Abstract
Libraries of phage-displayed β-lactamase mutants in which up to three loops have been engineered by genetic introduction of random peptide sequences or by randomization of the wild-type sequence have been submitted to selection protocols designed to find mutants in which binding of transition metal ions to the engineered secondary binding site leads to significant effects on the enzymatic activity. A double-selection protocol was applied: The phage-displayed libraries were first selected for transition metal ions affinity by panning on IMAC support, then a second selection step was applied to isolate mutants that have retained significant catalytic activity. The analysis of the kinetic properties of mutants in the presence of nickel, copper, or zinc ions allowed isolation of a few mutants whose activity was either enhanced or inhibited by factors up to three and >10, respectively, in a metal-specific manner. A remarkable mutant exhibiting differential allosteric regulation depending on the metal was found. Its activity was activated by nickel ion binding, inhibited by cupric ion binding, and nearly unaffected by zinc ions. These observations point to an interesting potential for up- or down-regulation of activity within a monomeric enzyme by binding to an “allosteric site” relatively remote from the active site.
Keywords: regulation, β-lactamase, engineered allosteric site, metal binding
The catalytic activity of many enzymes is regulated by binding of ligands to allosteric sites relatively remote from the active site. The importance of this property has led to the development of a research effort whose purpose was to engineer new binding sites for predefined ligands within the scaffold of nonregulated enzymes with the expectation that ligand binding would modulate their activity. In the early engineering experiments, the strategy was to mutagenize specific residues identified by computer modeling or to graft defined epitopes. For instance, the specific mutation into histidines of one or two residues on a loop bordering the active site has allowed a complete inhibition of the activity of trypsin by binding of Cu2+, Ni2+, or Zn2+ ions to the mutated residues as well as to the essential histidine (Higaki et al. 1990, 1992; McGrath et al. 1993). The addition of two histidine residues to glycogen phosphorylase has allowed enzymatic activation by transition metals in a cooperative and allosteric manner that mimics the natural activation by phosphorylation (Browner et al. 1994). Much progress has been made in the last years in the rational design of metal binding sites into protein scaffolds (Lu et al. 2001).
On the other hand, grafting of viral epitopes within the scaffold of enzymes has also been used to create enzymes whose activity would be regulated by antibody binding to these epitopes. These enzymes act as molecular sensors by competitive binding since the free enzyme activity can be recovered in the presence of free haptens or viral ligands (Brennan et al. 1994, 1995; Benito et al. 1996). In all of these cases, the design and the construction of a new mutant are necessary for each molecule to be detected.
Directed evolution strategies have also been used to create new binding sites into proteins: Large libraries of mutants are built from which mutants that have acquired an affinity for a target molecule are selected. This has been successfully accomplished on several protein scaffolds (Mathonet and Fastrez 2004; Binz et al. 2005). When new binding sites are created in an enzyme, they can become regulatory sites. Legendre et al. (1999) have inserted random peptides in some loops of the enzyme TEM-1 β-lactamase; selection with anti-PSA monoclonal antibodies (anti-PSA-mAb) led to the identification of mutants whose activity was regulated by anti-PSA-mAb binding. In vitro selection has also been used to obtain allosteric ribozymes whose activity is triggered by the binding of certain metal ions (Zivarts et al. 2005).
While it is frequently considered that allosteric regulation is a property of multimeric enzymes, recent work has pointed to the existence of natural allosteric sites even in monomeric enzymes (Kern and Zuiderweg 2003; Hardy and Wells 2004). Indeed, the fact that in some of the examples mentioned above ligand binding to an engineered site in a monomeric enzyme led not only to inhibition but also activation effects suggests that there is a potential for allosteric regulation within monomeric enzymes that deserves to be investigated. The goal of the work reported here is to test the possibility of regulation of an enzymatic activity by ligand complexation to a site remote from the active site. For this purpose, libraries of β-lactamase mutants in which three contiguous loops have been randomized have been submitted to selection protocols to find mutants whose activity could be regulated by metal ion binding. Metal ions were selected as initial target ligands since the type of residues involved in binding should normally be easily identified and since the affinity for metal ions is likely to be sufficiently high as a consequence of the strength of the coordination bonds involved.
