Abstract
As part of a functional analysis of archaeal Sm-related proteins, we have studied the oligomerization behavior of the Sm-2 type protein from the euryarchaeon Archaeoglobus fulgidus using gel filtration chromatography and noncovalent mass spectrometry. Our experiments show that the oligomeric state of the protein depends on the pH and presence of RNA. The protein forms a hexamer at acidic pH in the absence of RNA. The addition of RNA (oligo U10) induces the formation of a heptamer over the whole pH range studied. The stability of both the hexamer and the RNA-bound heptamer increases at lower pH.
Keywords: archaea, Sm-like protein, Sm fold, RNA binding, heptamer, hexamer, noncovalent mass spectrometry
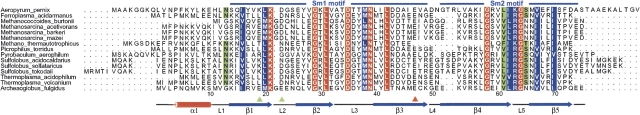
The Sm protein family is characterized by a conserved bipartite sequence motif of ∼70 amino acids, which is called the Sm domain. The domain contains two conserved sequence segments, known as the Sm1 and Sm2 motifs, separated by a loop region which differs in length and sequence among the family (Séraphin 1995; Salgado-Garrido et al. 1999). β-Strands 1, 2, and 3 constitute the first motif, and β-strands 4 and 5 constitute the second motif of the Sm domain. The overall architecture of the Sm monomer, a barrel-type OB-fold structure, is completed by the N-terminal α-helix (Fig. 1). In eukaryotes, seven distinct Sm or Sm-like (Lsm) proteins, together with various RNAs that contain the short, single-stranded, uridine-rich Sm site, form the core domain of the small nuclear ribonucleoprotein particles (snRNPs), which are essential for several RNA-processing events such as mRNA decapping and degradation (Bouveret et al. 2000; He and Parker 2000; Tharun et al. 2000; Kufel et al. 2004), pre-mRNA splicing (Mattaj et al. 1993; Hermann et al. 1995; Will and Lührmann 2001), and telomerase activity (Seto et al. 1999). Sm-related proteins have also been identified in archaea and eubacteria through database searches (Salgado-Garrido et al. 1999; Møller et al. 2002). The in vivo function of the archaeal Sm proteins is widely unknown; however, experimental results from our laboratory suggest their potential involvement in transfer RNA (tRNA) maturation (Törö et al. 2001). Archaeal Sm proteins share the common Sm fold, bind to U-rich RNA sequences, and form ring-shaped homo-hexameric or -heptameric complexes. The assembly of the protein complexes is primarily maintained through interactions between the β4 and β5 strands of adjacent monomers (Collins et al. 2001; Törö et al. 2002). The maximum number of distinct Sm proteins found in archaeal species is currently three. At present, there are only four archaeal species known (Pyrobaculum aerophilum, Sulfolobus acidocaldarius, Sulfolobus solfataricus, and Sulfolobus tokodaii) that contain all three Sm-type proteins. The Sm1-type is most abundant among the archaeal species and forms stable, heptameric ring-structures. The recently identified Sm3-type proteins contain an additional C-terminal domain (Mura et al. 2003).
Figure 1.
Sequence alignment of presently known archaeal Sm2-type proteins. Numbering and secondary structure assignment are depicted for A. fulgidus Sm2 (shown at the bottom). Fully conserved residues (e.g., K21 and G29) are shown in red; almost fully conserved residues are shown in blue; highly conserved residues are marked in green. Green arrows indicate the glutamates that interact between neighboring monomers of AF-Sm2, and the red arrow indicates the residue that is in close proximity to these two residues in the hexamer.
Among the currently studied archaeal Sm proteins, Archaeoglobus fulgidus Sm2 (AF-Sm2) is the only protein that can exist in hexameric or heptameric forms (Achsel et al. 2001; Törö et al. 2002). Previously, it was suggested that this unusual behavior of the AF-Sm2 protein could be explained by the interaction of residues E19 and E23 (Fig. 1; shown by green arrows) at the interface of adjacent monomers (Törö et al. 2002). At neutral pH, the carboxylate groups of E19 and E23 are deprotonated, destabilizing the hexameric species due to the repulsive interaction of the closely spaced negative charges. When the pH is lowered, these groups are protonated, which eliminates the repulsive interaction, and in turn hexamer formation becomes possible.
