Abstract
Remyelination of focal areas of the central nervous system (CNS) in animals can be achieved by transplantation of glial cells, yet the source of these cells in humans to similarly treat myelin disorders is limited at present to fetal tissue. Multipotent precursor cells are present in the CNS of adult as well as embryonic and neonatal animals and can differentiate into lineage-restricted progenitors such as oligodendroglial progenitors (OPs). The OPs present in adults have a different phenotype from those seen in earlier life, and their potential role in CNS repair remains unknown. To gain insights into the potential to manipulate the myelinating capacity of these precursor and/or progenitor cells, we generated a homogenous culture of OPs from neural precursor cells isolated from adult rat subependymal tissues. Phenotypic characterization indicated that these OPs resembled neonatal rather than adult OPs and produced robust myelin after transplantation. The ability to generate such cells from the adult brain therefore opens an avenue to explore the potential of these cells for repairing myelin disorders in adulthood.
Remyelination of the central nervous system (CNS) in patients where host remyelination fails or where the endogenous myelinating cells are genetically impaired may be achieved, at least focally, by glial cell transplantation. It has been assumed that human fetal brain will be the only viable source of myelinating cells for human transplantation, because oligodendroglial progenitors (OPs) derived from embryonic animals have a greater capacity for myelination than mature cells after transplantation (1, 2). However, the availbility of human fetal tissues remains a practical and ethical concern, and it would be preferable if the neonatal or adult human brain could be used as a source of myelinating cells. It has been established that OPs are present in adult human brain (3). Such cells have also been described in patients with multiple sclerosis (4) and in rodents with chronic experimental allergic encephalomyelitis (5). Despite their presence in chronic multiple sclerosis lesions, remyelination may be inadequate (6, 7), and either exogenous myelinating cells must be targeted to lesions or host cells must be recruited to aid in repair.
Two types of OP [also designated in vitro as oligodendrocyte type-2 astrocyte (O2A) progenitor] exist in the CNS; the neonatal OP (O2Aperinatal) that appears in the rat postnatally and disappears about 6 weeks after birth, and the adult OP (O2Aadult) (8, 9). The O2Aadult, which is identified by the mAb O4 in situ and in vitro, has a phenotype that distinguishes it from its neonatal counterpart. The most thoroughly characterized O2Aadult cells are those isolated from adult rat optic nerves, although similar cells are found in other parts of the CNS such as the spinal cord (10). Unlike the O2Aperinatal, the O2Aadult does not express the intermediate filament vimentin or a ganglioside recognized by the mAb A2B5. The O2Aadult cells also have a longer cell cycle time (65 ± 18 h) and are less motile (4 ± 1 μm/h) than O2Aperinatal (9). These characteristics suggest that they would only have a limited capacity to remyelinate demyelinated areas of the brain. In fact, it is not yet known whether these cells produce myelin in vivo, for example, after transplantation.
The OPs are generally thought to be derived from multipotent neural precursor cells or early progenitor cells in the CNS. Neural stem cells, which can give rise to both neurons and glia, have been found in the CNS of both embryonic and mature animals (11, 12). Clonal analyses suggest that the stem cells from adult CNS are similar to those of embryonic origin (11). At least, these adult stem cells can differentiate into neurons, astrocytes, and oligodendrocytes in vitro. It is not yet known whether adult stem cells differentiate into O2Aadult directly.
We have been studying the transition from multipotent precursor cells to lineage-restricted OPs and have shown that it is possible to generate a large number of self-renewing OPs from neural precursor cells derived from embryonic and neonatal brain (13, 14). Because multipotent stem cells exist in adult CNS, we sought to explore whether the OPs derived from adult neural stem or precursor cells have the capacity for extensive myelination. If this were proven in the rodents, a similar approach could provide cells for transplantation or suggest means for the induction of endogenous progenitors to enhance host repair in humans.
MATERIALS AND METHODS
Cell Culture.
The neural precursor cells in suspension culture (“neurospheres”) were prepared from subependymal striata of Wistar rats aged 3 and 16 months according to a protocol detailed previously (13, 15). The culture medium was DMEM/F-12 (1:1) supplemented with insulin (25 μg/ml), transferrin (100 μg/ml), progesterone (20 nM), putrescine (60 μM), and sodium selenite (30 nM). The above medium, referred to as “neurosphere medium,” was supplemented with 20 ng/ml human recombinant epidermal growth factor (EGF) or EGF plus 20 ng/ml of basic fibroblast growth factor (bFGF) (Collaborative Biomedical Products, Bedford, MA). In the initial week of culture, B27 (GIBCO) was added to the above medium. The cultures were incubated in a humidified atmosphere of 5% CO2/95% air with a partial medium change every other day.
The B104 neuroblastoma cells were cultured according to Louis et al. (16), and the conditioned medium (B104CM) was collected and filtered after 3 days of conditioning the B104 cells with serum-free “neurosphere medium.”
BrdUrd Incorporation Assay.
