Abstract
Previously, we have shown that residues 73–92 (sequence DRFSVNLDVKHFSPEELKVK) in αB-crystallin are involved in preventing the formation of light scattering aggregates by substrate proteins. In this study, we made single substitutions of three conserved amino acid residues (H83 → A, F84 → G, and P86 → A) and a nonconserved amino acid residue (K90 → C) in the functional region of αB-crystallin and evaluated their role in anti-aggregation activity. Mutation of conserved residues led to changes in intrinsic tryptophan intensity, bis-ANS binding, and in the secondary and tertiary structures. The H83A mutation led to a twofold increase in molar mass, while the other mutants did not produce significant changes in the molar mass when compared to that of wild-type protein. The chaperone-like activity of the H83A mutant was enhanced by 15%–20%, and the chaperone-like activity of F84G and P86A mutants was reduced by 50%–65% when compared to the chaperone-like activity of wild-type αB-crystallin. The substitution of the nonconserved residue (K90 → C) did not induce an appreciable change in the structure and function of the mutant protein. Fluorescence resonance energy transfer (FRET) assay demonstrated that destabilized ADH interacted near the K90 region in αB-crystallin. The data show that F84 and P86 residues are essential for αB-crystallin to effectively prevent the aggregation of substrate proteins. This study further supports the involvement of the residues in the 73–92 region of αB-crystallin in substrate protein binding and chaperone-like action.
Keywords: αB-crystallin, mutation, structure, chaperone, light scattering
α-Crystallin, a major refractive protein in the vertebrate lens, belongs to the group of small heat shock proteins (sHsps) and has a molecular mass of 300–1200 kDa (Bloemendal et al. 2004). The α-crystallin assembly is made of two subunits, αA- and αB-crystallin, in the ratio of 3:1 and contains ∼15–60 subunits (Horwitz 2003). The crystal structure of vertebrate α-crystallin remains unelucidated, mainly because of the polydispersed size distribution of the protein (Groenen et al. 1994). However, several hypothetical models for the quaternary structure of α-crystallin have been proposed (Tardieu et al. 1986; Augusteyn and Koretz 1987; Wistow 1993; Carver et al. 1994; Groth-Vasselli et al. 1995). Recombinant αA- and αB-crystallins and lens α-crystallin subunits upon separation and purification assemble to form homo-oligomers (Li and Spector 1973; Horwitz et al. 1998). The homo-oligomers exhibit a secondary structure similar to that of native α-crystallin, but show variability in oligomeric assembly (Horwitz et al. 1998). The crystallin subunits have considerable sequence similarity between them and with other members of the sHsp family (Ingolia and Craig 1982; de Jong et al. 1998). The core of the α-crystallin subunits, called the α-crystallin domain, constitutes a highly conserved stretch of 80–100 amino acids (Caspers et al. 1995). The conserved α-crystallin domain is flanked by a disordered N-terminal domain and a flexible C-terminal tail (de Jong et al. 1998). αA-crystallin is present mainly in the lens, with very low levels in other tissues (Srinivasan et al. 1992). In contrast with αA-crystallin, αB-crystallin is present in many non-lenticular tissues (Iwaki et al. 1990), and it may play a role in numerous diseases (Shinohara et al. 1993; van Noort et al. 1995; Vicart et al. 1998). α-Crystallin can function as a molecular chaperone (Horwitz 1992; Rao et al. 1995; Wang and Spector 1995). Both homo- and hetero-oligomers of αA- and αB-crystallins function as chaperones by interacting with and preventing the aggregation of structurally perturbed proteins (Muchowski et al. 1997; Brady et al. 2001; Rajaraman et al. 2001; Reddy et al. 2001; Santhoshkumar and Sharma 2001a). Age-related modifications of α-crystallin, such as deamidation (Gupta and Srivastava 2004), truncation (Thampi et al. 2002; Takeuchi et al. 2004), and glycation (Sharma and Ortwerth 1995), diminish the ability of the crystallin subunits to function as molecular chaperones (Harding 2002). In addition to their interaction with denatured proteins, α-crystallin subunits also interact with native molecules such as membrane proteins, Golgi matrix protein, structural proteins, nuclear proteins, and DNA (Cobb and Petrash 2002; Horwitz 2003; Bullard et al. 2004; Gangalum et al. 2004; Maddala and Rao 2005). The nature and significance of these interactions are not clear.
The components essential for chaperone-like activity and the factors that influence the chaperoning efficiency of α-crystallin have been the focus of numerous studies (Carver et al. 1995; Das et al. 1997; Koretz et al. 1997; Raman and Rao 1997; Sharma et al. 1997, 2000; Santhoshkumar and Sharma 2001b; Bera et al. 2002; Srinivas et al. 2002, 2005; Kumar et al. 2005). These studies implicate structure, hydrophobicity, and ionic charge as factors involved in the chaperone-like activity of α-crystallins. We have identified the hydrophobic sites in αA- and αB-crystallin by using the environment-sensitive probe 1,1′-bi(4-anilino)naphthalene-5,5′- sulfonic acid (bis-ANS ) (Sharma et al. 1998a,b). Our studies with bis-ANS and hydrophobic protein mellitin have shown that the chaperone site and the hydrophobic site overlap in αA-crystallin (Sharma et al. 2000). In addition to hydrophobic interactions, ionic pairing between the chaperone and the substrate protein is important for strengthening the chaperone–substrate complex (Bova et al. 1999; Kumar et al. 1999; Shroff et al. 2000; Bera et al. 2002). Replacement of charged residues in the flexible C-terminal tail decreases the chaperone-like activity of α-crystallin most likely by decreasing the solubility of the complex (Smulders et al. 1996). Positive charges on R21, R49, and R103 in αA-crystallin are shown to be important for chaperone function (Biswas et al. 2006). Mutation of a charged residue, R116, in αA-crystallin causes congenital cataracts (Litt et al. 1998), while mutation of R120 in αB-crystallin is linked to desmin-related myopathy as well as cataracts (Vicart et al. 1998; Bova et al. 1999).
Studies to identify the amino acids involved in substrate protein binding during α-crystallin chaperone-like action have demonstrated that significant chaperone activity exists in truncated versions of human α-crystallins that lack either the entire or parts of the N-terminal domain (Pasta et al. 2003; Yang et al. 2005) or the C-terminal extension (Takemoto 1994; Thampi and Abraham 2003; Aquilina et al. 2005) or both (Feil et al. 2001). These observations suggest that the region for substrate binding resides mainly in the α-crystallin domain. We determined that a 19-amino-acid residue from αA-crystallin (residues 70–88) containing bis-ANS-binding sequences possesses anti-aggregating properties similar to those of αA-crystallin (Sharma et al. 2000). Furthermore, using an F71G mutant, we have shown that this region is important in chaperone function at physiological temperatures (Santhoshkumar and Sharma 2001b). We found that residues 73–92 in αB-crystallin, called mini-αB-crystallin, can independently prevent the aggregation of denatured substrate proteins similar to the action of native αB-crystallin (Bhattacharyya et al. 2006).
In this study, we have done a BLAST search to look for sHsps having a region similar to the human αB-crystallin chaperone-site sequence (residues 73–92). The sequences were manually aligned to identify the conserved residues and to predict the critical residues involved in substrate protein interaction. We decided to substitute three conserved residues that would contribute to the ionic charge, hydrophobicity, and structure of the chaperone region. Accordingly, H83 (charged), F84 (hydrophobic), and P86 (β-sheet breaker) in human αB-crystallin were replaced individually to study their role in chaperone-like action of the protein. A nonconserved residue, K90, was also replaced, and the structural and functional consequences of mutation were compared with those of wild-type human αB-crystallin.