In order to study the oligomerization behavior and to unambiguously determine the oligomerization state of the AF-Sm2 protein we have used noncovalent mass spectrometry. This application for mass spectrometry (MS) emerged in the early 1990s, allowing, for the first time, the study of specific noncovalent macromolecular complexes using electrospray ionization (Katta and Chait 1991). Since then, an increasing number of publications have shown the reliability of this technique (for review, see Loo 2000; Van den Heuvel and Heck 2004). In fact, under carefully controlled experimental conditions it is possible to transfer intact noncovalent complexes into the gas phase of the mass spectrometer and analyze the binding stoichiometry of protein–protein (Sanglier et al. 2002) and protein–ligand (Stehlin-Gaon et al. 2003) complexes. Through noncovalent MS, it is also possible to monitor dynamics of complexes under various experimental conditions such as temperature and pH effects on the stability of multiprotein assemblies (Fändrich et al. 2000; Benesch et al. 2003; Liang et al. 2003).
Using gel filtration chromatography and noncovalent MS we show in this paper that the oligomerization behavior and complex stability of the A. fulgidus Sm2-type protein is pH and RNA dependent.
Results
Formation of the AF-Sm2 oligomer is pH and RNA dependent, as confirmed by gel filtration chromatography
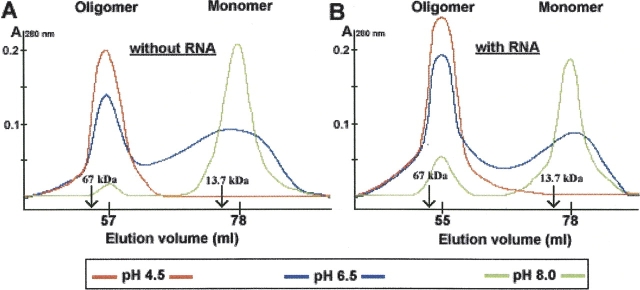
Purification profiles of AF-Sm2 using gel filtration columns reveal that the protein complex formation in the absence of RNA is strongly affected by pH. At pH 8, for example, AF-Sm2 is present as a monomer, and almost no oligomers are observed. Lowering the pH to an acidic value shifts the monomer/oligomer ratio in favor of oligomer formation (Fig. 2A). Gel filtration profiles of AF-Sm2 in the presence of RNA (oligo U10) reveal a similar stabilizing effect of the pH. Addition of RNA shifts the equilibrium toward more oligomer formation (Fig. 2B). At pH 4.5, all AF-Sm2 monomers, with or without RNA, assemble into oligomers, and there is no detectable free monomer left. Clearly, the effects of low pH and the presence of RNA are additive, and shift the equilibrium further toward the oligomeric species.
Figure 2.
Gel filtration profiles of AF-Sm2 with and without RNA. (A) In the absence of RNA, oligomerization behavior of AF-Sm2 protein is pH dependent. A shift from pH 8.0 to pH 4.5 favors the oligomer formation. (B) In the presence of RNA (monomer-to-RNA ratio is 7:1), oligomerization behavior of the AF-Sm2 protein is still pH dependent, and a shift from pH 8.0 to pH 4.5 favors the oligomer formation even more. Arrows indicate the elution volumes for Ribonuclease A (13.7 kDa) and Albumin (67 kDa) as 73 mL and 52.5 mL, respectively.
Determination of the oligomerization status of AF-Sm2 complexes by noncovalent MS
To unambiguously assess the stoichiometry of the different oligomeric species observed in the gel filtration profiles, we performed noncovalent MS analysis. In the absence of RNA and at pH 6.5, electrospray ionization mass spectrometry (ESI-MS) under nondenaturing conditions revealed that AF-Sm2 is mostly present as a monomer (Fig. 3A) The main ion series in the m/z range 1000–2000 lead to a molecular weight of 8609.9 ± 0.2 Da, which is in agreement with the molecular weight calculated from the amino acid sequence of AF-Sm2 (8608 Da). A second minor ion series with a molecular weight of 17,216.5 ± 0.3 Da corresponds to a dimeric form of AF-Sm2. No significant ion series can be observed in the upper m/z region of the ESI mass spectra, which suggests that AF-Sm2 mostly exists as a monomer and dimer in the absence of RNA at pH 6.5.
Figure 3.
