Abstract
Prior research has demonstrated that antisocial behavior, substance-use disorders, and personality dimensions of aggression and impulsivity are indicators of a highly heritable underlying dimension of risk, labeled externalizing. Other work has shown that individual trait constructs within this psychopathology spectrum are associated with reduced self-monitoring, as reflected by amplitude of the error-related negativity (ERN) brain response. In this study of undergraduate subjects, reduced ERN amplitude was associated with higher scores on a self-report measure of the broad externalizing construct that links these various indicators. In addition, the ERN was associated with a response-locked increase in anterior theta (4–7 Hz) oscillation; like the ERN, this theta response to errors was reduced among high-externalizing individuals. These findings suggest that neurobiologically based deficits in endogenous action monitoring may underlie generalized risk for an array of impulse-control problems.
Recent research examining patterns of diagnostic co-morbidity in community-epidemiological samples indicates that conduct disorder in children, antisocial behavior in adults, and substance-use disorders—along with personality traits related to behavioral disinhibition—are indicators of a common underlying vulnerability factor, labeled externalizing (Krueger, 1999; Krueger, McGue, & Iacono 2001). Behavior genetic research has revealed that this shared externalizing factor is highly (>80%) heritable (Krueger et al., 2002), suggesting that general vulnerability toward the development of impulse-control problems has a strong neurobiological basis. Clarifying the brain mechanisms that underlie this broad vulnerability will be an important step toward a more complete understanding of this spectrum of high-impact disorders. Prior work has revealed that reduced amplitude of the P300 brain potential is related to the diagnostic (Costa et al., 2000) and personality-trait (Justus, Finn, & Steinmetz, 2001) indicators of externalizing, and recent research has established a direct association between reduced P300 and the broad externalizing factor that links these various indicators (Patrick et al., 2006). The present study was designed to further elucidate the neurobiological underpinnings of the externalizing psychopathology factor by examining its association with a brain measure that has clear functional relevance to impulse-control problems, namely, the error-related negativity (ERN).
The adult externalizing construct originally emerged through factor analyses of psychiatric disorders in epidemiological samples (Krueger, 1999). This work revealed that two broad, higher-order psychopathology factors underpin the most commonly occurring mental disorders. One factor, labeled internalizing, is associated with anxiety and unipolar mood disorders; the other factor, labeled externalizing, accounts for the covariance among childhood conduct problems, adult antisocial behavior, and substance-use disorders. Subsequent research suggested that basic personality dimensions such as aggressiveness and lack of behavioral constraint may also be markers of externalizing vulnerability (Krueger, 2002; Krueger et al., 2001; Lynam, Leukefeld, & Clayton, 2003). Interestingly, the covariance among these diverse diagnostic and personality indicators is highly heritable (Kendler, Prescott, Myers, & Neale, 2003; Krueger et al., 2002; Young, Stallings, Corley, Krauter, & Hewitt, 2000), suggesting that there is a coherent genetic basis to externalizing. Recent work has also focused on developing a comprehensive quantitative model of the externalizing spectrum; this line of research aims to extend existing nosological frameworks by including a broad range of psychopathological symptoms and normal-range personality traits as indicators of the externalizing construct (Krueger, Markon, Patrick, Benning, & Kramer, in press; Krueger, Markon, Patrick, & Iacono, 2005). This effort has led to the development of the Externalizing Inventory (Krueger et al., in press), a self-report instrument designed to systematically index externalizing vulnerability via empirically derived behavioral and personality-trait indicators.
A common behavioral pattern reflected in many manifestations of externalizing is an apparent failure to learn from experience—harmful behaviors are repeated continually despite an awareness of negative consequences for self or others. This raises the possibility that externalizing may involve a deficit in the ability to self-monitor ongoing behavior for errors or situationally inappropriate actions. Prior research suggests that a reliable brain index of this self-monitoring process is the ERN (Falkenstein, Hohnsbein, & Hoormann, 1991; Gehring, Goss, Coles, Meyer, & Donchin, 1993), a response-locked negative deflection of the brain event-related potential that is observed following errors in laboratory performance tasks. The ERN typically peaks within 100 ms of the commission of an error and has a frontocentral scalp distribution. Evidence indicates that the primary neural generator of the ERN is the anterior cingulate cortex (Dehaene, Posner, & Tucker, 1994), a brain structure that is widely believed to be involved in self-monitoring and behavioral regulation (Bush, Luu, & Posner, 2000). The ERN is thought to reflect activation of the brain’s mechanism for on-line monitoring of its own performance, either through detection of errors (Falkenstein, Hoormann, Christ, & Hohnsbein, 2000) or through response conflict (Carter et al., 1998). The ERN response is maximal when accuracy is emphasized over speed in task instructions and has been linked to several indices of behavioral compensation for errors; for example, greater ERN amplitude is associated with less forceful error responses, higher probability of error correction, and greater slowing of response on trials following errors (Gehring et al., 1993). On the basis of this evidence, Gehring et al. postulated that the ERN provides input to multiple brain systems that act to inhibit or correct errors in progress, as well as prevent future errors via enhanced cognitive control strategies.