Results
In vitro selection for transition metal ions affinity
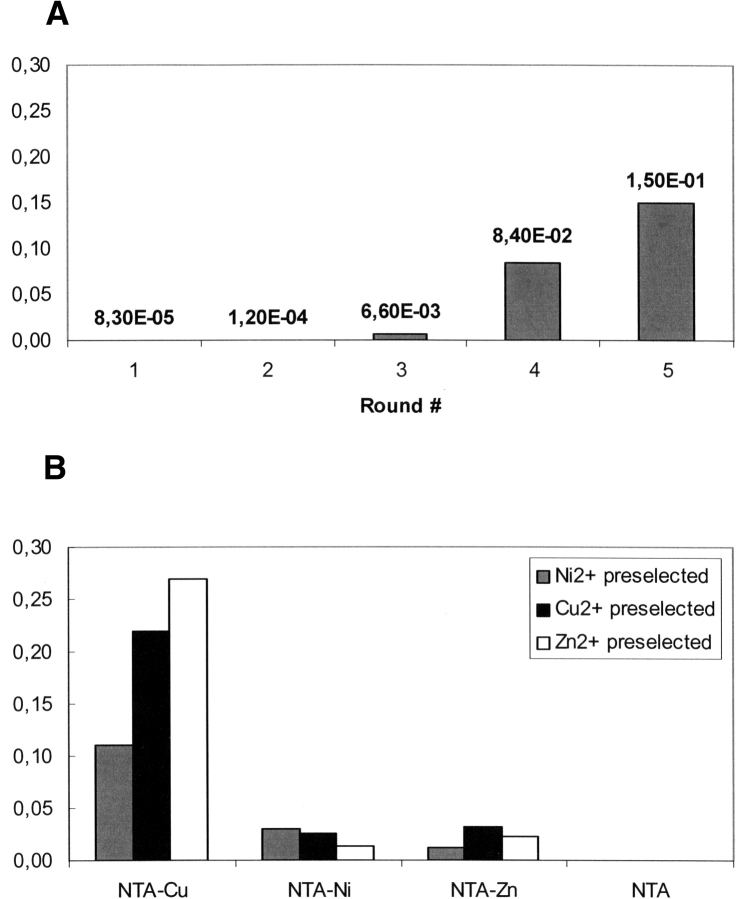
The library of 3 × 108 β-lactamase-displaying phage-clones in which the wild-type residues of three contiguous loops connecting, respectively, strands β3 and β4 (loop L1), the N-terminal helix α1 to strand β1 (loop L2), and strand β5 to the C-terminal helix α11 (loop L3) have been randomized and/or extended (see accompanying paper in this issue, Mathonet et al. 2006) was submitted to immobilized metal affinity chromatography (IMAC) selection protocols to isolate mutants with an affinity for Ni2+, Cu2+, or Zn2+ metal ions (Porath et al. 1975). The library was incubated with agarose magnetic beads linked to nitrilotriacetic acid (NTA), a strong metal chelating group, loaded with the transition metal. Phages with weak or nonspecific affinity were discarded by washes with a buffer containing a low concentration of imidazole (20 mM) and Tween 20, a detergent that prevents phage agglomeration. The bound phages were recovered by elution with a buffer containing a high concentration of imidazole (250 mM). Five successive rounds of selection were performed for the Ni2+, Cu2+, or Zn2+ metal ions. The yields of the selection rounds were determined by titration of input and output phages. Results obtained with Ni2+ are shown in Figure 1A. The yield is increasing with selection rounds, which indicates that the population of phage-enzymes is enriched in mutants having an affinity for the metal ion. Similar results are obtained with the other metal ions (data not shown).
Figure 1.
Ratios between the numbers of phages eluted versus the numbers of phages loaded on metal ion–NTA-coated magnetic beads. (A) Increase of the elution ratios with selection rounds on NTA–Ni2+ beads. (B) Elution ratios for libraries preselected on NTA–Cu, NTA–Ni, or NTA–Zn and reselected on the same or a different support.
Sequencing of selected clones indicated that all of them had several histidine residues in the engineered loops as expected for metal ion binding sites (Table 1A). This was particularly true in the engineered loop replacing Thr 271. The presence of many proline residues is also worth pointing out. They may rigidify the loops and/or favor the correct orientation of the histidines.
Table 1.
Sequences of the peptides inserted in the engineered loops in replacement of wild-type sequences

Metal ion specificity
The specificity of the selection process was assessed by determination of the yields of a sixth round of selection in which phages selected on Ni–NTA were incubated with Cu–NTA and Zn–NTA agarose beads and NTA beads as negative control. Similar analyses were performed with phages selected five times on Cu–NTA and Zn–NTA beads. The yields obtained (ratio output phages over input phages) for each one of the different metal ion complexes are shown in Figure 1B. In all cases, the binding affinities are higher for the Cu–NTA complex independent of the fact that the phages had been selected previously on different metal NTA complexes. Similar cross affinities were reported by Kjaergaard et al. (2001) in their attempts to select novel Zn2+-chelating peptides from fibria-displayed random peptide libraries. A weak selectivity is nevertheless observed with Ni–NTA or Zn–NTA preselected libraries for their respective ions. The yield observed with the negative control is low, in the background range.
In vivo selection for activity
As the diversity of the selected population seemed to be high, an in vivo selection for catalytic activity was then performed. Bacterial cells infected by the population of phage-enzymes selected for Ni2+, Cu2+, or Ni2+ affinity were spread on plates containing 20 mg/L ampicillin, a concentration at which clones reaching 5% of the wild-type phage-enzyme activity can grow. An average of 5% of the clones was observed to be resistant. A comparison of the sequences of active clones with those not selected for activity shows that the active ones present a more homogenous distribution of histidines in the three engineered loops (Table 1B). On the other hand, three clones (A-4, A-9, and A-21) show a deletion of the insertion in loop L3 leaving one remaining residue, the wild-type methionine in the case of clone A-4 and A-9 and a serine for clone A-21. When the original combinatorial library was selected for activity, it was also observed that a large portion of the sequenced clones did not contain a hexapeptide in replacement of Thr 271 (see accompanying paper in this issue, Mathonet et al. 2006).
