Abstract
Research has documented a strong association between early adolescent problem behavior and adult disinhibitory psychopathology, leading some to suggest that the latter can be reduced by preventing or delaying the former. But the prevention implications of this association necessarily depend upon the causal mechanisms that produce it. The current study was designed to test implications of a model that posits that early problem behavior and disinhibitory psychopathology are associated because they are both manifestations of a common inherited liability. At their age-17 assessment, 1080 twins from the older cohort of the Minnesota Twin Family Study reported whether and the age at which they first: drank alcohol, used tobacco, used illicit drugs, had sexual intercourse, and had police contact. An Early Problem Behavior index was computed by summing the number of these experiences each participant reported having before age 15. Outcome measures of disinhibitory psychopathology were assessed by clinical interview at the age-20 follow-up and included number of symptoms of nicotine dependence, alcohol abuse and dependence, drug abuse and dependence, and adult antisocial behavior. Biometric analysis of the multivariate twin data showed that: (1) early adolescent problem behavior is weakly heritable (approximately 20%), (2) the common factor underlying disinhibitory psychopathology is strongly heritable (approximately 75%), and (3) the phenotypic correlation between early adolescent problem behavior and disinhibitory psychopathology was strong (approximately 0.60) and accounted for primarily by genetic factors common to the two domains. Findings are discussed in the context of research on the prevention and developmental nature of substance use disorders and related psychopathology.
Keywords: Adolescent problem behavior, disinhibitory psychopathology, externalizing, twin studies
Behavioral genetic research has established the importance of both heritable and non-heritable influences on the development of adult substance use disorders (SUDs) including: nicotine dependence (Heath and Madden, 1995; Kendler et al., 1999), alcohol dependence (Heath, 1995; Kendler et al., 1992; McGue, 1999; Prescott and Kendler, 1999), and illicit substance abuse and dependence (Ball and Collier, 2002; Kendler and Prescott, 1998; Lynskey et al., 2002; McGue et al., 2000; Tsuang et al., 1996). Current behavioral genetic research aims to build on these findings by identifying the specific genetic and environmental factors that affect SUD risk and by characterizing their joint mechanisms of actions (Rutter et al., 2001). While much of this effort appropriately involves the use of molecular approaches to identify the specific genes that contribute to SUD risk (Goldman et al., 2005), complementary approaches aimed at characterizing the complex interplay of genetic and environmental influences within a developmental context are also needed to understand the origins of adult SUDs.
An observation critical to understanding the developmental nature of SUDs concerns the strong association of early substance use with SUD risk. Using data from a large U.S. epidemiological survey, Grant and Dawson (1997) reported that individuals who had used alcohol prior to age 15 were four times more likely to develop alcohol dependence in adulthood than individuals who had first tried alcohol after age 20. This finding, which suggests that risk for alcoholism is for some individuals established very early in life, has had a major impact on the conceptualization of both effective preventions and the origins alcoholism (Baumeister and Tossmann, 2005; Pitkänen et al., 2005; Stewart et al., 2005). Nonetheless, the implications of this research for prevention and developmental models of alcoholism depend on the causal basis for the association of early drinking with later alcoholism. Several alternative mechanisms have been advanced. First, early exposure to alcohol may alter the course of adolescent development, diminishing the likelihood that adolescents associate with the socializing agents that encourage sobriety and law abidingness (e.g., parents and schools) and increasing the likelihood they affliate with groups and individuals that model and encourage substance use and deviance (Dewit et al., 2000). In this case, adolescents who start drinking early are at an increased risk of developing alcohol dependence by early adulthood because their experiences foster a developmental course characterized by the rapid escalation of drinking throughout middle and late adolescence. Findings from neuroscience research provide additional, albeit indirect, support for the altered-course-of-adolescent-development model. Animal research, for example, has shown that the brain is more sensitive to the cognitive-impairing effects of alcohol but less sensitive to its sedative effects during adolescence than at other life stages (Spear, 2002; White and Swartzwelder, 2004). Recent research with humans further suggests that heavy alcohol exposure in adolescence may lead to neurocognitive changes that themselves may increase the likelihood of subsequent alcohol abuse (Brown and Tapert, 2004; De Bellis et al., 2000). Thus, the altered-course-of-adolescent-development model hypothesizes that early alcohol use increases the likelihood of adult alcoholism because adolescents who use alcohol early in life experience both increased social and biological risk as a consequence of that early exposure.
Alternatively, the association of early alcohol use with adult alcoholism may be non-causal. Prescott and Kendler (1999) hypothesized that a common inherited liability underlies both early alcohol use and alcoholism and thus accounts for their association. Support for this hypothesis comes for their analysis of data from a large cohort of nearly 9000 twins showing that the association between early alcohol use and alcoholism was predominantly genetically and not environmentally mediated. The common-inherited-liability model further predicts that because early use of alcohol is a manifestation of a general liability for disinhibitory behavior and psychopathology, it should predict a multitude of adult behavioral problems in addition to alcoholism (McGue and Iacono, 2004). Consistent with this expectation, we have shown that early use of alcohol predicts a wide range of adult behavioral problems including drug abuse, adult antisocial behavior, and academic underachievement (McGue et al., 2001a). We further found that early alcohol use is associated with personality and psychophysiological markers of behavioral disinhibition, is predicted by measures of pre-existing childhood disinhibitory psychopathology (e.g., attention deficit hyperactivity disorder), and evidences familial aggregation with indicators of parental disinhibitory psychopathology (McGue et al., 2001b). Taken together, these findings suggest that alcohol use prior to age 15 is an early manifestation of a generalized vulnerability that manifests in adulthood as a range of disorders including alcoholism, drug abuse, and antisocial personality disorder (ASPD) (cf. Hicks et al., 2004; Krueger et al., 2002; Young et al., 2000)
We have recently provided additional support for this model by showing that early alcohol use is strongly associated with other indicators of early adolescent problem behavior including experimentation with nicotine and illicit drugs, precocious sexual experience, and early contact with the police, and that taken together these indicators can define a factor of early adolescent problem behavior (McGue and Iacono, 2005). Importantly, these indicators of early adolescent problem behavior predicted a broad range of adult psychopathology including alcoholism, drug abuse, ASPD, nicotine dependence, and depression, both individually and in aggregate. The total number of problem behaviors (out of 5) an adolescent had engaged in prior to age 15 was strongly associated with risk of adult disinhibitory psychopathology. Males who had engaged in 4 or 5 problem behaviors before age 15, for example, had by age 20 rates of nicotine dependence, alcohol abuse or dependence, drug abuse or dependence, ASPD, and major depression that were respectively 92%, 92%, 100%, 92%, and 33%. The comparable rates in 20-year-old women were 82%, 61%, 84%, 35%, and 57%, respectively. The association of early alcohol use with alcoholism thus appears to be a specific instance of a general relationship between early adolescent problem behavior and adult psychopathology.