The coverslip cultures were incubated in 10 μM BrdUrd (Sigma) for various periods (see Results), fixed in acidic ethanol, and immunostained with anti-BrdUrd antibody (Amersham Pharmacia) at a dilution of 1:10, followed by fluorescein-labeled secondary antibody. For cell cycle time estimation, the cultures were exposed to BrdUrd for a period of 0.5, 1, 3, 5 and up to 16–24 h. The BrdUrd-labeled cells and the total cells stained with Hoechst were counted under a fluorescent microscope. The percentage of the labeled cells was plotted against the time the cells were pulsed, and the cell cycle time was estimated according to the graphic method of Sasaki et al. (17).
Assay of Cell Migration.
A single sphere was plated onto ornithine-coated 35-mm dishes in a drop of medium. After the sphere attached (10–15 min), 1.5 ml of medium was added gently. Only the samples with successfully attached sphere and without floating cells were followed at 4, 8, and 24 h postplating. The outgrowth of the sphere was examined under the phase-contrast microscope, and the images were photographed and stored in a computer. The longest distance from the edge of a sphere to the cell body in each quarter of the outgrowth was measured and the average distance of cells moved out of a single sphere at specific time points was calculated (13). At least 8 spheres were followed throughout the period of each individual experiment, and the experiment was repeated twice.
Immunocytochemistry.
Free-floating spheres or coverslip cultures were immunolabeled with fluorescein-tagged secondary antibodies (Jackson ImmunoResearch) according to the procedure detailed previously (13). The following primary antibodies were used. Monoclonal antibody anti-nestin (IgG) was a supernatant of mouse hybridoma rat401 (diluted 1:5), provided by Developmental Studies Hybridoma Bank (The Johns Hopkins University, Baltimore). A2B5 was a culture medium of mouse hybridoma clone 105 (American Type Culture Collection, CRL-1520, used at 1:100 dilution). O4 and O1 (both were IgM) were provided by M. Schachner. Anti-myelin basic protein (MBP, mouse IgG, 1:100) was from Boehringer Mannheim. Anti-vimentin (mouse IgG) and anti-βIII-tubulin (rabbit IgG) were purchased from Sigma (1:100). Polyclonal antibodies anti-glial fibrillary acidic protein (GFAP, 1:200) was purchased from Dako, and anti-platelet-derived growth factor receptor α (PDGFRα, 1:100) was from Santa Cruz Biotechnology.
Transplantation of Oligosphere Cells.
The oligospheres were triturated into single cells and were then concentrated to 50,000 cells per microliter. One microliter of cell suspension was transplanted into the spinal cord of postnatal day 6–8 myelin-deficient (md) rats according to the procedure described (13, 18). The injection site was marked with sterile charcoal before the incision was sutured.
Twelve to fourteen days after transplantation, the recipient rats were anesthetized with pentobarbital (i.p.) and perfused with 4% formaldehyde. The spinal cord was dissected and the white streak representing myelin made by the transplanted cells was measured. The spinal cords were then trimmed for immunostaining with anti-proteolipid protein (PLP, a gift from I. R. Griffiths, University of Glasgow) or for resin-embedding as described (13, 14).
RESULTS
Establishment of OP Cultures from Adult Rats.
The OPs were generated from neural precursors by using the approach described (13, 14). In the present study, cultures of neurospheres were initiated from subependymal striata of adult Wistar rats (aged 3 and 16 months). When cultured in the presence of EGF and absence of substrate, scattered phase-bright cells were found among debris at 4–7 days in vitro (DIV). These few cells grew into spheres in the subsequent 2–3 weeks. These spheres were triturated into single cells and expanded in the presence of EGF alone or EGF plus bFGF. Expanded neurosphere cells were immunopositive for nestin (Fig. 1 a and b), an intermediate filament protein mainly expressed by stem or precursor cells (19). When plated on poly(ornithine)-coated coverslips in the presence of 1% FBS but the absence of EGF or bFGF, the neurosphere cells migrated out and differentiated into a mixture of mainly astroglia (GFAP+) with flattened cell bodies and thick processes and some oligodendroglia (O4+). Some spheres also contained neurons that were βIII-tubulin+ (data not shown). The neurospheres were triturated into single cells and passaged in neurosphere medium with the presence of EGF and bFGF. These observations suggest that neurosphere cells are undifferentiated neural precursor cells, similar to those isolated from embryonic and neonatal striatum (13, 14).
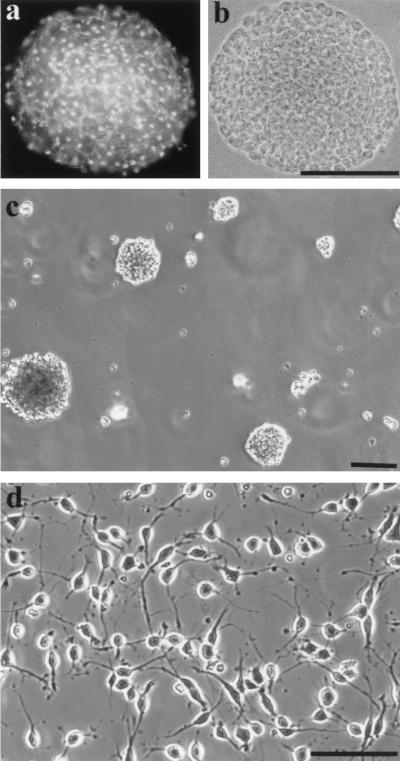
Figure 1.