Screening for activity modulation upon metal binding
Twenty clones picked at random from each M2+–NTA and activity-selected library were sequenced. Thirty of them had different sequences. They were isolated to evaluate the effect of metal ion binding (Ni2+, Cu2+, and Zn2+) on their catalytic activity. For 66% of them and for the wild-type enzyme, no significant effect was observed.
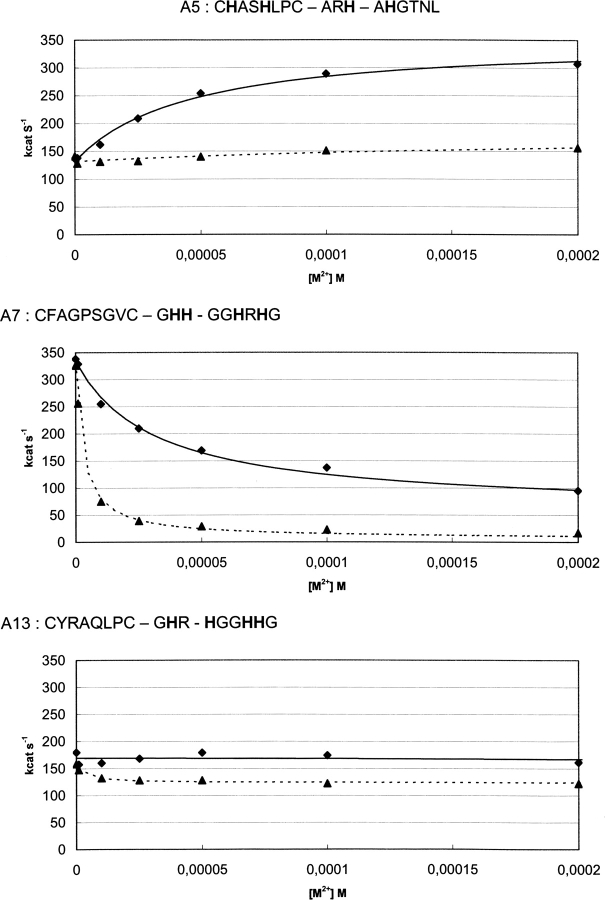
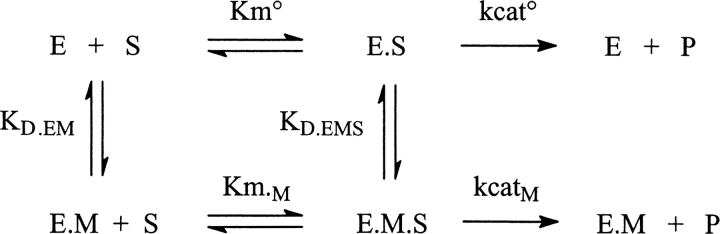
Five clones were investigated in more detail by following the kinetics of phage-enzymes catalyzed benzyl-penicillin (PenG) hydrolysis at several concentrations of Ni2+ or Zn2+ ions up to 0.5 mM. Values of k cat and K m were extracted from progress curves. As indicated from measurements of independent experiments, k cat values are reproducible within 10%; K m values are determined with a lower accuracy (±35%), particularly when below 10 μM, as a consequence of the small absorbance change (Δɛ) associated with PenG hydrolysis. Plots of k cat as a function of Ni2+ or Zn2+ concentration are given in Figure 2 for mutants A5, A7, and A13 sharing the characteristic that four histidine residues are present in the engineered loops but distributed differently. The metal ions dependence of activity is very different for the different mutants; it depends also markedly on the nature of the ion: strong inhibition, activation, or negligible effects are observed. Mutants A6 and A12 behave somewhat like mutant A7 except that the inhibition effects are smaller. Saturation curves are observed in all cases; they can be analyzed in terms of Scheme 1.
Figure 2.
Effect of Ni2+ (♦) or Zn2+ (▴) ion concentrations on activities (k cat) of mutants A5, A7, and A13 in which four histidine residues are distributed differently in the three engineered loops. The lines were calculated using Equation 2 and the parameters given in Table 2.
Scheme 1.
The metal ion concentration dependence of the kinetic parameters is analyzed like the pH dependence of these parameters (Fersht 1999). The rate of the reaction is described by Equation 1:
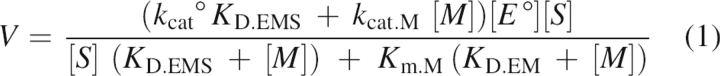 |
At saturating substrate concentration, Equation 1 simplifies to Equation 2:
 |
which indicates that the metal ion dependence of k cat is ruled by K D.EMS
Rearrangement of Equation 1 allows defining K m.app:
 |
from which, taking into account the relationship between binding constants:
it is easily shown that K m.app is also ruled by K D.EMS, since when [M] = K D.EMS,
When [S] << K m.app, Equation 1 simplifies to Equation 6:
 |
which indicates that (k cat/K m)app is ruled by K D.M, the free enzyme affinity for the metal ions. Values of k cat°, k cat.M, and K D.EMS obtained by fitting the experimental data are given in Table 2.
Table 2.
