Abstract
Glutathione S-transferase of the malarial parasite Plasmodium falciparum (PfGST) represents a novel class of GST isoenzymes. Since the architecture of the PfGST substrate binding site differs significantly from its human counterparts and there is only this one isoenzyme present in the parasite, PfGST is considered a highly attractive target for antimalarial drug development. Here we report the mechanistic, kinetic, and structural characterization of PfGST as well as its interaction with different ligands. Our data indicate that in solution PfGST is present as a tetramer that dissociates into dimers in the presence of glutathione (GSH). Fluorescence spectroscopy shows that in the presence of GSH GST serves as ligandin for parasitotoxic ferriprotoporphyrin IX with a high- and a low-affinity binding site. This is supported by a clear uncompetitive inhibition type. Site-directed mutagenesis studies demonstrate that neither Cys 86 nor Cys 101 contribute to the peroxidase activity of the enzyme, which is thus performed GSH-dependently at the active site. Tyr 9 is responsible for the deprotonation of GSH and Lys 15, but also Gln 71 are involved in GSH binding. We furthermore report the 2.4 Å resolution X-ray structure of PfGST cocrystallized with the inhibitor S-hexylglutathione. In comparison with a previously reported structure obtained by crystal soaking, differences occur at the C-terminal end of helix α4 and at the S-hexylmoiety of the inhibitor. We furthermore show that, in contrast to previous reports, the antimalarial drug artemisinin is not metabolized by PfGST.
Keywords: artemisinin, catalytic mechanism, glutathione, heme binding, malaria, X-ray structure
Glutathione S-transferases (GSTs) are a family of phase II detoxification enzymes catalyzing the conjugation of glutathione (GSH) to a large variety of electrophilic substrates (Mannervik and Danielson 1988). The enzymes occur abundantly in most organisms investigated, ranging from prokaryotes to mammals. The less toxic and more hydrophilic products of GST catalyzed reactions can be partially metabolized and excreted (Salinas and Wong 1999), thereby protecting cells against cytotoxic and genotoxic compounds. Besides conjugation reactions, some GSTs can catalyze GSH-dependent reduction of hydroperoxides generated, e.g., during oxidative stress. In addition to their enzymatic functions, GSTs are able to bind a wide range of endogenous and exogenous ligands, such as hormones, heme, and bilirubin as well as drugs and pesticides, which often impairs the catalytic activity of the enzyme (Salinas and Wong 1999; Sheehan et al. 2001; Deponte and Becker 2005a). Over the last years GST research has focused on resistance phenomena in insect strains (Sheehan et al. 2001) and multidrug-resistant tumor cells (Salinas and Wong 1999). Due to their isoenzyme-specific overexpression in different tumors GSTs have emerged as promising therapeutic targets (Townsend and Tew 2003), and a rationale to utilize GST inhibitors in combination with alkylating agents to circumvent resistance has been established (Tew et al. 1997). An example for GSTs being involved in resistance to insecticides are the DDT-metabolizing enzymes of the malaria vector Anopheles (for review, see Deponte and Becker 2005a).
Tropical malaria, which is caused by the protozoan parasite Plasmodium falciparum, is responsible for ~515 million clinical cases (Snow et al. 2005) and one to three million deaths annually (Sachs and Malaney 2002). The emergence and spread of drug resistance to commonly used chemotherapeutics are major factors contributing to this increasing burden. Thus, the characterization of alternative drug targets is urgently required (Olliaro 2001; Greenwood and Mutabingwa 2002; Ridley 2002).
GST activity has been detected in all Plasmodium species studied so far as well as in all intraerythrocytic stages of the parasite (Deponte and Becker 2005a). PfGST has been estimated to represent >1% of the total cellular protein (Harwaldt et al. 2002; Liebau et al. 2002). A role of GST from P. falciparum (PfGST) in the development of drug resistance in malarial parasites has been postulated and is controversially discussed (Harwaldt et al. 2002; Deponte and Becker 2005a). The primary structure as well as the three-dimensional X-ray structure of PfGST differ significantly from human GSTs, and indicate that PfGST cannot be assigned to any of the previously known GST classes, thus representing a novel GST isoform (Fritz-Wolf et al. 2003). Since, furthermore, the parasite harbors only one GST and inhibition of PfGST is expected to disturb GSH-dependent conjugation processes, to enhance levels of cytotoxic peroxides and to increase the concentration of toxic ferriprotoporphyrin IX (FP), PfGST is a most promising drug target (Deponte and Becker 2005a).
Here, we characterize the catalytic mechanisms of conjugase and peroxidase activity of PfGST employing site-directed mutagenesis and kinetic studies. In addition, the oligomerization and FP binding behavior of the protein was analyzed by gel filtration chromatography and fluorescence spectroscopy. We furthermore studied PfGST as drug target reporting its X-ray structure after cocrystallization with S-hexylglutathione, the possible role of PfGST in the metabolism of artemisinin, as well as the inhibitory effects of clinically used antimalarials on the native and recombinant PfGST enzyme.
Results and Discussion
Oligomerization of PfGST
With respect to the binding of physiological ligands and the potential accessibility for enzyme inhibitors, the oligomerization behavior of PfGST was studied under different conditions. Nonreducing SDS-PAGE analysis of wild-type PfGST, which was freshly purified in the absence of additional reducing agents, showed that the protein is monomeric under denaturing conditions. However, under native conditions the enzyme forms noncovalently linked higher aggregates as confirmed by gel filtration chromatography (Supplemental Table 1). In the presence of 2 mM GSH, PfGST is α2 dimeric, whereas in the absence of GSH the MRGSH6GS-tagged protein forms (α2)2 tetrameric (98%) and presumably octameric (2%) oligomers under non-reducing and reducing (2 mM 1,4-dithiothreitol (DTT)) conditions. This indicates that the disaggregation of the tetramer is not based on the reducing properties of GSH, but rather on its high affinity for the substrate binding site. These results can be explained by the X-ray structures of PfGST in absence and presence of S-hexylglutathione (see below). The apparent molecular masses of dimeric, tetrameric, and octameric PfGST are smaller than the calculated theoretical masses. This phenomenon is often observed in gel filtration and might be indicative for a rapid monomer–dimer equilibrium in solution, which prevents separation of these two enzyme species using gel filtration chromatography. In contrast, conversion of α2 dimers into (α2)2 tetramers or even higher aggregates occurs slowly.