Interestingly, recent research has revealed that both states and traits related to behavioral disinhibition are associated with reduced amplitude of the ERN response. For instance, with respect to disinhibitory states, ERN amplitude is reduced during acute alcohol intoxication (Ridderinkhof et al., 2002). With regard to trait variables, Dikman and Allen (2000) reported that individuals scoring low (i.e., bottom 3% of a large prescreened sample) on Gough’s (1960) socialization scale (reflecting high levels of rebelliousness, aggression, and impulsivity) exhibited reduced ERN amplitude during the punishment-avoidance condition of an experimental task, relative to a reward phase. Similarly, Pailing and Segalowitz (2004) reported that individuals scoring low on a measure of the Big Five personality dimension of Conscientiousness (a construct that reflects tendencies toward dutifulness, responsibility, and reliability) showed reduced ERN amplitude in response to errors that were not relevant to reward contingencies—a result suggesting reduced self-monitoring when explicit incentives were absent.
Recent work has also revealed associations between reduced ERN amplitude and other indices of disinhibitory personality, including impulsiveness (Pailing, Segalowitz, Dywan, & Davies, 2002; Potts, George, Martin, & Barratt, 2006) and Eysenck’s psychoticism dimension (Santesso, Segalowitz, & Schmidt, 2005). Thus, individuals who are low in socialization or high in psychoticism (traits related to antisocial behavior), who are low in Conscientiousness or high in impulsiveness (traits related to a lack of behavioral self-control), or who are acutely intoxicated with alcohol tend to show diminished neural responses to errors. In contrast, obsessive-compulsive disorder (which is marked by tendencies toward rumination and excessive self-monitoring) is positively associated with ERN amplitude (Gehring, Himle, & Nisenson, 2000). ERN amplitude is associated negatively, rather than positively, with the broad personality factor of negative emotionality (Luu, Collins, & Tucker, 2000), which encompasses traits of aggression and alienation, as well as trait anxiousness (Patrick, Curtin, & Tellegen, 2002). This finding is notable because negative emotionality (in particular, its aggression and alienation facets) shows a reliable positive association with the externalizing construct (Krueger, Caspi, Moffitt, Silva, & McGee, 1996).
Collectively, these studies point to a connection between impaired self-monitoring, as evidenced by reduced amplitude of the ERN, and a variety of constructs related to externalizing. This raises the possibility that reduced ERN is in fact related to the broad externalizing vulnerability that links these traits, rather than to the discrete trait indicators themselves. However, a definitive link has yet to be established between ERN response and the broad externalizing factor itself, as opposed to discrete trait indicators of externalizing. Thus, the major aim of the present study was to directly examine the relation between externalizing and ERN response in subjects selected to be either low or high on the broad externalizing factor as indexed by the newly developed Externalizing Inventory. We also examined the relation between externalizing and brain-wave oscillations associated with the ERN that follow the commission of errors. We performed these analyses on the basis of recent evidence that a brief increase in anterior theta (4–7 Hz) wave amplitude underlies the ERN response (Bernat, Williams, & Gehring, 2005; Luu, Tucker, & Makeig, 2004;however, see Yordanova, Falkenstein, Hohnsbein, & Kolev, 2004). An additional benefit of this type of analysis is that it allows the isolation of variance that is unique to the discrete theta-wave response from separate sources of slow-wave activity (e.g., motor response potential, stimulus processing) that might overlap in time with the ERN component. On the basis of prior research, we hypothesized that individuals high in externalizing would show significant reductions in both ERN and anterior theta response relative to individuals low in externalizing.
METHOD
Subjects
Subjects in this study were selected from a larger sample of undergraduate students (N = 1,637) in introductory psychology courses at the University of Minnesota who completed the Externalizing Inventory as a prescreening instrument. Individuals falling within the lowest and highest quartiles of the distribution of scores on this inventory were oversampled to form low-externalizing and high-externalizing groups for inclusion in our primary statistical analyses. In addition, individuals falling within the middle 50% of scores on the Externalizing Inventory were sampled to allow supplementary correlational analyses in which the full distribution of scores was represented. Ninety-five subjects were recruited; 3 individuals were excluded from the analyses because of recording-equipment malfunction. Thus, the final sample for this study consisted of 92 subjects: 36 (12 male) in the high-externalizing group, 28 (9 male) in the low-externalizing group, and 28 (13 male) in the intermediate group.
Measures
The Externalizing Inventory (Krueger et al., in press) is a 415-item self-report measure of externalizing, developed to assess a broad range of behavioral and personality characteristics associated with the externalizing spectrum of psychopathology. It includes 23 subscales designed to measure facet-level indicators of the externalizing construct, including physical-relational-destructive aggression, boredom proneness, irresponsibility, problematic impulsivity, drug and alcohol use or problems, theft, fraud, rebelliousness, alienation, and blame externalization. The measure was developed across three waves of data collection in large samples (total N = 1,787) drawn from undergraduate and prison populations. For purposes of large-scale prescreening in the present study, we utilized a subset of the full inventory consisting of the 100 items that possessed the strongest psychometric properties across all three rounds of data collection. Scores on the 100-item measure are highly correlated (r = .98) with scores on the full 415-item Externalizing Inventory. As a check on the construct validity of this 100-item measure, we also administered the following self-report measures that have conceptual or empirical links to externalizing: the Multidimensional Personality Questionnaire–Brief Form (MPQ-BF; Patrick et al., 2002); the Socialization scale (Gough, 1960); the Behavior Report on Rule-Breaking, a self-report measure of adolescent and adult antisocial behaviors composed of items from several other published measures (Clark & Tifft, 1966; Hindelang, Hirschi, & Weis, 1981; Nye & Short, 1957); the Alcohol Dependence Scale (H.A. Skinner & Allen, 1982); and the Short Drug Abuse Screening Test (A. Skinner, 1982).