Kinetic parameters of enzymes whose activity on benzyl-penicillin is modulated by Ni2+ or Zn2+ binding
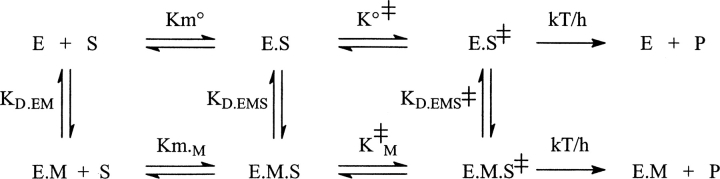
The activation or inhibition effects observed indicate that the affinity of the transition state for the ions is respectively higher or lower than that of the metal-free enzyme substrate complex as suggested by Scheme 2:
Scheme 2.
The transition state affinity for metal ions (KD.EMS≠) can be calculated from the ratios of k cat or k cat/K m in the presence and absence of metal ions according to Equations 7 and 8:
 |
 |
As shown by Equation 7, if the affinity of the transition state for M is weaker than that of the enzyme substrate complex, metal binding leads to a decrease in k cat, the reaction is inhibited. Values of the ratios k cat°/k cat.M, K m°/K m.M, (k cat/K m)°/(k cat/K m)M, and the binding constants to the enzyme and enzyme substrate complex are given in Table 3 for mutants A5 and A7. They were used to calculate affinities of metal ions for the enzyme, the enzyme substrate complex, and the transition state given in Table 4. To check that the effect of metal ions was independent of the presence of the phage, the gene encoding A5 was recloned and the free enzyme was expressed and purified. The enzymatic activity was measured as a function of metal ion concentrations at an enzyme concentration 40-fold higher than with the corresponding phage-enzyme. The following results were obtained: for Ni2+ ions, k cat.M/k cat° = 3.5 ± 0.4 and K D.EMS = 2.5 × 10−5 M, and for Zn2+ ions, k cat.M/k cat° = 1.6 ± 0.2 and K D.EMS = 5.7 × 10−5 M in satisfactory agreement with the results obtained with the phage-enzyme. These results suggest further that the metal ion effect on activity does not imply a dimerization of the enzyme since similar results are observed at different enzyme concentrations.
Table 3.
Ratios of kcat, Km, and kcat/Km for the hydrolysis of benzyl-penicillin catalyzed by β-lactamase variants in the presence and absence of metal ion
Table 4.
Binding constants of metal ions to the free enzyme, the enzyme substrate complex, and the transition state
The effect of Cu2+ was measured on the hydrolysis of nitrocefin since cupric ion absorption in the far ultraviolet range prevents following the hydrolysis of PenG, which can only be measured at 232 nm. The cupric ion concentration was limited to 40 μM by the solubility of cupric salts. A lag phase was always observed during hydrolysis in the presence of cupric ions, which prevented a reliable determination of kcat and Km parameters without a detailed study of the origin of the lag phase. An inhibition of nitrocefin hydrolysis was observed with the six mutants investigated (data not shown).
Discussion
The selection
The observation of the sequences of clones selected by binding to immobilized metal ion–NTA complexes indicates that clones containing sequences of successive histidine residues were preferentially selected. These proteins contain “His-tags” inserted into the most permissive C-terminal loop. After in vivo selection for activity, however, these clones are removed from the metal ion binding sublibrary and histidine residues are clearly better distributed within the engineered loops. Two, not mutually exclusive, interpretations of the difference between both classes of clones are possible. A high concentration of histidine residues in loop L3 is detrimental to activity. Alternatively, a partial proteolytic degradation of the displayed enzyme during phage morphogenesis could leave an unconstrained polyhistidine sequence on the peptide remaining attached to the phage, which would direct their selection. Despite the fact that the transition metal ions used for the selection also have a good affinity for cysteine residues, no cysteine was present in the loops. This is a result of the in vivo counterselection of peptides containing free cysteines during phage morphogenesis (Kay et al. 1993).
Inspection of the sequences of the active clones points to another feature: The same sequence is observed more than once either in loop L2 or loop L3. This is a likely consequence of the strategy used for the hierarchical construction of the library: independent construction of library L1-L2 (5 × 105 active clones) and library L3 (5 × 106 active clones) followed by a combination of them. In the combination step, many sequences are lost. Then, the two orthogonal selection steps to extract mutants endowed with both affinity for metal ions and activity leave relatively few possibilities. Similarities detected in the sequences may be useful at a later stage when the mechanism of modulation of the activity by ions binding will be investigated.
During the selection process, there is little specificity for particular ions; cupric ions appear to bind better, independent of the fact that a preselection has been made on zinc or nickel ions. This result might be contrasted with that of a selection experiment run on a library of unconstrained random peptides using zinc ions immobilized on agarose beads through an iminodiacetic acid (IDA) chelating group (Matsubara et al. 2003). All phages isolated appeared to have a better affinity for zinc than for copper as judged from the importance of ELISA signal generated when phages were incubated on metal–IDA-coated support. Good nickel binding was also frequently observed by these authors. The difference with our results may result from the difference in support used for the selections, metal–NTA versus metal–IDA leaving, respectively, two or three free coordination sites. The clones selected in our work, however, have the advantage that the affinity for the ions is significantly better, in the micromolar versus the millimolar range.
