Abstract
An existing preoperative nomogram predicts the probability of prostate cancer recurrence, defined by prostate-specific antigen (PSA), at 5 years after radical prostatectomy based on clinical stage, serum PSA, and biopsy Gleason grade. In an updated and enhanced nomogram, we have extended the predictions to 10 years, added the prognostic information of systematic biopsy results, and enabled the predictions to be adjusted for the year of surgery. Cox regression analysis was used to model the clinical information for 1978 patients treated by two high-volume surgeons from our institution. The nomogram was externally validated on an independent cohort of 1545 patients with a concordance index of 0.79 and was well calibrated with respect to observed outcome. The inclusion of the number of positive and negative biopsy cores enhanced the predictive accuracy of the model. Thus, a new preoperative nomogram provides robust predictions of prostate cancer recurrence up to 10 years after radical prostatectomy.
Predictive models for recurrence of prostate cancer after definitive therapy are essential for patient counseling, the rational application of neoadjuvant therapy, and clinical trial design. In 1998, we developed a nomogram based on serum prostate-specific antigen (PSA), clinical stage, and biopsy Gleason grade that calculates the 5-year freedom from PSA-defined progression after radical prostatectomy (1). The nomogram has been independently validated in diverse patient populations (2-4). Currently, it is the most widely used disease-specific prediction tool in oncology (5). Nomograms also exist for prostate cancer recurrence after external-beam radiotherapy and brachytherapy (6, 7).
Despite the robust prognostic information contained within the nomogram, it has several limitations. The 5-year progression-free probability overestimates a man's probability of long-term cancer control because a substantial number of men will experience disease recurrence after maintaining an undetectable PSA level for 5 years or more after radical prostatectomy (8). Since 1998, an association between the extent of cancer in systematic prostate biopsy specimens and disease recurrence has been reported (9) . Last, widespread PSA screening has resulted in a stage migration such that patients of a similar grade and stage treated recently have an improved prognosis compared with longer ago (8, 10, 11). We have addressed these limitations in an updated and enhanced model by extending the predictions to 10 years, by including systematic biopsy results, and by enabling the predictions to be adjusted for the year of surgery. The 10-year progression-free probability more closely estimates the likelihood of cure because patients with an undetectable PSA level for 10 years after radical prostatectomy have a long-term progression rate of only 3% (8).
Multivariable Cox proportional hazards regression analysis was used to model the clinical information for patients treated by radical prostatectomy alone for tumor stage 1C–3 prostate cancer by two high-volume surgeons at Baylor College of Medicine (n = 1086) and Memorial Sloan-Kettering Cancer Center (n = 1134) between 1983 and 2002 (12). Complete information was available in 1978 patients pertaining to PSA levels, biopsy Gleason grade, 1992 American Joint Committee on Cancer clinical stage (12), and systemic biopsy results (Table 1). An external validation cohort consisted of 1545 patients who were treated by other surgeons at our institution during this time. The schedule by which patients were followed postoperatively has been described previously (8). Disease progression was defined as a serum PSA value of 0.4 ng/mL or greater (confirmed by a second PSA value higher than the first by any amount), secondary therapy, clinical recurrence, or aborted radical prostatectomy for lymph node metastases (13).
Table 1.
Clinical characteristics of patients in the modeling and validation cohorts*
| Patient characteristics | Modeling cohort, N = 1978 | Validation cohort, N = 1545 |
|---|---|---|
| Year of surgery, n (%) | ||
| 1987–1992 | 294 (15) | 40 (3) |
| 1993–1998 | 649 (33) | 794 (51) |
| 1999–2003 | 1036 (52) | 711 (46) |
| Median preoperative PSA, ng/mL (IQR) | 6.1 (4.4–9.0) | 6.6 (4.7–9.7) |
| 1992 AJCC clinical stage, n (%) | ||
| T1C | 803 (41) | 809 (52) |
| T2A | 509 (26) | 299 (19) |
| T2B | 335 (17) | 307 (20) |
| T2C | 244 (12) | 113 (7) |
| T3 | 88 (4) | 17 (1) |
| Biopsy Gleason grade, n (%) | ||
| Gleason 2–6 | 1348 (68) | 1024 (66) |
| Gleason 7 (3+4) | 397 (20) | 295 (19) |
| Gleason 7 (4+3) | 130 (7) | 130 (8) |
| Gleason 8–10 | 104 (5) | 96 (6) |
| Median positive biopsy cores (IQR) | 2 (2–4) | 2 (1–3) |
| Median total biopsy cores (IQR) | 6 (6–9) | 7 (6–10) |
| Median follow-up, mo (IQR) | 54 (30–96) | 68 (41–103) |
| Follow-up >5/>10 years, n (%) | 976 (49)/391 (18) | 1202 (78)/361 (23) |
| Lost to follow-up at 5/10 years, n (%) | 252 (13)/321 (16) | 297 (19)/307 (20) |
| Prostate cancer-specific death, n (%) | 43 (2.2) | 24 (1.6) |
IQR = interquartile range; AJCC = American Joint Committee on Cancer.