Results
It is well established that the anti-aggregation activity of sHsps resides in the conserved α-crystallin domain (Feil et al. 2001). Residues 70–88 (▶) in αA-crystallin possess the anti-aggregation property, with residue F71 being one of the critical residues for chaperone-like function of the protein (Santhoshkumar and Sharma 2001b). Using mini-αB-crystallin, we have demonstrated that the corresponding region in αB-crystallin, residues 73–92, can also prevent the aggregation of destabilized substrates (Bhattacharyya et al. 2006). To identify the conserved residues in the chaperone site of αB-crystallin, a protein BLAST (NCBI, http://www.ncbi.nlm.nih.gov/BLAST/) search was done using the human αB-crystallin chaperone site sequence (residues 73–92) as query. Proteins having various levels of sequence similarity were picked and manually aligned (▶). Residues F74, V76, D80, V81, H83, F84, P86, V91, and K92 in αB-crystallin were found conserved among the sHsp family of various animals. Three of the highly conserved residues and a nonconserved residue were replaced in αB-crystallin (▶) to determine their roles in the chaperone-like action of the protein. Recombinant wild-type αB-crystallin and its mutants were purified under nondenaturing conditions using size exclusion and ion-exchange chromatographic techniques. Because α-crystallin structure and function can vary with pH, buffer conditions, protein concentration, and storage in buffer solution, all purified crystallins were concentrated (5 mg/mL), freeze-dried along with buffer salts, and stored at −20°C until use. The frozen samples were resuspended in water prior to the analysis.
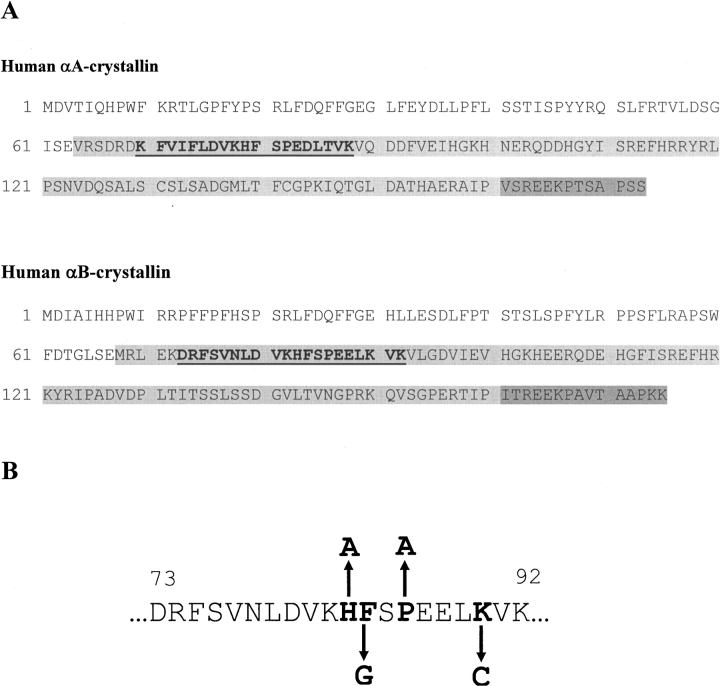
Figure 1.
(A) Amino acid sequence of αA- and αB-crystallin in humans showing the regions responsible for chaperone-like action (bold and underlined). The chaperone site is in the α-crystallin domain (light shaded), which is flanked by an N-terminal region (no shading) and a C-terminal tail (dark shaded). (B) Human αB-crystallin chaperone region sequence, showing the residues replaced in this study.
Figure 2.
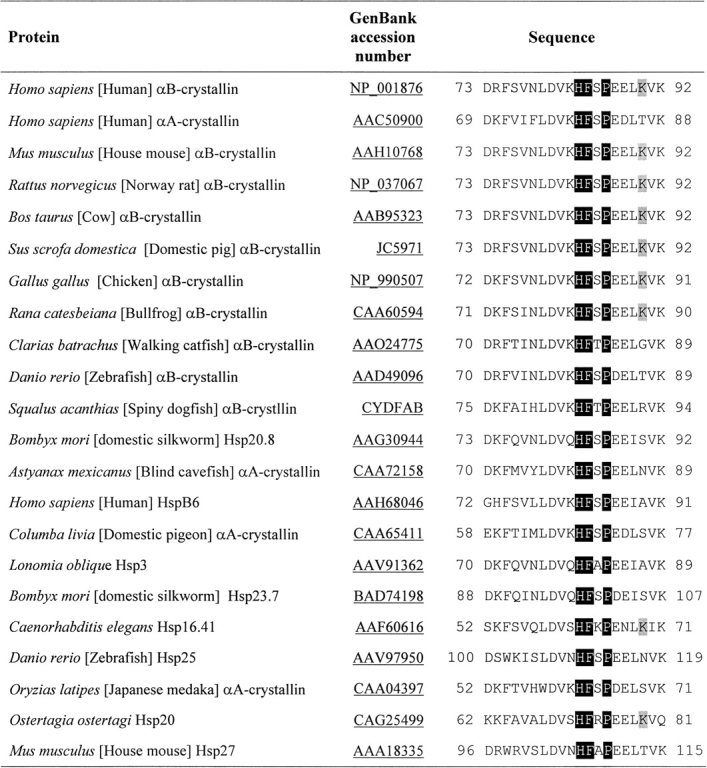
Comparison of chaperone region sequences of αB-crystallin with various sHsps. A protein–protein BLAST search (NCBI) of human αB-chaperone region sequence was done. Proteins having various levels of sequence identity were picked and manually aligned. Shaded letters correspond to residues that were mutated in this study.
Structural characterization of recombinant proteins
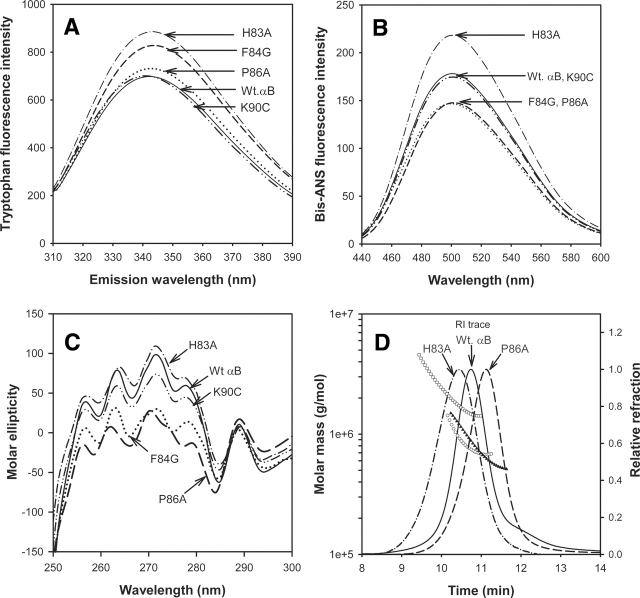
Tryptophans of proteins have a fixed solvent accessibility, and any change in their environment alters their fluorescence pattern and intensity (Colon 1999). We measured the intrinsic tryptophan fluorescence of the purified proteins to characterize structural changes. The tryptophan fluorescence intensity of K90C did not change, while other mutants showed a 5%–30% increase in tryptophan fluorescence when compared to wild-type αB tryptophan intensity (▶). One of the two tryptophan residues, Trp60 of αB-crystallin, is close to the chaperone site sequence, 73–92. Tryptophan fluorescence intensity was seen to increase as the mutation was introduced closer to this tryptophan residue.
Figure 3.
Structural characterization of wild-type αB-crystallin and its mutants. (A) Intrinsic tryptophan fluorescence intensity of wild-type and αB-crystallin mutants. The emission spectra were measured using 100 μg of recombinant proteins in 1 mL of phosphate buffer (pH 7.4). The samples were excited at 295 nm. The spectra shown are the average of three scans. (B) Interaction of bis-ANS with mutants and wild-type protein. The recombinant proteins (100 μg) were incubated for 30 min at 37°C with bis-ANS. The samples were excited at 385 nm, and the emission spectra were recorded between 400 and 600 nm. (C) Near-UV CD spectra (tertiary structure) of wild-type αB-crystallin and its mutants. Spectra were recorded at a protein concentration of 3 mg/mL. (D) Molar mass distribution across refractive index trace of wild-type αB (○), H83A (□), and P86A (▴), determined by using dynamic light scattering measurements. Profiles of F84G and K90C are not shown. The quaternary structural parameters determined from light scattering data are given in ▶.
