal linear and quadratic contrasts to test for group differences in the effects of emotional valence and arousal, respectively, on each measure. In all of these analyses, picture valence was the within-subjects factor and psychopathy group was the between-subjects factor. Twins who were in the same group had their data weighted by .5 to account for their statistical dependence on one another. An α level of .05 was used for all extreme groups analyses.
Supplementary regression analyses
To examine whether effects in the extreme group analyses were present in the sample as a whole, and to evaluate the effects of fearless dominance and impulsive antisociality simultaneously on participants’ psychophysiology, significant Group effects and Group × picture Valence interactions in the MANOVA analyses were followed up with regression analyses in which both estimated PPI factors were entered as predictors of psychophysiological response patterns.2 All coefficients were adjusted for the dependence of one twin’s scores on his co-twin’s scores with hierarchical linear modeling (Liang & Zeger, 1986) using the PROC MIXED routine from the Statistical Analysis System software package (The SAS Institute, 2000). An α level of .05 was used to evaluate the significance of all regression analyses.
Results
Affective Ratings
Manipulation check
Within the sample as a whole, there was a main effect for picture valence on affective ratings of valence and arousal, Fs(2,346)>540, ps<.001, partial η2s>.75. Valence ratings were greater for pleasant pictures than for aversive pictures, linear F(1,347) = 2380, p<.001, partial η2 = .873 (quadratic F [1,347] = 53.3, p<.001, partial η2 = .133). Pleasant and aversive pictures were both rated as more arousing than neutral pictures, quadratic F(1,347) = 741, p<.001, partial η2 = .681, although pleasant pictures were also rated as more arousing than aversive pictures, linear F(1,347) = 125, p<.001, partial η2 = .265.
Extreme groups
Participants high versus low on fearless dominance did not differ significantly in their ratings of picture valence or arousal, nor did groups high versus low on impulsive antisociality, Fs(2,67)<1.55, ps>.2, partial η2<.05.
Startle Blink
Manipulation check
There was a main effect of picture valence on startle blink magnitude, F(2,305) = 99.8, p<.001, partial η2 = .396. Aversive pictures were associated with larger startle blink magnitudes than pleasant pictures, linear F(1,306) = 177, p<.001, partial η2 = .366. The magnitude difference for pleasant versus neutral pictures (i.e., blink inhibition) tended to be greater in general than the difference for aversive versus neutral pictures (i.e., blink potentiation), quadratic F(1,306) = 8.18, p = .005, partial η2 = .026.
Extreme groups
Figure 1 plots the results of the extreme groups MANOVAs for startle blink magnitude, which revealed that fearless dominance scores moderated the emotional modulation of the startle reflex, Group × Valence F(2,59) = 3.79, p = .029, partial η2 = .113. Specifically, though participants low in fearless dominance exhibited fear-potentiated startle, those high in fearless dominance did not: Group × Aversive-Neutral F(1,60) = 6.80, p = .012, partial η2 = .102. It is important to note that modulation patterns for standardized startle scores need to be interpreted in relative rather than absolute terms (i.e., means for each picture valence condition reflect reactivity in comparison to reactivity in the other conditions; cf. Patrick et al., 1993). Thus, because these groups did not differ in their raw startle blink magnitudes during neutral pictures, t(60) = 1.23, p>.2, these results indicate that individuals high in fearless dominance exhibited a deficit in fear-potentiated startle (i.e., comparative reactivity for aversive vs. neutral pictures), rather than an exaggerated response to neutral pictures.
Figure 1.