Internal and external validation of the nomogram was performed using methods described previously (1). Predictive accuracy was assessed using the concordance (c-) index (discrimination) (14) and a visual inspection of the plots comparing the predicted probability of recurrence with the observed outcome (calibration). Internal validation was performed using bootstrapping analysis (15). External validation was performed by applying the nomogram to patients in the validation cohort. All statistical analyses were two-sided and were performed using S-Plus software (PC version 3.3, Redmond, WA) with additional functions (called Design) (15).
Disease progression was observed in 220 patients in the modeling cohort, and the 10-year progression-free probability was 77% (95% confidence interval = 73% to 80%). A nomogram containing PSA (P≤.001), clinical stage (P≤.001), primary (P≤.001) and secondary Gleason grade (P≤.001), year of surgery (P≤.001), and number of positive (P = .25) and negative (P = .094) biopsy cores had c-indices of 0.76 and 0.79 in internal and external validation, respectively. The nomogram estimates the 1- to 10-year progression-free probability after radical prostatectomy alone (Fig. 1, A). A model that did not include systematic biopsy results had an inferior predictive accuracy in external validation (c-index = 0.77). The c-index value, which is similar to the area under the receiver operating characteristic curve, indicates that the nomogram performs slightly better than midway between a perfect model and a coin flip (c-indices = 1.0 and 0.5, respectively).
Fig. 1.
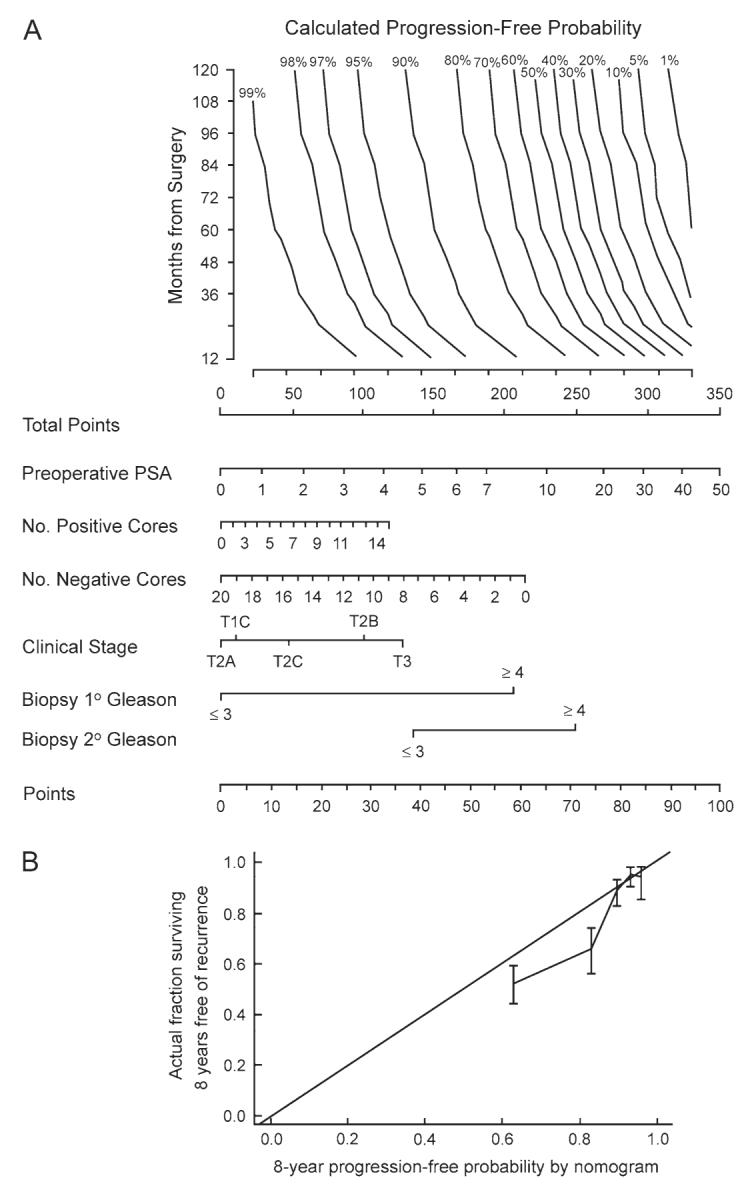
A) Preoperative nomogram estimating the 1- to 10-year progression-free probability after radical prostatectomy alone. B) Calibration plot of the nomogram in external validation. The 45° line represents an ideal model in which estimates of recurrence are perfectly calibrated with outcome. Vertical bars are 95% confidence intervals for quintiles in the validation set.