Modulation of activity
The μM affinities imply that more than one histidine is involved in binding because the affinities of the tested transition metal ions for imidazole are in the mM range (pKD1 = 4.33 for Cu2+, 3.36 for Ni2+, and 2.58 for Zn2+; Sillen and Martell 1964). This has the consequence that metal binding will frequently induce a local conformational change either within a loop or between two loops and a possible broader transmission of new conformational constraints. This will frequently affect the activity. The observed effects are mainly on k cats, sometimes also on K ms or both parameters, although the lower accuracy on this parameter limits the detectability of small changes. While the selection protocol did not allow selecting clones binding selectively to zinc, nickel, or copper ions, a clear differentiation appears when investigating the effects on activities. Different ions induce different effects and different mutants show different behaviors. Mutant A5, for instance, is activated by nickel ion binding, nearly unaffected by zinc ion binding, and inhibited by cupric ion binding. Mutant A7, on the other hand, is strongly inhibited by zinc ion binding, more weakly inhibited by nickel ion binding, and again inhibited by cupric ions.
The nearly systematic differences of responses observed with the different metal ions arise from differences in the ligand field geometry imposed in the complexes by d-electron repulsions and differences in bond strength reflected in the dissociation constants of imidazole complexes (see above). Cupric ions form the highest affinity complexes with imidazole, they systematically inhibit the enzyme and, more interestingly, they appear to block the enzyme in an inactive or a less active conformation that is slowly converted into a more active one during the steady state. This indicates that these ions have a higher affinity for the free enzyme than for the enzyme substrate complex and that there is a significant barrier to the conformational change restoring the activity. No similar effects are observed with the other ions.
Altogether, these observations emphasize the main finding of this work, that there is a potential for up- or down-regulation of activity within a monomeric enzyme by binding to an “allosteric site” relatively remote from the active site. At this stage, a clear description of the mechanisms of activity modulation remains out of scope. Several guidelines can nevertheless be envisaged for future research. The surprising observation of an inhibition of β-lactamase activity as a consequence of binding to a natural “allosteric site,” 16 Å from the active site pocket and in the area where we have engineered loops, already suggests that the perturbation of the packing of secondary structures may lead to significant effects on activity by relocation of important residues.
Although it is clear that the present capacities of model building have not yet reached the stage where the conformation of loops could be predicted with sufficient accuracy to allow the definition of ion binding sites, models, whatever their accuracy, can point to constraints on the interpretation. In Figure 3, a model of mutant A5 generated by automatic modeling (Guex and Peitsch 1997; Schwede et al. 2003) draws attention to the following facts. Loop L1 (between strands β3 and β4) on the rim of the active site likely adopts the wild-type enzyme conformation because no insertion was allowed there. Loop L2 is of intermediate rigidity since a disulfide bridge limits the conformational fluctuations at the foot of the loop; it is also too far away from loop L1 to allow an easy formation of a metal ion binding site made up of histidines contributed by L1 and L2. Loop L3, made up of ∼10 residues, is likely to be highly mobile and histidine residues located on this loop can most probably interact with those in loops L1 and L2 except if they are at the extremity of L3, that is, close to the C-terminal helix. Because of its size, loop L3 contributes also to prevent easy interactions between L1 and L2. Additional guidelines can be extracted from rules governing the architecture of metal coordination sites in proteins: In metal ion binding sites contributed by two (or more) histidine residues, the observed separation between them is one, two, or three residues (sequences HXH, HX2H, HX3H), binding by two adjacent histidines being very rarely observed (Harding 2004).
Figure 3.
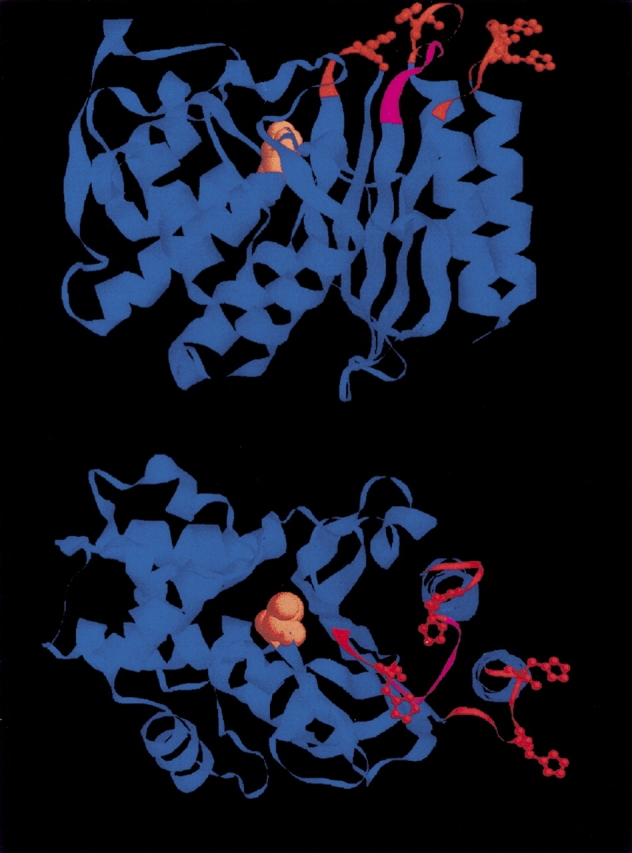
Model of mutant A5. The engineered loops are shown in red with the histidine residue side chains displayed as balls and sticks. The segment of loop L3 containing the original residues is shown in magenta. The nucleophilic serine of the active site is displayed in space filling. Two orthogonal views are shown.