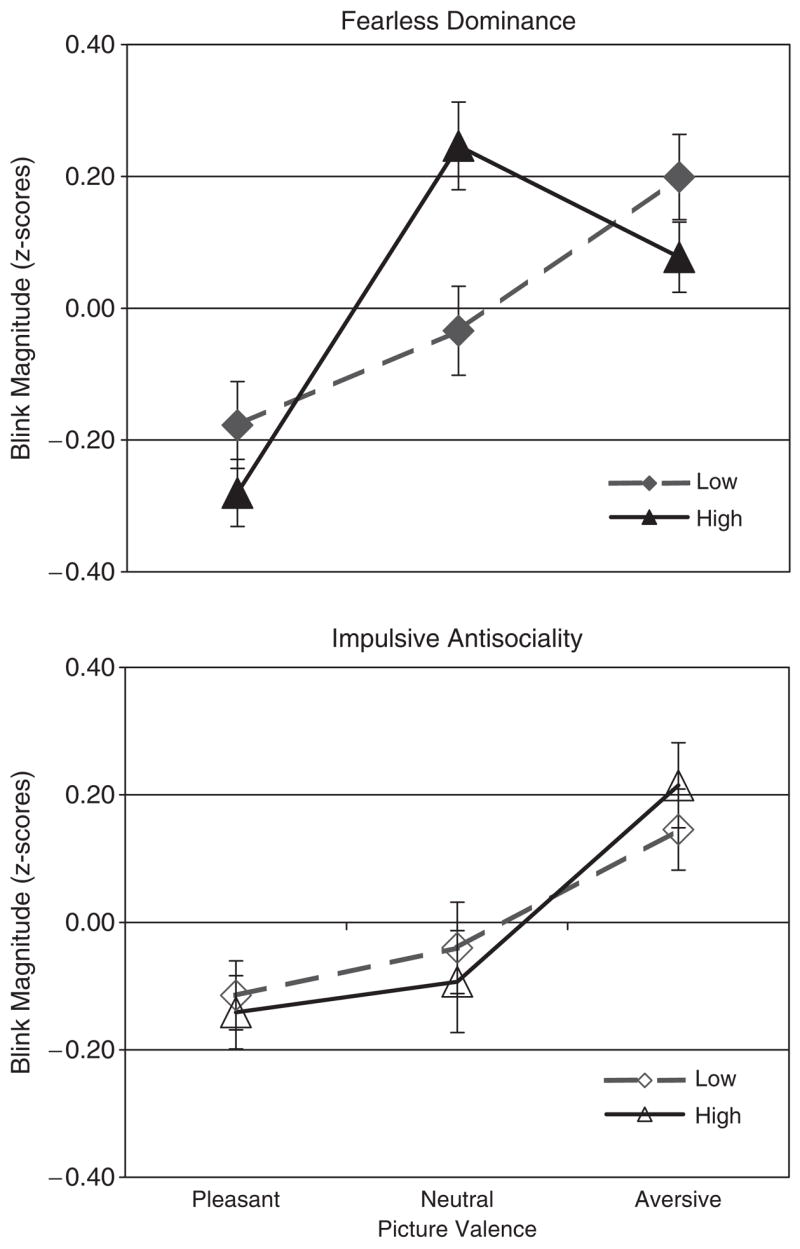
Startle blink means ( ± SE) by extreme 10% psychopathy factor groups (n = 31 in each group).
In contrast, the impulsive antisociality extreme groups did not exhibit a significant Group × Valence interaction, F(2,59) = 0.604, p = .550, partial η2 = .020, or significant differences in fear-potentiated startle, Group × Aversive-Neutral F(1,60) = 0.394, p = .533, partial η2 = .007.
Regression analyses
Within the regression model including both psychopathy factors as predictors of startle reflex potentiation, fearless dominance scores emerged as a significant predictor of the difference between startle magnitudes for aversive versus neutral pictures, β = − .14, p = .014, whereas impulsive antisociality scores did not emerge as a significant predictor, β = − .01, p = .914.
Skin Conductance Response
Manipulation check
Picture valence had a main effect on skin conductance magnitude, F(2,353) = 35.7, p<.001, partial η2 = .168. Paralleling the arousal ratings pattern for the sample as a whole, skin conductance responses during pleasant and aversive pictures were greater than those during neutral pictures, F(1,354) = 51.8, p<.001, partial η2 = .128, and pleasant pictures generated larger skin conductance responses than aversive pictures, F(1,354) = 61.2, p<.001, partial η2 = .147.
Extreme groups
Unlike participants low in fearless dominance, participants high in fearless dominance showed less of a tendency to generate larger skin conductance responses to aversive pictures than to neutral pictures, Group × Aversive-Neutral F(1,70) = 3.74, p = .057, partial η2 = .051 (see Figure 2). Most notably, those high in fearless dominance had significantly smaller skin conductance responses to aversive pictures than did those low in fearless dominance, t(70) = 2.95, p = .004. In addition, participants high in fearless dominance tended to show smaller skin conductance response magnitudes overall than those low in fearless dominance, Group F(1,70) = 3.81, p = .055, partial η2 = .052.
Figure 2.