We also investigated the structural differences among the proteins by analyzing bis-ANS binding. This dye is known to bind exposed hydrophobic surfaces and is helpful in analyzing relative hydrophobicity and conformational changes of the proteins (Sharma et al. 1998a). The H83A mutation yielded a 30% increase in bis-ANS binding when compared to wild-type αB-crystallin, whereas F84G and P86A mutants showed 22% less dye binding than the wild-type αB (▶). The K90C mutation in αB-crystallin did not alter bis-ANS binding.
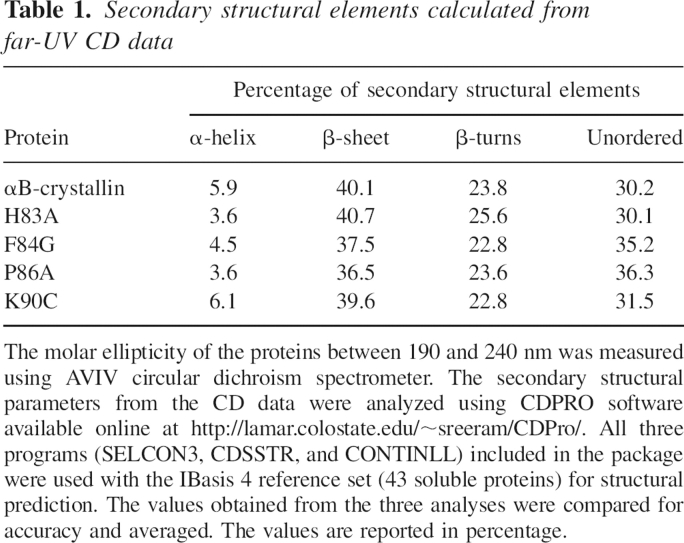
UV-CD spectroscopic analysis of the structural differences between the wild-type αB and the mutants revealed that near-UV CD spectra had no appreciable change in the tertiary structure of H83A and K90C mutants. In contrast, F84G and P86A mutants showed increased negative ellipticity when compared to wild-type protein (▶). The far-UV profile of the wild-type and mutant αB-crystallins displayed a characteristic β-sheet conformation. The spectral data (190–240 nm) were used to calculate the secondary structural elements using the CDPRO software (Sreerama and Woody 2000). A set of 43 soluble proteins included in the software package was used as a base of reference. The secondary structural elements calculated from the data are listed in ▶ The wild-type αB-crystallin had 40.1% β-sheet, 23.8% β turns, 5.9% α-helix, and 30.2% unordered structure. Compared with wild-type αB, the F84G and P86A mutants exhibited a marginal decrease in β-sheet and an increase in unordered content. H83A and K90C did not show changes in their secondary structures when compared with wild-type αB-crystallin.
Table 1.
Secondary structural elements calculated from far-UV CD data
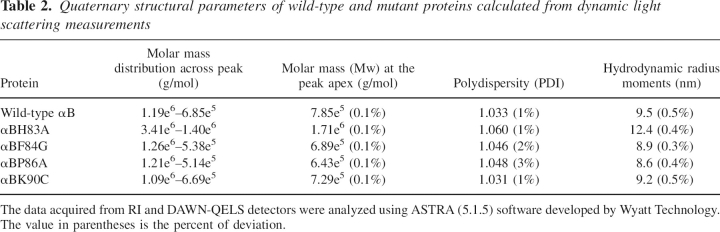
The oligomeric masses of the wild-type and mutant proteins were determined using HPLC fitted with a gel filtration column and refractive index detector, coupled to a dynamic light scattering instrument. Multi-angle light scattering coupled with size exclusion chromatography (SEC) permits the determination of polydispersity and an estimate of the absolute molar mass and size, which cannot be obtained by conventional chromatographic detection methods (Wyatt 1993). ▶ shows the elution profile and mass distribution across the peaks of wild-type αB and its mutants. The H83A mutant exhibited a wider molar mass distribution than the wild-type αB and other mutants. The molar mass (Mw), polydispersity index (PDI), and hydrodynamic radius (Rh) of wild-type αB and its mutants calculated from dynamic light scattering measurements are given in ▶. The wild-type αB-crystallin had an average Mw of 7.85e5 g/mol. Compared to wild-type αB, the H83A mutant exhibited about a twofold higher Mw at the peak apex. These data suggest that the average number of subunits in H83A is 81, whereas wild-type αB has 36 subunits. The F84G and P86A mutants showed a slight decrease in Mw, and the K90C mutant showed no significant difference in the average Mw or the number of subunits estimated. The PDI is the measure of the distribution of molecular weights in a given protein sample. The PDI always has a value >1, but as the proteins move toward having a uniform molecular weight across the peak, the PDI approaches unity. Wild-type αB-crystallin had a PDI of 1.033. The PDI did not change significantly when K90 was replaced with Cys. However, the other mutants showed an increase in PDI. The Rh is calculated from the translational diffusion constant of the protein in a solvent of known viscosity and temperature. Protein samples with a larger Rh diffuse more slowly through a solvent than do protein samples with a smaller Rh. The H83A mutation in αB-crystallin increased the Rh from 9.5 nm, which was the Rh of wild-type αB, to 12.4 nm, whereas F84A and P86A mutations decreased the Rh values to 8.9 and 8.6 nm, respectively. The Rh of K90C was 9.2 nm, not a significant difference from the Rh of wild-type αB-crystallin.
Table 2.
Quaternary structural parameters of wild-type and mutant proteins calculated from dynamic light scattering measurements
Functional characterization of recombinant proteins
The relative percentage of aggregation by ADH in the presence of mutants was compared with that of wild-type αB-crystallin at the 45-min time point (▶). The wild-type αB-crystallin and the mutants showed suppression of ADH aggregation, which increased with their increasing concentration in the assay tube. Approximately 12 μg of wild-type αB-crystallin and 12 μg of K90C were required to prevent the aggregation of 200 μg of ADH by 50% at 45 min. To provide the same level of protection against aggregation, 9.5 μg of H83A, 27 μg of F84G, and 24.5 μg of P86A were required.
Figure 4.
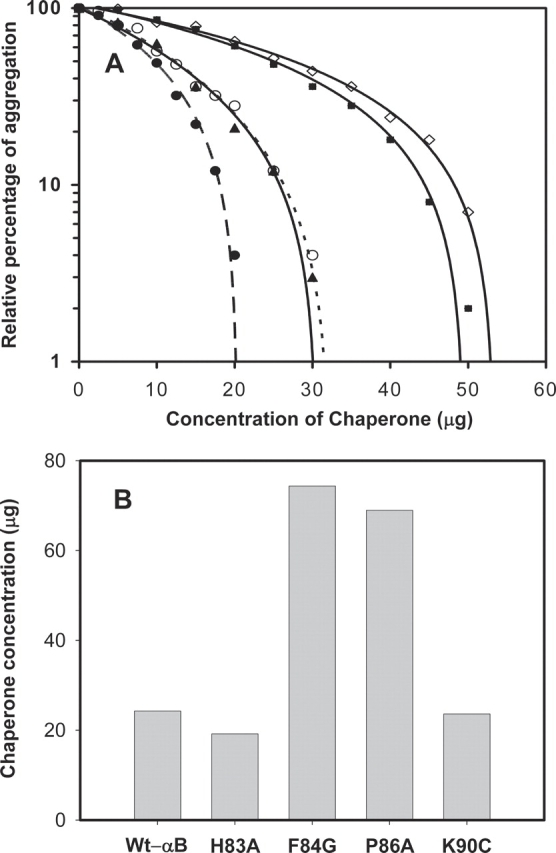
Comparison of anti-aggregation activities of wild-type and mutant αB-crystallins at 37°C using ADH and insulin as substrates. (A) Chaperone-like activity of wild-type αB-crystallin and mutants using ADH as the substrate. Aggregation of ADH (0.2 mg) was induced by 100 mM EDTA in the presence of various concentrations of chaperone protein. The percentage of aggregation at the 45-min time point was compared. (○) Wild-type wt.αB; (•) H83A; (◊) F84G; (▪) P86A; and (▴) K90C. (B) Chaperoning efficiency of wild-type αB-crystallin and mutants against insulin substrate. Aggregation of insulin at the 40-min time point in the presence of different concentrations of wild-type and mutant protein was determined. The data were analyzed by regression analysis, as in A. To compare the chaperoning efficiency, the concentration of the chaperone protein required to prevent the aggregation of insulin by 50% was calculated and plotted.