A neurosphere (from 16-month-old rat) grown in the presence of EGF immunostained with nestin indicated that all cells were nestin+ (a). b shows the phase-contrast image of a. (c) New spheres were generated from disaggregated oligosphere cells. (d) Disaggregated oligosphere cells displayed bipolar or tripolar morphology in the presence of B104CM. (Bar = 100 μm.)
To generate OPs from neurospheres, we gradually changed the EGF-containing medium to B104CM-containing medium by replacing one-fourth of the former medium with the latter medium every other day. During the transition period (1–2 weeks), the number and size of spheres did not increase. This is similar to the phenomenon observed in the neurosphere cultures from neonatal rat (13). By week two, the size and number of spheres began to increase. Three to four weeks later, the cultures were passaged in medium containing B104CM (30%) but no EGF or bFGF by plating 1 × 106 cells into a 75-cm2 flask. New spheres with various sizes formed in 1 week (Fig. 1c). When the spheres were triturated into single cells and plated onto ornithine-coated coverslips, all cells displayed bipolar or tripolar morphology, typical of O2A progenitors (Fig. 1d). Therefore, the spheres were now referred to as “oligospheres,” a term that was first used by Evercooren and colleagues (24). Similar results were obtained when generating oligospheres from neurospheres that were derived from both 3-month- and 16-month-old rat brains by using the same protocol.
Antigenic Expression of Oligosphere Cells.
The O2Aperinatal displays a bipolar morphology and is positive for A2B5, whereas the O2Aadult is unipolar and O4+ (9). In contrast to the O2Aadult previously derived from the adult optic nerve, all oligosphere cells exhibited bi- or tripolar morphology and expressed vimentin, A2B5, and PDGFRα (Fig. 2 a–c) when the oligospheres were disaggregated and cultured on ornithine-coated coverslips at a density of 1 × 105 per coverslip in the presence of B104CM. These cells were negative for O4 (Fig. 2d). Within a week, the cultures were confluent. Similar results were obtained when the cells were cultured in the presence of both PDGF (10 ng/ml) and bFGF (20 ng/ml) except that they did not reach confluency until about 10 DIV. When the cells were cultured in the presence of PDGF alone with addition of PDGF every other day for 7 DIV, many cells were still bipolar or tripolar (Fig. 2e) and the majority were positive for A2B5 (90.9 ± 2.4%; n = 5), vimentin, and PDGFRα. A small number of cells (5.2 ± 3.0%; n = 5), however, became multiprocess-bearing and O4+. In addition, some cells were round without processes. These round cells were positive for A2B5 and vimentin but negative for O4, similar to those seen in the presence of B104CM.
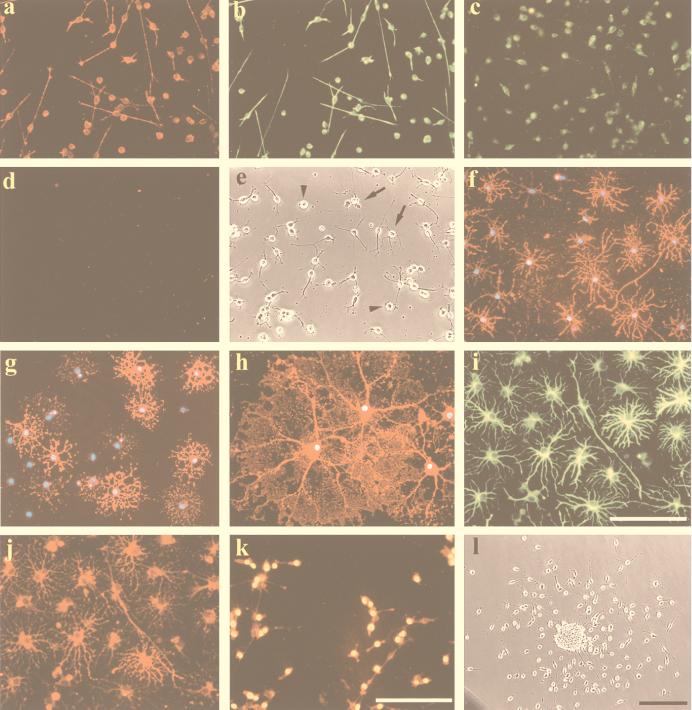
Figure 2.