Procedure
The experimental task was a modified version of the Eriksen flanker task (Eriksen & Eriksen, 1974) in which subjects made right- or left-hand button-press responses to indicate the middle letter in a five-letter stimulus array. Target characters consisted of the letters S or H; distracting flanker letters were either congruent or incongruent with the center target, resulting in a set of four target arrays that occurred with equal frequency across the task as a whole: “SSSSS,” “HHHHH,” “SSHSS,” and “HHSHH.” Subjects were instructed to respond to the target letter S with one hand and the target letter H with the opposite hand. Button-press responses were registered on a response box positioned on the subject’s lap. To enhance the difficulty of the task and thereby ensure a sufficient number of errors, we presented nontarget stimulus arrays (in which the center letter was an X, and subjects made no response) on 14% of the trials. On 50% of these no-go trials, the flanking letters were congruent with the center X (i.e., “XXXXX”), and on the other 50%, the flanking letters were incongruent (i.e., “SSXSS” and “HHXHH”).
Each stimulus array was presented for 150 ms, followed by a 1,000-ms response window; responses that occurred prior to stimulus offset were not recorded. A fixation point appeared in the center of the screen throughout each intertrial interval (ITI). ITIs varied in duration from 1,500 to 2,500 ms, with a mean of 2,000 ms. Subjects were instructed to respond as quickly and accurately as possible to each target array. They were also instructed that they could self-correct any errors aside from errors of commission on no-go trials. Prior to beginning the task, each subject completed a series of 40 practice trials. The main task consisted of six blocks of 100 trials each, for a total of 600 trials. Short breaks were included between blocks, and during each break, on-screen feedback regarding cumulative accuracy was provided (“Your performance so far: N% correct”). To make the task more difficult and thereby further enhance the frequency of errors, we reversed hand-letter assignment following the completion of each block of trials; initial hand-letter assignment was counterbalanced across subjects.
Psychophysiological Data Acquisition and Reduction
Electrodes for recording electroencephalographic (EEG) signals were placed at 64 scalp lead sites according to the guidelines of the International 10–20 system. Electrodes were also placed above and below the left eye to monitor ocular activity. Impedances were kept below 10 kΩ. All EEG signals were digitized on-line at 1000 Hz, then epoched off-line from 500 ms before response onset to 1,000 ms after response, rereferenced to linked mastoids, and resampled to 128 Hz. Trial-level EEG data were then corrected for ocular and movement artifacts. A 1-Hz high-pass filter was applied to trial-level data to reduce the effect of slow-wave motor potentials that can contaminate response-locked brain potentials. Data were then averaged across trials within condition (correct vs. error trials) for the purpose of ERN and time-frequency analyses.
The ERN was defined as the maximum negative-voltage peak, relative to a −250– to −50-ms preresponse baseline, that occurred within a window beginning with the onset of an incorrect button-press response and terminating 200 ms after the response. To further investigate the nature of the ERN response, we also performed a principal-components analysis of time-frequency brain-response data to quantify changes in EEG activity occurring at various frequencies across time (for a detailed description of this method, including illustrations of applicability to ERN data, see Bernat et al., 2005). This method is designed to disaggregate brain-wave responses that oscillate at different frequencies, so that these distinct EEG signals can be analyzed separately.
RESULTS
Questionnaire Data
Correlations between the 100-item Externalizing Inventory and self-report criterion measures are presented in Table 1. Evidence of criterion-related validity was demonstrated by robust positive associations between externalizing scores and measures of antisocial behavior (in children and adults), alcohol dependence, and illicit drug abuse (cf. Krueger et al., 2002). In addition, higher externalizing scores were associated with lower scores for constraint and higher scores for negative emotionality on the MPQ-BF (cf. Krueger et al., 1996) and lower scores on socialization.
TABLE 1.
Correlations Between Scores on the 100-Item Externalizing Inventory and Criterion Measures
| Measure | n | r |
|---|---|---|
| MPQ | ||
| Positive Emotionality | 80 | .04 |
| Negative Emotionality | 80 | .74 |
| Constraint | 80 | −.54 |
| Socialization scale | 91 | −.61 |
| Behavior Report on Rule-Breaking | ||
| Total | 91 | .83 |
| Adult | 91 | .75 |
| Adolescent | 91 | .76 |
| Alcohol Dependence Scale | 91 | .64 |
| Short Drug Abuse Screening Test | 90 | .61 |
Note. MPQ = Multidimensional Personality Questionnaire.