A reduction in insulin results in the separation of subunits and precipitation of the B chain, which can be monitored by measuring light scattering. The presence of αB-crystallin in the assay prevents the aggregation of insulin B chain, and the solution remains clear. The insulin aggregation assay revealed that wild-type αB-crystallin and mutant αB-crystallins prevented insulin B-chain aggregation in a concentration-dependent manner. The percentage of insulin aggregation at 45 min in each concentration of the chaperone protein was calculated to determine the activity of wild-type and mutant proteins. ▶ depicts the concentrations of wild-type and mutant αB-crystallins required to reduce the aggregation of insulin B chain by 50%. H83A was slightly better than wild-type αB-crystallin in preventing the aggregation of insulin B chain. F84G and P86A mutants were less effective when compared with wild-type αB-crystallin. This pattern is the same as that observed with the ADH substrate. The K90C mutation did not alter the ability of the protein to suppress insulin B-chain aggregation.
Interaction of substrate proteins at residue 90 in αB-crystallin during chaperone action
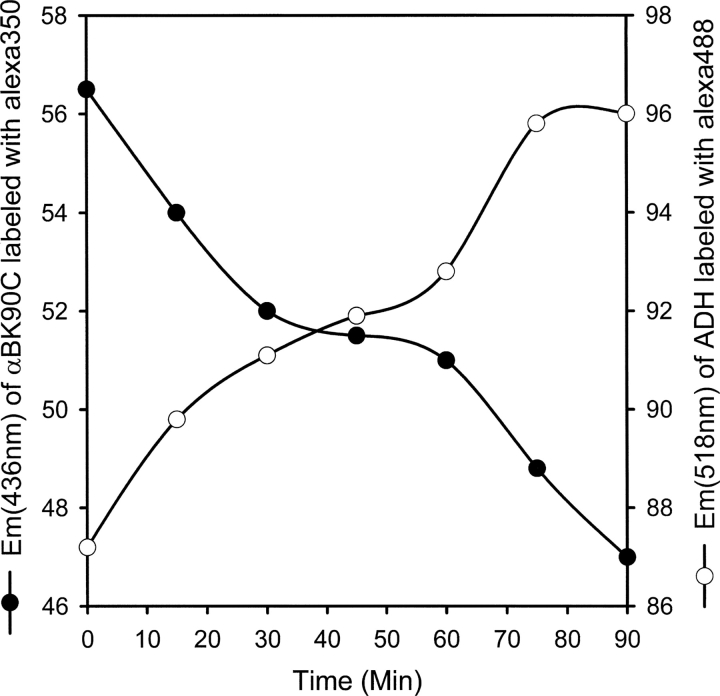
We used the FRET assay to demonstrate the interaction of ADH at residue 90 of αB-crystallin. The K90C mutant did not show any change in structure and function, and we took advantage of the lone Cys residue of the K90C mutant to attach an energy donor molecule (Alexa 350). The amino groups in ADH were labeled with acceptor molecule (Alexa 480). Labeling with Alexa dye did not affect the chaperone activity of K90C and the aggregation of ADH (data not shown). During chaperone assay, if the labeled Lys residue in ADH comes in close proximity to the K90 residue in αB-crystallin, the energy will be transferred from the donor molecule to the acceptor (Selvin 1995). Indeed, a small (18%) and progressive decrease occurred in the emission intensity of K90C350, with a concomitant increase in the emission intensity of ADH488 (▶). A FRET assay performed in absence of EDTA (nondenaturing) did not show any change in the emission maxima of labeled K90C and ADH, suggesting that αB-crystallin interacts only when the structure of ADH is destabilized. This was also confirmed by analyzing the incubation mixtures of labeled samples on a gel permeation chromatography wherein they eluted separately in the absence of EDTA (data not shown).
Figure 5.
Interaction of ADH at K90 region in αB-crystallin as demonstrated by FRET assay. Alexa 480 (amino group reactive)-labeled ADH (0.2 mg) was treated with Alexa 350 (thiol reactive)-labeled K90C (0.1 mg) at 37°C. ADH aggregation was induced by 100 mM EDTA. The change in emission intensity at 436 and 518 nm was measured up to 1.5 h.
Discussion
In this study, single amino acid substitutions were made in the chaperone region of αB-crystallin to identify the critical residues involved in the anti-aggregation activity of the native protein. We replaced three conserved amino acid residues—H83 → A, F84 → G, and P86 → A—and a nonconserved amino acid residue, K90 → C, in human αB-crystallin to test the role of these residues in the chaperone-like activity. The precise structural requirement for chaperone-like activity of α-crystallin cannot be delineated because of the polydisperse nature and variable quaternary structure of the protein (Haley et al. 1998). Furthermore, chaperone-like activity is governed by multiple factors (temperature, buffer condition, pH, and substrate protein used) and is subject to variation. Under such circumstances, extreme care must be exercised when comparing the chaperone activity of two proteins. To minimize the effects of these variables, we used substrates that aggregate at physiological temperature and measured the chaperone activity of all mutants at the same time using a single stock of substrate protein. We also used multiple concentrations of chaperone proteins that show various levels of protection and applied regression analysis to determine the chaperone protein concentration required for preventing aggregation of the substrate protein by 50%.
The chaperoning efficiency of α-crystallin depends on its ability to recognize substrates undergoing denaturation, interact with the substrate, and keep the complex in soluble form. An event that alters any of these factors would result in a protein with altered chaperone-like function. Mutation of K90C did not cause an appreciable change in the structure and function of αB-crystallin. On the other hand, mutation of F84G and P86A produced subtle changes in the αB-crystallin structure and a significant decrease in anti-aggregation activity against ADH and insulin substrates. The H83A mutant did not result in much variation in the secondary and tertiary structures but showed a marginal increase in chaperone activity.
One could argue that the loss in chaperone activity of mutants is due to structural alterations as a consequence of the mutation. However, the minor changes in the secondary and tertiary structures of F84G and P86A mutants are unlikely to have caused such a significant decrease in chaperone activity. It has been proposed that hydrophobic sites in α-crystallin play a role in chaperone-like activity. The F84G and P86A mutants showed 22% less bis-ANS binding than wild-type αB-crystallin, which correlated with their decrease in anti-aggregation activity. On the other hand, the H83A mutant showed a 30% increase in bis-ANS binding, with a 10%–15% increase in chaperone activity. It is difficult to judge whether increased activity of H83A is significant because of the wide variations in the chaperone assay. Regardless, the increased chaperone activity would be expected when His is substituted with a relatively hydrophobic residue. However, substitution of His with Ala did not increase the chaperone activity of mini-αB-crystallin (Bhattacharyya et al. 2006). The magnitude of the increase in chaperone activity is less than the increase in bis-ANS binding by H83A, because hydrophobic probes such as bis-ANS also interact at regions that do not contribute to chaperone-like function (Sharma et al. 1998b). Additionally, increased bis-ANS binding is not always accompanied by enhanced chaperone activity (Santhoshkumar and Sharma 2001b; Bhattacharyya et al. 2002; Kumar et al. 2005). Substitution of Met68 with hydrophobic residues Val and Ile has been shown to increase the chaperone activity, whereas substitution with a hydrophilic Thr residue decreased the chaperone activity of αB-crystallin (Shroff et al. 2001).
The intrinsic tryptophan fluorescence intensity increased as the mutations were introduced closer to the tryptophan residue. The data give qualitative information on the tryptophan microenvironment in the protein. However, in this study the intensity of tryptophan fluorescence did not correlate with the chaperone activity of mutants. Moreover, the two tryptophan residues in αB-crystallin are found in the N-terminal domain.