Table 1.
Kinetic parameters of wild-type PfGST and G-site mutants of PfGST determined using the CDNB conjugation assay (columns 2–4) and the peroxidase assay (column 5)
| PfGSTa | KmGSH(μM)b | kcatGSH(min−1)b | kcatGSH/KmGSH (M−1sec−1)b | Specific activityCu-OOH (nmol min−1 mg−1)c |
| Wild-type | 108 ± 4 (100%) | 6.3 ± 0.01 (100%) | 978 ± 34 (100%) | 11.4 ± 1.5 (100%) |
| Y9F | 101 ± 12 (94%) | 0.2 ± 0.01 (3%) | 32.4 ± 1.5 (3%) | 0.8 ± 0.2 (7%) |
| K15E | 994 ± 150 (922%) | 0.1 ± 0.00 (2%) | 2.0 ± 0.3 (0.2%) | 0.5 ± 0.1 (4%) |
| Q71E | 281 ± 27 (260%) | 3.4 ± 0.04 (54%) | 203 ± 18 (21%) | 5.4 ± 0.7 (47%) |
| C86A | 123 ± 3 (114%) | 5.6 ± 0.2 (88%) | 756 ± 48 (77%) | 10.0 ± 0.3 (88%) |
| C101A | 246 ± 6 (228%) | 4.5 ± 0.2 (71%) | 305 ± 3 (31%) | 8.3 ± 1.3 (73%) |
| Y211F | 128 ± 2 (119%) | 7.6 ± 0.4 (120%) | 985 ± 42 (101%) | 24.1 ± 2.7 (211%) |
a Given are mean values of two independent determinations and standard deviations. In parentheses is the percentage of the respective wild-type value.
b The Km for GSH as well as the kcat and the catalytic efficiency were determined using the CDNB conjugation assay.
c The last column gives the specific activities in the peroxidase assay with cumene hydroperoxide and glutathione as substrates (see Materials and Methods for details).
Tertiary and quaternary structure of PfGST cocrystallized with S-hexylglutathione
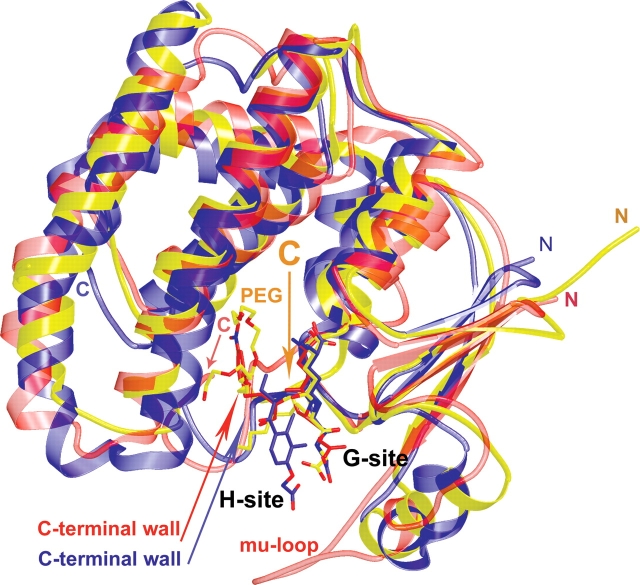
The three-dimensional structure of PfGST has recently been solved, and a structure which had been obtained by crystal soaking with the inhibitor S-hexylglutathione has been reported (Fritz-Wolf et al. 2003; Perbandt et al. 2004). Like other members of the GST superfamily PfGST adopts the canonical GST fold. The active site of GSTs is located in the cleft between the two domains of each monomer (Fig. 1), and is composed of two binding sites: the G-site, which binds reduced glutathione, and the more variable H-site, which can accommodate a variety of substrates.
Figure 1.
Structural comparison of GST enzymes. Superposition of the enzyme–inhibitor structures of PfGST (yellow), mu-class human GST (red, 1c72), and pi-class human GST (blue, 11gs). All inhibitors are drawn in ball and stick, with oxygens red and nitrogens blue. PfGST with bound S-hexylglutathione and a polyethylene glycol (PEG) molecule (carbons: yellow). Human mu-class GST with a bound glutathione conjugate (1-hydroxy-2-S-glutathionyl-3-paranitrophenoxypropane) (carbons: red). Human pi-class GST with the bound glutathione conjugate of ethacrynic acid (carbons: blue). The figure was drawn using the programs molscript and raster3d (Kraulis 1991; Merritt and Bacon 1997).
Since soaking of a preformed crystal with an inhibitor often results in a different structure than cocrystallization experiments, and since S-hexylglutathione represents one of the most important lead compound for the development of specific PfGST inhibitors, we carried out an X-ray crystallographic analysis of PfGST cocrystallized with S-hexylglutathione. The obtained structure reveals the biological dimer in the asymmetric unit of the crystal (monomers A and A′). The two active sites are occupied by S-hexylglutathione and by a polyethylene glycol (PEG) molecule (Figs. 1, 2B). Superposition of S-hexylglutathione-modified PfGST with liganded GSTs from the pi-class (Oakley et al. 1997) and the mu-class (Chern et al. 2000) shows a similar binding mode for glutathione (GSH, γ-Glu-Cys-Gly) (Fig. 1), while the conjugated moiety stretches out into different directions (Fig. 1) of the H-site.
Figure 2.
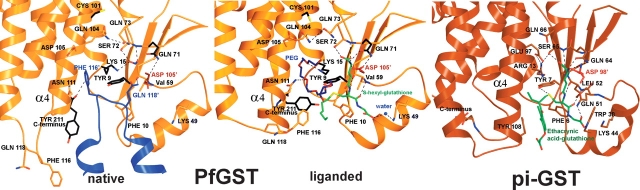
Equivalent views of the H-site. Close-up of native and liganded PfGST (A, B, gold) and a pi-GST (C, brown, 11gs), focusing on the active site. The active site is occupied by glutathione conjugate moieties (green, B, C). In PfGST, H-site residues interact with a PEG molecule (blue, B) or with the loop of monomer B (blue, A), which protrudes into monomer A resulting in a marked change in the position of Phe 116. Mutated residues are shown in black. The figure was drawn using the programs molscript and raster3d (Kraulis 1991; Merritt and Bacon 1997).