Behavioral Data
Mean reaction time (RT) and accuracy data are presented in Table 2. Behavioral data were missing for 2 subjects because of equipment failure; thus, 90 subjects were included in these analyses. There were no differences in accuracy or RT between the high- and low-externalizing groups (Fs < 1); furthermore, correlations between continuous externalizing scores and accuracy, r(88) = −.14, and RT, r(88) = .05, were negligible. Flanker interference effects on task performance were observed, as indicated by a main effect of stimulus type (congruent vs. incongruent) on overall accuracy, F(1, 89) = 5.34, p = .023, prep = .92, , reflecting reduced accuracy on incongruent trials. For RT, there was a Stimulus Type × Response Accuracy (error vs. correct) interaction, F(1, 77) = 45.66, p <.001, prep > .99, , reflecting longer RTs for incongruent stimuli on correct trials, t(89) = −18.04, p < .001, prep > .99, d = −0.62, and shorter RTs for incongruent stimuli on error trials, t(77) = 3.87, p <.001, prep > .99, d = 0.48. Overall, RTs were longer on error trials than on correct trials, t(89) = −6.93, p <.001, prep > .99, d = −0.63.1 None of these effects interacted with externalizing group (Fs < 1).
TABLE 2.
Task Performance Measures by Condition and Externalizing Group
| Reaction time (ms)
|
Accuracy
|
|||
|---|---|---|---|---|
| Group and condition | Error trials | Correct trials | Total errors | % correct |
| High-externalizing group | ||||
| Congruent | 728 (113) | 622 (73) | 9.0 (8.7) | 89.8 (8.0) |
| Incongruent | 677 (126) | 672 (72) | 10.9 (13.6) | 88.3 (10.4) |
| Low-externalizing group | ||||
| Congruent | 741 (171) | 618 (73) | 7.1 (8.2) | 91.9 (7.8) |
| Incongruent | 661 (140) | 662 (75) | 9.1 (13.4) | 90.0 (9.3) |
Note. Standard deviations are given in parentheses.
Brain Response Data
ERN Peak Scores
Figure 1a presents average response-locked waveforms recorded at FCz for the high- and low-externalizing groups. The ERN is evident as a sharp negative deflection in the error waveform that peaks approximately 50 ms post-response. The scalp topography map in Figure 1b reveals that ERN amplitude was maximal at FCz; this finding is consistent with a medial-frontal source within the brain.
Fig. 1.
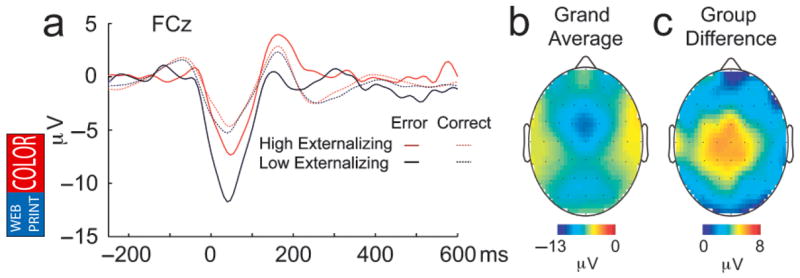
Response-locked brain potential responses to correct and error trials. The graph (a) displays waveforms for correct and error trials for the high- and low-externalizing groups separately; 0 ms marks the time of the button-press response. The topographical maps present the scalp distribution of the error-related negativity (ERN), computed using data from all 64 electrodes; the front of the head is at the top of the illustrations. The map in (b) presents the distribution of the grand-average difference waveform for error trials minus correct trials, and the map in (c) presents the distribution of group differences (high-externalizing group minus low-externalizing group) in the amplitude of the difference waveform.
Inspection of the scalp distribution illustrated in Figure 1c revealed that externalizing-group differences in ERN amplitude were greatest at frontocentral electrodes. To test for group differences in ERN amplitude at FCz, we conducted a 2 (response accuracy: error vs. correct) × 2 (externalizing group: high vs. low) repeated measures analysis of variance (ANOVA). There was a robust main effect of response accuracy, F(1, 62) = 53.29, p < .001, prep >.99, ηp2 = .462, reflecting greater negativity on error than on correct trials. A Response Accuracy × Externalizing Group interaction, F(1, 62) = 8.06, p = .006, prep = .96, ηp2 = .115, revealed that ERN amplitude was reduced in the high-externalizing group, and that this effect was specific to error trials, as illustrated in Figure 2. This effect was also reflected in the correlation between ERN peak amplitude and continuous externalizing scores across the sample as a whole on error trials, r(90) = .29, p = .006, prep = .96; the effect was nonsignificant for correct trials, r(90) = .08, p = .473, prep = .52.
Fig. 2.