Another feature of α-crystallins thought to be important for chaperone function is their ability to form oligomers (Giese and Vierling 2002; Narberhaus 2002). A reduction in the oligomeric mass is known to affect the chaperone activity of many sHsps (Giese and Vierling 2002; Kelley and Abraham 2003). However, the native oligomeric state of α-crystallin may not be essential to suppress aggregation of substrates (Horwitz et al. 2004; Saha and Das 2004). Our investigation of oligomeric mass, PDI, and Rh of the mutants using dynamic light scattering measurements demonstrated a 12%–17% decrease in the oligomeric mass and an increase in PDI of F84G and P86A when compared to wild-type αB-crystallin (▶). However, these mass values are comparable to the reported oligomeric mass values of polydisperse recombinant αB-crystallin determined by the gel permeation chromatography method (Kumar et al. 1999). Only the H83A mutant exhibited a significantly higher mass distribution across the peak and had a higher Rh value when compared to other proteins. Whether the higher mass distribution and Rh accounts for the marginal increase in chaperone activity of the H83A mutant is not known.
While a total loss in chaperone activity of F84G and P86A mutants did not occur, the data are sufficient to conclude that F84 and P86 residues in the substrate binding domain of αB-crystallin are essential to prevent ADH and insulin aggregation. A Phe residue is found in the binding interfaces of many amyloidogenic proteins and plays a key role in diseases such as Alzheimer's, type II diabetes, age-related aortic medial amyloid deposition, Finnish hereditary amyloidosis, and chronic inflammatory amyloidosis (Azriel and Gazit 2001). We showed in a previous study that replacement of the F75 residue in mini-αB abolishes the chaperone activity of the peptide against ADH (Bhattacharyya et al. 2006). Other investigators have shown that the chaperone activity of an αBF28S mutant is unaffected at 37°C (Kelley and Abraham 2003). Together these findings lead us to conclude that only specific Phe residues in αB-crystallin are involved in substrate protein interaction.
The FRET assay is widely used to study subunit exchange and oligomeric organization of sHsps. In the present study we demonstrate for the first time, using the FRET assay, that destabilized ADH interacts near residue 90 in αB-crystallin during chaperone action. We saw a decrease in the emission intensity of the K90C350, and a corresponding increase in the emission intensity of ADH480. However, unlike in the subunit exchange assays, the transfer of energy was very small and continued until the end of the assay. This finding is expected, since we have previously observed that only 10%–20% of ADH precipitates after incubation for 1 h at 37°C and that α-crystallins interact with <6% of the primary sequence of ADH (Santhoshkumar and Sharma 2002). Furthermore, substrate proteins unfold gradually when subjected to heat stress, and α-crystallins interact with substrate proteins that are partially unfolded (Rajaraman et al. 1998). Small energy transfer can also be seen when an ADH molecule interacts slightly away from the K90 residue.
The present study involving site-directed mutagenesis and FRET assay confirms our previous findings that residues in the 73–92 region are involved in the chaperone action of αB-crystallin. Studies conducted by others suggest that additional sites are present in αB-crystallin for substrate protein interaction and for efficient chaperone action. Recently, the pin array study confirmed the involvement of residues DRFSVNLDVKHFS (73–85) in the chaperone action of αB-crystallin and identified sequences 9–20, 43–58, 113–120, 131–138, 141–158, and 157–164 in αB as potential target protein binding sites (Ghosh et al. 2005). However, only peptides 73–85 and 131–141 displayed chaperone activity, and the chaperone activity of sequence 131–141 was less than that of the 73–85 sequence. It is yet to be determined if sequence 131–141 in αB-crystallin oligomers is accessible to the substrate proteins. The same laboratory has earlier showed that substitutions of S139, G141, T144, G147, and P160 cause a modest decrease in αB-crystallin chaperone function without inducing any major structural changes (Muchowski et al. 1999). Several studies suggest the role of the R120 residue in αB-crystallin chaperone activity. The αB R120G mutation causes desmin-related myopathy in humans (Vicart et al. 1998). The aggregation of desmin or other substrates is not due to the inability of the αBR120G to interact with substrates but because of altered interaction resulting in insolubilization of the complex and enhanced aggregation (Treweek et al. 2005).
In conclusion, this study reveals that the conserved residues F84 and P86 in αB-crystallin are essential for preventing protein aggregation. Using the FRET assay, we demonstrated for the first time that substrate binding occurs near the K90 region in αB-crystallin. This study also confirms our previous study, which showed that sequence 73–92 plays a role in the substrate protein binding and chaperone function of αB-crystallin.
Materials and Methods
Preparation of wild-type and mutant αB-crystallins
The H83A, F84G, P86A, and K90C mutants were constructed using the QuikChange site-directed mutagenesis kit (Stratagene). Human αB-crystallin cDNA cloned in pET23d (Novagen-EMD Biosciences) was used as the template. The mutation was confirmed by DNA sequencing. Expression of proteins was achieved in Escherichia coli BL21(DE3)pLysS cells (Invitrogen). The cell pellet (from a 1 L culture) was resuspended in 5 mL of ice-cold lysis buffer (50 mM Tris, 2 mM EDTA, 100 mM NaCl at pH 7.5) and treated with 50 μL of protease inhibitor cocktail III (Calbiochem-EMD Biosciences), lysozyme (1 mg), and DTT (10 mM final concentration). The cell suspension was treated with 1 μL (25 units) of benzonase (Novagen-EMD Biosciences) and incubated for 30 min at 37°C on a shaking platform. The extract was centrifuged at 17,000g for 1 h, and the supernatant was treated with 45% ammonium sulfate to precipitate αB-crystallin or its mutants. The protein precipitate was resolubilized in 3 mL of phosphate buffer and initially purified by the gel filtration chromatography technique on a Hiload 16/60 Superdex 200 column (GE Healthcare Bio-Sciences Corp.) using 0.05 M phosphate buffer containing 0.15 M NaCl (pH 7.4). The proteins were further purified on a High Q anion exchange column (Bio-Rad Laboratories, Inc.) using a stepwise gradient of NaCl (0, 0.1, 0.25, 0.5, and 1 M) in 20 mM Tris-HCl (pH 8). The purity of the proteins was checked by sodium dodecylsulfate polyacrylamide gel electrophoresis (SDS-PAGE), and the molecular mass was determined by Matrix Assisted Laser Desorption/Ionization Time of Flight (MALDI-TOF) mass spectrometry.
Structural characterization of αB-crystallin and its mutants
The structural variations between wild-type and mutant αB-crystallins were determined by spectroscopic analysis. Phosphate buffer (0.05 M) containing 0.15 M NaCl and 0.02% sodium azide (pH 7.4) was used in all measurements unless otherwise specified.
The intrinsic tryptophan fluorescence spectra of the wild-type and mutant αB-crystallins were recorded using a Jasco FP-750 spectrofluorometer at room temperature. The excitation wavelength was set to 295 nm, and the emission was recorded between 300 nm and 400 nm. Protein samples of 100 μg/mL in phosphate buffer were used.
The stock solution (14.8 mM) of bis-ANS (Molecular Probes, Inc.) was prepared in 95% ethanol. Bis-ANS stock solution, 10 μL, was added to 100 μg of wild-type and mutant αB-crystallins in 1 mL of phosphate buffer and incubated for 30 min at 37°C. The interactions of bis-ANS with wild-type and mutant αB-crystallins were examined by recording the emission spectra between 400 nm and 600 nm. The samples were excited at 385 nm.
Changes in secondary and tertiary protein structures were investigated by far-UV and near-UV circular dichroism (CD) measurements using a Model 202 circular dichroism spectrophotometer (AVIV Biomedical Inc.) at room temperature. Protein concentrations of 3.0 mg/mL and 0.20 mg/mL in phosphate buffer were used for near- and far-UV CD measurements, respectively. The path length was 5 mm. The proteins were scanned eight times, the spectra were averaged, and the molar ellipticity of the mutants was compared with that of wild-type αB crystallin. The secondary structural elements were calculated on the basis of the far-UV (190–240 nm) CD data using the CDPro software available online at http://lamar.colostate.edu/∼sreeram/CDPro/ (Sreerama and Woody 2000).
The quaternary structural parameters were determined by dynamic light scattering measurements. Purified proteins were incubated for 1 h at 37°C in phosphate buffer and then injected (150 μg) into a TSK G5000PWXL (Tosoh Bioscience) size-exclusion column fitted to an HPLC with RID detector (Shimadzu Scientific Instruments, Inc.) and equilibrated with phosphate buffer. The HPLC was coupled to multi-angle light scattering (DAWN) and quasi-elastic light scattering detectors (Wyatt Technology Corporation). The molar mass, polydispersity, and hydrodynamic radius of the samples were determined using ASTRA (5.1.5) software developed by Wyatt Technology.