With these guidelines in mind, the following comparisons can be made. When histidines are present only in loop L2 (mutant 21) or are too far away to interact with histidines in L1 (mutants A4 and A9), the activity is hardly affected by metal ion binding. This is reasonable because ion binding within L2 will not easily affect its conformation already constrained by the disulfide bridge. Mutants A7 and A13 have no histidine in L2; mutant A7 is strongly inhibited by zinc ion binding, whereas the activity of mutant A13 is hardly affected. A possible interpretation is that interaction between H239 and H271c (H271e is too far away, the numbering of the residues in loop L3 is X271a to X271f) limits the flexibility of the enzyme or blocks it in a less active conformation in mutant A7. The interaction between H239 and H271a would be easier and less detrimental to activity in A13 (again H271d and H271e are too far away). When there is no histidine in L1, and histidines are present in L2 and L3 (mutants A6, A8, and A12), the activity is moderately inhibited by nickel or zinc ion binding. Finally, the sequence of A5 is similar to that of mutants A10, A11, and A17 with histidines present in the three loops, A5 is moderately activated on nickel binding while the others are not. No simple interpretation can be proposed for these differences since the free energy differences between binding to the free enzyme, the enzyme substrate complex, or the transition state are rather small.
Engineered allosteric enzymes whose activity is controlled by binding of small molecules or ions may offer new possibilities of design of biosensors for use in different areas like clinical biosensors, toxin sensors, environmental biosensors, and food biosensors (Nakamura and Karube 2003). This is presently under investigation.
Materials and methods
General
T4 DNA ligase, Taq polymerase, and restriction enzymes were provided by Roche. Synthetic oligonucleotides were provided by Eurogentec. Ampicillin and benzylpenicillin were from Sigma, and nitrocefin from Oxoid.
Oligonucleotides
The oligonucleotides used for sequencing and recloning for expression of clone A5 as free enzyme were:
fdP3S: 5′-TAT-TCG-CAA-TTC-CTT-TAG-TTG-T-3′
AB348: 5′-TTC-TGT-ATG-AGG-TTT-TGC-TAA-3′
BA40: 5′-GCG-GGG-TAC-CTC-ATG-AAA-AAA-TTA-TTA-TTC-G-3′
BAstop: 5′-TGC-GCT-GTC-ATT-CTA-GAC-CCC-AAT-GCT-TAA-TC-3′
Phage production and purification
Phages from libraries or individuals clones were amplified by infection of 25 mL of E. coli TG1 [K-12 Δ(lac-pro) supE thi hsdDS/F’ traD36 proA+B+ lacI lacZM15] culture. Infected cells were then transferred in 250 mL of LB-Tet medium (LB containing 7.5 μg/mL tetracycline) and incubated overnight at 37°C. After centrifugation, the bacterial pellet was resuspended in 250 mL of fresh LB-Tet medium, and a fast culture was performed by incubating at 30°C for 3–4 h. Phages were then purified by two successive polyethylene glycol precipitations and were resuspended in TBS buffer (50 mM Tris, 150 mM NaCl at pH 7.5). Between the first and the second precipitations, the phage solutions were filtered through a 0.45-μm filter. Stock phage concentrations were determined by measuring their absorbance at 265 nm using 8.4 × 107 M−1 cm−1 as extinction coefficient.
In vitro selection for affinity and in vivo selection for catalytic activity
The phage display libraries were resuspended in TBS buffer and selected for affinity for different transition metal ions using Ni–NTA magnetic agarose beads (QIAGEN). The Ni–NTA beads were incubated with 5 × 1012 phages for 2 h in 1 mL of washing buffer (WB = 50 mM NaH2PO4, 300 mM NaCl, 20 mM imidazole, 0.1% Tween 20 at pH 7.8). Nonspecific phages were discarded by several washings with 1 mL of WB; finally, elution was performed by incubating the beads in 1 mL of elution buffer (EB = 50 mM NaH2PO4, 300 mM NaCl, 250 mM imidazole, 0.1% Tween 20 at pH 7.8) for 1 min. The eluted phages were amplified by infecting log phase TG1 cells. Eluted phages were titrated by plating the infected bacteria on LB-Tet medium. Successive rounds of selection were performed in this way but the number of washes was increased as selection progressed (Smith and Petrenko 1997; Willats 2002).
The enzymatically active mutants were then selected by spreading bacterial cells infected by phages eluted in the last round of metal affinity selection on plates containing 20 mg/L ampicillin and incubating overnight at 30°C.
For the selections of Cu2+ and Zn2+ binders, the Ni2+ ion was replaced by the other transition metals. One hundred microliters of Ni–NTA beads were washed three times with WB. The ion in complex with the NTA was removed by incubation for 10 min with 100 mM EDTA, and the beads were washed three times again with WB to remove the excess of EDTA. The NTA agarose beads were then charged with the different metallic ions using a 100 mM solution of their sulfate salt and incubated for 1 hr at room temperature. The unbound metal ions were discarded and the beads washed three times with WB.