The purpose of the present paper is to more fully explore the association between early adolescent problem behavior and adult psychopathology from a behavioral genetic perspective. Specifically, we make use of a cohort of 1252 male and female twins to test two implications of the common-inherited-liability model: (1) early adolescent problem behavior is heritable, and (2) the relationship between early adolescent problem behavior and adult psychopathology is predominantly genetically and not environmentally mediated.
METHODS
Sample
The sample consisted of twin participants from the older cohort of the Minnesota Twin Family Study (MTFS). The MTFS is a longitudinal study of a community-based sample of two cohorts of twins. The older (i.e., age-17) cohort had a mean age of 17.5 years (SD=0.45) at the intake assessment and consisted of 626 pairs of like-sex twins (189 monozygotic male [MZM], 100 dizygotic male [DZM], 223 MZ female [MZF] and 114 DZ female [DZF]). A complete description of the recruitment of the MTFS sample as well as evidence of sample representativeness is given in Iacono et al. (1999). The present study is based on those twins from the older cohort who had valid early problem behavior data from their intake assessment and completed a follow-up assessment at age 20 (mean of 20.7, SD=0.57). Of the 1252 twins who completed the intake assessment, 1111 (89%) completed the first follow-up at age 20, of which 31 did not have valid early problem behavior data (see below). This left a sample of 1080 twins (472 male and 608 female) available for the present study (149 MZM pairs, 81 DZM pairs, 200 MZF pairs, 109 DZF pairs, and 33 twins whose cotwin is not part of the sample).
Measures
Early Problem Behavior Index
Five indicators of adolescent problem behavior were obtained through self-report at the twins’ age-17 intake assessment. These were: (a) tobacco use (“Have you ever tried tobacco?”), (b) alcohol use (“Have you ever used alcohol without parental permission?”), (c) police contact (“Other than for traffic violations, have you ever been in trouble with the police?”), (d) sexual intercourse (“Have you ever had intercourse?”), and (e) use of any of 10 illicit substances (separate assessment for marijuana, amphetamines, barbiturates, tranquilizers, cocaine, heroin, opiates, PCP, psychedelics, and inhalants). Adolescents reported whether they had ever engaged in each of the activities and, if they had, the age of first occurrence. To integrate the current findings with those from the literature on early problem behavior (e.g., Dewit et al., 2000), each indicator was scored positive if the adolescent reported having engaged in the activity prior to age 15. A total Early Problem Behavior index was computed by summing the five individual indicators. Because the resulting Early Problem Behavior index was positively skewed, it was log-transformed (after adding 1) for computation of statistical tests and in the biometric analyses.
Symptom Outcome Data
Members of a twin pair were interviewed separately by different interviewers to assess lifetime symptoms of mental disorders according to the DSM-III-R criteria, the diagnostic standard current at the time the MTFS began. Clinical interviewers had either a bachelor’s or master’s degree in psychology or related discipline and had completed an extensive program of training that ended with their satisfying proficiency criteria before they were allowed to interview study participants. The clinical assessments included the expanded substance abuse module (SAM), developed by Robins et al. (1987) as a supplement to the World Health Organization’s Composite International Diagnostic Interview (CIDI, Robins et al., 1988) for substance use disorders, and the ASPD section from the Structured Clinical Interview for DSM-III-R (Spitzer et al., 1987), which was modified to include additional probes and follow-up questions to cover symptoms of adult antisocial behavior (AAB). Interview data were reviewed in case conferences by at least two graduate students with advanced training in descriptive psychopathology and differential diagnosis. Members of the review team needed to achieve a consensus before any symptom was coded as positive. Rather than diagnoses, we made use of symptom count variables for each of the clinical outcomes. Symptom counts provide a more sensitive measure of the underlying construct than do discrete diagnoses (MacCallum et al., 2002), and reflect our conceptualization of disinihibitory psychopathology as a dimensional rather than categorical construct(Kraemer et al., 2004; Krueger et al., 2005). Four symptom count variables were computed by summing the number of positive symptoms for each of the following DSM-III-R diagnoses: (1) Nicotine Dependence, (2) Alcohol Abuse or Dependence, (3) Drug Abuse or Dependence (i.e., abuse or dependence on any of the following: amphetamines, cannabis, cocaine, hallucinogens, inhalants, opiates, PCP, and sedatives), and (4) AAB (i.e., the ASPD symptoms of adult antisocial behavior occurring since age 15). To minimize the impact of skewness, symptom count variables were log-transformed (after adding 1) for all statistical and biometric analyses.
Statistical Methods
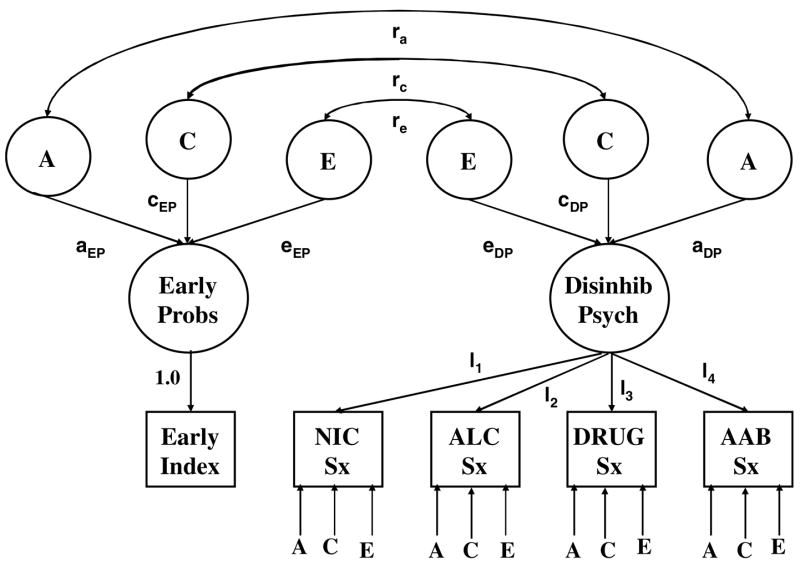
To account for missing data, robust estimates of the MZ and DZ twin correlations for the log-transformed early problem index and symptom scales were obtained using the EM (Expectation-Maximization) algorithm (Dempster et al., 1977) as implemented in SPSS for Windows (version 11.01). In effect, EM gives maximum likelihood estimates for the relevant parameters assuming a multivariate normal distribution with unobserved data assumed to be missing at random (Schafer, 1997). Correlation estimation was followed by biometric analysis of the twin data using standard methods of analysis (Neale and Cardon, 1992). That is for any scale, we assumed that the total phenotypic variance (P) could be decomposed into additive genetic (A), shared environmental (C) and non-shared environmental (E) components (i.e., P = A+C+E). We began by fitting a five-variable Cholesky model to establish a base model against which the relative fit of other models was judged and to obtain estimates of the proportion of variance in each phenotype associated with genetic (a2), shared environmental (c2), and non-shared environmental factors (e2). We next fit a bivariate factor model (Fig. 1), in which the first factor loaded exclusively on the Early Problem Behavior index and the second, or Disinhibitory Psychopathology, factor loaded on the four symptom scales. In the factor model, genetic and environmental contributions were modeled at both the factor and residual levels. Because the first factor loaded exclusively on the Early Problem Behavior index, however, there were no residual genetic and environmental contributions for that variable. The relationship between the two factors was modeled in terms of correlations in underlying additive genetic (rA), shared environmental (rC) and non-shared environmental (rE) effects.
