Abstract
Ca2+ channel β subunits regulate trafficking and gating (opening and closing) of voltage-dependent Ca2+ channel α 1 subunits. Based on primary sequence comparisons, they are thought to be modular structures composed of five domains (A–E) that are related to the large family of membrane associated guanylate-kinase (MAGUK) proteins. The crystal structures of the β subunit core, B–D, domains have recently been reported; however, very little is known about the structures of the A and E domains. The N-terminal A domain is a hypervariable region that differs among the four subtypes of Ca2+ channel β subunits (β1–β4). Furthermore, this domain undergoes alternative splicing to create multiple N-terminal structures within a given gene class that have distinct effects on gating. We have solved the solution structure of the A domain of the human β4a subunit, a splice variant that we have shown previously to have α 1 subunit subtype-specific effects on Ca2+ channel trafficking and gating.
Keywords: Ca2+ channel, β4a subunit, nuclear magnetic resonance, alternative splicing, membrane-associated guanylate-kinase protein, protein structure, domains and motifs, exon/intron relationship, ion channel
Voltage-gated Ca2+ channels open in response to membrane depolarizations induced by propagating action potentials and thereby regulate excitation–contraction coupling in skeletal and heart muscle cells and excitation-transmitter release coupling in neurons (Catterall 2000). Their influence over cytosolic Ca2+ levels also gives them a prominent role in Ca2+-mediated signal transduction and gene expression. Voltage-gated Ca2+ channel complexes include four subunits, α 1, α 2/δ , and β, that are assembled in a 1:1:1:1 ratio (Dalton et al. 2005). Ten genes code for α 1 subunits (180–240 kDa) and are classified into three main families, Cav1–Cav3. The α 1 subunit forms the pore and can function without α 2/δ and β; however, the gating properties of α 1 subunits alone do not match those of native channels. Four genes code for α 2/δ subunits (~150 kDa), consisting of two proteins attached by multiple disulfide bonds (Arikkath and Campbell 2003). The δ subunit is inserted into the plasma membrane, while the heavily glycosylated α 2 subunit is entirely extracellular. In contrast, β subunits, encoded by four genes, β1–β4, are located on the cytosolic surface of the complex and bind to a well-characterized inter-action domain (AID) on the intracellular loop of α 1 subunits (Pragnell et al. 1994; Richards et al. 2004). The β subunits mediate α 1/α 2 trafficking and surface expression and regulate gating kinetics via multiple contacts with the α 1 subunit (Maltez et al. 2005).
The β subunits contain five proposed structural domains (A–E) (Hanlon et al. 1999). These domains show striking similarity to a number of membrane-associated guanylate kinase (MAGUK) proteins (McGee et al. 2004), indicating that they have evolved from a common ancestor. Based on X-ray crystallography (Chen et al. 2004; Opatowsky et al. 2004; Van Petegem et al. 2004) and sequence alignments with MAGUK proteins (McGee et al. 2004), the B domain (~66 residues) resembles an SH3 fold, and the D domain (~190 residues), a guanylate-kinase fold. These domains form the core structure of the β subunit. Primary sequence alignments of the different β subunits indicates a high level of conservation in the core SH3 (>60%) and GK (>67%) domains, suggesting that the core domain has similar functions in all β subunits. The hypervariable A, C, and E domains are not well-conserved between β subunit subtypes and undergo extensive alternative splicing. It is likely that these variable domains serve cell-type and α 1 subunit-specific roles in regulating Ca2+ channel function.
We have shown previously that the β4 subunit undergoes alternative splicing to generate A domains of either 58 (β4a) or 92 (β4b) residues that precede the SH3 fold (Helton and Horne 2002). Functional studies indicate that this alternative splicing event results in differential gating of P/Q type Ca2+ channels (Helton et al. 2002). To date, only the SH3 and GK domains have been shown to bind directly to the α 1 subunit (Maltez et al. 2005), raising the possibility that β subunit A domains may regulate channel properties through protein–protein interactions with non-Ca2+ channel proteins. To further understand the role of the β subunit A domain in Ca2+ channel gating and synaptic transmission, we determined the three-dimensional solution structure of the β4a-A domain. Our results show that the β4a-A domain is an independently folded module positioned away from the β subunit core and α 1 subunit and support the idea that the β4a-A domain is involved in protein–protein interactions.