In PfGST, the α-amino group of GSH interacts with the strictly conserved residues Gln 71 and Asp 105′ of the second monomer (Fig. 2). The α-carboxyl group of the γ-glutamyl moiety is aligned by two bonds involving main-chain and side-chain atoms of Ser 72. The backbone of the Cys moiety of GSH forms two hydrogen bonds with the backbone of Val 59. The carboxyl group of the Gly moiety of GSH interacts with Lys 49 via a water molecule. The SH-group of the Cys moiety is stabilized in alpha-, mu-, and pi-class enzymes by a conserved tyrosine that corresponds to Tyr 9 in PfGST. In the inhibited PfGST-structure the OH-group of Tyr 9 is 3.3 Å away from the SH-group of GSH. The hexyl moiety points to the solvent, and is surrounded by Tyr 211, Leu 115, and Phe 116 and is in hydrophobic interaction with Phe 116 (Fig. 2B).
The upper part of the H-site is occupied by a PEG molecule, which interacts in both monomers with Lys 15, while in monomer A′ it is additionally bound to Asn 111 (Fig. 2B). The PEG molecules protrude into the interface of the dimer.
Comparison of liganded and native PfGST structures reveals a similar overall tertiary structure but the crystal packing of the molecules is different. In the absence of ligands two biological dimers (AA′ and BB′) form a tetramer, these homodimers are interlocked with each other by a loop (residues 113–120) of monomer B (B′), which occupies the H-site of monomer A (A′) (Fritz-Wolf et al. 2003). Upon binding of S-hexylglutathione, the H-site loop 113–120 rearranges (Fig. 2A,B), residues Asn 114, Leu 115, and Phe 116 form an additional coil in helix α4 and the side chains of Asn 111, Phe 116, and Tyr 211 flip into the H-site (Fig. 2A,B). The changed course of the residues 113–120 in the liganded enzyme prevents the interlocking of the dimers; as a consequence, the molecules are packed as dimers (monomers A and A′). The change in the position of Phe 116 upon ligand binding becomes particularly evident when comparing Figure 2A and B.
In the X-ray structure by Perbandt et al. 2004, PfGST was not cocrystallized with S-hexylglutathione; instead, native PfGST crystals had been soaked with S-hexylglutathione. The soaked structure reveals no rearrangement of loop 113–120, and the monomers are still packed as interlocked dimers. Differences between the soaked structure and our cocrystallized structure occur mainly at the C-terminal end of helix α4 and at the S-hexylmoiety of S-hexylglutathione. The binding mode of the glutathione part is similar, except for the Gly moiety of GSH, which is bound by Thr 121 of the second dimer in the soaked structure but interacts with Lys 49 via a water molecule in the cocrystallized structure.
In comparison to other GSTs, and in particular to the human isoforms, PfGST possesses a shorter C-terminal section resulting in a more solvent accessible binding site for the hydrophobic and amphiphilic substrates (Fritz-Wolf et al. 2003). X-ray analysis of the native and the inhibited structure reveals different conformations of the protein chains, lining the lower part of the H-site, where the hexylmoiety is located. This rearrangement of H-site residues shows that the shape of the H-site is very flexible and that large compounds could be bound. The hexylmoiety of S-hexylglutathione is not very spacious, and indeed, it sparely interacts with the enzyme, in accordance with the finding that S-hexylglutathione is not a very potent inhibitor in solution (Ki = 35 μM) (Harwaldt et al. 2002). However, the upper H-site pocket, occupied by a PEG molecule in the inhibited structure, is less flexible, and seems to be suitable for the design of specific PfGST inhibitors.
These data provide valuable hints for interactions of ligands with H-site residues, which are currently exploited for the design of more potent inhibitors. PfGST is active as a dimer with two distinct active centres. In the S-hexylglutathione-PfGST structure (Fig. 2) the H-sites are occupied by PEG molecules, which protrude into the dimer interface and indicate the presence of a cavity. This demonstrates that the H-sites can indeed be linked to each other. This strongly suggests to further improve the optimized H-site inhibitors by linking them to a G-site inhibitor or by linking two H-site inhibitors to one bivalent inhibitor.
Function of the active site residues of PfGST
In order to further elucidate the PfGST catalyzed reactions and their respective mechanisms we carried out site-directed mutagenesis studies focusing on the active site residues. For this purpose six PfGST mutants were generated which were expected to influence substrate binding and thus conjugation and/or peroxidase activity.
Since the glutathione binding sites in GSTs are highly conserved, also the glutathione moiety of S-hexylglutathione is bound very similarly in different GSTs (Fig. 1) and a conserved tyrosine residue at the G-site is essential for the catalytic activity (Tyr 6 for mu, Tyr 7 for pi, and Tyr 8 for alpha classes) (Kolm et al. 1992; Liu et al. 1992; Caccuri et al. 1998). It has been proposed that the tyrosine hydroxyl group acts as a hydrogen bond donor to stabilize the deprotonated thiolate anion during catalysis (Patskovsky et al. 1999). According to the crystal structure of PfGST, Tyr 9 and Tyr 211 are both in hydrogen bond distance to the thiolate anion (Fig. 2). Phenylalanine mutants of PfGST show that Tyr 9 but not Tyr 211 is essential for both the conjugation activity with 1-chloro-2,4-dinitrobenzene (CDNB) and the peroxidase activity with cumene hydroperoxide (CuOOH) (Table 1). Probably, deprotonation of GSH becomes the rate-limiting step for both enzymatic reactions in the case of the Y9F mutant. Mutation of Tyr 211 to the more hydrophobic phenylalanine leads to an increase of kcat in the CDNB conjugation assay and to a doubling of the specific activity with CuOOH. Both substrates are relatively hydrophobic, demonstrating a role of Tyr 211 in substrate binding at the H-site.