Functional characterization of αB-crystallin and its mutants
The ability of wild-type and mutant αB-crystallins to prevent protein aggregation was determined by using ADH and insulin as substrates. Aggregation of target proteins was monitored by measuring light scattering at 360 nm as a function of time, using a Shimadzu spectrophotometer equipped with a temperature-regulated multi-cell holder.
Aggregation of ADH (250 μg; Biozyme Laboratories) was induced by the addition of 0.1 M EDTA (final concentration) in 1 mL of phosphate buffer in the presence of various amounts of wild-type and mutant proteins, kept for 1 h at 37°C.
An insulin aggregation assay was conducted using 200 μg of insulin (Sigma) in 1 mL of 0.01 M phosphate buffer containing 0.10 M NaCl (pH 7.2). Aggregation was induced by the addition of 20 μL of 1 M dithiothreitol (Research Products International Corp.) and monitored in the presence of various amounts of wild-type and mutant proteins for 1 h at 37°C.
Demonstration of ADH interaction near residue 90 of αB-crystallin
FRET assay was performed to demonstrate the interaction of ADH near residue 90 of αB-crystallin, using the K90C mutant. The lone cysteine residue in αBK90C was labeled with Alexa Fluor 350 C5-maleimide, as described by the supplier (Molecular Probes, Inc.). In brief, the K90C protein (2 mg/mL phosphate buffer) was treated with reducing agent TCEP to eliminate any disulfide bonds in the protein. A 20 mM stock solution of the dye was prepared in DMSO. The reduced K90C was treated with the dye (20 mol/1 mol protein). The reaction was carried out for 2 h at room temperature in the dark. The reaction was quenched by the addition of excess DTT, and the mixture was dialyzed extensively against phosphate buffer to remove free dye and reducing agents. ADH was labeled using amine-reactive Alexa Fluor 488 carboxylic acid TFP ester, as described above except the reducing agent was not used and the reaction was quenched using Tris instead of DTT. For the FRET assay, 200 μg of Alexa 488-labeled ADH (ADH488) was treated with 100 μg of Alexa 350-labeled K90C (K90C350). The ADH–αB interaction was induced by the addition of EDTA (100 mM) in a final volume of 1 mL. The sample was incubated at 37°C and excited at 346 nm, and the emission was monitored at 436 nm and 518 nm for up to 1.5 h on a JASCO FP-750 spectrofluorometer (JASCO). The excitation and emission bandwidths were set at 10 nm and 5 nm, respectively. The concentration of K90C350 used in the assay completely prevented the scattering of light by ADH, which otherwise would have interfered with the fluorescence measurements.
Acknowledgments
Acknowledgments
We thank Jing Wang and Elizabeth Cheney for technical assistance. This work is supported in part by National Institutes of Health grants EY11981 and EY14975 and an unrestricted grant-in-aid from Research to Prevent Blindness to the Department of Ophthalmology. We thank Sharon Morey for help with preparation of the manuscript.
Footnotes
Reprint requests to: K. Krishna Sharma, Mason Eye Institute, University of Missouri, Columbia, MO 65212, USA; e-mail: sharmak@health.missouri.edu; fax: (573) 884-4100.
Abbreviations: sHsps, small heat shock proteins; Bis-ANS, 1,1′-bi(4-anilino)naphthalene-5,5′-disulfonic acid; FRET, fluorescence resonance energy transfer; ADH, alcohol dehydrogenase; DTT, dithothreitol; CD, circular dichroism; TCEP, tris-(2-carboxyethyl)phosphine, hydrochloride; DMSO, dimethyl sulfoxide; HPLC, high pressure liquid chromatography; MALS, multi-angle light scattering.
Article and publication are at http://www.proteinscience.org/cgi/doi/10.1110/ps.062338206.
References
- Aquilina, J.A., Benesch, J.L., Ding, L.L., Yaron, O., Horwitz, J., and Robinson, C.V. 2005. Subunit exchange of polydisperse proteins: Mass spectrometry reveals consequences of αA-crystallin truncation. J. Biol. Chem. 280 14485–14491. [DOI] [PubMed] [Google Scholar]
- Augusteyn, R.C. and Koretz, J.F. 1987. A possible structure for α-crystallin. FEBS Lett. 222 1–5. [DOI] [PubMed] [Google Scholar]
- Azriel, R. and Gazit, E. 2001. Analysis of the minimal amyloid-forming fragment of the islet amyloid polypeptide. An experimental support for the key role of the phenylalanine residue in amyloid formation. J. Biol. Chem. 276 34156–34161. [DOI] [PubMed] [Google Scholar]
- Bera, S., Thampi, P., Cho, W.J., and Abraham, E.C. 2002. A positive charge preservation at position 116 of αA-crystallin is critical for its structural and functional integrity. Biochemistry 41 12421–12426. [DOI] [PubMed] [Google Scholar]
- Bhattacharyya, J., Srinivas, V., and Sharma, K.K. 2002. Evaluation of hydrophobicity versus chaperonelike activity of bovine αA- and αB-crystallin. J. Protein Chem. 21 65–71. [DOI] [PubMed] [Google Scholar]
- Bhattacharyya, J., Padmanabha Udupa, E.G., Wang, J., and Sharma, K.K. 2006. Mini-αB-crystallin: A functional element of αB-crystallin with chaperone-like activity. Biochemistry 45 3069–3076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswas, A., Miller, A., Oya-Ito, T., Santhoshkumar, P., Bhat, M., and Nagaraj, R.H. 2006. Effect of site-directed mutagenesis of methylglyoxal-modifiable arginine residues on the structure and chaperone function of human αA-crystallin. Biochemistry 45 4569–4577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloemendal, H., de Jong, W., Jaenicke, R., Lubsen, N.H., Slingsby, C., and Tardieu, A. 2004. Ageing and vision: Structure, stability and function of lens crystallins. Prog. Biophys. Mol. Biol. 86 407–485. [DOI] [PubMed] [Google Scholar]
- Bova, M.P., Yaron, O., Huang, Q., Ding, L., Haley, D.A., Stewart, P.L., and Horwitz, J. 1999. Mutation R120G in αB-crystallin, which is linked to a desmin-related myopathy, results in an irregular structure and defective chaperone-like function. Proc. Natl. Acad. Sci. 96 6137–6142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady, J.P., Garland, D.L., Green, D.E., Tamm, E.R., Giblin, F.J., and Wawrousek, E.F. 2001. αB-Crystallin in lens development and muscle integrity: A gene knockout approach. Invest. Ophthalmol. Vis. Sci. 42 2924–2934. [PubMed] [Google Scholar]
- Bullard, B., Ferguson, C., Minajeva, A., Leake, M.C., Gautel, M., Labeit, D., Ding, L., Labeit, S., Horwitz, J., and Leonard, K.R., et al. 2004. Association of the chaperone αB-crystallin with titin in heart muscle. J. Biol. Chem. 279 7917–7924. [DOI] [PubMed] [Google Scholar]
- Carver, J.A., Aquilina, J.A., and Truscott, R.J. 1994. A possible chaperone-like quaternary structure for α-crystallin. Exp. Eye Res. 59 231–234. [DOI] [PubMed] [Google Scholar]
- Carver, J.A., Guerreiro, N., Nicholls, K.A., and Truscott, R.J. 1995. On the interaction of α-crystallin with unfolded proteins. Biochim. Biophys. Acta 1252 251–260. [DOI] [PubMed] [Google Scholar]