Results
Structure of the human Ca2+ channel β4a-A domain
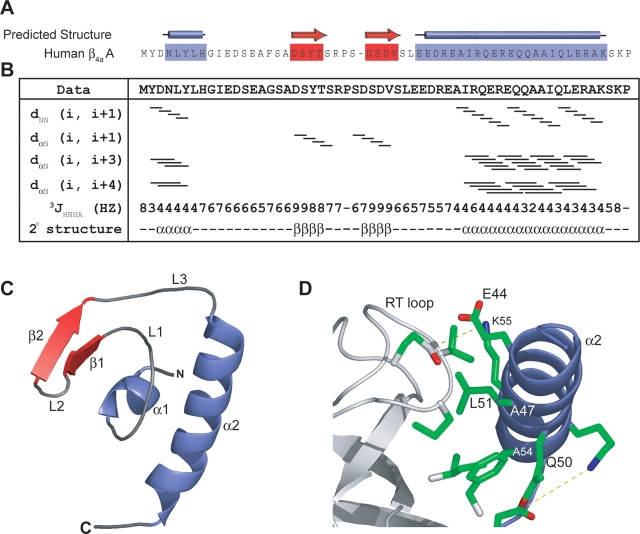
The sequence and predicted secondary structure of the β4a-A domain are aligned in Figure 1A. The prediction indicates that the A domain contains 2 α-helices and 2 β-strands. Nuclear magnetic resonance (NMR) spectroscopic methods were used to determine the high-resolution three-dimensional structure of the protein. Sequence-specific chemical shift assignments of β4a-A were accomplished using standard triple-resonance experimental procedures outlined in the Materials and Methods section. The locations of secondary structural elements were identified by chemical shift indices, HNHα coupling constants, and NOE interaction patterns (Fig. 1B). Based on NOESY and dihedral angle restraint data (HNHα coupling constants), and in agreement with the secondary structure prediction, the β4a-A domain is composed of two anti-parallel β-sheets (residues 19–22 and 26–29) and two α-helices (residues 2–7 and 40–55). These results and preliminary NOE constraint data were used to generate a number of hydrogen bonds used in the final structure calculations of the β4a-A domain.
Figure 1.
(A) Secondary structure predictions performed with the PSA server (http://bmerc-www.bu.edu/psa; White et al. 1994) indicate that the β4a-A domain has a mixture of α-helix and β-sheet. (B) Secondary structural characterization by multidimensional NOESY-HSQC and HNHA J coupling experiments. Solid bars for dNN (i, i + 1) and dα N (i, i + 1) represent continuous cross peaks observed in 15N-edited NOESY-HSQC experiments run with a 50-msec mixing time. Lines for dα N (i, i + 3) and dα N (i, i + 4) represent NOE cross-peaks between residues three and four amino acids away, respectively. The 3JHNHA coupling constants provide further information on the secondary structure boundaries. These results outline the secondary structural elements present in the β4a-A domain and correlate with predicted structural elements. (C ) Solution structure of the β4a-A domain determined with a backbone RMSD of 0.73 A °(Table 1). The β4a-A domain is independently folded with the first helix (α 1) coordinating two β-strands (β1 and 2), three loops (L1–3), and a C-terminal α-helix (α 2) to create a globular, hydrophobic core. (D) The α 2 helix is involved in packing against the SH3 domain (Opatowsky et al. 2004). Conserved residues are involved in hydrophobic packing (A54, L51, and A47 on the A domain, and V80, F100, W104, and I106 on the SH3 domain) and formation of salt bridges (R53–E97 and K55–E115) between the A and the SH3 domains of β4a.
The β4a-A domain solution structure (Fig. 1C) reveals that the short N-terminal helix (α 1) packs against the conserved C-terminal helix (α 2). Between the two helices there are three loop structures (L1, L2, and L3) and a pair of short, anti-parallel β-strands (β1 and β2). The first helix (α 1) is positioned orthogonal to the second helix (α 2) and is packed between α 2 and the β elements. Tyr21 of β1 packs against Leu5, Tyr6, and Leu7 on α 1 to create a small hydrophobic core for the β4a-A domain.