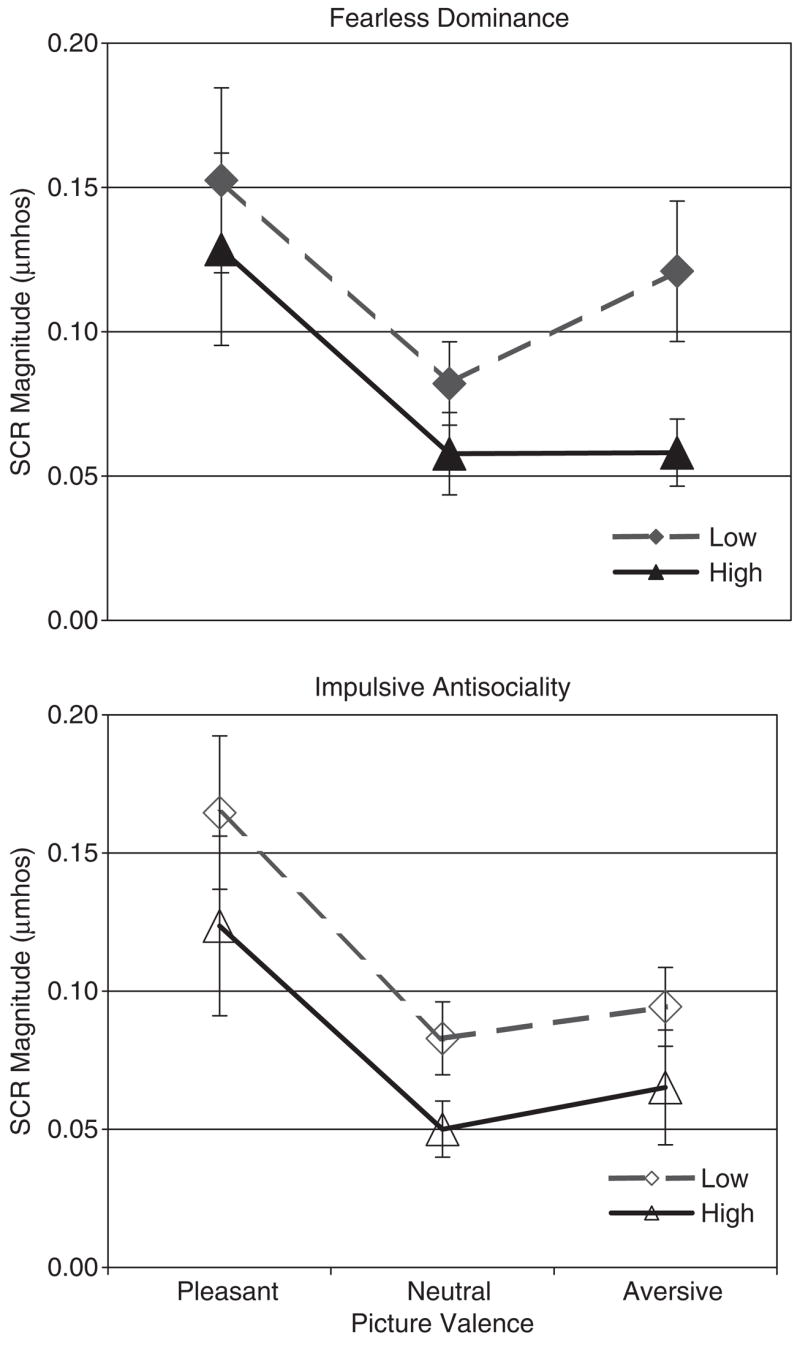
Skin conductance response means ( ± SE) by extreme 10% psychopathy factor groups (n = 36 in each group).
Though the extreme impulsive antisociality groups showed similar patterns of skin conductance modulation by picture valence, Group × Aversive-Neutral F(1,70) = 0.001, p = .970, partial η2 = .000, participants high in impulsive antisociality also tended to show significantly smaller overall skin conductance magnitudes than those low in impulsive antisociality, Group F(1,70) = 2.96, p = .090, partial η2 = .041 (see Figure 2). In particular, those high in impulsive antisociality had smaller skin conductance responses in response to neutral pictures than those low in impulsive antisociality, t(70) = 2.26, p = .027.
Regression analyses
Fearless dominance scores tended to be related negatively to the difference in skin conductance magnitude between aversive and neutral pictures, β = − .11, p = .053 (β for impulsive antisociality = − .01, p = .790). However, scores on impulsive antisociality showed a robust negative association with overall skin conductance magnitude, β = − .15, p = .006, whereas fearless dominance scores were unrelated to overall skin conductance magnitude, β = − .07, p = .200.
Discussion
This study examined the effects of self-reported fearless dominance and impulsive antisociality components of psychopathy in young adult men recruited from the community. Normal-range personality traits were used to assess prospectively these facets of psychopathy among participants in a picture-viewing paradigm similar to that used in prior studies of psychopathy in prisoners. Consistent with predictions, only participants high on fearless dominance showed deficient fear-potentiated startle. Participants high on impulsive antisociality exhibited deficient overall skin conductance magnitudes, whereas the highest-scoring participants on fearless dominance showed evidence of a specific deficiency in skin conductance magnitudes to aversive pictures. These relations were observed despite a 3-year lag between the assessment of these community participants’ personality and their participation in the picture-viewing task, suggesting some stability of these components of psychopathic personality from adolescence to young adulthood.