- Caspers, G.J., Leunissen, J.A., and de Jong, W.W. 1995. The expanding small heat-shock protein family, and structure predictions of the conserved “α-crystallin domain.”. J. Mol. Evol. 40 238–248. [DOI] [PubMed] [Google Scholar]
- Cobb, B.A. and Petrash, J.M. 2002. α-Crystallin chaperone-like activity and membrane binding in age-related cataracts. Biochemistry 41 483–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colon, W. 1999. Analysis of protein structure by solution optical spectroscopy. Methods Enzymol. 309 605–632. [DOI] [PubMed] [Google Scholar]
- Das, B.K., Liang, J.J., and Chakrabarti, B. 1997. Heat-induced conformational change and increased chaperone activity of lens α-crystallin. Curr. Eye Res. 16 303–309. [DOI] [PubMed] [Google Scholar]
- de Jong, W.W., Caspers, G.J., and Leunissen, J.A. 1998. Genealogy of the α-crystallin–small heat-shock protein superfamily. Int. J. Biol. Macromol. 22 151–162. [DOI] [PubMed] [Google Scholar]
- Feil, I.K., Malfois, M., Hendle, J., van Der Zandt, H., and Svergun, D.I. 2001. A novel quaternary structure of the dimeric α-crystallin domain with chaperone-like activity. J. Biol. Chem. 276 12024–12029. [DOI] [PubMed] [Google Scholar]
- Gangalum, R.K., Schibler, M.J., and Bhat, S.P. 2004. Small heat shock protein αB-crystallin is part of cell cycle-dependent Golgi reorganization. J. Biol. Chem. 279 43374–43377. [DOI] [PubMed] [Google Scholar]
- Ghosh, J.G., Estrada, M.R., and Clark, J.I. 2005. Interactive domains for chaperone activity in the small heat shock protein, human αB crystallin. Biochemistry 44 14854–14869. [DOI] [PubMed] [Google Scholar]
- Giese, K.C. and Vierling, E. 2002. Changes in oligomerization are essential for the chaperone activity of a small heat shock protein in vivo and in vitro. J. Biol. Chem. 277 46310–46318. [DOI] [PubMed] [Google Scholar]
- Groenen, P.J., Merck, K.B., de Jong, W.W., and Bloemendal, H. 1994. Structure and modifications of the junior chaperone α-crystallin. From lens transparency to molecular pathology. Eur. J. Biochem. 225 1–19. [DOI] [PubMed] [Google Scholar]
- Groth-Vasselli, B., Kumosinski, T.F., and Farnsworth, P.N. 1995. Computer-generated model of the quaternary structure of α crystallin in the lens. Exp. Eye Res. 61 249–253. [DOI] [PubMed] [Google Scholar]
- Gupta, R. and Srivastava, O.P. 2004. Deamidation affects structural and functional properties of human αA-crystallin and its oligomerization with αB-crystallin. J. Biol. Chem. 279 44258–44269. [DOI] [PubMed] [Google Scholar]
- Haley, D.A., Horwitz, J., and Stewart, P.L. 1998. The small heat-shock protein, αB-crystallin, has a variable quaternary structure. J. Mol. Biol. 277 27–35. [DOI] [PubMed] [Google Scholar]
- Harding, J.J. 2002. Viewing molecular mechanisms of ageing through a lens. Ageing Res. Rev. 1 465–479. [DOI] [PubMed] [Google Scholar]
- Horwitz, J. 1992. α-Crystallin can function as a molecular chaperone. Proc. Natl. Acad. Sci. 89 10449–10453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz, J. 2003. α-Crystallin. Exp Eye Res. 76 145–153. [DOI] [PubMed] [Google Scholar]
- Horwitz, J., Huang, Q.L., Ding, L., and Bova, M.P. 1998. Lens α-crystallin: Chaperone-like properties. Methods Enzymol. 290 365–383. [DOI] [PubMed] [Google Scholar]
- Horwitz, J., Huang, Q., and Ding, L. 2004. The native oligomeric organization of α-crystallin, is it necessary for its chaperone function? Exp. Eye Res. 79 817–821. [DOI] [PubMed] [Google Scholar]
- Ingolia, T.D. and Craig, E.A. 1982. Four small Drosophila heat shock proteins are related to each other and to mammalian α-crystallin. Proc. Natl. Acad. Sci. 79 2360–2364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwaki, T., Kume-Iwaki, A., and Goldman, J.E. 1990. Cellular distribution of αB-crystallin in non-lenticular tissues. J. Histochem. Cytochem. 38 31–39. [DOI] [PubMed] [Google Scholar]
- Kelley, P.B. and Abraham, E.C. 2003. Thermally induced disintegration of the oligomeric structure of αB-crystallin mutant F28S is associated with diminished chaperone activity. Mol. Cell. Biochem. 252 273–278. [DOI] [PubMed] [Google Scholar]
- Koretz, J.F., Doss, E.W., and Reid, G.H. 1997. Analysis of the factors involved in the loss and restoration of the chaperone-like function of α-crystallin. Biochem. Biophys. Res. Commun. 231 270–276. [DOI] [PubMed] [Google Scholar]
- Kumar, L.V., Ramakrishna, T., and Rao, C.M. 1999. Structural and functional consequences of the mutation of a conserved arginine residue in αA and αB crystallins. J. Biol. Chem. 274 24137–24141. [DOI] [PubMed] [Google Scholar]
- Kumar, M.S., Kapoor, M., Sinha, S., and Reddy, G.B. 2005. Insights into hydrophobicity and the chaperone-like function of αA- and αB-crystallins: An isothermal titration calorimetric study. J. Biol. Chem. 280 21726–21730. [DOI] [PubMed] [Google Scholar]
- Li, L.K. and Spector, A. 1973. The reaggregation of purified subunits of α-crystallin. Exp. Eye Res. 15 179–183. [DOI] [PubMed] [Google Scholar]
- Litt, M., Kramer, P., LaMorticella, D.M., Murphey, W., Lovrien, E.W., and Weleber, R.G. 1998. Autosomal dominant congenital cataract associated with a missense mutation in the human α crystallin gene CRYAA. Hum. Mol. Genet. 7 471–474. [DOI] [PubMed] [Google Scholar]
- Maddala, R. and Rao, V.P. 2005. α-Crystallin localizes to the leading edges of migrating lens epithelial cells. Exp. Cell Res. 306 203–215. [DOI] [PubMed] [Google Scholar]
- Muchowski, P.J., Bassuk, J.A., Lubsen, N.H., and Clark, J.I. 1997. Human αB-crystallin. Small heat shock protein and molecular chaperone. J. Biol. Chem. 272 2578–2582. [DOI] [PubMed] [Google Scholar]
- Muchowski, P.J., Wu, G.J., Liang, J.J., Adman, E.T., and Clark, J.I. 1999. Site-directed mutations within the core “α-crystallin” domain of the small heat-shock protein, human αB-crystallin, decrease molecular chaperone functions. J. Mol. Biol. 289 397–411. [DOI] [PubMed] [Google Scholar]
- Narberhaus, F. 2002. α-Crystallin-type heat shock proteins: Socializing minichaperones in the context of a multichaperone network. Microbiol. Mol. Biol. Rev. 66 64–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasta, S.Y., Raman, B., Ramakrishna, T., and Rao Ch, M. 2003. Role of the conserved SRLFDQFFG region of α-crystallin, a small heat shock protein. Effect on oligomeric size, subunit exchange, and chaperone-like activity. J. Biol. Chem. 278 51159–51166. [DOI] [PubMed] [Google Scholar]
- Rajaraman, K., Raman, B., Ramakrishna, T., and Rao, C.M. 1998. The chaperone-like α-crystallin forms a complex only with the aggregation-prone molten globule state of α-lactalbumin. Biochem. Biophys. Res. Commun. 249 917–921. [DOI] [PubMed] [Google Scholar]
- Rajaraman, K., Raman, B., Ramakrishna, T., and Rao, C.M. 2001. Interaction of human recombinant αA- and αB-crystallins with early and late unfolding intermediates of citrate synthase on its thermal denaturation. FEBS Lett. 497 118–123. [DOI] [PubMed] [Google Scholar]
- Raman, B. and Rao, C.M. 1997. Chaperone-like activity and temperature-induced structural changes of α-crystallin. J. Biol. Chem. 272 23559–23564. [DOI] [PubMed] [Google Scholar]