The C-terminal helix (α 2) shown in the NMR structure corresponds to the N-terminal helix of the β2a core (SH3-GK) X-ray crystal structure (Opatowsky et al. 2004). In this helix, Glu44, Ala47, Gln50, Leu51, Ala54, and Lys55 of β4a are conserved in all β subunits. The β2a crystal structure indicates that these residues make contacts with a surface distal to the canonical SH3 PXXP binding pocket (Fig. 1D). The conservation of these residues on α 2 of the A domain suggests that these residues are likely important for stabilizing the A domain and orienting it away from the Ca2+ channel α 1 subunit interaction domain (AID).
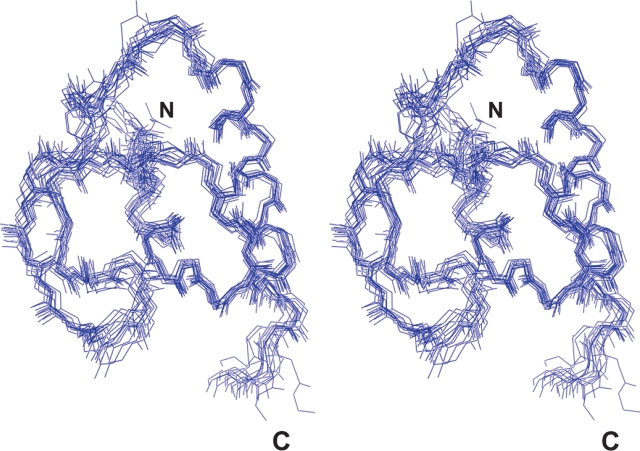
A total of 1156 restraints were used to calculate a family of 15 representative structures (Fig. 2) with a root-mean-square deviation (RMSD) of 0.74 Å for the backbone atoms and 1.35 Å for all atoms (Table 1). Ramachandran plot analysis of the secondary elements of the β4a-A domain yields 79% of residues in most favored regions, 16.3% in additional allowed regions, 4.7% in generously allowed regions, and 0% in disallowed regions. Further statistical information for the set of 15 structures of the β4a-A domain is found in Table 1. Atomic coordinates for the human β4a-A domain have been deposited with the protein database (PDB) at Rutgers University (accession code 2D46). Structural analysis using several PDB programs revealed that the β4a-A domain has a unique fold.
Figure 2.
Stereo view of the 15 lowest energy solution structures of the human Ca2+ channel β4a subunit A domain. Shown are the backbone traces of the β4a-A domain with the highest convergence in the structured residues 2–7 (α 1), 19–22 (β1), 26–29 (β2), and 40–55 (α 2).
Table 1.
Summary of structural statistics for the human β4a-A domain, ensemble of the 15 lowest energy structures
| NOE upper distance limits | 963 |
| Intraresidue | 472 |
| Sequential | 196 |
| Medium range (1 < |i − j| ≥ 4) | 87 |
| Long range (|i − j| > 4) | 208 |
| Dihedral angle constraints | 143 |
| φ | 52 |
| ψ | 52 |
| χ | 39 |
| Hydrogen bonds | 2 × 25 |
| RMSD from experimental constraints | |
| Distances (Å) | 0.0339 ± 0.001 |
| Dihedrals (°) | 0.7455 ± 0.134 |
| Average number of NOE distance constraint violations | |
| >0.5 Å | 0.00 ± 0.00 |
| >0.2 Å | 2.27 ± 1.39 |
| RMSD from idealized covalent geometry | |
| Bonds (Å) | 0.0054 ± 0.0003 |
| Angles (°) | 0.6689 ± 0.0333 |
| Impropers (°) | 0.6105 ± 0.0426 |
| Atomic RMSD values (Å) for β4a-A domain residues 1–55 | |
| Backbone atoms | 0.74 ± 0.11 Å |
| All atoms | 1.35 ± 0.17 Å |
| Ramachandran plot (%)a,b | |
| Most favored regions | 79.0% |
| Additional allowed regions | 16.3% |
| Generously allowed regions | 4.7% |
| Disallowed regions | 0.0% |
Structure statistics are reported as averages of the 15 lowest energy structures calculated from the CNS program.
a Glycines and prolines are excluded.
bResidues 1–11, 19–22, and 27–55.