The oligosphere cells cultured on ornithine-coated coverslips in the presence of B104CM were positive for vimentin (a), A2B5 (b), and PDGFRα (c) but negative for O4 (d). In the presence of PDGF alone for 7 DIV, the oligosphere cells were largely bipolar or tripolar. There were also round cells (arrowheads) and a few multiprocess-bearing cells (arrows) (e). In the presence of 0.5% FBS for 2 DIV, all cells were O4+ (f) and many cells were O1+ (g). At 7 DIV, cells were MBP+ (h). In the presence of 10% FBS, almost all cells were positive for GFAP (i) and A2B5+ (j). Incubation of the culture with BrdUrd for 20 h indicated that the majority of cells were labeled in the nuclei (yellow in k). All cells were A2B5+ (red in k). (l) A single sphere plated on ornithine-coated dish in the presence of B104CM for 24 h shows that bipolar cells migrated out of the sphere. The nuclei of cells in (f–h) were stained with 4′,6-diamidino-2-phenylindole (DAPI). (Bar = 100 μm.)
Differentiation of Oligosphere Cells.
The O2Aadult cells differentiate more slowly than their neonatal counterparts (9). To assess the potential and speed of differentiation, oligosphere cells were cultured in the medium consisting of DMEM and 0.5% FBS. The cultures were immunostained with O4, O1, and anti-MBP antibodies, which recognize progressively later developmental stages of oligodendroglial lineage. At 2 DIV, virtually all of the cells were O4+ (Fig. 2f). At the same time, 57.5 ± 4.4% (n = 6) of the cells were already O1+, although the staining was mainly in the cell bodies and main processes (Fig. 2g). At 3 DIV, the majority of cells were O1+. At 5–7 DIV, most cells were positive for MBP, displaying membrane-like structures (Fig. 2h). In the presence of high concentrations of FBS (5–10%), the majority of cells were flattened, with star-shaped processes, and expressed both GFAP (Fig. 2i) and A2B5 (Fig. 2j). Similar results were obtained when oligosphere cells of passage 4 or 12 from both ages were examined.
Proliferation Potential.
When 1 × 106 oligosphere cells were plated in the presence of 30% B104CM, (8.8 ± 1.2) × 106 (n = 3) cells were obtained in 7 DIV. A similar number of cells were generated when oligospheres from passage 2–12 were examined. The oligospheres could also be expanded in the presence of PDGF plus bFGF, although the yield was lower.
The cell cycle time for oligosphere cells in the presence of B104CM was estimated by using the graphic method described by Sasaki (17). The phase of DNA synthesis was deduced as 6.8–8.4 h from the linear regression of BrdUrd incorporation over incubation time (based on three independent experiments). The total cell cycle time was estimated to be about 20 hours. Incubation of the cells with BrdUrd for 20 h led to ≈92% of the cells labeled with anti-BrdUrd (Fig. 2k).
To assess the proliferation potential of oligosphere cells in response to growth factors, oligospheres (passage 4 and 10) were triturated and cultured for 3 days on coated coverslips in the presence of B104CM (30% vol/vol), bFGF (20 ng/ml), PDGF (10 ng/ml), and PDGF plus bFGF. The cultures were then exposed to BrdUrd for 4 h, and the incorporation of BrdUrd into nuclei was assessed. Without the presence of B104CM or above growth factors, cells differentiated into oligodendrocytes (O1+) and did not incorporate BrdUrd. In the presence of B104CM or growth factors, cells incorporated BrdUrd into their nuclei. The highest percentage of cells incorporating BrdUrd were the cells treated with B104CM (46%), followed by bFGF plus PDGF, bFGF, and PDGF (Table 1). This pattern of growth response of oligosphere cells is similar to that of O2Aperinatal cells in response to growth factors (20, 21).
Table 1.
BrdUrd incorporation by oligosphere cells
| PDGF | bFGF | PDGF/bFGF | B104CM | |
|---|---|---|---|---|
| BrdUrd+, % | 29.5 ± 2.84 | 34.2 ± 2.27 | 36.9 ± 3.25 | 46 ± 4.13 |
BrdUrd+ cells and total cells were counted in four optic fields of each coverslip. Each group consisted of at least four coverslips. Total cell counts in each group were 3,300–3,965. The data were from the experiment with passage 4 cells derived from a 3-month-old rat.
To examine whether a single cell can renew itself and regenerate an oligosphere, a single sphere cell was plated in each well of a 96-well plate containing 200 μl of B104CM (30%)-containing neurosphere medium (13). After 7 days, the plates were reexamined, and the wells containing sphere(s) were marked. The percentage of the cells able to generate new sphere(s) was ≈29% (32/111). The clonally expanded cells retained the same potential to differentiate into oligodendroglia or type-2 astroglia in vitro (see above). Similar results were obtained when a single cell was plated into ornithine-coated 96-well plate except that the generated cells did not form a sphere (data not shown).
Migration of Oligosphere Cells.
After the oligosphere attached, individual cells migrated out of the sphere within 1 h. At 4 h post-plating, cells were found surrounding the whole sphere. The migration velocity was calculated based on the average distance of cells moving away from the sphere at 4, 8, 12, and 24 hours post-plating. Migration velocity was 25 ± 5.4 μm/h (n = 10) in the presence of B104CM and 13.5 ± 1.7 μm/h (n = 8) in the presence of PDGF (10 ng/ml) for oligospheres (passage 8) derived from the 3-month-old rat. Cells migrating out of the sphere were bipolar (Fig. 2l). Unlike the oligosphere cells derived from neonatal rat, the pattern of migration was not always radially oriented. Similar results were obtained when spheres from the 16-month-old rat (passage 6) were examined.