Fig. 1.
Bivariate factor model fit to the twin data. Note that because there is only one indicator for the Early Problem Behaviors factor, that factor is equivalent to the observed Early Problem Behavior Index, for which there are no residual effects.
Biometric models were fit to the EM estimated twin variance–covariance matrices using the maximum likelihood procedure implemented in the Mx software system (Neale et al., 1999). Fit of the general factor model was judged relative to the fit of a general Cholesky model (Neale and Cardon, 1992), which places no constraints on the observed variances and covariances beyond those implied by intraclass structure (e.g., the variance for the first twin should be the same as the variance for the second twin) and the equality of total effects across the MZ and DZ samples (e.g., MZ and DZ variances should be the same.) Three indexes were used to evaluate model fit: (1) the χ2 goodness-of-fit test, (2) the Akaike Information Criterion (AIC= χ2-2df), and (3) the root mean squared error of approximation (RMSEA). The AIC is an information index that balances model fit (χ2) against parsimony (df) (Akaike, 1987). In a fully saturated model where there are as many parameters as input statistics, AIC=0. Negative AICs correspond to models that improve on this saturated model. RMSEA indexes the extent to which the model-implied variance–covariance structure matches the variance–covariance structure estimated for the population from which the sample was drawn. RMSEA values less than 0.08 are considered to indicate good model fit, while values less than 0.05 indicate excellent fit (McDonald, 1989).
RESULTS
Attrition Analysis
Among the total of 1252 twins who comprised the 17-year-old intake MTFS sample, 172 could not be included in the present investigation. There were two reasons for non-inclusion: (1) non-participation at follow-up, and (2) failure to complete the Early Problem Behavior index at intake. To evaluate the effect of attrition we first compared the log-transformed Early Problem Behavior index scores of individuals who did or did not participate at follow-up. Non-participants had significantly higher index scores than participants in both the female (standardized effect size (ES) for log-transformed scale=0.38, p<0.05) and male (ES=0.22, p<0.05) samples. We next compared the four follow-up log-transformed symptom count scales for those who did or did not have a valid Early Problem Behavior index score at intake. In the female sample, none of the four comparisons was statistically significant. In the male sample, non-completers scored significantly higher than completers on all scales except the nicotine dependence scale, with ESs ranging from 0.78 to 0.91. Participants in the present study thus appear to be on average better adjusted than non-participants, although given the overall high rate of participation (89%) sample attrition is not likely to have a large effect on the results presented here.
Descriptive Analysis
Table I gives the frequency of each of the early problem behavior indicators in the male and female samples. As expected, rates of problem behavior prior to age 15 were highest for tobacco and alcohol use and lowest for sexual intercourse and illicit drug use. Males were significantly more likely than females to report early use of tobacco and alcohol and to have had early police contact. Rate of early illicit drug use and sexual intercourse did not vary significantly by gender. Table II gives the tetrachoric correlations among the five problem indicators in the pooled male and female sample. The correlations range from approximately 0.50–0.80, underscoring the strong commonality that exists among the multiple indicators. When broken down by gender, the tetrachoric correlations among the multiple problem indicators were still consistently high, albeit somewhat lower than in the pooled sample reflecting the consistent gender difference in prevalence. For females, tetrachoric correlations ranged from 0.48 (early alcohol – early police contact) to 0.74 (early smoking – early drug use) and averaged 0.64. For males, the correlations ranged from 0.42 (early alcohol—early police contact) to 0.71 (early sexual intercourse—early drug use) and averaged 0.55.
Table I.
Rates of Adolescent Problem Behavior Prior to Age 15 in Male and Female Samples
| Males
|
Females
|
Gender Comparison
|
||||
|---|---|---|---|---|---|---|
| Early Problem Indicator | N | % | N | % | χ2(1 df) | p |
| Tobacco | 480 | 56 | 625 | 40 | 26.2 | <0.001 |
| Alcohol | 480 | 24 | 625 | 19 | 4.26 | 0.04 |
| Illicit Drugs | 480 | 4 | 624 | 5 | 0.40 | 0.53 |
| Sexual Intercourse | 459 | 3 | 593 | 5 | 2.44 | 0.12 |
| Police Contact | 475 | 13 | 571 | 4 | 30.14 | <0.001 |
Table II.
Tetrachoric Correlations Among Multiple Indicators of Early Adolescent Problem Behavior
| Tobacco | Alcohol | Illicit Drugs | Sexual Intercourse | Police Contact | |
|---|---|---|---|---|---|
| Tobacco | 1.00 | ||||
| Alcohol | 0.81 | 1.00 | |||
| Illicit Drugs | 0.82 | 0.77 | 1.00 | ||
| Sexual Intercourse | 0.58 | 0.68 | 0.61 | 1.00 | |
| Police Contact | 0.55 | 0.48 | 0.47 | 0.46 | 1.00 |
Each early problem indicator was coded positive if expressed prior to age 15 and negative otherwise. Pairwise Ns vary from 1022 (Police Contact and Sexual Intercourse) to 1080 (Alcohol and Illicit Drug). All correlations are statistically significant at p<0.001.
The distribution of the Early Problem Behavior score is given in Figure 2, additional descriptive information for this index and the four outcome symptom count scales is given in Table III. The average number of early problem behaviors was 0.99 for males and 0.74 for females, a difference that is statistically significant at p<0.001. As is evident from the figure, the early problem index is very positively skewed, with relatively few males or females engaging in 2 or more problem behaviors prior to age 15. The symptom outcome scales are also positively skewed, with a relatively large proportion of both the male and the female samples having no symptoms on each of the symptom scales. As with the Early Problem Behavior scale, males reported significantly more symptoms of alcohol dependence, drug dependence and adult antisocial behavior than females. The two groups did not, however, differ significantly in reported symptoms of nicotine dependence.