Physiological Indicators of Fearlessness and Underarousal in Psychopathy
These results lend additional support to the notion that fearless dominance and impulsive antisociality are distinct facets of psychopathy, with unique and divergent correlates. Consistent with the traits of low Stress Reaction, low Harm Avoidance, and high Social Potency that characterize it, fearless dominance appears to index a physiological deficit in trait anxiety and fearfulness (cf. Lykken, 1957, 1995), similar to the lack of anxiety (Harpur et al., 1989) and deficient fear-potentiated startle (Levenston et al., 2000; Patrick, 1994; Patrick et al., 1993) exhibited by offenders high on Factor 1 of the PCL-R and by those low in Cloninger’s (1987) Harm Avoidance (Corr, Kumari, Wilson, Checkley, & Gray, 1997; Corr et al., 1995), an analogue of Gray’s (1987) behavioral inhibition system. Indeed, the deficits in fear-potentiated startle and skin conductance potentiation to aversive pictures, but not in startle inhibition and skin conductance potentiation to pleasant pictures, suggest that affective response differences associated with fearless dominance are specific to aversive emotional states and do not reflect a general blunting of emotional reactivity (cf. Cleckley, 1941/1988). A possible implication of these results, arising from prior research on incarcerated psychopaths (Patrick, 1994), is that individuals exhibiting the interpersonal–emotional features of psychopathy may be deficient in defensive system (including amygdala) reactivity to fear-inducing situations, which may allow such individuals to approach stimuli and situations from which others would quickly and fearfully withdraw. People high on this trait dimension accordingly may lack the social fears and apprehension (Benning et al., 2005) that tend to inhibit deceptive and guileful behavior in individuals who are average or low on this dimension. Furthermore, their lack of fear about the consequences of their actions might dispose them toward enjoyment of risky activities (Benning et al., 2005), including antisocial acts (Benning et al., 2003). Hence, people high in fearless dominance may be relatively immune to internalizing disorders (Kagan & Snidman, 1999) at the cost of being vulnerable to externalizing symptoms
Conversely, emotional modulation of physiological responses to both pleasant and aversive pictures appears to be intact in individuals high in impulsive antisociality. Instead, impulsive antisociality was associated with general underarousal in absolute levels of visceral-electrodermal (but not reflexive) reactivity to pictures, although this relationship was weaker than that between fearless dominance and reduced startle potentiation. Nevertheless, this pattern is similar to the electrodermal underarousal observed in chronically antisocial individuals (Raine, 1997), people with antisocial personality disorder (Raine, Lencz, Bihrle, LaCasse, & Coletti, 2000), those low in socialization (Waid, 1976), and individuals who exhibit “acquired sociopathy” after damage to the ventromedial prefrontal cortex (Damasio, Tranel, & Damasio, 1990; Sanchez-Navarro, Martinez-Selva, & Roman, 2005). Chronic somatic underarousal has been theorized to play an important role in the relatively uncontrolled behavior in which antisocial people are prone to engage (Damasio, 1996). In this view, somatic “tags,” which help to create links between behavior and subsequent emotional reactions and that facilitate correct decision making in social situations in normal people, are not formed. Hence, persons without these somatic tags may suffer from an overload of information when confronted with a decision that must be made in real time. Consequently, they may act antisocially based on limited, impulsive considerations of possible outcomes, with attendant undesirable effects on others and themselves. Additionally, these individuals’ proneness to boredom (Benning et al., 2005) may lead them to engage in more antisocial activities to provide them relief from negative affect.
Notably, previous research has largely entailed the use of special diagnostic, incarcerated, or brain-damaged participants to study the mechanisms underpinning constructs analogous to fearless dominance and impulsive antisociality. However, the current study suggests that individuals with similar psychophysiological characteristics may be found in community samples, using an omnibus inventory of normal personality traits to assess these facets of psychopathy. Moreover, fearless dominance and impulsive antisociality exhibited the predicted relations with psychophysiological variables despite being assessed prospectively 3 years prior to the picture-viewing task. Hence, it appears that participants selected from the community may be used to study the physiological responses and mechanisms that produce fearless dominance and impulsive antisociality in psychopathy. These results also indicate that there are continuities between community and incarcerated participants in the biological mechanisms that underlie the interpersonal–emotional and antisocial deviance facets of psychopathy, suggesting that participants from either population may be useful in understanding the etiologies of these elements of psychopathy.
Limitations and Future Directions
This study used only one picture order, which is atypical for picture viewing studies and may limit the generalizability of these findings. Also, this study was limited in its psychophysiological tasks and measures. Thus, one avenue for future research would be to examine these facets of psychopathy in relation to other tasks, such as the “countdown” procedure (Hare, 1978; Ogloff & Wong, 1990) and social stressor tasks (Ishikawa et al., 2001), in which heart rate modulation has been examined in relation to psychopathy. It might be predicted that individuals high in fearless dominance would show reduced skin conductance and startle blink reactivity to the stressors in both tasks, although they may show anticipatory heart rate accelerations before stressor onset, reflecting an active coping mechanism that reduces the aversiveness of a stressor (Fowles, 1980; Hare, 1978). In contrast, participants high in impulsive antisociality would be predicted to have overall smaller electrodermal and heart rate acceleration magnitudes in both tasks, although they would be expected to show normal (or perhaps even exaggerated) increases in electrodermal activity to predictable stressors during “countdown” and social stressor tasks.