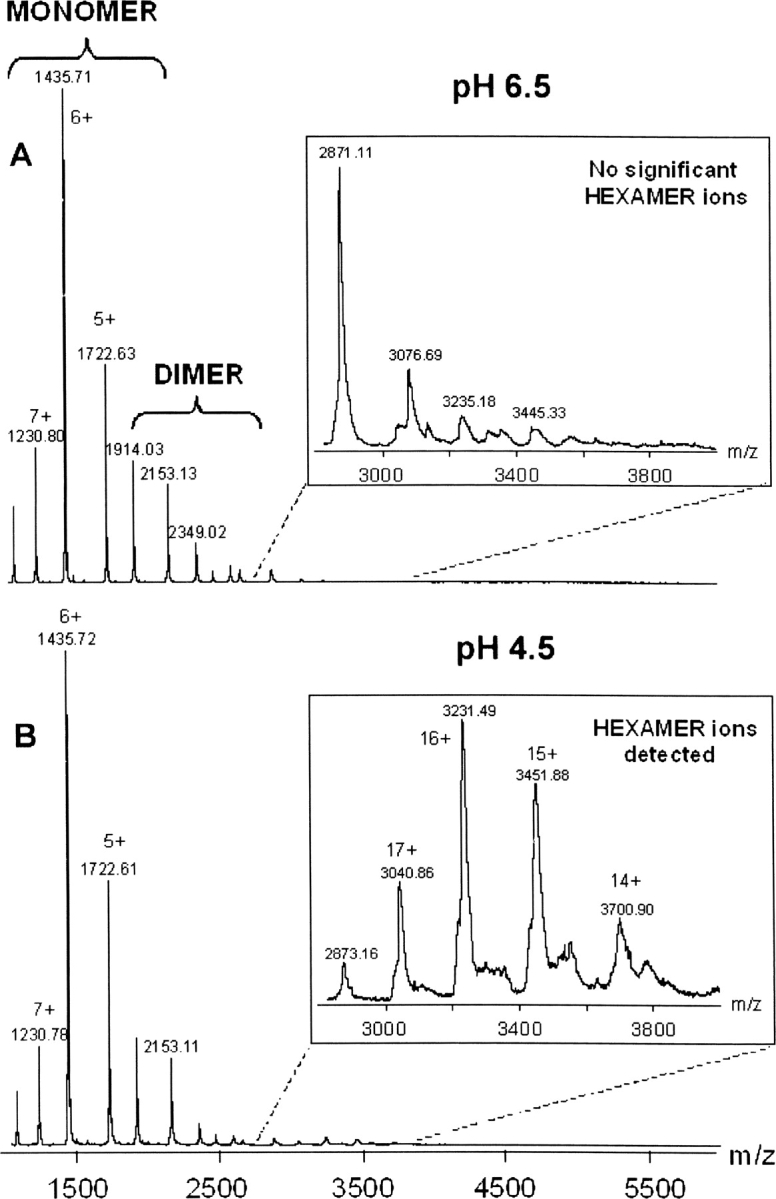
ESI mass spectra obtained under nondenaturing conditions for AF-Sm2 in the absence of RNA. (A) At pH 6.5, the only signals detected correspond to monomeric and dimeric forms of AF-Sm2. No higher oligomeric form can be observed in the upper m/z range of the mass spectrum. (B) At pH 4.5, the most intense ions are still monomers and dimers of AF-Sm2, but an additional ion series in the m/z range 2500–4000 can clearly be detected. These additional species correspond to the association of six AF-Sm2 subunits with a molecular weight of 51,670.1 ± 17.7 Da.
At pH 4.5, the most intense ion series still corresponded to monomeric and dimeric AF-Sm2 (Fig. 3B). Additional very low intensity signals were detected in the m/z range of 2500–4000, corresponding to species with a molecular weight of 51,670.1 ± 17.7 Da, which is consistent with the theoretical molecular weight of the hexamer (51,648 Da).
The reason for the discrepancy between noncovalent MS and gel filtration results at pH 6.5 could be the hydrophobic interactions mainly responsible for the cohesion of the hexameric complex. In fact, special instrumental conditions were necessary for the detection of hexameric ions (the pressure in the interface of the instrument was increased to 7.3 mbar, and an accelerating voltage <80 V was applied). Such experimental data strongly suggest that the AF-Sm2 hexameric complex can be easily destabilized in the interface of the mass spectrometer. These applied instrumental parameters and the low intensity of the signals corresponding to the hexamer can also explain the low mass accuracy for the mass measurement of the hexamer. However, despite all of these difficulties, it can be deduced from noncovalent MS results that AF-Sm2 forms a hexamer at pH 4.5.