Fig. 2.
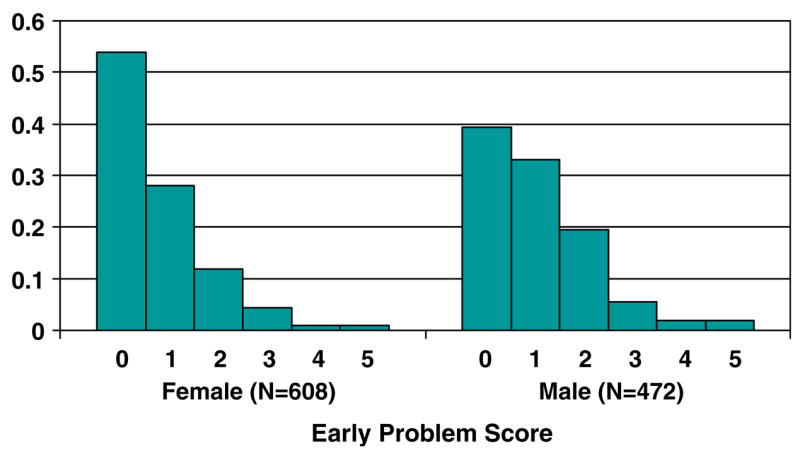
Distribution of early problem score in the female and male samples. Vertical axis gives proportion with that score.
Table III.
Descriptive Statistics for Early Problem Behavior and Follow-up Symptom Count Outcome Measures
| Males
|
Females
|
Gender Comparison
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Scale | N | % Zero | Mean (SD) | N | % Zero | Mean (SD) | ES | t | df | p |
| Early Problem | 472 | 39.4 | 0.99 (1.04) | 608 | 53.8 | 0.74 (1.01) | 0.28 | 4.07 | 1078 | <0.001 |
| Nicotine Symptoms | 477 | 61.4 | 1.38 (2.01) | 629 | 67.6 | 1.31 (2.09) | 0.13 | 0.55 | 1104 | 0.58 |
| Alcohol Symptoms | 480 | 47.9 | 1.70 (2.20) | 628 | 79.1 | 0.78 (1.68) | 0.99 | 7.91 | 1106 | <0.001 |
| Illicit Drug Symptoms | 481 | 76.9 | 1.27 (3.34) | 630 | 88.3 | 0.75 (2.58) | 0.76 | 8.52 | 1109 | 0.004 |
| Adult Antisocial Symptoms | 481 | 37.1 | 1.60 (1.62) | 628 | 61.5 | 0.79 (1.18) | 0.65 | 9.65 | 1107 | <0.001 |
Note: In all cases, gender comparisons based on the log-transformed scales. ES=standardized effect size computed here as (male mean–female mean)/(pooled standard deviation) on the log-transformed scale. % Zero gives the percent of individuals who had a zero score on that scale.
Biometric Analysis
Twin correlations for the Early Problem Behavior index and the four symptom count scales are reported in Table IV. The MZ correlation for the Early Problem Behavior index was greater than the DZ twin correlation in both the male and female samples, although the magnitude of these differences is small suggesting moderate heritable effects and the possibility of shared environmental influences. In all except one case, the MZ twin correlation for the symptom scales was also greater than the corresponding DZ correlation. The somewhat lower MZ correlations for the symptom scales in the female as compared to the male samples suggest that there may be gender differences in heritable effects, a possibility we test formally using biometric methods.
Table IV.
Twin Correlations and Variance Component Estimates for Early Problem Index and Age-20 Symptom Count Scales
| Males
|
Females
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| MZ | DZ | a2 | c2 | e2 | MZ | DZ | a2 | c2 | e2 | |
| Early Problem | 0.57 | 0.50 | 0.23 (0.05, 0.52) | 0.36 (0.08, 0.54) | 0.41 (0.33, 0.50) | 0.60 | 0.48 | 0.32 (0.09, 0.62) | 0.30 (0.01, 0.51) | 0.38 (0.32, 0.47) |
| Nicotine Symptoms | 0.66 | 0.31 | 0.56 (0.31, 0.70) | 0.10 (0.00, 0.32) | 0.34 (0.27, 0.44) | 0.56 | 0.40 | 0.45 (0.19, 0.65) | 0.14 (0.00, 0.38) | 0.41 (0.33, 0.50) |
| Alcohol Symptoms | 0.64 | 0.29 | 0.58 (0.37, 0.70) | 0.07 (0.00, 0.26) | 0.35 (0.27, 0.44) | 0.36 | 0.39 | 0.15 (0.01, 0.47) | 0.26 (0.00, 0.42) | 0.59 (0.49, 0.70) |
| Illicit Drug Symptoms | 0.61 | 0.28 | 0.46 (0.24, 0.64) | 0.15 (0.01, 0.35) | 0.39 (0.31, 0.49) | 0.40 | 0.39 | 0.31 (0.09, 0.52) | 0.15 (0.01, 0.35) | 0.54 (0.44, 0.65) |
| Adult Antisocial Behavior | 0.58 | 0.35 | 0.42 (0.21, 0.60) | 0.17 (0.01, 0.37) | 0.41 (0.33, 0.51) | 0.57 | 0.40 | 0.48 (0.22, 0.65) | 0.12 (0.00, 0.36) | 0.40 (0.32, 0.49) |
Note: Symptom count scales and early problem index were log transformed prior to correlation estimation. Correlations were estimated using the Expectation-Maximization (EM) algorithm that included intake symptom count scales to take into account differential rates of non-response at follow-up. Biometric parameter estimates obtained from a 5-factor Cholesky model that used as input the EM estimated variance-covariance matrices. Parenthetical values are the 95% confidence interval estimates of the variance component parameters.
The five-variable Cholesky model fit the twin data moderately well (χ2 = 226.9, 130 df, p<0.001, AIC = −33.1, RMSEA=0.074). Constraining parameters to be equal in the male and female samples resulted in a model that fit slightly more poorly than the full model (i.e., AIC = −31.1). Based on the slight superiority of the gender differences model as well as a pattern of correlations suggesting greater heritability in the male as compared to the female sample, all subsequent models fit to the data allowed for gender differences in parameter estimates. Estimates of the proportion of phenotypic variance associated with additive genetic (a2), shared environmental (c2), and non-shared environmental factors (e2) from the full Cholesky model are given in Table IV. The Early Problem Behavior index is moderately but significantly (as judged by the confidence intervals) heritable in both the male and female samples. Estimates of shared environmental effects are also moderate and statistically significant. Consistent with the twin correlations, heritability estimates for the symptom scales were consistently larger in the male as compared to the female sample, and estimates of shared environmental influences on the symptom count scales were consistently small, especially in the male sample.