Discussion
The structures of Ca2+ channel β subunits have eluded investigators for >15 yr following the cloning and sequencing of the first skeletal muscle β1 subunit (Ruth et al. 1989). Progress toward solving their structures began only when it was recognized that these proteins were members of the large family of modular MAGUK proteins (Hanlon et al. 1999). Post-synaptic-density protein 95 (PSD-95) served as a model for approaching the structure of β subunits. The X-ray crystal structure of the core SH3 and GK domains of PSD-95 was solved after removing its three N-terminal PDZ domains (McGee et al. 2001). A similar approach proved successful in solving the crystal structures of the core SH3 and GK domains of Ca2+ channel subunits β2a, β3, and β4 (Chen et al. 2004; Opatowsky et al. 2004; Van Petegem et al. 2004). Difficulties in crystallizing full-length β subunits likely arise from the dynamic structures of the N-terminal A and C-terminal E domains. This is supported by the fact that in the present study low temperatures were used to stabilize the β4a-A domain fold and to provide better resolved data. Circular dichroism studies of the β4a-A domain reveal a partial unfolding of the structure with increasing temperature (A. Vendel, unpubl.). This may be due to loss of stabilizing interactions of the α 2 helix with the SH3 domain present in the native β4a structure (Fig. 1D). However, yeast two-hybrid studies carried out at 30° C indicate that the β4a-A domain is capable of protein–protein interactions (N. Iverson, unpubl.), suggesting that the conformation of the A domain could be stabilized through protein–protein interactions. Our ongoing studies are addressing these possibilities.
This is the first report showing that residues in a domain outside the core of the Ca2+ channel β subunit are capable of independent folding. This is especially interesting in light of the fact that these are the regions that undergo alternative splicing. It has become increasingly apparent in recent years that alternative splicing, especially in the nervous system, has evolved to increase the number of unique proteins from what is a surprisingly limited gene pool (Lipscombe 2005). The results presented in this protein structure report are an important step toward understanding the structural and functional consequences of one of these alternative splicing events.
Materials and methods
Protein expression and purification
To determine the structure of the β4a-A domain, a PCR fragment encoding residues 1–58 of the full-length human β4a gene (accession no. NP_001005747) was cloned into a bacterial expression vector containing an N-terminal His-tag sequence (pET-15b, Novagen). The resulting construct was sequenced prior to expression and purification. The pET-15b β4a-A expression vector was transformed into Escherichia coli strain BL21-CodonPlus (DE3)-RIL (Stratagene). Cells were grown at 37° C to an optical density of 0.6 at 600 nm and induced with 1 mM isopropyl-β-D-thiogalactopyranoside (IPTG) (Anatrace) for 4 h. Cells were harvested by centrifugation and lysed by sonication in 50 mM sodium phosphate, 500 mM NaCl, and 10 mM imidazole (pH 8) (Ni2+-load buffer). Histidine-tagged β4a-A (His-β4a-A) was removed from the soluble fraction by Ni2+ affinity chromatography (His·Bind; Novagen), washed with Ni2+-load buffer to remove contaminants, and eluted with 50 mM sodium phosphate, 300 mM NaCl, and 400 mM imidazole (pH 8.4) (Ni2+-elute buffer). Excess imidazole was removed by dialyzing His-β4a-A against 50 mM sodium phosphate, 150 mM NaCl, and 10 mM imidazole (pH 8.4). The His-tag was removed by thrombin cleavage overnight at room temperature. The cleaved His-tag was separated from β4a-A by Ni2+ affinity chromatography. The flowthrough containing β4a-A was dialyzed against 50 mM sodium phosphate (pH 7.0) overnight at 4° C, prior to final purification by anion exchange chromatography (UNO Q6 column and Biologic DuoFlow System, BioRad) using a linear salt gradient from 0 to 1 M NaCl. Purified β4a-A was dialyzed against 16 L of water and stored as lyophilized powder. The identity and the purity of the β4a-A domain was confirmed with electrospray mass spectrometry, and the observed and the expected mass agreed to within 1 Da.