When interpreting the Km values of the six mutants as a measure of affinity for GSH (Table 1), it can be concluded that Lys 15, Gln 71, and Cys 101 are directly or indirectly involved in GSH binding, whereas Tyr 9, Cys 86, and Tyr 211 are not. The mutation K15E is likely to cause a direct repulsion of the α-carboxylate group of the γ-glutamyl residue of GSH. In our X-ray structure Lys 15 also interacts with a PEG molecule, which is bound in the upper pocket of the H-site. This makes it likely that Lys 15 is also involved in binding of H-site substrates. For example, mutation of Arg 15 of human GST A1-1 to lysine lead to decreased kcat for a phospholipid hydroperoxide but not for CDNB, suggesting that the correct topography of the GSH site is more critical for the phospholipid substrate (Hurst et al. 1998). The interaction of Gln 71 with the α-amino group of GSH in the crystal structure as well as the high GSH concentration in the assay could furthermore explain why the residual activity of Q71E is still high in comparison with the K15E mutant. The increased Km for C101A is surprising, and might be caused by an interaction between Cys 101′ and Gln 71/Asp 105′, thus indirectly influencing the binding of the α-amino group of GSH (Fig. 2B).
In contrast to, e.g., peroxiredoxins (Deponte and Becker 2005b), the cysteine residues of PfGST (Cys 86 and Cys 101) do not seem to be directly involved in the peroxidase activity. Taking together the results of all mutants characterized in this work, it is likely that in comparison with the conjugation reaction the first part of the peroxidase reaction—the nucleophilic attack of GSH at the electrophilic peroxide bridge—occurs with a similar mechanism at the same active site.
Ferriprotoporphyrin IX binding to PfGST wild type and mutants
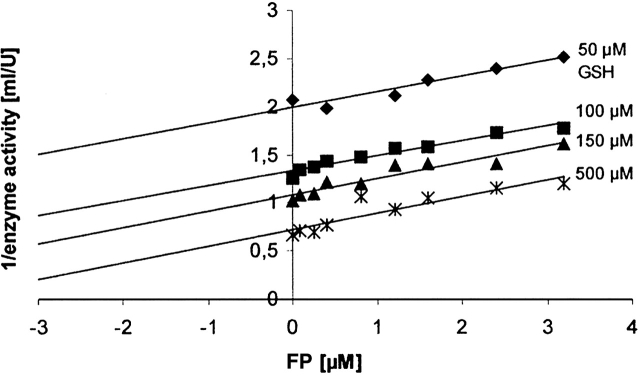
Since the malarial parasite resides in red blood cells and digests large quantities of erythrocyte hemoglobin, it subsequently has to deal with high amounts of potentially toxic FP. As shown in previous studies (Harwaldt et al. 2002), PfGST as an important intracellular ligandin is able to efficiently bind FP. We thus studied the binding of FP to PfGST and the different enzyme mutants in more detail using steady-state kinetics and intrinsic fluorescence quenching (Figs. 3, 4). Binding experiments were carried out in the presence of 1 mM GSH. Under these conditions, which are close to the in vivo GSH concentration in Plasmodium faciparum, PfGST is in the native and active α2 dimeric form as shown by gel filtration chromatography (Supplemental Table 1). Furthermore, our inhibition studies of PfGST by FP clearly revealed an uncompetitive inhibition type towards GSH, which indicates that FP preferentially binds to a preformed PfGST–GSH complex (Fig. 3).
Figure 3.
Inhibition of PfGST by ferriprotoporphyrin IX. The parallel lines in a Dixon plot indicate an uncompetitive inhibition type of PfGST by FP. The corresponding Cornish-Bowden diagram ([S]/v against [I]) yields a Ki value of 3 μM (data not shown).
Figure 4.
Binding of ferriprotoporphyrin IX to wild-type and mutant PfGST monitored by fluorescence quenching. Quenching of the intrinsic fluorescence of wild-type and mutant PfGST following successive additions of FP in the presence of 1 mM GSH. All titrations have been reproduced at least three times yielding values which differed by <3%.
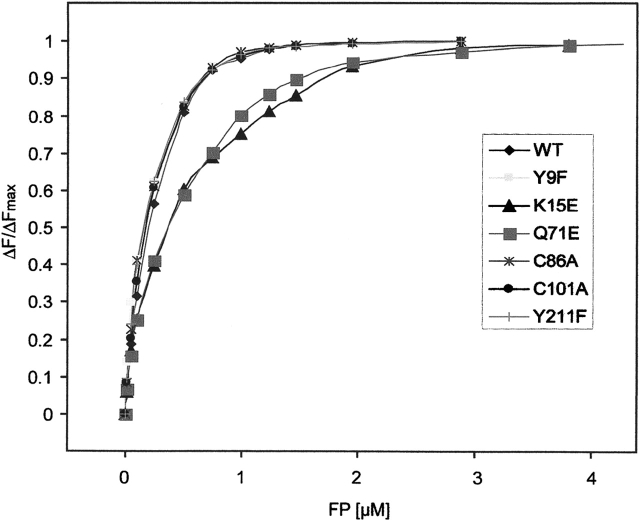
For the wild-type PfGST enzyme as well as the PfGST variants Y9F, C86A, C101A, and Y211F, the fluorescence binding data show a stoichiometry of one FP per monomer (Fig. 4). These titrations were conducted in a concentration range in which c(FP) >>Kass. In this range the stoichiometry of binding can be determined quite precisely; however, Kass can only be estimated. The stoichiometry of 1:1 was thus unambiguously detectable for the wild type and the mutants Y9F, C86A, C101A, and Y211F, as indicated by the fact that at 1 μM FP saturation of an equimolar amount of PfGST was reached, however not at 0.5 or 2 μM. Based on these measurements, a high-affinity binding site with a KD of about 30 nM was determined for these enzyme species. These data on wild-type PfGST are in good agreement with the observations made recently by Liebau et al. (2005).
The mutants Q71E and K15E, however, showed a different FP binding behavior and were not yet saturated at 1 mM GSH. This is also reflected by the increased Km values for glutathione (Table 1). The binding data for those two mutants fit best a two binding site model with KD values of about 0.25 μM (high-affinity binding site) and 2.5 μM (low-affinity binding site). These data support the prerequisite of GSH binding for efficient FP binding.