Instructions for physician: Locate patient's preoperative prostate-specific antigen (PSA) on the Preoperative PSA axis. Draw a straight line down to the Points axis to determine how many points toward disease recurrence that patient receives. Repeat this process for each of the remaining variable axes. Sum the points for each predictor and locate this sum on the Total Points axis. Draw a line straight up from the Total Points axis until it intersects with the horizontal line drawn from Months from Surgery, corresponding to the time point in the future within which the risk of recurrence is wished to be calculated. The progression-free probability can be estimated at 12 to 120 months after radical prostatectomy. The slanted vertical line that crosses this intersection point corresponds to the calculated progression-free probability within that point in time after radical prostatectomy.
Instructions for patient: Mr. X, if we had 100 men exactly like you, we would predict <predicted probability from nomogram×100> percent to remain free of disease progression at <specified months from surgery> following radical prostatectomy alone.
The model exhibited good calibration across the spectrum of predictions in internal validation (data not shown) but exhibited some optimism in external validation; patients with an 8-year progression-free probability between 70% and 85% had an observed rate of freedom from progression of 57%–72% (Fig. 1, B). Part of the optimism may be explained by the fact that the model was developed on patients treated by high-volume surgeons and that the validation cohort was managed by both high- and low-volume surgeons. We have previously reported that positive surgical margins, which are associated with a threefold increased risk of recurrence, are influenced by surgeon volume and individual technique (16, 17). Surgeon volume may also be an independent predictor of recurrence (18).
An added feature of the nomogram is the ability to estimate the probability of recurrence at any point in time from 1 to 10 years after radical prostatectomy. The ability to predict the risk of early recurrence may be important for neoadjuvant treatment strategies, because disease recurrence within 2–3 years of radical prostatectomy is associated with an increased risk of metastasis progression and cancer-specific mortality (19, 20).
The nomogram predictions are adjusted for year of surgery, enabling the model to account for the improved prognosis of patients treated more recently. Of note, the nomogram assumes the patient is treated in 2003 and thus, theoretically, its accuracy for patients treated after 2003 is uncertain. However, we previously demonstrated that the improved prognosis associated with year of surgery is most pronounced for the years prior to 1998 and the hazard ratio is similar for the years 2000–2003 (10). Thus, the nomogram predictions are likely to be valid for patients treated after 2003. The current nomogram is most appropriate to use in regions where PSA screening is widespread (21). The original nomogram may be better suited for use in non-PSA-screened patients given that it has been extensively validated in these populations (1, 2).
In a previous study, the percentage of positive biopsy cores did not improve the accuracy of the original nomogram (22). The addition of the number of positive and negative biopsy cores to the current nomogram modestly increased its predictive accuracy. Neither parameter was statistically significantly associated with recurrence in the model, but statistical significance is not a prerequisite for improved outcome prediction (23). We previously demonstrated that the length of cancer and noncancer in biopsy cores substantially enhanced the predictive accuracy of a nomogram for indolent prostate cancer (24). Incorporation of these parameters into future nomograms is anticipated.
Acknowledgments
Supported in part by funds from National Cancer Institute grant CA-92629 SPORE in prostate cancer and by a gift from the Leon Lowenstein Foundation. A. J. Stephenson is supported in part by the American Foundation for Urologic Disease and by National Institutes of Health grant T32 CA-82088.