Cloning, expression, and purification of β-lactamase A5
The β-lactamase gene of clone A5 was PCR amplified with primers BA40 and BAstop that introduced BspHI (compatible with NcoI site) and a stop codon after XbaI site. Fragments were restricted and cloned between NcoI and XbaI sites of pBAD/myc-HisB-Tet expression vector (the stop codon after the XbaI site prevents attachment of the mic and His tags to the enzyme). This vector is a derivative of pBAD/myc-HisB (Invitrogen) but the ampicillin resistance marker has been replaced by a tetracycline resistance gene. It was kindly provided by B. Hallet (Unité de Génétique, Université catholique de Louvain, Belgium). Ligation products were electroporated in TOP10 cells. DNA from transformants was analyzed by restriction and sequencing. Transformed E. coli Top 10 were grown in 1 L of Luria-Bertani broth medium supplemented with tetracycline (7.5 μg/mL) at 37°C until the A 600 reaches ∼0.6. Protein production was induced by addition of L-arabinose at a final concentration of 0.2% (w/v) for 4 h at 30°C. The harvest cells were resuspended with 33 mL of sucrose buffer (sucrose 20%-Tris 20 mM at pH 8) and 66 μL of EDTA 0.5M (pH 8) per L of culture. The medium was centrifuged at 4400g for 10 min and the supernatant was discarded; the periplasmic fraction was released by adding 40% of cool magnesium sulfate and centrifuged at 10,000g for 10 min. The supernatant was collected and dialyzed overnight at 4°C with buffer A (50 mM HEPES at pH 7.5, 0.5 M NaCl, and 10 mM imidazole) using a Spectra/Por membrane (Mr cut-off, 10,000). Following passage through a 0.22-μM filter, the proteins were applied to a Ni2+-chelating column (HiTrap chelating, Amersham Biosciences) and washed with 10 column volumes of buffer A, and finally eluted with a 10–500 mM imidazole gradient in buffer A. Fractions were analyzed by SDS-PAGE and by nitrocefin activity in ELISA wells. Those fractions containing the pure β-lactamase protein were dialyzed using 50 mM HEPES and 200 mM NaCl (pH 7.4) buffer and concentrated with the filter Amicon Ultra 15 (from Millipore).
Kinetic measurements
The β-lactamase activity of phage-enzymes was determined at 25°C in 50 mM HEPES buffer (pH 7.4). Specific activities of phage-displayed enzymes were extracted from complete progress curves of substrate disappearance or product formation. The change in absorbance was measured as a function of time with penicillin G at 232 nm (Δɛ = −1040 M−1 cm−1) or nitrocefin at 468 nm (Δɛ = 15,900 M−1 cm−1). The V max and K m parameters were extracted by fitting the progress curves of substrate disappearance with results of numerical integration of the Michaelis-Menten equation. The first-order rate constant kcat (turnover number) was calculated by dividing V max by the total phage-enzyme concentration determined by the absorbance at 265 nm of the phage stock solution (ɛ = 8.4 × 107 M−1 cm−1). The specific activities of phage-displayed enzymes are determined per mole of phage. The effect of metallic ions on enzyme activity was determined by incubating phage-enzymes with various metal concentrations and following the substrate disappearance. For zinc ions, the contribution of metal-catalyzed reaction was subtracted; a linear correction was also applied for a small absorbance drift observed at the end of the reaction. Dissociation constants of complexes between free enzymes and the enzyme-substrate complexes and the metal ions were determined by curve fitting of metal ion concentration dependence of k cat, K m, and k cat/K m (Equations 2 , 3, and 6).
Acknowledgments
We thank Dr. B. Hallet for providing expression vectors. P.S. is a research associate of the Belgian Fonds National de la Recherche Scientifique. P.M. acknowledges a fellowship from the Fonds pour la formation à la Recherche dans l'Industrie et dans l'Agriculture. This work was supported by the Action de Recherches Concertées de la Communauté Française de Belgique (P.M., Ph.D. fellowship) and by the Interuniversity Attraction Poles Programme – Belgian State – Federal Office for Scientific, Technical and Cultural Affairs and the Fonds National de la Recherche Scientifique (H.B., postdoctoral fellowship).
Footnotes
Reprint requests to: Jacques Fastrez, Laboratoire de Biochimie Physique et des Biopolymères, Institut des Sciences de la Vie, Université catholique de Louvain, Place L. Pasteur, 1, B1348 Louvain la Neuve, Belgium; e-mail: fastrez@bioc.ucl.ac.be; fax: +32-10-47-28-20.
Article published online ahead of print. Article and publication date are at http://www.proteinscience.org/cgi/doi/10.1110/ps.062304406.
References
- Benito, A., Feliu, J.X., Villaverde, A. 1996. β-Galactosidase enzymatic activity as a molecular probe to detect specific antibodies. J. Biol. Chem. 271: 21251–21256. [DOI] [PubMed] [Google Scholar]
- Binz, H.K., Amstutz, P., Pluckthun, A. 2005. Engineering novel binding proteins from nonimmunoglobulin domains. Nat. Biotechnol. 23: 1257–1268. [DOI] [PubMed] [Google Scholar]
- Brennan, C., Christianson, K., Surowi, T., Mandecki, W. 1994. Modulation of enzyme activity by antibody binding to an alkaline phosphatase-epitope hybrid protein. Protein Eng. 7: 509–514. [DOI] [PubMed] [Google Scholar]
- Brennan, C.A., Christianson, K., La Fleur, M.A., Mandecki, W. 1995. A molecular sensor system based on genetically engineered alkaline phosphatase. Proc. Natl. Acad. Sci. 92: 5783–5787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browner, M.F., Hackos, D., Fletterick, R. 1994. Identification of the molecular trigger for allosteric activation in glycogen phosphorylase. Nat. Struct. Biol. 1: 327–333. [DOI] [PubMed] [Google Scholar]
- Fersht, A.R. 1999. The pH dependence of enzyme catalysis. In Structure and mechanism in protein science: A guide to enzyme catalysis and protein folding, pp. 173–179. Freeman, New York.