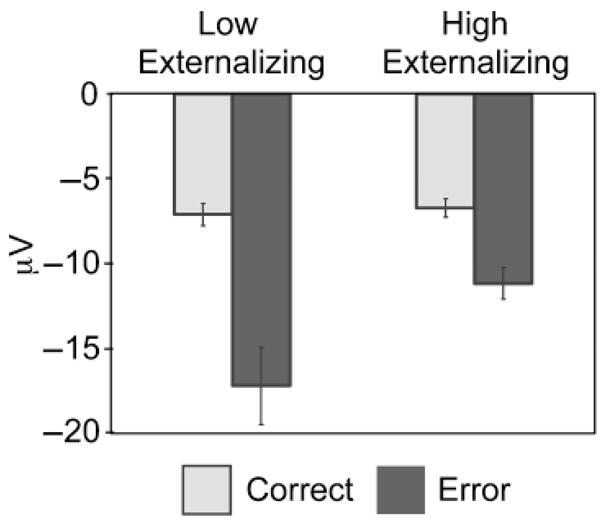
Average peak amplitudes of brain potential responses to correct and error trials at electrode FCz. Results are shown separately for the low- and high-externalizing groups. The data presented are average peak values ±1 SE.
Time-Frequency Component Scores
Results of the time-frequency analysis of averaged error-trial data are presented in Figure 3. As shown in Figure 3a, oscillatory brain responses to errors were characterized primarily by an increase in power within the theta (4–7 Hz) frequency band that peaked approximately 50 to 75 ms following incorrect responses. Following the methods outlined by Bernat et al. (2005), we further decomposed these data using principal-components analysis. Inspection of the resulting scree plot (Fig. 3b) indicated that a four-component solution best accounted for the observed patterns of covariance in the time-frequency data. The second component (PC-2; see Fig. 3c) reflected the unique variance related to an increase in anterior theta power, which was maximal at approximately 6 Hz. This theta component accounted for nearly 64% of variance in peak ERN amplitude. Furthermore, the peak of this response-locked increase in theta energy coincided in time with the ERN and had a similar frontocentral scalp maximum (see Fig. 3c), strongly suggesting that this component was the time-frequency representation of the ERN.
Fig. 3.
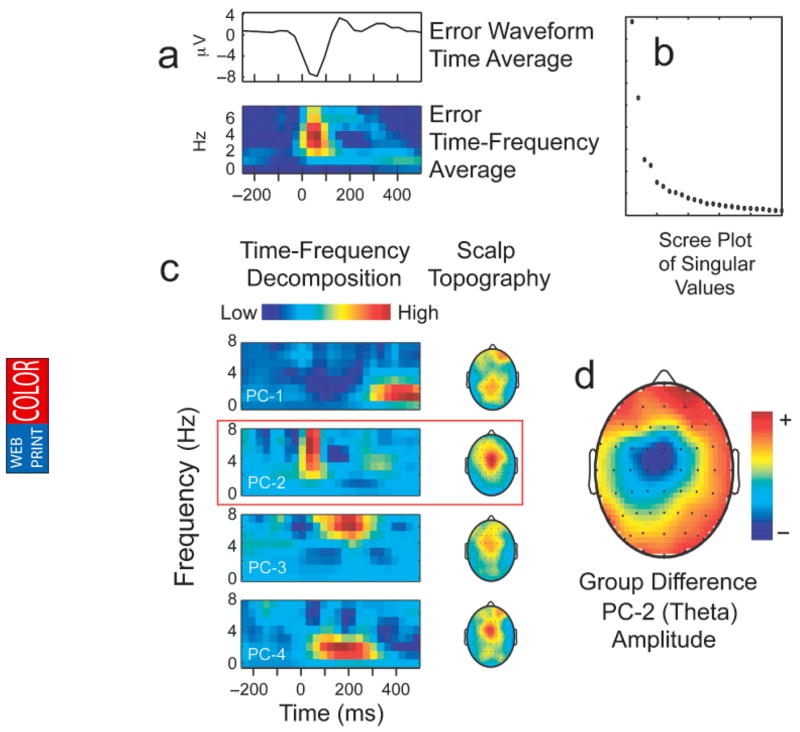
Decomposition of the time-frequency brain responses to errors via principal-component analysis: (a) the grand-average time waveform for error trials and the average time-frequency data surface, (b) the scree plot used to determine the number of components for extraction, (c) results of the four-component solution and scalp topographies of the time-frequency components, and (d) the scalp topography of externalizing-group differences (high-externalizing group minus low-externalizing group) in PC-2 amplitude.
Externalizing-group differences in theta power were maximal at frontocentral electrodes, as illustrated in Figure 3d. A one-way ANOVA was conducted to test for group differences in the magnitude of theta response to errors at FCz. As predicted, theta activity was attenuated in the high-externalizing group, F(1, 62) = 5.52, p = .022, prep = .92, ηp2 = .082. In the sample as a whole, there was a negative correlation between theta power and externalizing, r(90) = −.24, p = .022, prep = .92.
DISCUSSION
Externalizing is a broad trait-dispositional factor that reflects disinhibitory personality and proneness to an array of impulse-control problems. Previous work has demonstrated that a number of conceptually related trait constructs are associated with reduced ERN amplitude. In the present study, we found evidence of a link between reduced ERN response and a measure of the broad externalizing factor that unites these more narrowly defined traits. The implication is that constructs that have previously been linked to reduced ERN (e.g., socialization, psychoticism, Conscientiousness, impulsivity) may tap a common underlying neurobiological process that is associated more generally with externalizing vulnerability.