Artemisinin is not a substrate of PfGST
A reductive metabolization of the antimalarial drug artemisinin by human GST has been postulated by Mukanganyama et al. (2001). Artemisinin is a sesquiterpene lactone containing an endoperoxide bridge. It is of high clinical value and particularly employed against otherwise drug resistant strains of P. falciparum (Maitland et al. 2004). As shown by Mukanganyama and colleagues, artemisinin stimulated NADPH oxidation in cytosolic cell extracts as well as in assay systems containing human recombinant GSTs. The authors thus hypothesized that GSTs may contribute to the in vivo biotransformation of artemisinin and proposed a model, based on the known reactions of GSTs and sesquiterpenes, in which (1) artemisinin reacts with GSH resulting in oxidized glutathione; (2) the oxidized glutathione is then converted to reduced glutathione via glutathione reductase; and (3) the latter reaction may then result in the depletion of NADPH via glutathione reductase.
In case this reaction would be verified for PfGST, a role of the enzyme in drug metabolism and potential resistance mechanisms would have to be considered. We carried out a respective assay exactly as described by Mukanganyama et al. (2001). However, the addition of 1 μg/mL recombinant PfGST or human placenta GST, as described by the authors, did not result in enzymatic activity. On the contrary, when adding 1.5 mM artemisinin to the enzyme assay, the absorption at 340 nm increased, although it should decrease when NADPH is consumed. The absorption also increased in the absence of enzyme indicating a nonenzymatic background reaction. This reaction thus represents an artefact and is certainly not due to a GST-dependent turnover of artemisinin. The addition of 1.5 mM CuOOH instead of artemisinin in our control assays led to a time-dependent clear decrease in absorption characteristic for a reaction where NADPH is consumed. Furthermore, in our peroxidase assay system (see Materials and Methods) with up to 500 μg/mL PfGST, artemisinin was not found to be active as a substrate, either. Thus, according to our data, PfGST does not turn over artemisinin in biologically meaningful quantities and also for human placenta GST this reaction does not apply. Experiments with artesunate (data not shown) yielded comparable results.
PfGST is not a major target of presently available antimalarial drugs
PfGST has been discussed over the last years as potential target of clinically used antimalarial drugs (Becker et al. 2004). Indeed, Srivastava et al. (1999), reported a stage-dependent inhibition of GST in P. knowlesi extracts by 20 μM chloroquine (59–100% inhibition), artemisinin (20–41%), and primaquine (32–62%). The respective data are summarized in column 3 of Supplemental Table 2. We exactly reproduced the experiments of Srivastava et al. (1999), using fresh P. falciparum trophozoite extracts and detected a maximum of 5% inhibition at 20 μM of the three drugs (see column 4 of Supplemental Table 2). Increasing drug concentrations did not yield significant inhibition either. Comparable results were obtained when using artemisinin and primaquine in our CDNB assay optimized for PfGST or when studying the isolated recombinant enzyme (see columns 5 and 6 of Supplemental Table 2). Only for chloroquine at high concentrations of 70 μM a considerable inhibition approximating 50% was reached on isolated PfGST.
Table 2.
X-ray crystallographic data collection and structure refinement
| Space group | P212121 |
| Unit cell dimensions (Å ) | a = 61.17 b = 69.99 c = 123.69 agr;= β= γ = 90° |
| Resolution range (Å)a | 14.99–2.4 (2.55–2.4) |
| Completeness (%) | 97.6 (88.5) |
| Mean redundancy | 4.1 (3.1) |
| Rsymb (%) | 6.5 (20.0) |
| 〈I/σ〉 | 13.9 (5.3) |
| Reflections used in refinement | 20,845 |
| Protein atoms per dimer | 3519 |
| S-hexyl glutathione per dimer | 2 |
| Bound solvent molecules per dimer | 189 |
| RMSD bond (Å)/RMSD angles | 0.006/1.2 |
| Rcrystc (%)/Rfreed (%) | 19.4/23.7 |
a Values within parentheses are for data in the last resolution shell.
bRsym = 100 ∑ |Ih − 〈I〉|/∑ Ih.
cRcryst = 100 ∑ ||Fobs|−|Fcalc||/∑ ||Fobs| calculated for all observed data. No sigma cutoff was used.
dRfree calculated for 3% of randomly chosen unique reflections that were excluded from the refinement.
The data indicate that artemisinin, primaquine, and also chloroquine are most unlikely to lead to biologically meaningful PfGST inhibition in vivo. With respect to future drug development using PfGST as target these results are encouraging. Due to the increasing resistances of the parasites against the presently available drugs new drugs acting on novel targets are urgently required.
Materials and methods
Production of recombinant enzymes
Recombinant PfGST species were overexpressed in Escherichia coli and purified as described for the wild-type enzyme (Harwaldt et al. 2002). PfGST mutants were produced by site-directed mutagenesis. The PCRs were carried out with Pfu-DNA-polymerase (94°C, 1.5 min; 94°C, 30 sec; 55°C, 1 min; 70°C, 9 min; 18 cycles; 70°C, 9 min) (for details, see Supplemental Material). Correct insertions and sequences were confirmed by DNA sequencing.
Crystallization
Crystals of wild-type GST were grown at 22°C by the hanging-drop technique. The initial concentration in the drop was 60 mM CaCl2, 30 mM NaHEPES (pH 7.5), 8.4% PEG 400, 3.4 mg GST/mL, and 1.4 mM S-hexylglutathione. The reservoir contained 150 mM CaCl2, 75 mM NaHEPES (pH 7.5), and 21% PEG 400. Prior to the measurements crystals were soaked in mother liquor (150 mM CaCl2, 75 mM NaHEPES at pH 7.5, and 21% PEG 400, 3.5 mM S-hexylglutathione, and 1 M DL-threitol for stabilizing the crystals during the freezing process).