Table V gives the fit indices for the various factor models fit to the twin data. The full factor model fit better than the general Cholesky model by both AIC (−65.3 vs. −33.1) and RMSEA (0.067 vs. 0.074). Both the additive genetic and shared environmental correlation between the two factors could be set to 1.0 without loss of model fit. Residual shared environmental effects could be dropped from the model, although once these parameters were dropped the model could not be further simplified by dropping the residual additive genetic effects. The “best-fitting” factor model (χ2 = 284.8, 184 df, p<0.001, AIC= −83.2, RMSEA=0.063) fixed the additive genetic and shared environmental correlations to 1.0 and dropped the residual shared environmental effects. To characterize the “robustness” of this solution, two additional models were fit to the twin covariance matrices. First, we reexamined the statistical evidence for the existence of sex differences in parameter estimates first indicated in the fit of the Cholesky model by determining whether constraining parameter estimates to be equal in the male and female samples for this “best-fitting” model resulted in a better fitting model. As before, the no-sex-differences model did not fit the data as well (χ2 = 345.7, 200 df, p<0.001, AIC= −54.3, RMSEA=0.066) as a model that allowed for sex differences in parameter estimates. Second, to determine whether the association between the Early Problem Behavior index and symptom scales was properly accounted for at the factor level, we allowed for genetic and nonshared environmental correlations between the index and the genetic and environmental residuals for each of the four symptom scales. This model resulted in a non-significant decrease in χ2 (change in χ2 = 16.3, df=16, p=0.43) and an increase in AIC (from −83.2 to −67.5), suggesting that the relationship between the Early Problem Behavior index and the symptom scales was adequately accounted for by the disinhibitory psychopathology factor.
Table V.
Indexes of Model Fit
| Model | χ2 | df | p | AIC | RMSEA |
|---|---|---|---|---|---|
| 1. Full Cholesky | 226.9 | 130 | <0.001 | −33.1 | 0.074 |
| 1a. Male=Female Cholesky | 318.9 | 175 | <0.001 | −31.1 | 0.074 |
| 2. Full Factor, Male ≠ Female | 278.7 | 172 | <0.001 | −65.3 | 0.067 |
| 2a. ra= 1.0 | 279.0 | 174 | <0.001 | −69.0 | 0.066 |
| 2b. rc=1.0 | 279.4 | 174 | <0.001 | −68.6 | 0.066 |
| 2c. ra=rc=1.0 | 280.2 | 176 | <0.001 | −71.8 | 0.065 |
| 2d. No Residual Genetic Effects | 292.3 | 180 | <0.001 | −67.7 | 0.068 |
| 2e. No Residual Shared Environmental Effects | 283.0 | 180 | <0.001 | −77.0 | 0.064 |
| 2f. No Residual Genetic and Shared Environmental Effects | 397.5 | 188 | <0.001 | 21.5 | 0.088 |
| 2g. No Residual Shared Environmental Effects, ra=rc=1.0 | 284.8 | 184 | <0.001 | −83.2 | 0.063 |
Note: AIC=Akaike Information Criterion, RMSEA=Root Mean Square Error of Approximation.
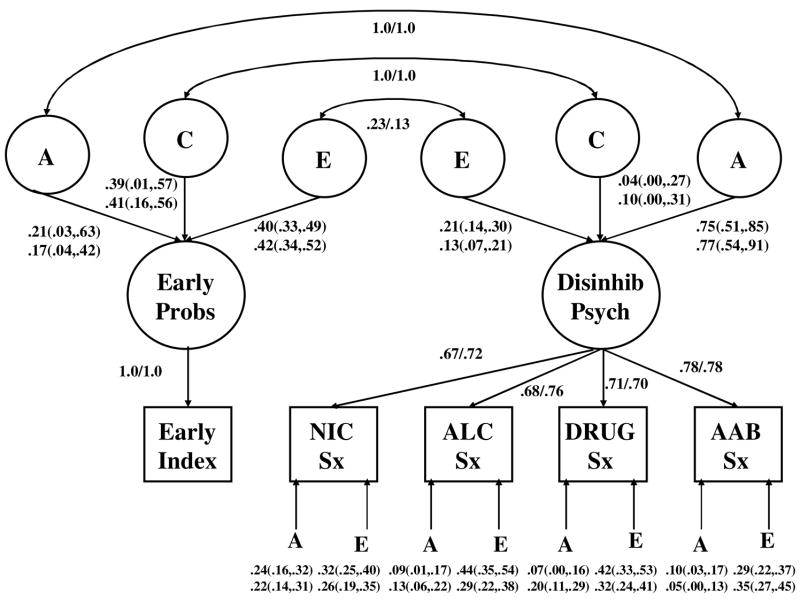
The standardized estimates for the best-fitting model with sex differences in parameter estimates are given in Figure 3. Even though a model that allowed for sex differences in parameter estimates fit better than a sex-invariant model, the standardized estimates are very similar in the male and female samples suggesting that variance differences may be the major source of the significant sex effect (a possibility for which we did not undertake a formal statistical test).
Fig. 3.
Standardized parameter estimates for the best-fitting biometric model (i.e., genetic and shared environmental correlations fixed to 1.0 and no residual shared environmental effects on the symptom scales). First value given is estimate in the female sample; second value is estimate for males. Additive genetic, shared environmental, and non-shared environmental values are standardized variance components rather than path coefficients. Parenthetical values give the 95% confidence intervals for the variance component parameters.
Based on the best-fitting factor model, the heritability of the Early Behavior Problem index was modest but significantly heritable (a2=0.21 and 0.17 in males and females, respectively). Corresponding estimates of shared environmental effects were larger and also statistically significant (c2=0.39 and 0.41 in males and females). In contrast, for the Disinhibitory Psychopathology factor, heritable effects were strong and significant (0.75 and 0.77 in females and males, respectively), while shared environmental influences were modest and non-significant (0.04 and 0.10). The correlation between the two factors, derived as a function of the correlations in the underlying genetic and environmental effects, equaled 0.59 in both samples. The bivariate heritability coefficient is a generalization of the univariate heritability and equals the proportion of the covariance between two phenotypes that can be accounted for by common genetic effects. In this case, the bivariate heritability between the Early Problem Behavior index and the Disinhibitory Psychopathology factor was 0.67 in the female and 0.61 in the male samples. Shared environmental factors accounted for, respectively, 21% and 34% of the correlation between the index and the factor, while nonshared environmental factors accounted for the remaining 11% and 5% of the correlation.