Myelination Potential by Oligosphere Cells.
Oligosphere cells of passage 8 from a 16-month-old rat and passage 4 and 12 derived from a 3-month-old rat were transplanted into the spinal cords of 24 md rats. Twelve to fourteen days after transplantation, a white streak, of average 4 mm (3.0–6.5 mm) in length, was present in the dorsal column of the spinal cord of the md rat, which is otherwise semitranslucent because of the lack of myelin (Fig. 3a). A white streak of 3.9 ± 1.25 mm (n = 7) formed by cells of passage 4 and 3.8 ± 1.5 mm (n = 8) formed by cells of passage 12 that were both derived from the 3-month-old rat. When cells (passage 8) from the 16-month-old rat were transplanted, a white streak of 4.2 ± 1.0 mm (n = 9) formed. There was no difference in the degree of longitudinal spread of transplanted cells and myelination by cells from both ages or cells from passage 4 and 12. A cross section of the spinal cord indicated that the white patch occupied most of the dorsal funiculus. Immunostaining of the spinal cord sections indicated that the myelin sheaths formed by the transplanted cells were positive for PLP (Fig. 3b) as well as for MBP (data not shown). The host spinal cord lacks PLP-positive myelin because of a mutation in the PLP gene (22), although PLP+ oligodendrocytes were detected in freshly prepared tissues (Fig. 3b). Toluidine blue-stained semithin sections (1 μm) confirmed that the majority of axons in the dorsal funiculus were myelinated (Fig. 3c). There was no obvious difference between the samples with cells from different ages in terms of the amount of myelin that are present in the transverse section.
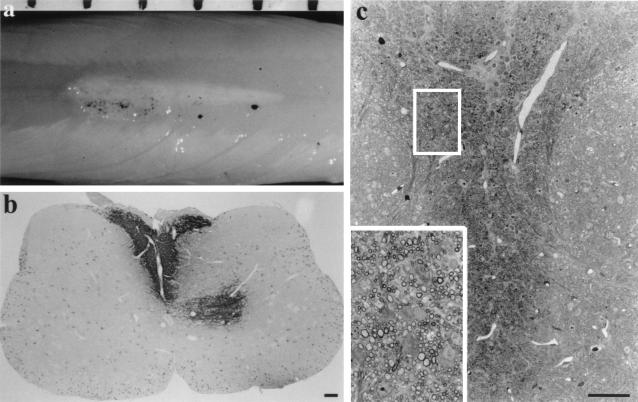
Figure 3.
Transplantation of oligosphere cells from a 16-month-old rat into md rats. Twelve to fourteen days later, a white streak of myelin was seen along the dorsal surface of the cord (a). The black dots are sterile charcoal marking the injection site. The space bar on top represents 1 mm. (b) Immunostaining of the transplanted cord showed PLP+ myelin in the dorsal funiculus with some myelin also appeared in the gray matter. Other areas of the spinal cord showed no PLP+ myelin except the PLP+ cell bodies. (c) Semithin sections stained with toluidine blue demonstrated that the dorsal funiculus was occupied by a large number of myelin sheaths. Inset is the enlargement of the boxed area in c. (Bar = 100 μm.)
DISCUSSION
The major finding of this study is that the adult brain can be used as a source of OPs with the O2Aperinatal phenotype and that these cells can be propagated extensively to generate a large number of progenies that maintain their myelinating potential. If similar approaches were feasible in humans, it would be possible to generate large numbers of cells by ex vivo manipulation with growth factors, before transplantation. Similarly, it raises the possibility that such cells might be induced to expand by in vivo growth factor application and be recruited to target areas of demyelination in the human brain.
Oligosphere Cells Derived from Adult Brain Resemble O2Aperinatal Cells.