In conclusion, the results of this study suggest that psychophysiological studies of fearless dominance and impulsive antisociality in community samples represent a potentially fruitful avenue of investigation. Using this approach, it should be possible to identify “successful psychopaths” via en masse screening of participants who have no criminal history or by studying the normal-range personality characteristics of individuals who have attained celebrity through their achievements in political, business, sporting, or artistic endeavors, but who have also achieved notoriety for their devious, underhanded, or brazenly disruptive behavioral patterns. It may be the case that these superficially charming but deeply deceptive individuals might show the same psychophysiological profiles as those high in fearless dominance and impulsive antisociality in the current study.
Acknowledgments
This work was supported by grants MH17069, MH48657, MH52384, andMH65137 from the National Institutes of Mental Health and by funds from the Graduate Research Partnership Program and the Hathaway endowment at the University of Minnesota–Twin Cities. We thank Edward Bernat and Steve M. Malone for their invaluable technical consultation and assistance in data reduction. Portions of this work were presented at the Developmental and Neuroscience Perspectives on Psychopathy conference in Madison, Wisconsin, July 17–19, 2003, at the 42nd meeting of the Society for Psychophysiological Research in Chicago, Illinois, October 29–November 2, 2003, and were submitted in partial fulfillment of Stephen D. Benning’s Master’s degree.
Footnotes
The pleasant pictures consisted of numbers 4180, 4680*, 4220*, 4660*, 8080*, 4310, 4210*, 4290, and 8510*. The neutral pictures were numbers 5500*, 1390, 7090*, 7060*, 2410, 7050*, 6150*, 7500, and 7130*. The aversive pictures comprised numbers 3000*, 2800, 3140*, 3010*, 3120, 9140*, 3170*, 3220*, and 3180.
None of the extreme group or regression analyses involving the interaction between the two factors of psychopathy (i.e., fearless dominance × impulsive antisociality scores) yielded significant results. In calculating the interaction term, centering the variables was not necessary because scores on the two factors were already essentially centered, M (SD) fearless dominance = 0.01 (0.70); M (SD) impulsive antisociality = 0.00 (0.72).
References
- Benning SD, Patrick CJ, Blonigen DM, Hicks BM, Iacono WG. Estimating facets of psychopathy from normal personality traits: A step toward community-epidemiological investigations. Assessment. 2005;12:3–18. doi: 10.1177/1073191104271223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benning SD, Patrick CJ, Hicks BM, Blonigen DM, Krueger RF. Factor structure of the Psychopathic Personality Inventory: Validity and implications for clinical assessment. Psychological Assessment. 2003;15:340–350. doi: 10.1037/1040-3590.15.3.340. [DOI] [PubMed] [Google Scholar]
- Benning SD, Patrick CJ, Salekin RT, Leistico AMR. Convergent and discriminant validity of psychopathy factors assessed via self-report: A comparison of three instruments. Assessment. 2005;12:270–289. doi: 10.1177/1073191105277110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blonigen DM, Hicks BM, Krueger RF, Patrick CJ, Iacono WG. Psychopathic personality traits: Heritability and genetic overlap with internalizing and externalizing psychopathology. Psychological Medicine. 2005;35:637–648. doi: 10.1017/S0033291704004180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley MM, Codispoti M, Cuthbert BN, Lang PJ. Emotion and motivation I: Defensive and appetitive reaction in picture processing. Emotion. 2001;1:276–298. [PubMed] [Google Scholar]
- Bradley MM, Cuthbert BN, Lang PJ. Pictures as prepulse: Attention and emotion in startle modification. Psychophysiology. 1993;30:541–545. doi: 10.1111/j.1469-8986.1993.tb02079.x. [DOI] [PubMed] [Google Scholar]
- Bradley MM, Lang PJ. Measuring emotion: The self-assessment manikin and the semantic differential. Journal of Behavioral Therapy and Experimental Psychiatry. 1994;25:49–59. doi: 10.1016/0005-7916(94)90063-9. [DOI] [PubMed] [Google Scholar]
- Carlson SR, Katsanis J, Iacono WG, McGue M. Emotional modulation of the startle reflex in twins: Preliminary findings. Biological Psychiatry. 1997;46:235–246. doi: 10.1016/s0301-0511(97)00014-8. [DOI] [PubMed] [Google Scholar]