- Rao, P.V., Huang, Q.L., Horwitz, J., and Zigler Jr., J.S. 1995. Evidence that α-crystallin prevents non-specific protein aggregation in the intact eye lens. Biochim. Biophys. Acta 1245 439–447. [DOI] [PubMed] [Google Scholar]
- Reddy, G.B., Reddy, P.Y., and Suryanarayana, P. 2001. αA- and αB-crystallins protect glucose-6-phosphate dehydrogenase against UVB irradiation-induced inactivation. Biochem. Biophys. Res. Commun. 282 712–716. [DOI] [PubMed] [Google Scholar]
- Saha, S. and Das, K.P. 2004. Relationship between chaperone activity and oligomeric size of recombinant human αA- and αB-crystallin: A tryptic digestion study. Proteins 57 610–617. [DOI] [PubMed] [Google Scholar]
- Santhoshkumar, P. and Sharma, K.K. 2001a. Analysis of α-crystallin chaperone function using restriction enzymes and citrate synthase. Mol. Vis. 7 172–177. [PubMed] [Google Scholar]
- Santhoshkumar, P. and Sharma, K.K. 2001b. Phe71 is essential for chaperone-like function in αA-crystallin. J. Biol. Chem. 276 47094–47099. [DOI] [PubMed] [Google Scholar]
- Santhoshkumar, P. and Sharma, K.K. 2002. Identification of a region in alcohol dehydrogenase that binds to α-crystallin during chaperone action. Biochim. Biophys. Acta 1598 115–121. [DOI] [PubMed] [Google Scholar]
- Selvin, P.R. 1995. Fluorescence resonance energy transfer. Methods Enzymol. 246 300–334. [DOI] [PubMed] [Google Scholar]
- Sharma, K.K. and Ortwerth, B.J. 1995. Effect of cross-linking on the chaperone-like function of α crystallin. Exp. Eye Res. 61 413–421. [DOI] [PubMed] [Google Scholar]
- Sharma, K.K., Kaur, H., and Kester, K. 1997. Functional elements in molecular chaperone α-crystallin: Identification of binding sites in αB-crystallin. Biochem. Biophys. Res. Commun. 239 217–222. [DOI] [PubMed] [Google Scholar]
- Sharma, K.K., Kaur, H., Kumar, G.S., and Kester, K. 1998a. Interaction of 1,1′-bi(4-anilino)naphthalene-5,5′-disulfonic acid with α-crystallin. J. Biol. Chem. 273 8965–8970. [DOI] [PubMed] [Google Scholar]
- Sharma, K.K., Kumar, G.S., Murphy, A.S., and Kester, K. 1998b. Identification of 1,1′-bi(4-anilino)naphthalene-5,5′-disulfonic acid binding sequences in α-crystallin. J. Biol. Chem. 273 15474–15478. [DOI] [PubMed] [Google Scholar]
- Sharma, K.K., Kumar, R.S., Kumar, G.S., and Quinn, P.T. 2000. Synthesis and characterization of a peptide identified as a functional element in αA-crystallin. J. Biol. Chem. 275 3767–3771. [DOI] [PubMed] [Google Scholar]
- Shinohara, H., Inaguma, Y., Goto, S., Inagaki, T., and Kato, K. 1993. αB crystallin and HSP28 are enhanced in the cerebral cortex of patients with Alzheimer's disease. J. Neurol. Sci. 119 203–208. [DOI] [PubMed] [Google Scholar]
- Shroff, N.P., Cherian-Shaw, M., Bera, S., and Abraham, E.C. 2000. Mutation of R116C results in highly oligomerized αA-crystallin with modified structure and defective chaperone-like function. Biochemistry 39 1420–1426. [DOI] [PubMed] [Google Scholar]
- Shroff, N.P., Bera, S., Cherian-Shaw, M., and Abraham, E.C. 2001. Substituted hydrophobic and hydrophilic residues at methionine-68 influence the chaperone-like function of αB-crystallin. Mol. Cell. Biochem. 220 127–133. [DOI] [PubMed] [Google Scholar]
- Smulders, R., Carver, J.A., Lindner, R.A., van Boekel, M.A., Bloemendal, H., and de Jong, W.W. 1996. Immobilization of the C-terminal extension of bovine αA-crystallin reduces chaperone-like activity. J. Biol. Chem. 271 29060–29066. [DOI] [PubMed] [Google Scholar]
- Sreerama, N. and Woody, R.W. 2000. Estimation of protein secondary structure from circular dichroism spectra: Comparison of CONTIN, SELCON, and CDSSTR methods with an expanded reference set. Anal. Biochem. 287 252–260. [DOI] [PubMed] [Google Scholar]
- Srinivas, V., Santhoshkumar, P., and Sharma, K.K. 2002. Effect of trifluoroethanol on the structural and functional properties of α-crystallin. J. Protein Chem. 21 87–95. [DOI] [PubMed] [Google Scholar]
- Srinivas, V., Raman, B., Rao, K.S., Ramakrishna, T., and Rao Ch, M. 2005. Arginine hydrochloride enhances the dynamics of subunit assembly and the chaperone-like activity of α-crystallin. Mol. Vis. 11 249–255. [PubMed] [Google Scholar]
- Srinivasan, A.N., Nagineni, C.N., and Bhat, S.P. 1992. αA-Crystallin is expressed in non-ocular tissues. J. Biol. Chem. 267 23337–23341. [PubMed] [Google Scholar]
- Takemoto, L. 1994. Release of α-A sequence 158–173 correlates with a decrease in the molecular chaperone properties of native α-crystallin. Exp. Eye Res. 59 239–242. [DOI] [PubMed] [Google Scholar]
- Takeuchi, N., Ouchida, A., and Kamei, A. 2004. C-terminal truncation of α-crystallin in hereditary cataractous rat lens. Biol. Pharm. Bull. 27 308–314. [DOI] [PubMed] [Google Scholar]
- Tardieu, A., Laporte, D., Licinio, P., Krop, B., and Delaye, M. 1986. Calf lens α-crystallin quaternary structure. A three-layer tetrahedral model. J. Mol. Biol. 192 711–724. [DOI] [PubMed] [Google Scholar]
- Thampi, P. and Abraham, E.C. 2003. Influence of the C-terminal residues on oligomerization of αA-crystallin. Biochemistry 42 11857–11863. [DOI] [PubMed] [Google Scholar]
- Thampi, P., Hassan, A., Smith, J.B., and Abraham, E.C. 2002. Enhanced C-terminal truncation of αA- and αB-crystallins in diabetic lenses. Invest. Ophthalmol. Vis. Sci. 43 3265–3272. [PubMed] [Google Scholar]
- Treweek, T.M., Rekas, A., Lindner, R.A., Walker, M.J., Aquilina, J.A., Robinson, C.V., Horwitz, J., Perng, M.D., Quinlan, R.A., and Carver, J.A. 2005. R120G αB-crystallin promotes the unfolding of reduced α-lactalbumin and is inherently unstable. FEBS J. 272 711–724. [DOI] [PubMed] [Google Scholar]
- van Noort, J.M., van Sechel, A.C., Bajramovic, J.J., el Ouagmiri, M., Polman, C.H., Lassmann, H., and Ravid, R. 1995. The small heat-shock protein αB-crystallin as candidate autoantigen in multiple sclerosis. Nature 375 798–801. [DOI] [PubMed] [Google Scholar]
- Vicart, P., Caron, A., Guicheney, P., Li, Z., Prevost, M.C., Faure, A., Chateau, D., Chapon, F., Tome, F., and Dupret, J.M., et al. 1998. A missense mutation in the αB-crystallin chaperone gene causes a desmin-related myopathy. Nat. Genet. 20 92–95. [DOI] [PubMed] [Google Scholar]
- Wang, K. and Spector, A. 1995. α-Crystallin can act as a chaperone under conditions of oxidative stress. Invest. Ophthalmol. Vis. Sci. 36 311–321. [PubMed] [Google Scholar]
- Wistow, G. 1993. Possible tetramer-based quaternary structure for α-crystallins and small heat shock proteins. Exp. Eye Res. 56 729–732. [DOI] [PubMed] [Google Scholar]
- Wyatt, P.J. 1993. Light scattering and the absolute characterization of macromolecules. Anal. Chim. Acta 272 1–40. [Google Scholar]
- Yang, C., Salerno, J.C., and Koretz, J.F. 2005. NH2-terminal stabilization of small heat shock protein structure: A comparison of two NH2-terminal deletion mutants of αA-crystallin. Mol. Vis. 11 641–647. [PubMed] [Google Scholar]