- Guex, N. and Peitsch, M.C. 1997. SWISS-MODEL and the Swiss-PdbViewer: An environment for comparative protein modeling. Electrophoresis 18: 2714–2723. [DOI] [PubMed] [Google Scholar]
- Harding, M.M. 2004. The architecture of metal coordination groups in proteins. Acta Crystallogr. D Biol. Crystallogr. 60: 849–859. [DOI] [PubMed] [Google Scholar]
- Hardy, J.A. and Wells, J.A. 2004. Searching for new allosteric sites in enzymes. Curr. Opin. Struct. Biol. 14: 706–715. [DOI] [PubMed] [Google Scholar]
- Higaki, J.N., Haymore, B.L., Chen, Q., Fletterick, R.J., Craik, C.S. 1990. Regulation of serine protease activity by an engineered metal switch. Biochemistry 29: 8582–8586. [DOI] [PubMed] [Google Scholar]
- Higaki, J.N., Fletterick, R.J., Craik, C.S. 1992. Engineered metalloregulation in enzymes. Trends Biochem. Sci. 17: 100–104. [DOI] [PubMed] [Google Scholar]
- Kay, B.K., Adey, N.B., He, Y.S., Manfredi, J.P., Mataragnon, A.H., Fowlkes, D.M. 1993. An M13 phage library displaying random 38-amino-acid peptides as a source of novel sequences with affinity to selected targets. Gene 128: 59–65. [DOI] [PubMed] [Google Scholar]
- Kern, D. and Zuiderweg, E.R.P. 2003. The role of dynamics in allosteric regulation. Curr. Opin. Struct. Biol. 13: 748–757. [DOI] [PubMed] [Google Scholar]
- Kjaergaard, K., Shembri, M.A., Klemm, P. 2001. Novel Zn chelating peptides selected from a fibria-displayed random peptide library. Appl. Environ. Microbiol. 67: 5467–5473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legendre, D., Soumillion, P., Fastrez, J. 1999. Engineering a regulatable enzyme for homogeneous immunoassays. Nat. Biotechnol. 17: 67–72. [DOI] [PubMed] [Google Scholar]
- Lu, Y., Berry, S.M., Pfister, T.D. 2001. Engineering novel metalloproteins: Design of metal-binding sites into native protein scaffolds. Chem. Rev. 101: 3047–3080. [DOI] [PubMed] [Google Scholar]
- Mathonet, P. and Fastrez, J. 2004. Engineering of non-natural receptors. Curr. Opin. Struct. Biol. 14: 505–511. [DOI] [PubMed] [Google Scholar]
- Mathonet, P., Deherve, J., Soumillion, P., Fastrez, J. 2006. Active TEM-1 β-lactamase mutants with random peptides inserted in three contiguous surface loops. Protein Sci. 15: (this issue). [DOI] [PMC free article] [PubMed]
- Matsubara, T., Hiura, Y., Kawahito, O., Yasuzawa, M., Kawashiro, K. 2003. Selection of novel structural zinc sites from a random peptide library. FEBS Lett. 555: 317–321. [DOI] [PubMed] [Google Scholar]
- McGrath, M.E., Haymore, B.L., Summers, N.L., Craik, C.S., Fletterick, R.J. 1993. Structure of an engineered, metal-actuated switch in trypsin. Biochemistry 32: 1914–1919. [DOI] [PubMed] [Google Scholar]
- Nakamura, H. and Karube, I. 2003. Current research activity in biosensors. Anal. Bioanal. Chem. 377: 446–468. [DOI] [PubMed] [Google Scholar]
- Porath, J., Carlsson, J., Olsson, I., Belfrage, G. 1975. Metal chelate affinity chromatography, a new approach to protein fractionation. Nature 258: 598–599. [DOI] [PubMed] [Google Scholar]
- Schwede, T., Kopp, J., Guex, N., Peitsch, M.C. 2003. SWISS-MODEL: An automated protein homology-modeling server. Nucleic Acids Res. 31: 3381–3385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, G.P. and Petrenko, V.A. 1997. Phage display. Chem. Rev. 97: 391–410. [DOI] [PubMed] [Google Scholar]
- Sillen, L.G. and Martell, A.E. In Stability constants of metal-ion complexes .1964. The Chemical Society, London.
- Willats, W.G. 2002. Phage display: Practicalities and prospects. Plant Mol. Biol. 50: 837–854. [DOI] [PubMed] [Google Scholar]
- Zivarts, M., Liu, Y., Breaker, R.R. 2005. Engineered allosteric ribozymes that respond to specific divalent metal ions. Nucleic Acids Res. 33: 622–631. [DOI] [PMC free article] [PubMed] [Google Scholar]