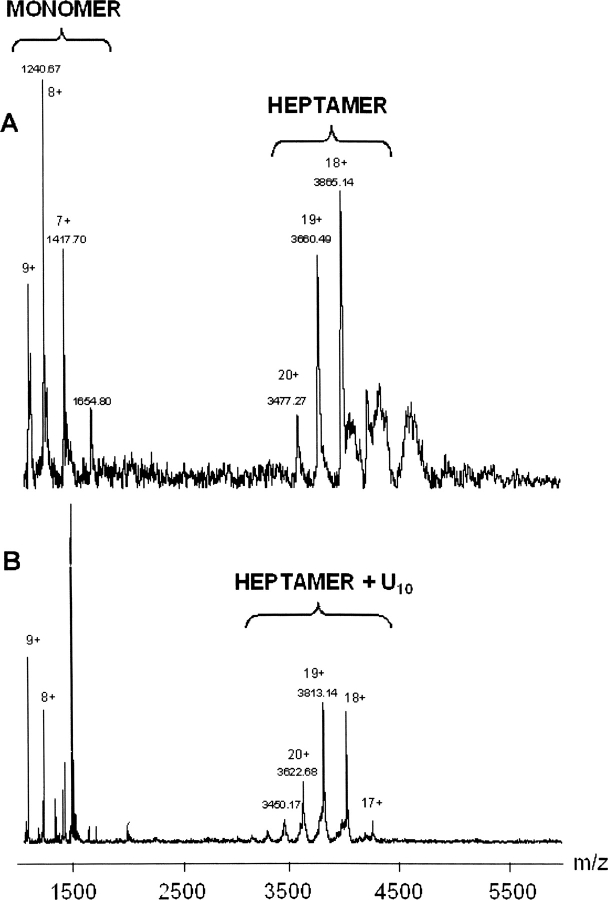
In the presence of RNA, ESI-MS spectra under nondenaturing conditions presented two ion series (Fig. 4). The main ion series in the lower m/z range of 1000–2000 corresponds to the AF-Sm2 monomer. Additionally, low intensity signals that lead to a molecular weight of 63,279.2 ± 8.8 Da could be clearly detected in the upper m/z range (3000–4000), which is in agreement with the association of seven AF-Sm2 subunits and one molecule of oligo U10 (the theoretical mass in total is 63,256 Da). This protein–RNA complex could be identified both at acidic pH (4.5, 5.5, and 6.5) and slightly basic pH (7.5). Thus, ESI-MS spectra under nondenaturating conditions indicate that the AF-Sm2–U10 complex is a specific noncovalent heptamer partly independent of pH. The stabilizing effect of pH on the protein–RNA complex is clearly observed by shifting the pH from 7.5 to 4.5 as the relative heptamer signals continuously increase, reflecting a strong stabilization of the heptameric complex upon pH decrease.
Figure 4.

ESI mass spectra obtained under nondenaturing conditions for AF-Sm2 in the presence of RNA. At pH 4.5, species with a molecular weight of 63,279.2 ± 8.8 Da are mainly detected and correspond to the association of seven subunits of AF-Sm2 with one molecule of oligo U10. By increasing the pH up to 7.5, relative signals corresponding to the AF-Sm2 heptamer continuously decrease, which indicates the stabilizing effect of acidic pH on the protein–RNA complex.
Effect of the three glutamic acids on the stability and behavior of AF-Sm2 by site-directed mutagenesis
To assess the pH dependence of the hexamer, we performed mutagenesis on the three glutamates (E19, E23, and E47). Through mutagenesis, each of the glutamates was mutated to alanine to eliminate their effect on the hexameric complex formation. The gel filtration profiles of the mutant AF-Sm2 samples indicated that the hexamer formation is favored upon mutating any of the three glutamates. Mutation of E19, E23, or E47 to alanine resulted in similar gel filtration profiles; in Figure 5, the gel filtration result of the E19A mutation is shown as a representation of all three mutation analyses. In Figure 5A, E19A mutation results in a more stable complex, which forms even at pH 8.0 and is less affected by a pH change to 6.5 compared to the wild type presented in Figure 2A. However, we still observe a full conversion of monomers to oligomers upon decreasing pH to 4.5 as we observed for wild-type AF-Sm2. Clearly, the gel filtration profiles of the mutant samples indicate that glutamates are involved in the pH dependency of the wild-type hexameric complex. Another difference of the mutant samples becomes apparent when RNA is added to protein. At pH 6.5, addition of RNA converts all the monomers to oligomers (Fig. 5B).