- Cleckley HM. The mask of sanity. 5. Augusta, GA: Emily S. Cleckley; 19411988. [Google Scholar]
- Cloninger CR. A systematic method for clinical description and classification of personality variants. Archives of General Psychiatry. 1987;44:573–588. doi: 10.1001/archpsyc.1987.01800180093014. [DOI] [PubMed] [Google Scholar]
- Corr PJ, Kumari V, Wilson GD, Checkley S, Gray JA. Harm avoidance and affective modulation of the startle reflex: A replication. Personality and Individual Differences. 1997;22:591–593. [Google Scholar]
- Corr PJ, Wilson GD, Fotiadou M, Kumari V, Gray NS, Checkley S, et al. Personality and affective modulation of the startle reflex. Personality and Individual Differences. 1995;19:543–553. [Google Scholar]
- Cronbach LJ, Meehl PE. Construct validity of psychological tests. Psychological Bulletin. 1955;52:281–302. doi: 10.1037/h0040957. [DOI] [PubMed] [Google Scholar]
- Damasio AR. The somatic marker hypothesis and the possible functions of the prefrontal cortex. Philosophical Transactions: Biological Sciences. 1996;351:1412–1420. doi: 10.1098/rstb.1996.0125. [DOI] [PubMed] [Google Scholar]
- Damasio AR, Tranel D, Damasio H. Individuals with sociopathic behavior caused by frontal damage fail to respond autonomically to social stimuli. Behavioural Brain Research. 1990;41:81–94. doi: 10.1016/0166-4328(90)90144-4. [DOI] [PubMed] [Google Scholar]
- Dengerink HA, Bertilson S. Psychopathy and physiological arousal in an aggressive task. Psychophysiology. 1975;12:682–684. doi: 10.1111/j.1469-8986.1975.tb00074.x. [DOI] [PubMed] [Google Scholar]
- Gough HG. Manual for the California Psychological Inventory. Palo Alto, CA: Consulting Psychologists Press; 1957. [Google Scholar]
- Gough HG. Theory and measurement of socialization. Journal of Consulting and Clinical Psychology. 1960;24:23–30. [Google Scholar]
- Gray JA. The psychology of fear and stress. New York: Cambridge University Press; 1987. [Google Scholar]
- Fowles DC. The three arousal model: Implications of Gray’s two-factor learning theory for heart rate, electrodermal activity and psychopathy. Psychophysiology. 1980;17:87–104. doi: 10.1111/j.1469-8986.1980.tb00117.x. [DOI] [PubMed] [Google Scholar]
- Frick PJ, Hare RD. The Antisocial Process Screening Device (APSD) Toronto, Ontario: Multi-Health Systems; 2001. [Google Scholar]
- Hare RD. Acquisition and generalization of a conditioned-fear response in psychopathic and nonpsychopathic criminals. Journal of Psychology. 1965a;59:367–370. doi: 10.1080/00223980.1965.10544625. [DOI] [PubMed] [Google Scholar]
- Hare RD. Temporal gradient of fear arousal in psychopaths. Journal of Abnormal Psychology. 1965b;70:442–445. doi: 10.1037/h0022775. [DOI] [PubMed] [Google Scholar]
- Hare RD. Psychopathy and physiological responses to adrenalin. Journal of Abnormal Psychology. 1972;79:138–147. doi: 10.1037/h0032725. [DOI] [PubMed] [Google Scholar]
- Hare RD. Electrodermal and cardiovascular correlates of psychopathy. In: Hare RD, Schalling D, editors. Psychopathic behavior: Approaches to research. Chichester, UK: Wiley; 1978. pp. 107–144. [Google Scholar]
- Hare RD. A research scale of the assessment of psychopathy in criminal populations. Personality and Individual Differences. 1980;1:111–119. [Google Scholar]
- Hare RD. Psychopathy and physiological activity during anticipation of an aversive stimulus in a distraction paradigm. Psychophysiology. 1982;19:266–271. doi: 10.1111/j.1469-8986.1982.tb02559.x. [DOI] [PubMed] [Google Scholar]
- Hare RD. Comparison of the procedures for the assessment of psychopathy. Journal of Clinical and Consulting Psychology. 1985;53:111–119. doi: 10.1037//0022-006x.53.1.7. [DOI] [PubMed] [Google Scholar]
- Hare RD. The Hare Psychopathy Checklist–Revised. Toronto, Ontario: Multi-Health Systems; 1991. [Google Scholar]
- Hare RD. The Hare Psychopathy Checklist–Revised. 2. Toronto, Ontario: Multi-Health Systems; 2003. [Google Scholar]
- Hare RD, Craigen D. Psychopathy and physiological activity in a mixed-motive game situation. Psychophysiology. 1974;11:197–206. doi: 10.1111/j.1469-8986.1974.tb00839.x. [DOI] [PubMed] [Google Scholar]
- Hare RD, Quinn MJ. Psychopathy and autonomic conditioning. Journal of Abnormal Psychology. 1971;77:223–235. doi: 10.1037/h0031012. [DOI] [PubMed] [Google Scholar]