15N-labeled and 15N, 13C-labeled β4a-A was prepared as described above, except cells were grown in M9 minimal medium containing 0.8 g/L 15NH4Cl and 3 g/L 13C-glucose (McIntosh and Dahlquist 1990), supplemented with 10% 15N and 15N, 13C-labeled Celtone media (Spectra). The final yield of purified 15N, 13C uniformly labeled β4a-A was 25 mg/L.
Protein concentration determination
Concentrations of protein stock solutions were determined by absorbance in 6 M GuHCl, 10 mM sodium phosphate, and 150 mM sodium chloride (pH 6.5) at 25° C using an extinction coefficient for β4a-A of 4350 M−1 cm−1 (Edelhoch 1967).
NMR spectroscopy
NMR spectra were acquired with a Varian Unity Inova spectrometer operating at 500.1 MHz for 1H, 125.7 MHz for 15N, and 50.6 MHz for 13C. Internal DSS (2,2-dimethyl-2-silapen-tane-5-sulphonic acid) was used to standardize 1H, 15N, and 13C chemical shifts based on IUPAC recommendations (Wishart and Case 2001). Data were processed with NMRPipe (Delaglio et al. 1995) and analyzed with NMRView (Johnson and Blevins 1994) and ANSIG for Windows (Helgstrand et al. 2000). All spectra were acquired at 5° C on samples prepared in 50 mM sodium phosphate, 150 mM NaCl, 10% D2O, and 100 μM sodium azide (pH 5.5).
Sequence specific assignments of the β4a-A backbone resonances were obtained using combinations of gradient sensitivity-enhanced HNCA, HN(CO)CA; HNCO, HN(CA)CO; HNCACB and CBCA(CO)NH experiments as described elsewhere (Cavanagh et al. 1996). Sequence-specific side-chain assignments were accomplished with HNCACB, CBCA(CO)NH, 15N-edited HCCONH and TOCSY-HSQC, and 13C-edited HCCH-TOCSY experiments (Cavanagh et al. 1996). Backbone φ angles of β4a-A were constrained using the 3JHNHA experiment (Kuboniwa et al. 1994) with T1 and T2 values of 7.5 and 12.5 msec, respectively. Experiments were run with spectral windows of 6982 and 5500 for 1H in the direct and the indirect dimensions, respectively; 1700 for 15N; and 10,063 for 13C. Linear prediction was applied to all data prior to apodization, zero filling, and Fourier transformation.
Structure determination
A total of 1156 structural restraints were used to calculate the final 15 representative solution structures of the β4a-A domain (Fig. 2). Nine hundred sixty-three NOE derived distance constraints were utilized in the calculation, of which 472 were intraresidue, 196 sequential, 87 medium-range (4 < |i − j|>1), and 208 long-range (|i −j| ≥ 4) (Table 1), providing an average of 15.8 NOE constraints/residue. NOE cross-peak intensities were classified into three categories: strong (1.8–2.5 Å), medium (2.6–3.5 Å), and weak (3.6–6.0 Å). A total of 104 φ and ψ dihedral angle constraints were obtained from a directly measured 3JHNHA experiment and the TALOS program (Cornilescu et al. 1999), respectively. Thirty-nine Chi (χ1) dihedral angles were restrained to one of three values (60°, 180°, −60°) based on measured HNHB coupling constants or α β2-α β3/NHβ2-NHβ3 NOE profiles. A total of 25 hydrogen bond restraints were used in structure calculations, based on 3JHNHA coupling constants, 15N-edited NOESY-HSQC spectral analysis, and preliminary structure calculations. Structure calculations were performed with Crystallography and NMR System (CNS) (Brunger et al. 1998). Initial high-temperature annealing was set at 50,000 K with 1000 dynamic steps of 15 fsec, and NOE and dihedral scaling factors of 150 and 100, respectively, followed by a primary torsion slow-cooling stage of 1000 steps of 15 fsec with a temperature gradient from 50,000 to 0 K in 250-K increments. A second cartesian slow-cooling stage was performed consisting of 3000 steps of 5 fsec, cooling from 2000 to 0 K. Final minimization consisted of 10 cycles of 200 steps with dihedral angle and NOE energy constants set to 400 and 200 kcal mol−1 Å−4, respectively.