What is the nature of this underlying neurobiological process? The ERN has been construed primarily as a reflection of the brain’s mechanism for detecting errors in behavior and is most likely centered in the anterior cingulate cortex (Dehaene et al., 1994). This process presumably plays a role in behavioral regulation by signaling other brain regions of the need for enhanced executive control and attentional deployment when errors are made (Gehring et al., 1993). A deficit in this mechanism would have clear functional relevance for externalizing, insofar as impairment in the brain’s error-detection network might impede the learning process by which future errors are avoided. The consequence of such an impairment would be a tendency to continually repeat maladaptive or harmful mistakes, especially in the absence of explicit reinforcement contingencies, when such internal sources of response feedback are most critical.
Alternatively, it has recently been suggested that the ERN (and the anterior cingulate activity with which it is associated) reflects an internal conflict-monitoring process that is initiated when mutually incompatible response pathways become activated simultaneously (Carter et al., 1998). This perspective is supported by neuroimaging research demonstrating increased anterior cingulate activity during correct responses in tasks that involve a high degree of conflict, such as the continuous-performance task (Carter et al., 1998). The conflict-detection system proposed by Carter et al., rather than detecting errors in performance per se, acts as an early warning system that signals other brain regions of the need for enhanced cognitive control under conditions of heightened response conflict, when errors are most likely to occur. A deficit in such a mechanism might give rise to externalizing tendencies via a decreased sensitivity to subtle response conflict (and subsequent failure to engage cognitive control), rather than a reduced capacity to recognize errors during on-line processing and adapt behavior accordingly. However, it is noteworthy that despite an apparent neural deficit in self-monitoring, high-externalizing subjects were able to maintain a level of task performance that was equivalent to that of the low-externalizing group for both congruent (low-conflict) and incongruent (high-conflict) stimuli. This may have been the case because the flanker task, which is perceptually and conceptually straightforward, does not place sufficient demands on processing resources for complex executive-control strategies or behavioral adjustments to be required for adequate performance. Given a more complex experimental task, or under real-world conditions, deficits in self-monitoring may have more substantial consequences for performance.
Another notable finding from the present study concerns self-monitoring as measured via the anterior theta oscillation. Prior work has suggested that the ERN reflects a brief increase in an ongoing frontal theta oscillation (Bernat et al., 2005), perhaps representing the moment of maximum phase-locking of this brain-wave component, that is, the point at which disparate neural generators of theta activity become maximally synchronized in response to a discrete event. It has previously been suggested that theta activity is associated with a distributed action-regulation system that includes the hippocampus, thalamus, and cingulate cortex (Luu, Tucker, Derryberry, Reed, & Poulsen, 2003). Data from the present study replicated prior findings indicating that the ERN is associated with a phase-locked increase in midline-frontal theta; in addition, this theta response was reduced among high-externalizing subjects.
One important question that remains to be addressed in future research concerns the potential moderating impact of motivation on the relation between self-monitoring and externalizing. Recent work has demonstrated that the ERN response is sensitive to experimental manipulations that vary the motivational salience of errors (Hajcak, Moser, Yeung, & Simons, 2005). This raises the possibility that high-externalizing individuals may show reduced ERN primarily in the absence of extrinsic (e.g., monetary) motivation to perform well, which would accord with prior research regarding trait indicators of externalizing and error monitoring under varying motivational conditions (Dikman & Allen, 2000; Pailing & Segalowitz, 2004). Another intriguing question is whether reduced ERN might represent a physiological trait marker or endophenotype of externalizing vulnerability, as P300 reduction does (Patrick et al., 2006). For instance, would reduced ERN manifest itself in the unaffected relatives of high-externalizing individuals, or would this feature be evident only in the context of active psychopathology? Future research in genetically informative (twin) samples will help to address this issue. Furthermore, extension of the present research to clinical samples, including incarcerated offenders exhibiting extreme levels of impulsivity and aggression, should provide further insights into the pathophysiology of externalizing.
In conclusion, the present study found that externalizing vulnerability, which represents generalized risk for aggression, antisocial behavior, substance-use problems, and a disinhibited personality style, is related to a brain-based deficit in on-line self-monitoring of behavior. In this context, the externalizing construct provides a useful conceptual framework for interpreting results from previous studies that have demonstrated links between discrete trait markers of the broad externalizing dimension and impaired self-monitoring, as measured by the ERN. Thus, the present findings shed additional light on the neurobiological underpinnings of this important and costly spectrum of psychopathology.
Acknowledgments
This research was supported by Grants MH65137, MHO72850, MH17069, and AA12164 from the National Institutes of Health and by funds from the Hathaway Endowment at the University of Minnesota. Jason R. Hall conducted this work in partial fulfillment of thesis requirements for his master’s degree. Preliminary findings were presented at the 45th Annual Meeting of the Society for Psychophysiological Research, Lisbon, Portugal, September 2005. We thank Benjamin Steffen, Noah Venables, Meredith Cadwallader, William Schoeppner, Jiyon Kim, and Hannah Kamke for their invaluable assistance.