Data collection and structure determination
All diffraction data were recorded by the rotation method and processed with XDS (Kabsch 1993). The crystals obey P212121 space group symmetry and contain two monomers in the asymmetric unit. The data set (Table 2) was collected at 100 K using synchrotron radiation at the ESRF (beamline ID14-4, rotation/ image: 1.0°, wavelength 0.93927 Å, Quantum4 CCD detector; ADSC). Reflection phasing and structure refinements were carried out using the CNS program suite (Brunger et al. 1998). The structure was solved by the Molecular Replacement method, using the structure of native PfGST as a search model (1OKT, Fritz-Wolf et al. 2003). After a rigid body refinement, which was used to improve the molecular solutions and to compensate for any possible changes in crystal packing, the initial Rfree factor was 35%. An initial atomic model of PfGST was fitted to this map using the interactive graphics program O (Jones et al. 1991); refinement of the model was completed after a few rounds of model correction. S-hexylglutathione could be clearly determined in the initial difference map. The structure was refined to 2.4 Å resolution. The asymmetric unit contains two monomers (A, A′), two S-hexylglutathione, two PEG molecules, and 189 water molecules. The overall temperature factor of the structure is 41.3 Å2. Except for the first three N-terminal-residues, residues 143–149 and the hexyl moiety of S-hexylglutathione, the structure is well defined by the electron density. The structure was refined to Rfree = 23.7%, the crossvalidated coordinate error is 0.3 Å (Table 2). The model displays good stereochemistry as verified by Procheck (Laskowski et al. 1993).
CDNB assays and kinetic studies
PfGST activity toward GSH and CDNB was determined spectrophotometrically at 340 nm on the basis of the extinction coefficient for the product S-(2,4-dinitrophenyl)glutathione (ɛ340nm = 9.6 mM −1cm−1). The assay mixture (500 μL) contained 0.5 mM CDNB and 1 mM GSH in 100 mM HEPES, 1 mM EDTA (pH 6.5). The reaction was started by addition of PfGST. One unit of GST activity was defined as the conjugation of 1 μmol of CDNB with GSH per minute at 25°C (Harwaldt et al. 2002). Specific activities (μmol/min/mg of protein) were calculated using the activities at 25°C and protein concentrations determined on the basis of ɛ280nm = 26.3 mM−1cm−1 for wild-type and most mutant enzymes and ɛ 280nm = 25.0 mM−1cm−1 for PfGST Y9F and Y211F. Putative PfGST inhibitors were tested in the assay by addition of enzyme to the inhibitor-containing assay mixture.
Km and kcat values for GSH in the CDNB conjugation assay were determined by duplicate measurements of activity at various GSH concentrations (10 μM–2 mM) and a constant starting concentration of CDNB (0.5 mM). Data analysis of the results was carried out using Lineweaver-Burk, Hanes and Eadie-Hofstee plots. The Km for CDNB on the wild-type enzyme is in the millimolar range and due to the absorbance of CDNB difficult to determine precisely. Thus, we did not include affinity tests for CDNB into the characterization of the mutants.
Peroxidase activity of PfGST with cumene hydroperoxide and artemisinin
GPx activity of PfGST towards CuOOH was determined by using the glutathione reductase (GR)-coupled assay. The enzyme was added to 100 mM HEPES, 1 mM EDTA (pH 6.5), which contained 1 mM GSH, 1 U/mL PfGR, and 100 μM NADPH. The reaction was started at 25°C by the addition of 200 μM CuOOH. The consumption of NADPH was followed spectrophotometrically (ɛ340 nm = 6.22 mM−1cm−1). One unit of GPx activity was defined as the consumption of 1 μmol of NADPH per minute. A blank containing no enzyme was used to correct for nonenzymatic GPx activity.
To test the peroxidase activity of PfGST with artemisinin as peroxide substrate, the standard GPx assay was carried out as described above using artemisinin instead of CuOOH. In direct comparison, the assay was carried out as described by Mukanganyama et al. (2001). Briefly, the assay contained 0.1 M phosphate buffer (pH 7.0), 1 mM EDTA, 1 U/mL PfGR, 0.2 mM NADPH, 1 mM GSH, 1 mM sodium azide, and 0.5 to 1 μg/mL recombinant PfGST or human placenta GST, respectively. The mixture was incubated for 5 min at 25°C before initiation of the reaction by addition of 1.5 mM artemisinin. The rate of oxidation of NADPH was assayed spectrophotometrically at 340 nm at 30°C.
Gel filtration chromatography and SDS-PAGE
Oligomerization of 30 to 60 nmol PfGST was studied by native gel filtration chromatography on a HiLoad 16/60 Superdex 200 prep grade column, which was connected to an ÄKTA-FPLC system (Amersham Pharmacia Biotech). The column was calibrated with a gel filtration standard (Amersham Pharmacia Biotech), and then equilibrated again with 50 mM sodium phosphate, 300 mM NaCl (pH 7.4). Alternatively the buffer was supplemented with 2 mM GSH or 2 mM DTT. FPLC fractions were analyzed photometrically, and peak areas and kAV values were evaluated using the software UNICORN 4.11 (Amersham Pharmacia Biotech). Protein containing FPLC-fractions were analyzed by reducing and nonreducing SDS-PAGE.
Fluorescence spectroscopy
Emission spectra of 1 μM wild-type or mutant PfGST in 100 mM HEPES, 1 mM EDTA, 1 mM GSH (pH 6.5), were recorded at 25°C on a Hitachi F4500 spectrofluorometer following successive additions of FP (0–5.6 μM final concentration). The samples were excited at 280 nm (slit, 5 nm), and the emission spectra were recorded in the wavelength range of 280–450 nm (slit, 5 nm). The FP-induced quenching of PfGST fluorescence was deduced from the decrease of fluorescence intensity at 327 nm. Spectral scans were repeated to test for time-dependent effects, and all samples were found to exhibit rapid equilibration. The fluorescence quenching induced by FP binding to PfGST indicates either (1) a direct interaction of the ligand with tryptophane residues of the protein or (2) conformational changes induced by FP binding which alter tryptophane fluorescence. Data analysis was performed using the program DYNAFIT (Kuzmic 1996).