Acknowledgments
This work was supported by a grant from the NIH (R01 NS42600) to W.A.H. We thank Nicole Iverson for assistance in protein purification, and Ryan McKay (NANUC, Alberta, Canada) and Pascal Mercier (University of Alberta, Canada) for helpful discussions relating to data analysis.
Abbreviations
AID, α 1 subunit interaction domain
DSS, 2-2-Di-methyl-2-silapentane-5-sulfonic acid
GK, guanylate-kinase
HSQC, heteronuclear single quantum coherence
MAGUK, membrane associated guanylate-kinase
NMR, nuclear magnetic resonance
NOE, nuclear Overhauser effect
NOESY, NOE spectroscopy
PCR, polymerase chain reaction
RMSD, root-mean-square deviation
SH3, src homology 3
TOCSY, total correlation spectroscopy
Article published online ahead of print. Article and publication date are at http://www.proteinscience.org/cgi/doi/10.1110/ps.051894506.
References
- Arikkath, J. and Campbell, K.P. 2003. Auxiliary subunits: Essential components of the voltage-gated Ca2+ channel complex. Curr. Opin. Neurobiol. 13: 298–307. [DOI] [PubMed] [Google Scholar]
- Brunger, A.T., Adams, P.D., Clore, G.M., DeLano, W.L., Gros, P., Grosse-Kunstleve, R.W., Jiang, J.S., Kuszewski, J., Nilges, M., and Pannu, N.S. 1998. Crystallography & NMR System: A new software suite for macromolecular structure determination. Acta Crystallogr. D Biol. Crystallogr. 54: 905–921. [DOI] [PubMed] [Google Scholar]
- Catterall, W.A. 2000. Structure and regulation of voltage-gated Ca2+ channels. Annu. Rev. Cell Dev. Biol. 16: 521–555. [DOI] [PubMed] [Google Scholar]
- Cavanagh, J., Fairbrother, W.J., Palmer III, A.G., and Skelton, N.J. 1996. Protein NMR spectroscopy: Principles and practice, p. 597. Academic Press, San Diego, CA.
- Chen, Y.H., Li, M.H., Zhang, Y., He, L.L., Yamada, Y., Fitzmaurice, A., Shen, Y., Zhang, H., Tong, L., and Yang, J. 2004. Structural basis of the α 1-β subunit interaction of voltage-gated Ca2+ channels. Nature 429: 675–680. [DOI] [PubMed] [Google Scholar]
- Cornilescu, G., Delaglio, F., and Bax, A. 1999. Protein backbone angle restraints from searching a database for chemical shift and sequence homology. J. Biomol. NMR 13: 289–302. [DOI] [PubMed] [Google Scholar]
- Dalton, S., Takahashi, S.X., Miriyala, J. and Colecraft, H.M. 2005. A single Cavβ can reconstitute both trafficking and macroscopic conductance of voltage-dependent Ca2+ channels. J. Physiol. 567 (Pt. 3): 757–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaglio, F., Grzesiek, S., Vuister, G.W., Zhu, G., Pfeifer, J., and Bax, A. 1995. NMRPipe: A multidimensional spectral processing system based on UNIX pipes. J. Biomol. NMR 6: 277–293. [DOI] [PubMed] [Google Scholar]
- Edelhoch, H. 1967. Spectroscopic determination of tryptophan and tyrosine in proteins. Biochemistry 6: 1948–1954. [DOI] [PubMed] [Google Scholar]
- Hanlon, M.R., Berrow, N.S., Dolphin, A.C., and Wallace, B.A. 1999. Modelling of a voltage-dependent Ca2+ channel β subunit as a basis for understanding its functional properties. FEBS Lett. 445: 366–370. [DOI] [PubMed] [Google Scholar]
- Helgstrand, M., Kraulis, P., Allard, P., and Hard, T. 2000. Ansig for Windows: An interactive computer program for semiautomatic assignment of protein NMR spectra. J. Biomol. NMR 18: 329–336. [DOI] [PubMed] [Google Scholar]