Footnotes
Note that degrees of freedom vary across these tests because not all subjects committed errors in each condition; that is, some subjects did not commit errors in the congruent condition (n = 8), whereas others committed no errors in the incongruent condition (n = 4).
References
- Bernat EM, Williams WJ, Gehring WJ. Decomposing ERP time-frequency energy using PCA. Clinical Neurophysiology. 2005;116:1314–1334. doi: 10.1016/j.clinph.2005.01.019. [DOI] [PubMed] [Google Scholar]
- Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulate cortex. Trends in Cognitive Sciences. 2000;4:215–222. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- Carter CS, Braver TS, Barch DM, Botvinick MM, Noll D, Cohen JD. Anterior cingulate cortex, error detection, and the online monitoring of performance. Science. 1998;280:747–749. doi: 10.1126/science.280.5364.747. [DOI] [PubMed] [Google Scholar]
- Clark JP, Tifft LL. Polygraph and interview validation of self-reported deviant behavior. American Sociological Review. 1966;31:516–523. [PubMed] [Google Scholar]
- Costa L, Bauer L, Kuperman S, Porjesz B, O’Connor S, Hesselbrock V, Rohrbaugh J, Begleiter H. Frontal P300 decrements, alcohol dependence, and antisocial personality disorder. Biological Psychiatry. 2000;47:1064–1071. doi: 10.1016/s0006-3223(99)00317-0. [DOI] [PubMed] [Google Scholar]
- Dehaene S, Posner MI, Tucker DM. Localization of a neural system for error detection and compensation. Psychological Science. 1994;5:303–305. [Google Scholar]
- Dikman ZV, Allen JJB. Error monitoring during reward and avoidance learning in high- and low-socialized individuals. Psychophysiology. 2000;37:43–54. [PubMed] [Google Scholar]
- Eriksen B, Eriksen C. Effects of noise letters upon the identification of a target letter in a non-search task. Perception & Psychophysics. 1974;16:143–149. [Google Scholar]
- Falkenstein M, Hohnsbein J, Hoormann J. Effects of crossmodal divided attention on late ERP components: II. Error processing in choice reaction tasks. Electroencephalography and Clinical Neurophysiology. 1991;78:447–455. doi: 10.1016/0013-4694(91)90062-9. [DOI] [PubMed] [Google Scholar]
- Falkenstein M, Hoormann J, Christ S, Hohnsbein J. ERP components on reaction errors and their functional significance: A tutorial. Biological Psychology. 2000;51:87–107. doi: 10.1016/s0301-0511(99)00031-9. [DOI] [PubMed] [Google Scholar]
- Gehring WJ, Goss B, Coles MGH, Meyer DE, Donchin E. A neural system for error detection and compensation. Psychological Science. 1993;4:385–390. [Google Scholar]
- Gehring WJ, Himle J, Nisenson LG. Action-monitoring dysfunction in obsessive-compulsive disorder. Psychological Science. 2000;11:1–6. doi: 10.1111/1467-9280.00206. [DOI] [PubMed] [Google Scholar]
- Gough HG. Theory and measurement of socialization. Journal of Consulting Psychology. 1960;24:23–30. [Google Scholar]
- Hajcak G, Moser JS, Yeung N, Simons RF. On the ERN/Ne and the significance of errors. Psychophysiology. 2005;42:151–160. doi: 10.1111/j.1469-8986.2005.00270.x. [DOI] [PubMed] [Google Scholar]
- Hindelang MJ, Hirschi T, Weis JG. Measuring delinquency. Beverly Hills, CA: Sage; 1981. [Google Scholar]
- Justus AN, Finn PR, Steinmetz JE. P300, disinhibited personality, and early alcohol problems. Alcoholism: Clinical and Experimental Research. 2001;25:1457–1466. doi: 10.1097/00000374-200110000-00008. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Prescott CA, Myers J, Neale MC. The structure of genetic and environmental risk factors for common psychiatric and substance use disorders in men and women. Archives of General Psychiatry. 2003;60:929–937. doi: 10.1001/archpsyc.60.9.929. [DOI] [PubMed] [Google Scholar]
- Krueger RF. The structure of common mental disorders. Archives of General Psychiatry. 1999;56:921–926. doi: 10.1001/archpsyc.56.10.921. [DOI] [PubMed] [Google Scholar]
- Krueger RF. Personality from a realist’s perspective: Personality traits, criminal behaviors, and the externalizing spectrum. Journal of Research in Personality. 2002;36:564–572. [Google Scholar]
- Krueger RF, Caspi A, Moffitt TE, Silva PA, McGee R. Personality traits are differentially linked to mental disorders: A multi-trait/multi-diagnosis study of an adolescent birth cohort. Journal of Abnormal Psychology. 1996;105:299–312. doi: 10.1037//0021-843x.105.3.299. [DOI] [PubMed] [Google Scholar]