- Harpur TJ, Hare RD, Hakstian AR. Two-factor conceptualization of psychopathy: Construct validity and assessment implications. Psychological Assessment. 1989;1:6–17. [Google Scholar]
- Hathaway SR, McKinley JC. The Minnesota Multiphasic Personality Inventory. Minneapolis, MN: University of Minnesota Press; 1942. [Google Scholar]
- Hawk LW, Cook EW., III Independence of valence modulation and prepulse inhibition of startle. Psychophysiology. 2000;37:5–12. [PubMed] [Google Scholar]
- Herpertz SC, Werth U, Lukas G, Qunaibi M, Schuerkens A, Kunert HJ, et al. Emotion in criminal offenders with psychopathy and borderline personality disorder. Archives of General Psychiatry. 2001;58:737–745. doi: 10.1001/archpsyc.58.8.737. [DOI] [PubMed] [Google Scholar]
- Hitchcock J, Davis M. Lesions of the amygdala, but not of the cerebellum or red nucleus, block conditioned fear as measured with the potentiated startle paradigm. Behavioral Neuroscience. 1986;100:11–22. doi: 10.1037//0735-7044.100.1.11. [DOI] [PubMed] [Google Scholar]
- Iacono WG, Carlson SR, Taylor J, Elkins IJ, McGue M. Behavioral disinhibition and the development of substance use disorders: Findings from the Minnesota Twin Family Study. Development and Psychopathology. 1999;11:869–900. doi: 10.1017/s0954579499002369. [DOI] [PubMed] [Google Scholar]
- Ishikawa SS, Raine A, Lencz T, Bihrle S, LaCasse L. Autonomic stress reactivity and executive functions in successful and unsuccessful criminal psychopaths from the community. Journal of Abnormal Psychology. 2001;110:423–432. doi: 10.1037//0021-843x.110.3.423. [DOI] [PubMed] [Google Scholar]
- Kagan J, Snidman N. Early childhood predictors of adult anxiety disorders. Biological Psychiatry. 1999;46:1536–1541. doi: 10.1016/s0006-3223(99)00137-7. [DOI] [PubMed] [Google Scholar]
- Krueger RF, Hicks BM, Patrick CJ, Carlson SR, Iacono W, McGue GM. Etiologic connections among substance dependence, antisocial behavior, and personality: Modeling the externalizing spectrum. Journal of Abnormal Psychology. 2002;111:411–424. [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. Emotion, attention, and the startle reflex. Psychological Review. 1990;97:377–395. [PubMed] [Google Scholar]
- Lang PJ, Greenwald MK, Bradley MM, Hamm AO. Looking at pictures: Affective, facial, visceral, and behavioral reactions. Psychophysiology. 1993;30:261–273. doi: 10.1111/j.1469-8986.1993.tb03352.x. [DOI] [PubMed] [Google Scholar]
- Levenson MR, Kiehl KA, Fitzpatrick CM. Assessing psychopathic attributes in a noninstitutional population. Journal of Personality and Social Psychology. 1995;68:151–158. doi: 10.1037//0022-3514.68.1.151. [DOI] [PubMed] [Google Scholar]
- Levenston GK, Patrick CJ, Bradley MM, Lang PJ. The psychopath as observer: Emotion and attention in picture processing. Journal of Abnormal Psychology. 2000;109:373–385. [PubMed] [Google Scholar]
- Liang KY, Zeger SL. Longitudinal data analysis using generalized linear models. Biometrika. 1986;73:13–22. [Google Scholar]
- Lilienfeld SO, Andrews BP. Development and preliminary validation of a self report measure of psychopathic personality traits in noncriminal populations. Journal of Personality Assessment. 1996;66:488–524. doi: 10.1207/s15327752jpa6603_3. [DOI] [PubMed] [Google Scholar]
- Lorber MF. Psychophysiology of aggression, psychopathy, and conduct problems: A meta-analysis. Psychological Bulletin. 2004;130:531–552. doi: 10.1037/0033-2909.130.4.531. [DOI] [PubMed] [Google Scholar]
- Lykken DT. A study of anxiety in the sociopathic personality. Journal of Abnormal and Social Psychology. 1957;55:6–10. doi: 10.1037/h0047232. [DOI] [PubMed] [Google Scholar]
- Lykken DT. The antisocial personalities. Mahwah, NJ: Lawrence Erlbaum; 1995. [Google Scholar]
- Lynam DR, Whiteside S, Jones S. Self-reported psychopathy: A validation study. Journal of Personality Assessment. 1999;73:110–132. doi: 10.1207/S15327752JPA730108. [DOI] [PubMed] [Google Scholar]
- MathWorks Inc. Matlab (Version 6.0.0.88) Natick, MA: Author; 2000. [Google Scholar]
- McKinley JC, Hathaway SR. The MMPI: V. Hysteria, hypomania and psychopathic deviate. Journal of Applied Psychology. 1944;28:153–174. [Google Scholar]