The funding agencies had no role in the study design, data collection, analysis, or interpretation of the data.
M. W. Kattan is a founder and the chairman of the scientific advisory board for Oncovance Technologies, the company that developed the predictive nomogram for 5-year freedom from PSA-defined progression of prostate cancer after radical prostatectomy.
Footnotes
This study received institutional review board approval and was conducted according to Health Insurance Portability and Accountability Act guidelines.
This study was presented in part at the 2005 American Urological Association Annual Meeting, May 21–26, in San Antonio, TX.
Nomogram software for personal computers and personal digital assistants is available in the public domain for free download at http://www.nomograms.org.
References
- 1.Kattan MW, Eastham JA, Stapleton AM, Wheeler TM, Scardino PT. A preoperative nomogram for disease recurrence following radical prostatectomy for prostate cancer. J Natl Cancer Inst. 1998;9:766–71. doi: 10.1093/jnci/90.10.766. [DOI] [PubMed] [Google Scholar]
- 2.Graefen M, Karakiewicz PI, Cagiannos I, Quinn DI, Henshall SM, Grygiel JJ, et al. International validation of a preoperative nomogram for prostate cancer recurrence after radical prostatectomy. J Clin Oncol. 2002;20:3206–12. doi: 10.1200/JCO.2002.12.019. [DOI] [PubMed] [Google Scholar]
- 3.Bianco FJ, Jr, Kattan MW, Scardino PT, Powell IJ, Pontes JE, Wood DP., Jr Radical prostatectomy nomograms in black American men: accuracy and applicability. J Urol. 2003;170:73–6. doi: 10.1097/01.ju.0000068037.57553.54. discussion 76–7. [DOI] [PubMed] [Google Scholar]
- 4.Greene KL, Meng MV, Elkin EP, Cooperberg MR, Pasta DJ, Kattan MW, et al. Validation of the Kattan preoperative nomogram for prostate cancer recurrence using a community based cohort: results from cancer of the prostate strategic urological research endeavor (capsure) J Urol. 2004;171(6 Pt 1):2255–9. doi: 10.1097/01.ju.0000127733.01845.57. [DOI] [PubMed] [Google Scholar]
- 5.Blumberg JW. PDA applications for physicians. ASCO News. 2004;16:S4–6. [Google Scholar]
- 6.Kattan MW, Zelefsky MJ, Kupelian PA, Scardino PT, Fuks Z, Leibel SA. Pretreatment nomogram for predicting the outcome of three-dimensional conformal radiotherapy in prostate cancer. J Clin Oncol. 2000;18:3352–9. doi: 10.1200/JCO.2000.18.19.3352. [DOI] [PubMed] [Google Scholar]
- 7.Kattan MW, Potters L, Blasko JC, Beyer DC, Fearn P, Cavanagh W, et al. Pretreatment nomogram for predicting freedom from recurrence after permanent prostate brachytherapy in prostate cancer. Urology. 2001;58:393–9. doi: 10.1016/s0090-4295(01)01233-x. [DOI] [PubMed] [Google Scholar]
- 8.Stephenson AJ, Scardino PT, Eastham JA, Bianco FJ, Jr, Dotan ZA, Diblasio CJ, et al. Postoperative nomogram predicting the 10-year probability of prostate cancer recurrence after radical prostatectomy. J Clin Oncol. 2005;23:7005–12. doi: 10.1200/JCO.2005.01.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.D'Amico AV, Whittington R, Malkowicz SB, Wu YH, Chen MH, Hurwitz M, et al. Utilizing predictions of early prostate-specific antigen failure to optimize patient selection for adjuvant systemic therapy trials. J Clin Oncol. 2000;18:3240–6. doi: 10.1200/JCO.2000.18.18.3240. [DOI] [PubMed] [Google Scholar]
- 10.Cagiannos I, Karakiewicz P, Graefen M, Eastham JA, Ohori M, Rabbani F, et al. Is year of radical prostatectomy a predictor of outcome in prostate cancer? J Urol. 2004;171(2 Pt 1):692–6. doi: 10.1097/01.ju.0000107260.98031.0e. [DOI] [PubMed] [Google Scholar]