- Krueger RF, Hicks BM, Patrick CJ, Carlson SR, Iacono WG, McGue M. Etiologic connections among substance dependence, antisocial behavior, and personality: Modeling the externalizing spectrum. Journal of Abnormal Psychology. 2002;111:411–424. [PubMed] [Google Scholar]
- Krueger RF, Markon KE, Patrick CJ, Benning S, Kramer M. Linking antisocial behavior, substance use, and personality: Toward a comprehensive quantitative model of the adult externalizing spectrum. Journal of Abnormal Psychology. doi: 10.1037/0021-843X.116.4.645. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger RF, Markon KE, Patrick CJ, Iacono WG. Externalizing psychopathology in adulthood: A dimensional-spectrum conceptualization and its implications for DSM-V. Journal of Abnormal Psychology. 2005;114:537–550. doi: 10.1037/0021-843X.114.4.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger RF, McGue M, Iacono WG. The higher-order structure of common DSM mental disorders: Internalization, externalization, and their connections to personality. Personality and Individual Differences. 2001;30:1245–1259. [Google Scholar]
- Luu P, Collins P, Tucker DM. Mood, personality, and self-monitoring: Negative affect and emotionality in relation to frontal lobe mechanisms of error monitoring. Journal of Experimental Psychology: General. 2000;129:43–60. doi: 10.1037//0096-3445.129.1.43. [DOI] [PubMed] [Google Scholar]
- Luu P, Tucker DM, Derryberry D, Reed M, Poulsen C. Electrophysiological responses to errors and feedback in the process of action regulation. Psychological Science. 2003;14:47–53. doi: 10.1111/1467-9280.01417. [DOI] [PubMed] [Google Scholar]
- Luu P, Tucker DM, Makeig S. Frontal-midline theta and the error-related negativity: Neurophysiological mechanisms of action regulation. Clinical Neurophysiology. 2004;115:1821–1835. doi: 10.1016/j.clinph.2004.03.031. [DOI] [PubMed] [Google Scholar]
- Lynam DR, Leukefeld C, Clayton RR. The contribution of personality to the overlap between antisocial behavior and substance use/misuse. Aggressive Behavior. 2003;29:316–331. [Google Scholar]
- Nye FI, Short JF., Jr Scaling delinquent behavior. American Sociological Review. 1957;22:326–331. [Google Scholar]
- Pailing PE, Segalowitz SJ. The error-related negativity as a state and trait measure: Motivation, personality, and ERPs in response to errors. Psychophysiology. 2004;41:84–95. doi: 10.1111/1469-8986.00124. [DOI] [PubMed] [Google Scholar]
- Pailing PE, Segalowitz SJ, Dywan J, Davies PL. Error negativity and response control. Psychophysiology. 2002;39:198–206. doi: 10.1017/S0048577202010247. [DOI] [PubMed] [Google Scholar]
- Patrick CJ, Bernat E, Malone SM, Iacono WG, Krueger RF, McGue M. P300 amplitude as a marker of externalizing in adolescent males. Psychophysiology. 2006;43:84–92. doi: 10.1111/j.1469-8986.2006.00376.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrick CJ, Curtin JJ, Tellegen A. Development and validation of a brief form of the Multidimensional Personality Questionnaire. Psychological Assessment. 2002;14:150–163. doi: 10.1037//1040-3590.14.2.150. [DOI] [PubMed] [Google Scholar]
- Potts GF, George MRM, Martin LE, Barratt ES. Reduced punishment sensitivity in neural systems of behavior monitoring in impulsive individuals. Neuroscience Letters. 2006;397:130–134. doi: 10.1016/j.neulet.2005.12.003. [DOI] [PubMed] [Google Scholar]
- Ridderinkhof KR, de Vlugt Y, Bramlage A, Spaan M, Elton M, Snel J, Band GPH. Alcohol consumption impairs detection of performance errors in mediofrontal cortex. Science. 2002;298:2209–2211. doi: 10.1126/science.1076929. [DOI] [PubMed] [Google Scholar]
- Santesso DL, Segalowitz SJ, Schmidt LA. ERP correlates of error monitoring in 10-year olds are related to socialization. Biological Psychology. 2005;70:79–87. doi: 10.1016/j.biopsycho.2004.12.004. [DOI] [PubMed] [Google Scholar]
- Skinner A. The Drug Abuse Screening Test. Addictive Behaviors. 1982;7:363–371. doi: 10.1016/0306-4603(82)90005-3. [DOI] [PubMed] [Google Scholar]
- Skinner HA, Allen BA. Alcohol dependence syndrome: Measurement and validation. Journal of Abnormal Psychology. 1982;91:199–209. doi: 10.1037//0021-843x.91.3.199. [DOI] [PubMed] [Google Scholar]
- Yordanova J, Falkenstein M, Hohnsbein J, Kolev V. Parallel systems of error processing in the brain. NeuroImage. 2004;22:590–602. doi: 10.1016/j.neuroimage.2004.01.040. [DOI] [PubMed] [Google Scholar]
- Young SE, Stallings MC, Corley RP, Krauter KS, Hewitt JK. Genetic and environmental influences on behavioral disinhibition. American Journal of Medical Genetics (Neuropsychiatric Genetics) 2000;96:684–695. [PubMed] [Google Scholar]