Cultivation of Plasmodium falciparum
Intraerythrocytic stages of the chloroquine resistant P. falciparum strain K1 were grown in continuous culture as described by Trager and Jensen (1976), with slight modifications. Parasites were maintained at 1–10% parasitaemia and 3.3% haematocrit in an RPMI 1640 culture medium supplemented with A+ erythrocytes, 4% A+ human serum, 0.2% lipid-rich bovine serum albumin (Albumax), 9 mM glucose, 0.2 mM hypoxanthine, 2.1 mM L-glutamin, and 22 μg/mL gentamycin. All incubations were carried out at 37°C, 3% O2, 3% CO2, and 94% N2. Parasites were synchronized to ring stages by the sorbitol method (Lambros and Vanderberg 1979). After 24 h, parasites in the trophozoite stage were isolated by suspending the red cells in a 20-fold volume of buffer containing 7 mM K2HPO4, 1 mM NaH2PO4, 11 mM NaHCO3, 58 mM KCl, 56 mM NaCl, 1 mM MgCl2, 14 mM glucose, and 0.02% saponin for 10 min at 37°C. The pellets were washed three times and the parasites were diluted in 150 μL of the same buffer and disrupted by three times freezing and thawing. After centrifugation, the supernatant was used for the various analyses.
Effects of chloroquine, artemisinin, and primaquine on recombinant PfGST and P. falciparum cell extracts
Protein concentration in the parasite extracts was determined according to Bradford. To study the effect of different anti-malarials on PfGST, an assay was carried out as described previously (Srivastava et al. 1999). Briefly, a typical assay mixture contained 1 mM glutathione, 1 mg/mL of cytosolic protein and the respective drug in 0.1 M phosphate buffer at pH 6.5. The mixture was preincubated for 10 min at 37°C before starting the assay at 37°C by addition of 1 mM CDNB. In comparison, the assay was also carried out under our standard conditions (0.5 mM CDNB and 1 mM GSH in 100 mM HEPES, 1 mM EDTA at pH 6.5) starting with either cell extract (without preincubation) or recombinant PfGST (with and without preincubation).
Electronic supplemental material
Electronic supplementary material contains a word file including additional information on primer design, enzyme kinetics, and gel filtration experiments.
Acknowledgments
We thank Stefan Rahlfs (Giessen University) and Heiner Schirmer (Heidelberg University) for helpful discussions. We also thank Monique Akoachere for her support in the P. falciparum cell culture. Elisabeth Fischer and Marina Fischer are highly acknowledged for their excellent technical assistance. The study was supported by the SFB 535, TP A12 from the Deutsche Forschungsgemeinschaft.
Abbreviations
CDNB, 1-chloro-2,4-dinitrobenzene
CuOOH, cumene hydroperoxide
DTT, 1,4-dithiothreitol
FP, ferriprotoporphyrinIX
GSH, glutathione
GST, glutathione S-transferase
PEG, polyethylene glycol
Pf, Plasmodium falciparum
Article published online ahead of print. Article and publication date are at http://www.proteinscience.org/cgi/doi/10.1110/ps.051891106.
Supplemental material: see www.proteinscience.org
References
- Becker, K., Tilley, L., Vennerstrom, J.L., Roberts, D., Rogerson, S., and Ginsburg, H. 2004. Oxidative stress in malaria parasite-infected erythrocytes: Host–parasite interactions. Int. J. Parasitol. 34: 163–189. [DOI] [PubMed] [Google Scholar]
- Brunger, A.T., Adams, P.D., Clore, G.M., DeLano, W.L., Gros, P., Grosse-Kunstleve, R.W., Jiang, J.S., Kuszewski, J., Nilges, M., Pannu, N.S., et al. 1998. Crystallography & NMR system: A new software suite for macromolecular structure determination. Acta Crystallogr. D Biol. Crystallogr. 54: 905–921. [DOI] [PubMed] [Google Scholar]
- Caccuri, A.M., Lo Bello, M., Nuccetelli, M., Nicotra, M., Rossi, P., Antonini, G., Federici, G., and Ricci, G. 1998. Proton release upon glutathione binding to glutathione transferase P1-1: Kinetic analysis of a multistep glutathione binding process. Biochemistry 37: 3028–3034. [DOI] [PubMed] [Google Scholar]
- Chern, M.K., Wu, T.C., Hsieh, C.H., Chou, C.C., Liu, L.F., Kuan, I.C., Yeh, Y.H., Hsiao, C.D., and Tam, M.F. 2000. Tyr115, gln165 and trp209 contribute to the 1, 2-epoxy-3-(p-nitrophenoxy)propane-conjugating activity of glutathione S-transferase cGSTM1–1. J. Mol. Biol. 300: 1257–1269. [DOI] [PubMed] [Google Scholar]
- Deponte, M. and Becker, K. 2005a. Glutathione S-transferase from malarial parasites—Structural and functional aspects. Methods Enzymol. 401: 240–252. [DOI] [PubMed] [Google Scholar]
- ———. 2005b. Biochemical characterization of Toxoplasma gondii 1-Cys peroxiredoxin 2 with mechanistic similarities to typical 2-Cys Prx. Mol. Biochem. Parasitol. 140: 87–96. [DOI] [PubMed] [Google Scholar]
- Fritz-Wolf, K., Becker, A., Rahlfs, S., Harwaldt, P., Schirmer, R.H., Kabsch, W., and Becker, K. 2003. X-ray structure of glutathione S-transferase from the malarial parasite Plasmodium falciparum. Proc. Natl. Acad. Sci. 100: 13821–13826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood, B. and Mutabingwa, T. 2002. Malaria in 2002. Nature 415: 670–672. [DOI] [PubMed] [Google Scholar]
- Harwaldt, P., Rahlfs, S., and Becker, K. 2002. Glutathione S-transferase of the malarial parasite Plasmodium falciparum: Characterization of a potential drug target. Biol. Chem. 383: 821–830. [DOI] [PubMed] [Google Scholar]