Figure 5.
Gel filtration profiles of the mutant AF-Sm2 protein (E19A) in the absence and presence of RNA. (A) In the absence of RNA, E19A mutation results in a more stable oligomer compared to wild type. (B) Addition of RNA at pH 6.5 results in complete oligomer formation. Arrows indicate the elution volumes for Ribonuclease A (13.7 kDa) and Albumin (67 kDa) as 73 mL and 52.5 mL, respectively.
Discussion
Hexamer formation of AF-Sm2 is pH dependent
It is clear from the noncovalent MS that the AF-Sm2 forms a hexamer at pH 4.5 in the absence of RNA. Although we could not observe any significant peaks corresponding to the hexameric species from the mass spectrum of pH 6.5, it was possible to extract peaks corresponding to hexameric (but not heptameric) species from the spectrum upon a magnification of ∼24 times as previously done by Törö et al. (2002). It is likely that such a magnification overestimates the oligomer peaks compared to monomer peaks. Nevertheless, in both of the MS spectra, the most intense signals correspond to the monomer and the signals corresponding to the hexameric species are negligible. That is why we conclude that the major and significant peaks at pH 6.5 in the absence of RNA refer to monomeric as well as dimeric species.
It is also clear from the gel filtration experiments that the hexamer formation shows a pH dependency. The explanation for this are the acidic residues present at the interface of the interacting monomers. In addition to the glutamates Glu19 and Glu23 as previously suggested (Törö et al. 2002), we analyzed an additional glutamate residue, Glu47, which is in close proximity to residues Glu19 and Glu23. This residue is at ∼4 Å distance from Glu23, and its position in the hexameric form is stabilized through a salt bridge with Arg55 (Fig. 6B). Glu47 seems to be conserved among all Sm2-type proteins (Fig. 1, indicated by a red arrow in the sequence alignment). The only exception in the sequence alignment is the Methanothermobacter thermautotrophicus Sm2 (MT-Sm2). However, a closer look indicates that this missing residue in the alignment could possibly be Glu45 or Glu46 in the MT-Sm2 sequence. Residue Arg55, on the other hand, which forms the salt bridge with Glu47, is only partially conserved. It seems that the pH dependence of the hexamer formation is a result of the negative environment created by the interaction of these three glutamic acid residues at the interface of the adjacent monomers.
Figure 6.
Interaction of residues Glu19/Glu23 and Glu47/Arg55 in the hexamer (Törö et al. 2002). (A) Arrangement of interacting residues in the hexamer. Each color represents one monomer. Residues Glu19 and Glu23 are found at the interface of adjacent monomers. (B) Close-up view of interacting residues. Upon protonation at acidic pH, residues Glu19 and Glu23 form hydrogen bonds (shown by green dashed lines with hydrogen bond distance of 3.04 Å) and contribute to the stability of the hexamer. Arg55 forms a salt bridge (shown by red dashed lines) with Glu47 and stabilizes its orientation. The salt bridge distance between residues Arg55 and Glu47 is 2.70 Å. An additional hydrogen bonding is observed between the main-chain nitrogen atom of Glu47 and main-chain oxygen atom of Arg55 (indicated by white dashed lines with a hydrogen bond distance of 2.66 Å).
The effect of these closely located glutamates in the hexamer structure is such that, when they are deprotonated at basic pH, their negatively charged carboxyl groups produce a repulsive effect that prevents the interaction of monomers to initiate the oligomerization. This repulsive effect is not observed when these residues are protonated at acidic pH, but instead, hydrogen bonds will further stabilize the complex (Fig. 6B). Comparison with other archaeal Sm2-type proteins shows that these acidic residues are not unique to the AF-Sm2 protein. Glu19 and Glu23 are partially conserved among the Sm2-type subfamily, whereas Glu47 is highly conserved (Fig. 1). In all the Methanosarcina species, for example, Glu19 is conserved and Glu23 is replaced by Asp23.