- Miller JD, Lynam DR, Widiger TA, Leukefeld C. Personality disorders as extreme variants of common personality dimensions: Can the five-factor model adequately represent psychopathy? Journal of Personality. 2001;69:253–276. doi: 10.1111/1467-6494.00144. [DOI] [PubMed] [Google Scholar]
- Ogloff JPR, Wong S. Electrodermal and cardiovascular evidence of a coping response in psychopaths. Criminal Justice and Behavior. 1990;17:231–254. [Google Scholar]
- Pastor MC, Molto J, Vila J, Lang PJ. Startle reflex modulation, affective ratings and autonomic reactivity in incarcerated Spanish psychopaths. Psychophysiology. 2003;40:934–938. doi: 10.1111/1469-8986.00111. [DOI] [PubMed] [Google Scholar]
- Patrick CJ. Emotion and psychopathy: Startling new insights. Psychophysiology. 1994;31:319–330. doi: 10.1111/j.1469-8986.1994.tb02440.x. [DOI] [PubMed] [Google Scholar]
- Patrick CJ, Berthot BD. Startle potentiation during anticipation of a noxious stimulus: Active versus passive response sets. Psychophysiology. 1995;32:72–80. doi: 10.1111/j.1469-8986.1995.tb03408.x. [DOI] [PubMed] [Google Scholar]
- Patrick CJ, Bradley MM, Lang PJ. Emotion in the criminal psychopath: Startle reflex modulation. Journal of Abnormal Psychology. 1993;102:82–92. doi: 10.1037//0021-843x.102.1.82. [DOI] [PubMed] [Google Scholar]
- Patrick CJ, Cuthbert BN, Lang PJ. Emotion in the criminal psychopath: Fear imagery processing. Journal of Abnormal Psychology. 1994;103:523–534. doi: 10.1037//0021-843x.103.3.523. [DOI] [PubMed] [Google Scholar]
- Raine A. Antisocial behavior and psychophysiology: A biosocial perspective and a prefrontal dysfunction hypothesis. In: Stoff DM, Breiling J, Maser JD, editors. Handbook of antisocial behavior. New York: Wiley; 1997. pp. 289–303. [Google Scholar]
- Raine A, Lencz T, Bihrle S, LaCasse L, Colletti P. Reduced prefrontal gray matter and autonomic activity in antisocial personality disorder. Archives of General Psychiatry. 2000;57:119–127. doi: 10.1001/archpsyc.57.2.119. [DOI] [PubMed] [Google Scholar]
- Ross SR, Benning SD, Patrick CJ, Thompson A, Thurston A. Factors of the Psychopathic Personality Inventory: Relationships with the Five-Factor Model of personality and criterion-related validity. 2005 doi: 10.1177/1073191108322207. Manuscript submitted for publication. [DOI] [PubMed] [Google Scholar]
- Russell JA. Affective space is bipolar. Journal of Personality and Social Psychology. 1979;37:345–356. [Google Scholar]
- Sanchez-Navarro JP, Martinez-Selva JM, Roman F. Emotional response in patients with frontal brain damage: Effects of affective valence and information content. Behavioral Neuroscience. 2005;119:87–97. doi: 10.1037/0735-7044.119.1.87. [DOI] [PubMed] [Google Scholar]
- The SAS Institute. The SAS System for Windows (Version 8.1) Cary, NC: Author; 2000. [Google Scholar]
- Smith SW. The scientist and engineer’s guide to digital signal processing. 2001 Retrieved January 25 2004 from http://www.dsponline.com/
- Sutton SK, Vitale JE, Newman JP. Emotion among females with psychopathy during picture perception. Journal of Abnormal Psychology. 2002;111:610–619. doi: 10.1037//0021-843x.111.4.610. [DOI] [PubMed] [Google Scholar]
- Tellegen A. Manual for the Multidimensional Personality Questionnaire. Minneapolis, MN: University of Minnesota Press; in press. [Google Scholar]
- Vanman EJ, Mejia VY, Dawson ME, Schell AM, Raine A. Modification of the startle reflex in a community sample: Do one or two dimensions of psychopathy underlie emotional processing? Personality and Individual Differences. 2003;35:2007–2021. [Google Scholar]
- Verschuere B, Crombez G, De Clercq A, Koster EHW. Psychopathic traits and autonomic responding to concealed information in a prison sample. Psychophysiology. 2005;42:239–245. doi: 10.1111/j.1469-8986.2005.00279.x. [DOI] [PubMed] [Google Scholar]
- Vrana SR, Spence EL, Lang PJ. The startle probe response: A new measure of emotion? Journal of Abnormal Psychology. 1988;97:487–491. doi: 10.1037//0021-843x.97.4.487. [DOI] [PubMed] [Google Scholar]
- Waid WM. Skin conductance response to both signaled and unsignaled noxious stimulation predicts level of socialization. Journal of Personality and Social Psychology. 1976;34:923–929. doi: 10.1037//0022-3514.34.5.923. [DOI] [PubMed] [Google Scholar]
- Zuckerman M. Sensation seeking: Beyond the optimal level of arousal. Hillsdale, NJ: Lawrence Erlbaum; 1979. [Google Scholar]