- Hurst, R., Bao, Y., Jemth, P., Mannervik, B., and Williamson, G. 1998. Phospholipid hydroperoxide glutathione peroxidase activity of human glutathione transferases. Biochem. J. 332: 97–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones, T.A., Zou, J.Y., Cowan, S.W., and Kjeldgaard, M. 1991. Improved methods for building protein models in electron density maps and the location of errors in these models. Acta Crystallogr. A 47: 110–119. [DOI] [PubMed] [Google Scholar]
- Kabsch, W. 1993. Automatic processing of rotation diffraction data from crystals of initially unknown symmetry and cell constants. J. Appl. Crystallogr. 26: 795–800. [Google Scholar]
- Kolm, R.H., Sroga, G.E., and Mannervik, B. 1992. Participation of the phenolic hydroxyl group of Tyr-8 in the catalytic mechanism of human glutathione transferase P1-1. Biochem. J. 285: 537–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraulis, P.J. 1991. Molscript: A program to produce both detailed and schematic plots of protein structures. J. Appl. Crystallogr. 24: 946–950. [Google Scholar]
- Kuzmic, P. 1996. Program DYNAFIT for the analysis of enzyme kinetic data: Application to HIV proteinase. Anal. Biochem. 237: 260–273. [DOI] [PubMed] [Google Scholar]
- Lambros, C. and Vanderberg, J.P. 1979. Synchronization of Plasmodium falciparum erythrocytic stages in culture. J. Parasitol. 65: 418–420. [PubMed] [Google Scholar]
- Laskowski, R.A., MacArthur, M.W., Moss, D.S., and Thornton, J.M. 1993. Procheck: A program to check the stereochemical quality of protein structures. J. Appl. Crystallogr. 26: 283–291. [Google Scholar]
- Liebau, E., Bergmann, B., Campbell, A.M., Teesdale-Spittle, P., Brophy, P.M., Luersen, K., and Walter, R.D. 2002. The glutathione S-transferase from Plasmodium falciparum. Mol. Biochem. Parasitol. 124: 85–90. [DOI] [PubMed] [Google Scholar]
- Liebau, E., De Maria, F., Burmeister, C., Perbandt, M., Turella, P., Antonini, G., Federici, G., Giansanti, F., Stella, L., Lo Bello, M., et al. 2005. Cooperativity and pseudo-cooperativity in the glutathione S-transferase from Plasmodium falciparum. J. Biol. Chem. 280: 26121–26128. [DOI] [PubMed] [Google Scholar]
- Liu, S., Zhang, P., Ji, X., Johnson, W.W., Gilliland, G.L., and Armstrong, R.N. 1992. Contribution of tyrosine 6 to the catalytic mechanism of isoenzyme 3-3 of glutathione S-transferase. J. Biol. Chem. 267: 4296–4299. [PubMed] [Google Scholar]
- Maitland, K., Makanga, M., and Williams, T.N. 2004. Falciparum malaria: Current therapeutic challenges. Curr. Opin. Infect. Dis. 17: 405–412. [DOI] [PubMed] [Google Scholar]
- Mannervik, B. and Danielson, U.H. 1988. Glutathione transferases—Structure and catalytic activity. CRC Crit. Rev. Biochem. 23: 283–337. [DOI] [PubMed] [Google Scholar]
- Merritt, E.A. and Bacon, D.J. 1997. Raster3D Version 2: Photorealistic molecular graphics. Methods Enzymol. 277: 505–524. [DOI] [PubMed] [Google Scholar]
- Mukanganyama, S., Naik, Y.S., Widersten, M., Mannervik, B., and Hasler, J.A. 2001. Proposed reductive metabolism of artemisinin by glutathione transferases in vitro. Free Radic. Res. 35: 427–434. [DOI] [PubMed] [Google Scholar]
- Oakley, A.J., Lo Bello, M., Mazzetti, A.P., Federici, G., and Parker, M.W. 1997. The glutathione conjugate of ethacrynic acid can bind to human pi class glutathione transferase P1–1 in two different modes. FEBS Lett. 419: 32–36. [DOI] [PubMed] [Google Scholar]
- Olliaro, P. 2001. Mode of action and mechanisms of resistance for anti-malarial drugs. Pharmacol. Ther. 89: 207–219. [DOI] [PubMed] [Google Scholar]
- Patskovsky, Y.V., Patskovska, L.N., and Listowsky, I. 1999. Functions of His107 in the catalytic mechanism of human glutathione S-transferase hGSTM1a-1a. Biochemistry 38: 1193–1202. [DOI] [PubMed] [Google Scholar]
- Perbandt, M., Burmeister, C., Walter, R.D., Betzel, C., and Liebau, E. 2004. Native and inhibited structure of a Mu class-related glutathione S-transferase from Plasmodium falciparum. J. Biol. Chem. 279: 1336–1342. [DOI] [PubMed] [Google Scholar]
- Ridley, R.G. 2002. Medical need, scientific opportunity and the drive for antimalarial drugs. Nature 415: 686–693. [DOI] [PubMed] [Google Scholar]
- Sachs, J. and Malaney, P. 2002. The economic and social burden of malaria. Nature 415: 680–685. [DOI] [PubMed] [Google Scholar]
- Salinas, A.E. and Wong, M.G. 1999. Glutathione S-transferases—A review. Curr. Med. Chem. 6: 279–309. [PubMed] [Google Scholar]
- Sheehan, D., Meade, G., Foley, V.M., and Dowd, C.A. 2001. Structure, function and evolution of glutathione transferases: Implications for classification of non-mammalian members of an ancient enzyme superfamily. Biochem. J. 360: 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snow, R.W., Guerra, C.A., Noor, A.M., Myint, H.Y., and Hay, S.I. 2005. The global distribution of clinical episodes of Plasmodium falciparum malaria. Nature 434: 214–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava, P., Puri, S.K., Kamboj, K.K., and Pandey, V.C. 1999. Glutathione-S-transferase activity in malarial parasites. Trop. Med. Int. Health 4: 251–254. [DOI] [PubMed] [Google Scholar]
- Tew, K.D., Dutta, S., and Schultz, M. 1997. Inhibitors of glutathione S-transferases as therapeutic agents. Adv. Drug Deliv. Rev. 26: 91–104. [DOI] [PubMed] [Google Scholar]
- Townsend, D.M. and Tew, K.D. 2003. The role of glutathione-S-transferase in anti-cancer drug resistance. Oncogene 22: 7369–7375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trager, W. and Jensen, J.B. 1976. Human malaria parasites in continuous culture. Science 193: 673–675. [DOI] [PubMed] [Google Scholar]