Heptamer formation of AF-Sm2 is RNA dependent
As clearly identified by noncovalent MS, the oligomeric state of the AF-Sm2–RNA complex is a heptamer. The stability of the heptameric protein–RNA complex is pH dependent, but heptamer formation is clearly observable at all pH values studied. It is not clear yet whether the heptamer formation of AF-Sm2 is due to some conformational changes induced by RNA binding. At present, there are no structural or functional data available on the other currently known archaeal Sm2-type proteins to answer the questions of whether the oligomerization behavior of the AF-Sm2 is unique or whether this behavior is due to other factors in addition to the protonation/deprotonation status of the interacting residues (Glu19/Glu23/Glu47).
However, our recent study of the Sm2-type protein from Sulfolobus solfataricus (SS-Sm2) using ESI-MS spectra under nondenaturing conditions suggests that the oligomerization behavior of AF-Sm2 is likely to be unique. Our results showed that SS-Sm2 is present as only one type of oligomer with or without RNA. The oligomerization status of SS-Sm2 at pH 6.5 is determined to be a heptamer independent of RNA (Fig. 7). Comparison of the sequences from AF-Sm2 with SS-Sm2 reveals that Glu19 in AF-Sm2 is replaced with Lys19 in SS-Sm2 (Fig. 1), suggesting that there will be no repulsive effect between adjacent monomers of SS-Sm2. In contrast, Lys19 can possibly form salt bridges with Asp23 (Glu23 in AF-Sm2) and Glu47, promoting the stability of the heptameric complex. While we could not observe the hexameric species of AF-Sm2 with ESI-MS at pH 6.5 (Fig. 3), we were able to identify the heptameric form of SS-Sm2 at pH 6.5 with and without RNA, showing the stability of the SS-Sm2 heptamer without such a disruptive effect between the monomers.
Figure 7.
ESI mass spectra obtained under nondenaturing conditions for S. solfataricus Sm2-type protein (SS-Sm2) in the absence and presence of RNA (oligo U10). (A) At pH 6.5, species with a molecular weight of 69,533.5 ± 9.4 Da are detected and correspond to the association of seven subunits of SS-Sm2. (B) In the presence of RNA (monomer-to-RNA ratio is 7:1), relative signals indicate the presence of an SS-Sm2 heptamer–RNA complex with a molecular weight of 72,433.6 ± 5.7 Da. Unlike the AF-Sm2 protein, SS-Sm2 is in the form of a heptamer in the presence and absence of RNA.
Materials and methods
Cloning, expression, and purification
The AF-Sm2 protein was purified from pETM30 transformed Escherichia coli BL21-CodonPlus (DE3) cells. The pETM30 vector contains an upstream sequence coding for a 6xHis-tag and GST-tag followed by a tobacco etch virus protease site (TEV site). The sequence-confirmed transformant was incubated in 6 L of Luria-Bertani (LB) media at 37°C until the OD600 reached ∼0.6. Cells were induced by adding isopropyl-β-D-thiogalactopyranoside (IPTG) at a final concentration of 1 mM for 3 h and harvested by centrifugation. Cell lysis was carried out using sonication in 50 mM Tris-HCl (pH 8.0), 0.3 M NaCl, 0.5% IGEPAL, 15 mM imidazole, and 10 mM β-mercaptoethanol. Cell lysate was loaded onto a Ni-NTA column for initial purification followed by a Sephadex g25 column for buffer exchange to 50 mM Tris-HCl (pH 8.0), 0.5 M ethylenediamine tetra-acetic acid (EDTA), and 1 mM dithiothreitol (DTT) for overnight digestion at 30°C using TEV protease. After cleavage, the protein solution was heated to 78°C for 10 min to yield homogeneous AF-Sm2 protein. Following the removal of denatured proteins by centrifugation, the supernatant was concentrated using a Millipore Amicon concentrator with a molecular weight cutoff (MWCO) of 10 kDa, and further analyzed for purity and molecular weight conformation by 18% SDS-PAGE and MS, respectively. The purification protocol of SS-Sm2 is the same as AF-Sm2 except for the expression vector, pETM11, which contains an upstream sequence coding for a 6xHis-tag only followed by a TEV site.
Mutagenesis
Mutations were introduced using the QuikChange Site-Directed Mutagenesis kit from Stratagene. Glutamates were mutated to alanine by point mutations as follows: GAA to GCA for E19A, GAG to GCG for E23A, and GAG to GCG for E47A. Resulting mutations were confirmed by sequencing as well as by MS after protein purification. The procedure for expression of the mutant AF-Sm2 proteins is the same as wild-type protein except that the expression volume is 3 L.