- Helton, T.D., and Horne, W.A. 2002. Alternative splicing of the β4 subunit has α 1 subunit subtype-specific effects on Ca2+ channel gating. J. Neurosci. 22: 1573–1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helton, T.D., Kojetin, D.J., Cavanagh, J., and Horne, W.A. 2002. Alternative splicing of a β4 subunit proline-rich motif regulates voltage-dependent gating and toxin block of Cav2.1 Ca2+ channels. J. Neurosci. 22: 9331–9339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson, B.A. and Blevins, R.A. 1994. NMRView: A computer program for the visualization and analysis of NMR data. J. Biomol. NMR 4: 603–614. [DOI] [PubMed] [Google Scholar]
- Kuboniwa, H., Grzesiek, S., Delaglio, F., and Bax, A. 1994. Measurement of HN-Hα J couplings in Ca2+-free calmodulin using new 2D and 3D water-flip-back methods. J. Biomol. NMR 4: 871–878. [DOI] [PubMed] [Google Scholar]
- Lipscombe, D. 2005. Neuronal proteins custom designed by alternative splicing. Curr. Opin. Neurobiol. 15: 358–363. [DOI] [PubMed] [Google Scholar]
- Maltez, J.M., Nunziato, D.A., Kim, J., and Pitt, G.S. 2005. Essential Cavβ modulatory properties are AID-independent. Nat. Struct. Mol. Biol. 12: 372–377. [DOI] [PubMed] [Google Scholar]
- McGee, A.W., Dakoji, S.R., Olsen, O., Bredt, D.S., Lim, W.A., and Prehoda, K.E. 2001. Structure of the SH3-guanylate kinase module from PSD-95 suggests a mechanism for regulated assembly of MAGUK scaffolding proteins. Mol. Cell. 6: 1291–1301. [DOI] [PubMed] [Google Scholar]
- McGee, A.W., Nunziato, D.A., Maltez, J.M., Prehoda, K.E., Pitt, G.S., and Bredt, D.S. 2004. Calcium channel function regulated by the SH3-GK module in β subunits. Neuron 42: 89–99. [DOI] [PubMed] [Google Scholar]
- McIntosh, L.P. and Dahlquist, F.W. 1990. Biosynthetic incorporation of 15N and 13C for assignment and interpretation of nuclear magnetic resonance spectra of proteins. Q. Rev. Biophys. 23: 1–38. [DOI] [PubMed] [Google Scholar]
- Opatowsky, Y., Chen, C.C., Campbell, K.P., and Hirsch, J.A. 2004. Structural analysis of the voltage-dependent Ca2+ channel β subunit. Neuron 42: 387–399. [DOI] [PubMed] [Google Scholar]
- Pragnell, M., De Waard, M., Mori, Y., Tanabe, T., Snutch, T.P., and Campbell, K.P. 1994. Calcium channel β subunit binds to a conserved motif in the I–II cytoplasmic linker of the α 1 subunit. Nature 368: 67–70. [DOI] [PubMed] [Google Scholar]
- Richards, M.W., Butcher, A.J., and Dolphin, A.C. 2004. Ca2+ channel β-subunits: Structural insights AID our understanding. Trends Pharmacol. Sci. 25: 626–632. [DOI] [PubMed] [Google Scholar]
- Ruth, P., Rohrkasten, A., Biel, M., Bosse, E., Regulla, S., Meyer, H.E., Flockerzi, V., and Hofmann, F. 1989. Primary structure of the β subunit of the DHP-sensitive Ca2+ channel from skeletal muscle. Science 245: 1115–1118. [DOI] [PubMed] [Google Scholar]
- Van Petegem, F., Clark, K.A., Chatelain, F.C., and Minor Jr., D.L. 2004. Structure of a complex between a voltage-gated Ca2+ channel β subunit and an α subunit domain. Nature 429: 671–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White, J.V., Stultz, C.M., and Smith, T.F. 1994. Protein classification by stochastic modeling and optimal filtering of amino-acid sequences. Math. Biosci. 119: 35–75. [DOI] [PubMed] [Google Scholar]
- Wishart, D.S. and Case, D.A. 2001. Use of chemical shifts in macromolecular structure determination. Methods Enzymol. 338: 3–34. [DOI] [PubMed] [Google Scholar]