DISCUSSION
The observation that youth who experiment with alcohol early in adolescence are at substantially increased risk of developing alcoholism as adults has prompted a call for renewed efforts at preventing early initiation of drinking in adolescence. Nonetheless, the belief that rates of adult alcoholism can be reduced by delaying the age of drinking initiation is based on the unproven hypothesis that the association of early drinking with alcoholism is causal (Harford, 2003). Prescott and Kendler (1999) have argued that the association may be noncausal, arising because both early alcohol use and alcoholism are manifestations of a common inherited liability. Findings from their large twin study showing that the association of early alcohol use with alcoholism is primarily genetically mediated provide support for their hypothesis. The purpose of the present study was to: (1) broaden the scope of the common-inherited-liability model by considering multiple indicators of early adolescent problem behavior and adult disinhibitory psychopathology, (2) estimate the contribution of genetic factors to early problem behavior, and (3) determine the extent to which the association between early problem behavior and disinhibitory psychopathology was genetically or environmentally mediated.
Consistent with research by Jessor and colleagues (Donovan and Jessor, 1985, 1977), we found that early alcohol use does not occur in isolation but rather is a specific instance of a broader pattern of behavior marked by a disregard for the rules parents, other adults, and socializing institutions have established for American adolescents. The vast majority of individuals will at some point in their life experience sexual intercourse and even experiment with tobacco (Johnston et al., 2004). That is, these behaviors are not viewed as being inherently wrong as much as they are seen as something that, in the U.S., society believes adolescents should not engage in. It is not surprising then to find that those American adolescents who are willing to defy conventions governing the propriety of adolescent alcohol use, tobacco use, and sexual experience are willing also to transgress more general social rules against illicit drug use and illegal behavior.
Consistent with previous research by us (Krueger et al., 2002; McGue and Iacono, 2005) as well as others (Kendler et al., 2003; Young et al., 2000), we found evidence for the existence of a highly heritable factor that underlies the association among multiple forms of disinhibitory or “externalizing” psychopathology. In both the male and female samples, the disinhibitory factor accounted for approximately 50–60% of the variance in each of the four age-20 symptom scales and was approximately 75% heritable. Moreover, estimates of shared environmental influence on the disinhibitory psychopathology factor were modest and non-significant. Although there was evidence of statistically significant residual genetic effects on all of the symptom scales, the corresponding estimates were generally modest (i.e., less than 20%) so that the majority of heritable influences on the individual symptom scales could be attributed to their relationship with the latent disinhibitory factor.
In contrast to findings with the disinhibitory psychopathology factor, the Early Problem Behavior index was only modestly, albeit significantly, heritable (in both sexes estimated value of approximately 20% from the factor model), and shared environmental influences were moderate and statistically significant (approximately 40%). Our finding that both genetic and shared environmental factors influence early adolescent problem behavior is consistent with numerous behavioral genetic studies of adolescent antisocial behavior (Rhee and Waldman, 2002; Rose et al., 2001). Despite the modest heritability of Early Problem Behavior, its association with age-20 disinhibitory psychopathology owed predominantly to common genetic effects. More than 60% of the phenotypic correlation of 0.59 between problem behavior and disinhibitory psychopathology could be attributed to genetic factors. We are thus faced with what may appear initially to be three incongruous results: (1) adolescent problem behavior is weakly heritable, (2) there is a strong phenotypic association between early problem behavior and disinhibitory psychopathology, and yet (3) this association appears to be predominantly genetically and not environmentally mediated. Statistically, the apparent anomaly can be accounted for by noting that the high heritability of disinhibitory psychopathology necessarily constrains the mechanisms that could account for the phenotypic correlation of 0.60. That is, a factor that accounts for 10% or less of the variance in one variable (e.g., the shared environmental effect on the disinhibitory psychopathology factor here) cannot account for much of a large correlation of that variable with another.
Conceptually, these findings suggest that the heritable influences that account for only a modest proportion of the variance in problem behavior prior to age 15 amplify to account for a majority of variance in the latent disinhibitory factor by age 20. The developmental mechanisms that underlie this amplification are unclear but we believe not necessarily incompatible with an essential role of the environment. Specifically, the amplification of genetic effects may reflect active genotype-environment correlation processes (Scarr and McCartney, 1983), such that individuals with an inherited vulnerability to develop disinhibitory psychopathology actively search out environments (e.g., peers, high-risk settings) that reinforce the expression of that vulnerability.
While the findings presented here argue against a simple causal model linking early adolescent alcohol use and alcoholism, there are several reasons why our findings should be interpreted cautiously. First, our analysis is based on cross-sectional data and proper evaluation of hypotheses concerning the influence of early problem behavior will require prospective observations. In particular, there is a need for longitudinal behavioral genetic data in which both environmental risk and disinhibitory psychopathology have been assessed from early ages. In the younger (i.e., age-11) cohort of the MTFS we have begun to collect such data. Other behavioral genetic research groups likely also have relevant longitudinal data. Second, certain features of our data appear to be poorly captured by the summary biometric model. In particular, twin correlations in the female sample suggest stronger shared environmental and weaker genetic effects than the estimates from the biometric models. While we have confirmed the numerical accuracy of the correlations and parameter estimates, this apparent discrepancy suggests that alternative formulations may be necessary. Third, our failure to find a strong environmental correlation between the Early Problem Behavior Index and the Disinhibitory Psychopathology factor may owe to measurement error. Specifically, while measurement error was removed statistically from the multiple-indicator Disinhibitory Psychopathology factor, it could not be similarly removed from the single-indicator Early Problem Behavior Index. Because measurement error is included in the non-shared environmental component of variance, unreliability in the index would attenuate estimates of the non-shared environmental but not the genetic correlation. We did provide evidence that the multiple problem behaviors are highly intercorrelated. Nonetheless, there is a clear need for more sensitive approaches to the assessment of early problem behavior. Finally, our results do not imply that efforts aimed at reducing adolescent drinking are without merit. Early drinking may interact with an inherited vulnerability towards disinhibitory psychopathology in ways we do not currently understand. Moreover, drinking by adolescents, especially heavy drinking, has a direct impact on a range of untoward outcomes including sexual victimization (Raghavan et al., 2004) and alcohol-related fatalities(Hingson et al., 2005), even if it does not directly influence the development of adult disinhibitory psychopathology.
Acknowledgments
We acknowledge the helpful comments by Richard Rose and an anonymous reviewer on an earlier version of this paper. Any errors that remain are of course our own. Supported in part by US-PHS grants # AA09367, MH65137, and DA05147.