O2Aperinatal cells can be isolated and expanded from neonatal rodents by using growth factors or conditioned media when the cells are in peak proliferation (23–25). We have explored alternative means of deriving such cells from multipotential neural precursor cells isolated from neonatal (13) or embryonic rat brains (S.-C.Z., unpublished data) by analogy to the hematopoietic cell lineage development (26). Because multipotential precursor cells exist in the CNS of adult (11, 15) as well as in embryonic stage (12), it is possible that OPs may be generated from adult CNS precursor cells as well. Therefore, the establishment of a homogeneous population of OPs from adult neural precursors was not unexpected. However, that all of the cells were positive for vimentin and A2B5 but negative for O4 contrasts with the antigenic phenotype of the O2Aadult as isolated directly from adult rat optic nerves (9). More importantly, the oligosphere cells proliferate much more vigorously and differentiate and migrate faster than the O2Aadult progenitors detailed in a series of studies performed by Noble and colleagues (9, 28–30). Therefore, the OPs from adult neural precursor cells resemble neonatal rather than adult O2As isolated directly from rat optic nerves. This conclusion is further supported by the antigenic expression and proliferation potential of oligosphere cells when they were cultured in the presence of PDGF instead of B104CM, a culture condition similar to that under which O2Aadult cells were characterized (9, 27). It should be noted that the population expansion does not parallel the cell cycle time of oligosphere cells. This is mainly due to cell death after mechanical disaggregation and death within spheres. The slower migration in the presence of PDGF alone is potentially accounted for by the techniques used and the growth factors present. Small et al. (27) measured the distance a cell moved (in all directions) directly by time-lapse cinematography. We could only measured the linear distance away from the sphere. In the presence of B104CM, the adult oligosphere cells migrated in a similar velocity as neonatal oligosphere cells (13). This result suggests that adult oligosphere cells are similarly motile to neonatal oligosphere cells and that factors other than PDGF also contribute to the migration of OPs. This is further supported by the similar extent of myelination by transplanted adult oligosphere cells as by neonatal oligosphere cells (13) or by the CG4 oligodendroglial progenitor cell line (18).
Oligosphere Cells Are Derived from Neural Precursor Cells.
The O2Aadult are derived from their neonatal counterparts (29, 31) and may regain the neonatal phenotype temporarily under certain circumstances, such as in the presence of both bFGF and PDGF (30, 32). Is the generation of neonatal-type OPs in the present study attributable to B104CM converting the adult OPs into neonatal progenitors? Our finding does not support this possibility, because the source cells (neurospheres) are nestin+ and the replacement of B104CM with PDGF in oligosphere cell cultures does not lead to the expression of the O2Aadult phenotype. We have attempted to generate oligospheres directly from (mechanically and enzymetically) dissociated adult (5-month-old) rat brain and optic nerves by using B104CM. The resultant culture contained floating cells that survived for up to 2 weeks in suspension but did not proliferate (data not shown). A recent observation also indicated that B104CM did not enhance the proliferation of purified O2Aadult progenitors (31) or convert the O2Aadult to O2Aperinatal (B. A. Barres, personal communication). B104CM is a potent mixture in selecting and propagating O2Aperinatal in culture (23–25). It may be speculated that some O2Aperinatal are selectively expanded by B104CM in the present study. The presence of O2Aperinatal in the adult CNS was reported based on their bipolar morphology and A2B5 positivity in a mixed culture (33). However, when the mixed glial cultures were irradiated, no O2Aperinatal developed (34), implying that in that study, O2Aperinatal cells were being generated de novo from A2B5-negative preprogenitor cells that were also present in the cultures. In a purified culture system, the O2A cells from adult (2-month-old) rat optic nerve displayed bipolar morphology and were immunoreactive to A2B5 (31), similar to those reported by Ffrench-Constant and Raff (33). Yet they had a very slow turnover rate (cell cycle time around 3 days), characteristic of O2Aadult cells. In our preparation of neurosphere cultures, these rare O2Aperinatal (if they are present) would be unlikely to survive in the condition without substrate and survival factors such as PDGF for a long time (>4 weeks). Our previous study (13) indicated that EGF is not a survival factor for OPs in suspension cultures. Our failure to generate oligospheres directly from dissociated adult brain and optic nerves suggests that either there are no O2Aperinatal present or such cells do not survive the procedure and culture condition. Therefore, the cells used for generating OPs are unlikely to contain cells that are already in the oligodendroglial lineage. Thus, the present study extends our previous argument that factors in B104CM may induce neural precursor cells to commit to oligodendroglial lineage while at the same time maintain the OPs in a state of self-renewal (13).
Multipotent Neural Precursor Cells as a Source for Remyelination.
The generation and extensive propagation of the neonatal type of OPs from the adult rat brain has an important impact on the design of strategies for promoting remyelination in vivo. In the first instance, as we show here, it may be possible to similarly derive progenitor cells of the neonatal phenotype from the adult human brain for transplantation. Extensive animal studies suggest that transplantation of myelinating cells, especially their progenitor cells, may be an effective approach (1, 2, 36, 37). In clinical human trials, however, cell availability becomes a problem if the cells are to be obtained from a source other than the patient. At present, human fetal tissues are the only source of immature neural cells. However, there are long-term practical and ethical concerns on the availability of such tissue, including stringent safety concerns. Here we show that it is possible to generate a large number of OPs from a small source of tissue in the rodent brain. A similar approach may be possible by biopsy from the human brain with ex vivo conversion of neural precursors to OPs with subsequent expansion. Such transplantation would therefore be autologous and obviate the need for immunosuppression.