- 11.Albertsen PC, Hanley JA, Barrows GH, Penson DF, Kowalczyk PD, Sanders MM, et al. Prostate cancer and the Will Rogers phenomenon. J Natl Cancer Inst. 2005;97:1248–53. doi: 10.1093/jnci/dji248. [DOI] [PubMed] [Google Scholar]
- 12.Bears OH. American Joint Committee on Cancer manual for cancer staging. 4th ed. Lippincott; Philadelphia (PA): 1992. [Google Scholar]
- 13.Stephenson AJ, Scardino PT, Eastham JA, Lilja H, Bianco FJ, Jr, Dotan ZA, et al. Defining biochemical recurrence of prostate cancer after radical prostatectomy: a proposal for a standardized definition. J Urol. 2005;173(4 Suppl):183. doi: 10.1200/JCO.2005.04.0756. abstract 671. [DOI] [PubMed] [Google Scholar]
- 14.Harrell FE, Jr, Califf RM, Pryor DB, Lee KL, Rosati RA. Evaluating the yield of medical tests. JAMA. 1982;247:2543–6. [PubMed] [Google Scholar]
- 15.Efron B, Tibshirani RJ. An introduction to the bootstrap. Chapman & Hall; New York (NY): 1993. [Google Scholar]
- 16.Eastham JA, Kattan MW, Riedel E, Begg CB, Wheeler TM, Gerigk C, et al. Variations among individual surgeons in the rate of positive surgical margins in radical prostatectomy specimens. J Urol. 2003;170(6 Pt 1):2292–5. doi: 10.1097/01.ju.0000091100.83725.51. [DOI] [PubMed] [Google Scholar]
- 17.Swindle P, Eastham JA, Ohori M, Kattan MW, Wheeler T, Maru N, et al. Do margins matter? The prognostic significance of positive surgical margins in radical prostatectomy specimens. J Urol. 2005;174:903–7. doi: 10.1097/01.ju.0000169475.00949.78. [DOI] [PubMed] [Google Scholar]
- 18.Bianco FJ, Jr, Scardino PT, Kattan MW, Rhee AC, Eastham JA. Surgeon volume is a predictor of improved outcomes in radical prostatectomy patients. J Urol. 2004;171(4 Suppl):211. abstract 796. [Google Scholar]
- 19.Pound CR, Partin AW, Eisenberger MA, Chan DW, Pearson JD, Walsh PC. Natural history of progression after PSA elevation following radical prostatectomy. JAMA. 1999;281:1591–7. doi: 10.1001/jama.281.17.1591. [DOI] [PubMed] [Google Scholar]
- 20.Freedland SJ, Humphreys EB, Mangold LA, Eisenberger M, Dorey FJ, Walsh PC, et al. Risk of prostate cancer-specific mortality following biochemical recurrence after radical prostatectomy. JAMA. 2005;294:433–9. doi: 10.1001/jama.294.4.433. [DOI] [PubMed] [Google Scholar]
- 21.Sirovich BE, Schwartz LM, Woloshin S. Screening men for prostate and colorectal cancer in the United States: Does practice reflect the evidence? JAMA. 2003;289:1414–20. doi: 10.1001/jama.289.11.1414. [DOI] [PubMed] [Google Scholar]
- 22.Kattan MW, Shariat SF, Andrews B, Zhu K, Canto E, Matsumoto K, et al. The addition of interleukin-6 soluble receptor and transforming growth factor beta1 improves a preoperative nomogram for predicting biochemical progression in patients with clinically localized prostate cancer. J Clin Oncol. 2003;21:3573–9. doi: 10.1200/JCO.2003.12.037. [DOI] [PubMed] [Google Scholar]
- 23.Kattan MW. Judging new markers by their ability to improve predictive accuracy. J Natl Cancer Inst. 2003;95:634–5. doi: 10.1093/jnci/95.9.634. [DOI] [PubMed] [Google Scholar]
- 24.Kattan MW, Eastham JA, Wheeler TM, Maru N, Scardino PT, Erbersdobler A, et al. Counseling men with prostate cancer: a nomogram for predicting the presence of small, moderately differentiated, confined tumors. J Urol. 2003;170:1792–7. doi: 10.1097/01.ju.0000091806.70171.41. [DOI] [PubMed] [Google Scholar]