Gel filtration analysis
Equal amounts of the purified AF-Sm2 sample were taken for buffer exchange to 50 mM AcONH4 (pH 4.5), 50 mM AcONH4 (pH 6.5), and 50 mM Tris-HCl (pH 8.0). Each sample was passed through a Superdex 75 gel-filtration column (Pharmacia Biotech) for analysis of oligomeric state. For the study of the oligomeric state in the presence of RNA, oligo U10 with a molecular weight of 2999.7 g/mol was dissolved in 50 mM Tris-HCl (pH 7.2) and added to the protein samples at a 7:1 ratio of monomer to RNA. After each gel filtration analysis, fractions containing the protein or protein–RNA complex were concentrated and stored at +4°C for MS. The procedure for gel filtration analysis of the mutant AF-Sm2 proteins was the same as for the wild-type protein.
Electrospray ionization mass spectrometry (ESI-MS) analysis
For noncovalent MS analysis, further desalting of the samples was achieved by single-use NAP-5 gel filtration columns (Amersham Biosciences) in 50 mM AcONH4 (pH 4.5, 6.5, and 7.5). Samples were subsequently concentrated using microconcentrators (MWCO 10 kDa Centricon, Millipore). ESI mass spectra were acquired on an ESI-TOF mass spectrometer fitted with a standard Z-spray source (LCT, Waters). In order to preserve the integrity of fragile noncovalent assemblies and enhance their detection sensitivity, the pressure in the interface was increased to 7.3 mbar and the cone voltage was optimized to 80 V. Samples were diluted to 20 μM in 50 mM AcONH4 buffer and directly introduced into the mass spectrometer using a syringe pump with a flow rate of 5 μL/min.
Acknowledgments
We thank Jérôme Basquin from EMBL-Heidelberg for providing us with the plasmid of wild-type AF-Sm2, and Cedric Atmanene from CNRS-Strasbourg for his help during mass spectrometry analysis.
Footnotes
Reprint requests to: Dietrich Suck, Structural and Computational Biology Programme, European Molecular Biology Laboratory (EMBL), Meyerhofstrasse 1, 69117 Heidelberg, Germany; e-mail: suck@embl.de; fax: 49-6221-387306.
Article published online ahead of print. Article and publication date are at http://www.proteinscience.org/cgi/doi/10.1110/ps.062191506.
References
- Achsel, T., Stark, H., Lührmann, R. 2001. The Sm domain is an ancient RNA-binding motif with oligo(U) specificity. Proc. Natl. Acad. Sci. 98: 3685–3689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benesch, J.L., Sobott, F., Robinson, C.V. 2003. Thermal dissociation of multimeric protein complexes by using nanoelectrospray mass spectrometry. Anal. Chem. 75: 2208–2214. [DOI] [PubMed] [Google Scholar]
- Bouveret, E., Rigaut, G., Shevchenko, A., Wilm, M., Séraphin, B. 2000. A Sm-like protein complex that participates in mRNA degradation. EMBO J. 19: 1661–1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins, B.M., Harrop, S.J., Kornfeld, G.D., Dawes, I.W., Curmi, P.M., Mabbutt, B.C. 2001. Crystal structure of a heptameric Sm-like protein complex from archaea: Implications for the structure and evolution of snRNPs. J. Mol. Biol. 309: 915–923. [DOI] [PubMed] [Google Scholar]
- Fändrich, M., Tito, M.A., Leroux, M.R., Rostom, A.A., Hartl, F.U., Dobson, C.M., Robinson, C.V. 2000. Observation of the noncovalent assembly and disassembly pathways of the chaperone complex MtGimC by mass spectrometry. Proc. Natl. Acad. Sci. 97: 14151–14155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He, W. and Parker, R. 2000. Functions of Lsm proteins in mRNA degradation and splicing. Curr. Opin. Cell Biol. 12: 346–350. [DOI] [PubMed] [Google Scholar]
- Hermann, H., Fabrizio, P., Raker, V.A., Foulaki, K., Hornig, H., Brahms, H., Lührmann, R. 1995. snRNP Sm proteins share two evolutionarily conserved sequence motifs which are involved in Sm protein–protein interactions. EMBO J. 14: 2076–2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katta, V. and Chait, B.T. 1991. Observation of the heme–globin complex in native myoglobin by electrospray-ionization mass spectrometry. J. Am. Chem. Soc. 113: 8534–8535. [Google Scholar]