The alternative approach is to recruit endogenous OPs to instigate repair. Cells that are responsible for remyelination in adults are mainly dividing “progenitor cells” (38, 39). The O4+ multiprocess-bearing cells that are regarded as the O2Aadult in vivo have been found in the CNS of normal and (myelin) diseased animals and humans (4, 5, 40). The apparent lack or limit of remyelination in terms of the universal existence of O4+ O2Aadult suggests that either the environment or the cells’ intrinsic properties (or both) is responsible. In the presence of (lysolecithin-indueced) demyelination, retrovirus-labeled proliferating progenitors failed to migrate even a short distance (<500 μm) over a period of 4 weeks to perform remyelination (38). Such a poor migration behavior may be intrinsic to the multiprocess-bearing O2Aadult rather than due to the nonpermissive environment, because transplanted neonatal OPs migrate a long distance and myelinate axons in dysmyelinated adult CNS (1, 36). Neuronal progenitors can also migrate a long distance from subependymal area to olfactory bulb in adult environment (41). In a separate study by Keirstead et al. (39), O2Aadult (identified by NG2 labeling) adjacent to focally demyelinated lesions decreased in number with time and were not mitotic, and they suggested therefore that the O2Aadult are inherently incapable of regeneration (39). Therefore, strategies designed to simply increase the number of O2Aadult, such as by delivering PDGF into the CNS (35), may not be effective. An alternative avenue to this strategy, therefore, is to promote the in vivo regeneration of the O2Aperinatal from host neural precursors or stem cells, in a similar fashion as suggested by the present study. Such cells are present in subependymal areas of the adult CNS and can differentiate into neurons and glia (11), therefore close to commonly affected areas in multiple sclerosis (6). The motility of O2Aperinatal might also indicate their ability to migrate to other parenchymal sites. The key to the application of these strategies in humans will be the identification of growth factors that have the biological effects both in vitro and in vivo on these precursor cells.
Acknowledgments
This work is supported by National Institutes of Health Grant NS33710.
ABBREVIATIONS
- CNS
central nervous system
- OP
oligodendroglial progenitors
- O2A
oligodendrocyte type 2 progenitor
- EGF
epidermal growth factor
- bFGF
basic fibroblast growth factor
- MBP
myelin basic protein
- GFAP
glial fibrillary acidic protein
- PLP
proteolipid
- DIV
days in vitro
References
- 1.Archer D R, Cuddon P A, Lipsitz D, Duncan I D. Nat Med. 1997;3:54–59. doi: 10.1038/nm0197-54. [DOI] [PubMed] [Google Scholar]
- 2.Warrington A E, Barbarese E, Pfeiffer S E. J Neurosci Res. 1993;34:1–13. doi: 10.1002/jnr.490340102. [DOI] [PubMed] [Google Scholar]
- 3.Armstrong R, Dorn H H, Kufta C V, Friedman E, Dubois-Dalcq M. J Neurosci. 1992;12:1538–1547. doi: 10.1523/JNEUROSCI.12-04-01538.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wolswijk G. J Neurosci. 1998;18:601–609. doi: 10.1523/JNEUROSCI.18-02-00601.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nishiyama A, Yu M, Drazba J A, Tuohy V K. J Neurosci Res. 1997;48:299–312. doi: 10.1002/(sici)1097-4547(19970515)48:4<299::aid-jnr2>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 6.Prineas J W, McDonald W I. In: Greenfield’s Neuropathology. 6th Ed. Graham D I, Lantos P L, editors. London: Arnold; 1997. pp. 813–881. [Google Scholar]
- 7.Raine C S. J Neuroimmunol. 1997;77:135–152. doi: 10.1016/s0165-5728(97)00073-8. [DOI] [PubMed] [Google Scholar]
- 8.Raff M C, Miller R H, Noble M. Nature (London) 1983;303:390–396. doi: 10.1038/303390a0. [DOI] [PubMed] [Google Scholar]
- 9.Wolswijk G, Noble M. Development (Cambridge, UK) 1989;109:691–698. doi: 10.1242/dev.109.3.691. [DOI] [PubMed] [Google Scholar]
- 10.Engel U, Wolswijk G. Glia. 1996;16:16–26. doi: 10.1002/(SICI)1098-1136(199601)16:1<16::AID-GLIA3>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 11.Weiss S, Dunne C, Hewson J, Wohl C, Wheatley M, Peterson A C, Reynolds B A. J Neurosci. 1996;16:7599–7609. doi: 10.1523/JNEUROSCI.16-23-07599.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reynolds B A, Weiss S. Dev Biol. 1996;175:1–13. doi: 10.1006/dbio.1996.0090. [DOI] [PubMed] [Google Scholar]