References
- Akaike H. Factor analysis and AIC. Psychometrica. 1987;52:317–332. [Google Scholar]
- Ball D, Collier D. Substance misuse. In: McGuffin P, Owen MJ, Gottesman II, editors. Psychiatric Genetics and Genomics. Oxford, England: Oxford University Press; 2002. pp. 267–302. [Google Scholar]
- Baumeister SE, Tossmann P. Association between early onset of cigarette, alcohol and cannabis use and later drug use patterns: An analysis of a survey in european metropolises. Eur Addict Res. 2005;11(2):92–98. doi: 10.1159/000083038. [DOI] [PubMed] [Google Scholar]
- Brown SA, Tapert SF. Adolescence and the trajectory of alcohol use: basic to clinical studies. Ann NY Acad Sci. 2004;1021:234–244. doi: 10.1196/annals.1308.028. [DOI] [PubMed] [Google Scholar]
- De Bellis MD, Clark DB, Beers SR, Solo PH, Boring AM, Hall J, et al. Hippocampal volume in adolescent-onset alcohol use disorders. Am J Psychiatry. 2000:737–744. doi: 10.1176/appi.ajp.157.5.737. [DOI] [PubMed] [Google Scholar]
- Dempster AP, Laird NM, Rubin DB. Maximum likelihood from incomplete data via the EM algorithm. J Roy Stat Soc, Ser B. 1977;39:1–38. [Google Scholar]
- Dewit DJ, Adlaf EM, Offord DR, Ogborne AC. Age at first alcohol use: a risk factor for the development of alcohol disorders. Am J Psychiatry. 2000;157:745–750. doi: 10.1176/appi.ajp.157.5.745. [DOI] [PubMed] [Google Scholar]
- Donovan JE, Jessor R. Structure of problem behavior in adolescence and young adulthood. J Consult Clin Psychol. 1985;53:890–904. doi: 10.1037//0022-006x.53.6.890. [DOI] [PubMed] [Google Scholar]
- Goldman D, Oroszi G, Ducci F. The genetics of addictions: uncovering the genes. Nat Rev Genet. 2005;6:521–532. doi: 10.1038/nrg1635. [DOI] [PubMed] [Google Scholar]
- Grant BF, Dawson DA. Age at onset of alcohol use and its association with DSM-IV alcohol abuse and dependence: results from the National Longitudinal Alcohol Epidemiologic Survey. J Subst Abuse. 1997;9:103–110. doi: 10.1016/s0899-3289(97)90009-2. [DOI] [PubMed] [Google Scholar]
- Harford TC. Early onset of alcohol use and health problems: spurious associations and prevention. Addiction. 2003;98:1483–1484. doi: 10.1046/j.1360-0443.2003.00555.x. [DOI] [PubMed] [Google Scholar]
- Heath AC. Genetic influences on drinking behavior in humans. In: Kissin HBB, editor. The Genetics of Alcoholism. New York: Oxford University Press; 1995. pp. 82–121. [Google Scholar]
- Heath AC, Madden PAF. Genetic influences on smoking behavior. In: Turner JT, Cardon LR, Hewitt JK, editors. Behavior Genetic Approaches in Behavioral Medicine. New York: Plenum; 1995. pp. 45–66. [Google Scholar]
- Hicks BM, Krueger RF, Iacono WG, McGue M, Patrick CJ. Externalizing disorders account for the genetic and environmental overlap between nicotine dependence and major depression. Arch Gener Psychiatry. 2004;61:922–928. doi: 10.1001/archpsyc.61.9.922. [DOI] [PubMed] [Google Scholar]
- Hingson R, Heeren T, Winter M, Wechsler H. Magnitude of alcohol-related mortality and morbidity among US college students ages 18–24: changes from 1998 to 2001. Ann Rev Public Health. 2005;26:259–279. doi: 10.1146/annurev.publhealth.26.021304.144652. [DOI] [PubMed] [Google Scholar]
- Iacono WG, Carlson SR, Taylor J, Elkins IJ, McGue M. Behavioral disinhibition and the development of substance use disorders: findings from the Minnesota Twin Family Study. Dev Psychopathol. 1999;11:869–900. doi: 10.1017/s0954579499002369. [DOI] [PubMed] [Google Scholar]
- Jessor R, Jessor SL. Problem Behavior and Psychosocial Development: A Longitudinal Study of Youth. New York: Academic Press; 1977. [Google Scholar]
- Johnston LD, O’Malley PM, Bachman JG, Schulenberg JE. Cigarette smoking among American teens continues to decline, but more slowly than in the past. Ann Arbor, MI: University of Michigan News and Information Services; 2004. [accessed 08/19/2005]. (Vol. Available: http://www.monitoringthefuture.org. [Google Scholar]
- Kendler KS, Heath AC, Neale MC, Kessler RC, Eaves LJ. A population-based twin study of alcoholism in women. JAMA. 1992;268(14):1877–1882. [PubMed] [Google Scholar]
- Kendler KS, Neale MC, Sullivan P, Corey LA, Gardner CO, Prescott CA. A population-based twin study in women of smoking initiation and nicotine dependence. Psychol Med. 1999;29(2):299–308. doi: 10.1017/s0033291798008022. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Prescott CA. Cannabis use, abuse, and dependence in a population-based sample of female twins. Am J Psychiatry. 1998;155(8):1016–1022. doi: 10.1176/ajp.155.8.1016. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Prescott CA, Myers J, Neale MC. The structure of genetic and environmental factors for common psychiatric and substance use disorders in men and women. Arch Gener Psychiatry. 2003;60:929–937. doi: 10.1001/archpsyc.60.9.929. [DOI] [PubMed] [Google Scholar]
- Kraemer HC, Noda A, O’Hara R. Categorical versus dimensional approaches to diagnosis: methodological challenges. J Psychiat Res. 2004;38:17–25. doi: 10.1016/s0022-3956(03)00097-9. [DOI] [PubMed] [Google Scholar]
- Krueger RF, Hicks BM, Patrick CJ, Carlson SR, Iacono WG, McGue M. Etiologic connections among substance dependence, antisocial behavior, and personality: modeling the externalizing spectrum. J Abnorm Psychol. 2002;111(3):411–424. [PubMed] [Google Scholar]
- Krueger RF, Markon KE, Patrick CJ, Iacono WG. Externalizing psychopathology in adulthood: a dimensional-spectrum conceptualization and its implications for DSM-V. J Abnorm Psychol. 2005;114(4):537–550. doi: 10.1037/0021-843X.114.4.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynskey MT, Heath AC, Nelson EC, Bucholz KK, Madden PAF, Slutske WS. Genetic and environmental contributions to cannabis dependence in a national young adult twin sample. Psycol Med. 2002;32:195–207. doi: 10.1017/s0033291701005062. [DOI] [PubMed] [Google Scholar]