- Kufel, J., Bousquet-Antonelli, C., Beggs, J.D., Tollervey, D. 2004. Nuclear pre-mRNA decapping and 5′ degradation in yeast require the Lsm2–8p complex. Mol. Cell. Biol. 24: 9646–9657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang, Y., Du, F., Sanglier, S., Zhou, B.R., Xia, Y., Van Dorsselaer, A., Maechling, C., Kilhoffer, M.C., Haiech, J. 2003. Unfolding of rabbit muscle creatine kinase induced by acid. A study using electrospray ionization mass spectrometry, isothermal titration calorimetry, and fluorescence spectroscopy. J. Biol. Chem. 278: 30098–30105. [DOI] [PubMed] [Google Scholar]
- Loo, J.A. 2000. Electrospray ionization mass spectrometry: A technology for studying noncovalent macromolecular complexes. Int. J. Mass Spectrom. 200: 175–186. [Google Scholar]
- Mattaj, I.W., Tollervey, D., Séraphin, B. 1993. Small nuclear RNAs in messenger RNA and ribosomal RNA processing. FASEB J. 7: 47–53. [DOI] [PubMed] [Google Scholar]
- Møller, T., Franch, T., Højrup, P., Keene, D.R., Bächinger, H.P., Brennan, R.G., Valentin-Hansen, P. 2002. Hfq: A bacterial Sm-like protein that mediates RNA–RNA interaction. Mol. Cell 9: 23–30. [DOI] [PubMed] [Google Scholar]
- Mura, C., Phillips, M., Kozhukhovsky, A., Eisenberg, D. 2003. Structure and assembly of an augmented Sm-like archaeal protein 14-mer. Proc. Natl. Acad. Sci. 100: 4539–4544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salgado-Garrido, J., Bragado-Nilsson, E., Kandels-Lewis, S., Séraphin, B. 1999. Sm and Sm-like proteins assemble in two related complexes of deep evolutionary origin. EMBO J. 18: 3451–3462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanglier, S., Ramström, H., Haiech, J., Leize, E., Van Dorsselaer, A. 2002. Electrospray ionization mass spectrometry analysis revealed a ∼310 kDa noncovalent hexamer of HPr kinase/phosphatase from Bacillus subtilis . Int. J. Mass Spectrom. 219: 681–696. [Google Scholar]
- Séraphin, B. 1995. Sm and Sm-like proteins belong to a large family: Identification of proteins of the U6 as well as the U1, U2, U4 and U5 snRNPs. EMBO J. 14: 2089–2098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seto, A.G., Zaug, A.J., Sobel, S.G., Wolin, S.L., Cech, T.R. 1999. Saccharomyces cerevisiae telomerase is an Sm small nuclear ribonucleoprotein particle. Nature 401: 177–180. [DOI] [PubMed] [Google Scholar]
- Stehlin-Gaon, C., Willmann, D., Zeyer, D., Sanglier, S., Van Dorsselaer, A., Renaud, J.P., Moras, D., Schüle, R. 2003. All-trans retinoic acid is a ligand for the orphan nuclear receptor RORβ. Nat. Struct. Biol. 10: 820–825. [DOI] [PubMed] [Google Scholar]
- Tharun, S., He, W., Mayes, A.E., Lennertz, P., Beggs, J.D., Parker, R. 2000. Yeast Sm-like proteins function in mRNA decapping and decay. Nature 404: 515–518. [DOI] [PubMed] [Google Scholar]
- Törö, I., Thore, S., Mayer, C., Basquin, J., Séraphin, B., Suck, D. 2001. RNA binding in an Sm core domain: X-ray structure and functional analysis of an archaeal Sm protein complex. EMBO J. 20: 2293–2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Törö, I., Basquin, J., Teo-Dreher, H., Suck, D. 2002. Archaeal Sm proteins form heptameric and hexameric complexes: Crystal structures of the Sm1 and Sm2 proteins from the hyperthermophile Archaeoglobus fulgidus . J. Mol. Biol. 320: 129–142. [DOI] [PubMed] [Google Scholar]
- Van den Heuvel, R.H. and Heck, A.J. 2004. Native protein mass spectrometry: From intact oligomers to functional machineries. Curr. Opin. Chem. Biol. 8: 519–526. [DOI] [PubMed] [Google Scholar]
- Will, C.L. and Lührmann, R. 2001. Spliceosomal UsnRNP biogenesis, structure and function. Curr. Opin. Cell Biol. 13: 290–301. [DOI] [PubMed] [Google Scholar]