- 13.Zhang S C, Lundberg C, Lipsitz D, O’Connor L T, Duncan I D. J Neurocytol. 1998;27:475–489. doi: 10.1023/a:1006953023845. [DOI] [PubMed] [Google Scholar]
- 14.Zhang S C, Lipsitz D, Duncan I D. J Neurosci Res. 1998;54:181–190. doi: 10.1002/(SICI)1097-4547(19981015)54:2<181::AID-JNR6>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 15.Reynolds B A, Weiss S. Science. 1992;255:1707–1710. doi: 10.1126/science.1553558. [DOI] [PubMed] [Google Scholar]
- 16.Louis J C, Magal E, Muir D, Manthorpe M, Varon S. J Neurosci Res. 1992;31:193–204. doi: 10.1002/jnr.490310125. [DOI] [PubMed] [Google Scholar]
- 17.Sasaki K, Murakami T, Takahashi M. Cytometry. 1987;8:526–528. doi: 10.1002/cyto.990080514. [DOI] [PubMed] [Google Scholar]
- 18.Tontsch U, Archer D R, Dubois-Dalcq M, Duncan I D. Proc Natl Acad Sci USA. 1994;91:11616–11620. doi: 10.1073/pnas.91.24.11616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lendahl U, Zimmerman L, McKay R D G. Cell. 1990;60:585–595. doi: 10.1016/0092-8674(90)90662-x. [DOI] [PubMed] [Google Scholar]
- 20.McKinnon R D, Matsui T, Dubois-Dalcq M, Aaronson S A. Neuron. 1990;5:603–614. doi: 10.1016/0896-6273(90)90215-2. [DOI] [PubMed] [Google Scholar]
- 21.McKinnon R D, Smith C, Behar T, Smith T, Dubois-Dalcq M. Glia. 1993;7:245–254. doi: 10.1002/glia.440070308. [DOI] [PubMed] [Google Scholar]
- 22.Duncan I D. In: Neuroglia. Ransom B R, Kettenmann H R, editors. Oxford: Oxford Univ. Press; 1995. pp. 990–1009. [Google Scholar]
- 23.Hunter S F, Bottenstein J E. Dev Brain Res. 1990;54:235–248. doi: 10.1016/0165-3806(90)90146-p. [DOI] [PubMed] [Google Scholar]
- 24.Avellana-Adalid V, Nait-Oumesmar B, Lachapelle F, Evercooren A B. J Neurosci Res. 1996;45:558–570. doi: 10.1002/(SICI)1097-4547(19960901)45:5<558::AID-JNR6>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 25.Juurlink B H J, Thorburne S K, Devon R M. In: Protocols for Neural Cell Culture. 2nd Ed. Fedoroff S, Richardson A, editors. Totowa, NJ: Humana; 1996. pp. 143–156. [Google Scholar]
- 26.Morrison S J, Uchida N, Weissman I L. Annu Rev Cell Dev Biol. 1995;11:35–71. doi: 10.1146/annurev.cb.11.110195.000343. [DOI] [PubMed] [Google Scholar]
- 27.Small R, Riddle P, Noble M. Nature (London) 1987;328:155–157. doi: 10.1038/328155a0. [DOI] [PubMed] [Google Scholar]
- 28.Wolswijk G, Riddle P N, Noble M. Glia. 1991;4:495–503. doi: 10.1002/glia.440040509. [DOI] [PubMed] [Google Scholar]
- 29.Wren D, Wolswijk G, Noble M. J Cell Biol. 1992;116:167–176. doi: 10.1083/jcb.116.1.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wolswijk G, Noble M. J Cell Biol. 1992;118:889–900. doi: 10.1083/jcb.118.4.889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shi J, Marinovich A, Barres B A. J Neurosci. 1998;18:4627–4636. doi: 10.1523/JNEUROSCI.18-12-04627.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hunter S F, Bottenstein J E. J Neurosci Res. 1991;28:574–582. doi: 10.1002/jnr.490280415. [DOI] [PubMed] [Google Scholar]
- 33.Ffrench-Constant C, Raff M C. Nature (London) 1986;319:499–502. doi: 10.1038/319499a0. [DOI] [PubMed] [Google Scholar]
- 34.Noble M, Murray K. EMBO J. 1984;3:2243–2247. doi: 10.1002/j.1460-2075.1984.tb02122.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ijichi A, Noel F, Sakuma S, Weil M M, Tofilon P J. Gene Ther. 1996;3:389–395. [PubMed] [Google Scholar]
- 36.Duncan I D, Grever W E, Zhang S C. Mol Med Today. 1997;3:554–561. doi: 10.1016/S1357-4310(97)01162-3. [DOI] [PubMed] [Google Scholar]
- 37.Blakemore W F, Franklin R J M, Noble M. In: Glial Cell Development: Basic Principles and Clinical Relevance. Jessen K R, Richardson W D, editors. Oxford: Oxford Univ. Press; 1996. pp. 209–220. [Google Scholar]
- 38.Gensert J M, Goldman J E. Neuron. 1997;19:197–203. doi: 10.1016/s0896-6273(00)80359-1. [DOI] [PubMed] [Google Scholar]
- 39.Keirstead H S, Levine J M, Blakemore W F. Glia. 1998;22:161–170. [PubMed] [Google Scholar]
- 40.Reynolds R, Hardy R. J Neurosci Res. 1997;47:455–477. doi: 10.1002/(sici)1097-4547(19970301)47:5<455::aid-jnr1>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 41.Goldman S A, Luskin M B. Trends Neurosci. 1998;21:107–114. doi: 10.1016/s0166-2236(97)01191-0. [DOI] [PubMed] [Google Scholar]