- MacCallum RC, Zhang S, Preacher KJ, Rucker DD. On the practice of dichotomization of quantitative variables. Psychol Methods. 2002;7(1):19–40. doi: 10.1037/1082-989x.7.1.19. [DOI] [PubMed] [Google Scholar]
- McDonald RP. An index of goodness-of-fit based on noncentrality. J Classif. 1989;6:97–103. [Google Scholar]
- McGue M. Behavioral genetic models of alcoholism and drinking. In: Leonard KE, Blane HT, editors. Psychological Theories of Drinking and Alcoholism. New York: Guilford Publications; 1999. pp. 372–421. [Google Scholar]
- McGue M, Elkins I, Iacono WG. Genetic and environmental influences on adolescent substance use and abuse. Am J Med Genet (Neuropsychiat Genet) 2000;96:671–677. doi: 10.1002/1096-8628(20001009)96:5<671::aid-ajmg14>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- McGue M, Iacono WG. The initiation of substance use in adolescence: A behavioral genetic perspective. In: DiLalla LF, editor. Development, Personality, and Psychopathology. Washington, DC: American Psychological Association; 2004. pp. 41–57. [Google Scholar]
- McGue M, Iacono WG. The association of early adolescent problem behavior with adult psychopathology. Am J Psychiatry. 2005;162(6):1118–1124. doi: 10.1176/appi.ajp.162.6.1118. [DOI] [PubMed] [Google Scholar]
- McGue M, Iacono WG, Legrand LN, Elkins I. The origins and consequences af age at first drink. I Associations with substance-use disorders, disinhibitory behavior and psychopathology, and P3 amplitude. Alcoholism: Clin Exp Res. 2001a;25:1156–1165. [PubMed] [Google Scholar]
- McGue M, Iacono WG, Legrand LN, Elkins I. Origins and consequences of age at first drink. II Familial risk and heritability. Alcoholism: Clin Exp Res. 2001b;25:1166–1173. [PubMed] [Google Scholar]
- Neale MC, Boker SM, Xie G, Maes HH. Mx: Statistical Modeling. 5. Richmond, Virginia: Department of Psychiatry, Medical College of Virginia; 1999. [Google Scholar]
- Neale MC, Cardon LR. Methodology for Genetic Studies of Twins and Families. Dordrecht, The Netherlands: Kluwer; 1992. [Google Scholar]
- Pitkänen T, Lyyra AL, Pulkkinen L. Age of onset of drinking and the use of alcohol in adulthood: a follow-up study from age 8–42 for females and males. Addiction. 2005;100(5):652–661. doi: 10.1111/j.1360-0443.2005.01053.x. [DOI] [PubMed] [Google Scholar]
- Prescott CA, Kendler KS. Age at first drink and risk for alcoholism: a noncausal association. Alcoholism: Clin Exp Res. 1999;23:101–107. [PubMed] [Google Scholar]
- Prescott CA, Kendler KS. Genetic and environmental contributions to alcohol abuse and dependence in a population-based sample of male twins. Am J Psychiatry. 1999;156(1):34–40. doi: 10.1176/ajp.156.1.34. [DOI] [PubMed] [Google Scholar]
- Raghavan R, Bogart LM, Elliott MN, Vestal KD, Schuster MA. Sexual victimization among a national probability sample of adolescent women. Perspectives Sexual Reproductive Health. 2004;36(6):225–232. doi: 10.1363/psrh.36.225.04. [DOI] [PubMed] [Google Scholar]
- Rhee SH, Waldman ID. Genetic and environmental influences on antisocial behavior: a meta-analysis of twin and adoption studies. Psychol Bull. 2002;128:490–529. [PubMed] [Google Scholar]
- Robins LM, Baber T, Cottler LB. Composite International Diagnostic Interview: Expanded Substance Abuse Module. St. Louis: Authors; 1987. [Google Scholar]
- Robins LN, Wing J, Wittchen HU, Helzer JE, Babor TF, Burke J. The composite international diagnostic interview. Arch Gener Psychiatry. 1988;45:1069–1077. doi: 10.1001/archpsyc.1988.01800360017003. [DOI] [PubMed] [Google Scholar]
- Rose RJ, Dick DM, Viken RJ, Pulkkinen L, Kaprio J. Drinking or abstaining at age 14? A genetic epidemiological study. Alcoholism: Clin Exp Res. 2001;25(11):1594–1604. [PubMed] [Google Scholar]
- Rutter M, Pickles A, Murray R, Eaves LJ. Testing hypotheses on specific causal effects on behavior. Psychol Bull. 2001;127:291–324. doi: 10.1037/0033-2909.127.3.291. [DOI] [PubMed] [Google Scholar]
- Scarr S, McCartney K. How people make their own environments: a theory of genotype ⇒ environment effects. Child Dev. 1983;54:424–435. doi: 10.1111/j.1467-8624.1983.tb03884.x. [DOI] [PubMed] [Google Scholar]
- Schafer JL. Analysis of Incomplete Multivariate Data. London: Chapman and Hall; 1997. [Google Scholar]
- Spear LP. The adolescent brain and the college drinker: biological basis of propensity to use and misuse alcohol. J Stud Alcohol Suppl. 2002;14:71–81. doi: 10.15288/jsas.2002.s14.71. [DOI] [PubMed] [Google Scholar]
- Spitzer RL, Williams JBW, Gibbon M. Structured Clinical Interview for DSM-III-R. New York: Biometrics Research Department, New York State Psychiatric Institute; 1987. [Google Scholar]
- Stewart SH, Conrod PJ, Marlatt GA, Comeau MN, Thush C, Krank M. New developments in prevention and early intervention for alcohol abuse in youths. Alcoholism: Clin Exp Res. 2005;29(2):278–286. doi: 10.1097/01.alc.0000153547.34399.e8. [DOI] [PubMed] [Google Scholar]
- Tsuang MT, Lyons MJ, Eisen SA, Goldberg J, True W, Lin N. Genetic influences on DSM-III-R drug abuse and dependence: a study of 3,372 twin pairs. Am J Med Genet (Neuropsychiat Genet) 1996;67(5):473–477. doi: 10.1002/(SICI)1096-8628(19960920)67:5<473::AID-AJMG6>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- White AM, Swartzwelder HS. Hippocampal function during adolescence—A unique target of ethanol effects. Ann NY Acad Sci. 2004;1021:206–220. doi: 10.1196/annals.1308.026. [DOI] [PubMed] [Google Scholar]
- Young SE, Stallings MC, Corley RP, Krauter KS, Hewitt JK. Genetic and environmental influences on behavioral disinhibition. Am J Med Genet (Neuropsychiat Genet) 2000;96(5):684–695. [PubMed] [Google Scholar]