Abstract
The dissolution process of model insoluble peptide sequences was investigated in view of the electron acceptor (AN) and electron donor (DN) solvent properties. The Alzheimer's disease-inducing (1–42) Aβ-amyloid peptide and its (1–21) fragment, the (66–97) transmembrane bradykinin B2 receptor sequence, and the strongly aggregated VVLGAAIV were selected as models of insoluble peptides. Solvents presenting similar AN and DN values failed, despite their polarities, to dissociate peptide chains (free in solution or bound to a polymer). The maximum solubility of these aggregated sequences was attained in solvents presenting the highest possible (AN–DN) values (in positive or negative mode). The AN–DN values ranged from approximately −20 to +80 and, notably, the lowest dissociation power was ascribed to solvents presenting values of approximately +40. The strong hydrogen bond donor water is located in this region, indicating that, for dissociation of specific insoluble segments, the solvent should appropriately combine its acid/base strength with the potential for van der Waals interactions. We also observed a sequence-dependent pH effect on peptide solubility confirmed through circular dichroism spectroscopy. This approach also revealed a complex but, in many cases, consistent influence of peptide conformation on its solubility degree, even when structure-inducing solvents were added. In conclusion, the random method of selecting solvents to dissolve insoluble and intractable peptide sequences, still in use by some, could be partially supplanted by the strategy described herein, which may be also applicable to other solute dissociation processes.
Keywords: peptide, solvent property, solubility, polarity, Alzheimer's disease
Although extremely relevant, the problem of insolubility of peptides and proteins is still far from being completely understood. For instance, many diseases share the common characteristic of aggregation of these types of macromolecules (Chen and Wetzel 2001; Xing and Higuchi 2002; Gordon and Meredith 2003). A high propensity for assembly into insoluble aggregates is a common yet serious limitation to the production and use of many peptides and proteins in a wide range of biotechnological and pharmaceutical applications. In addition, the elucidation of the peptide chain dissolution mechanism is of utmost relevance for successful peptide and protein synthesis, in either solution (Mutter and Bayer 1980) or solid-phase methodologies (Barany and Merrifield 1980; Fields and Noble 1990).
The number of pharmacologically active peptides and proteins under development for the prevention and treatment of human disorders is increasing, as is, consequently, the pressure to overcome problems associated with aggregation and stability (Lansbury 1992; Chi et al. 2003; Fowler et al. 2005). Approximately 95% of all drug candidates are discarded during preclinical or clinical trials, often because of problems related to low solubility or aggregation. Many peptide-based drugs with great therapeutic potential are rendered ineffective simply because of an unacceptable propensity for irreversible precipitation (Caldwell et al. 2001). Aggregation is also one of the most significant obstacles to the development of protein-based drugs due to the possibility that it will not only compromise their bioavailability and therapeutic effect but may also increase the risk of immunogenic reactions (Cleland et al. 1993; Schellekens 2002).
The insolubility of peptides and proteins, as well as of many other macromolecules, is, in fact, a consequence of a specific process of association between molecules of the solute under certain conditions and in the presence of components of the medium. Physicochemically, the type and intensity of electrostatic, hydrogen bonding, and van der Waals interactions between and among all molecules of the solution seem to govern the degree of solute molecule aggregation.
There are innumerable structural studies in the literature using various spectroscopic methods to better understand the complex chain–chain aggregation processes of many types of peptides (Milton et al. 1990; Szabó et al. 1999; Srisailam et al. 2003; Pawar et al. 2005). In a departure from the majority of these classical approaches, our group initially decided to evaluate this aggregation process by selecting peptide–polymers as models of complex solutes for interaction with different solvent systems (Cilli et al. 1996; Marchetto et al. 2005).
In addition to the establishment of some rules for peptide polymer solvation, a 1:1 ratio between Gutman's electron acceptor number (AN) and electron donor number (DN) (Gutmann 1976, 1978) could, based on this solvent effect study, be proposed as an alternative dimensionless and more accurate solvent polarity scale which ranges from zero to about 130 (Malavolta et al. 2002). In contrast to other polarity scales composed of a single term, such as the dielectric constant (Parker 1969), Dimroth-Reichardt's ET(30) (Dimroth et al. 1963) or Hildebrand's δ solubility parameters (Hildebrand 1949), the (AN + DN) polarity term is a combination of two opposite physicochemical concepts, and is therefore designated amphoteric constant.
Following through with this approach, we soon decided to consider the electrophilic (AN) and nucleophilic (DN) properties of a solvent system, in isolated or combined forms, aiming to contribute to a deeper understanding of the peptide chain's association process. In this sense, we have demonstrated that a mixture of highly electrophilic and highly nucleophilic solvents tends to fail in disrupting aggregated peptide chains, spread throughout, for instance, a resin network (Cilli et al. 1996; Malavolta et al. 2002). This occurs as a consequence of the fact that the two components of the mixture tend to interact with each other than to disrupt solute–solute interaction, mainly under strongly aggregated conditions. This is typically the case with heterogeneous mixed trifluoroethanol (TFE)/dimethyl sulfoxide (DMSO) or TFE/dimethyl formamide (DMF) solutions, which are composed of highly electrophilic and highly nucleophilic molecules, respectively, and were unable to solvate the model peptide resins properly.
This same rationale that is applied to mixed solvents was recently extended to single solvents (Malavolta and Nakaie 2004). In this case, the solvents must, of necessity, present quite similar electrophilicity (AN) and nucleophilicity (DN), which may facilitate the occurrence of an internal self-neutralization effect in their molecules, thus weakening their solvation capacity of the solute. Acetonitrile (MeCN) and acetone presenting AN/DN values of 18.9/14.1 and 12.5/17.0, respectively, were preliminarily tested, revealing a total lack of dissociation capacity of peptide chains bound to a resin or free in solution (Malavolta and Nakaie 2004).
To pursue this interpretation of solute–solvent interaction based mainly on the influence of acidic and basic properties of solvent molecules, the focus of the present study was deliberately shifted to the specific evaluation of the dissolution process of highly insoluble peptide sequences. The main goal was to establish a clearer and more consistent strategy for the dissolution of insoluble peptides that would be applicable to many other macromolecules. Initially, chain dissociations will be compared among these peptides when still attached to a polymeric matrix, and those of the same peptides free in solution will be compared later. In addition, circular dichroism (CD) technique will be applied, varying the pH and the amount of secondary structure-inducing solvents in order to tentatively evaluate the degree of solubility and conformation features of these model aggregated peptides. The well-known (1–42) Aβ-amyloid peptide, which is involved in the neurodegenerative Alzheimer's disease (Soto and Frangione 1995; Koo et al. 1999; Lynn and Meredith 2000; Cruz et al. 2004; De-Felice et al. 2004; Ferrao-Gonzales et al. 2005), its minor (1–21) fragment, the 34-mer (66–97) transmembrane fragment of the bradykinin B2 receptor (McEarchen et al. 1991) and, finally, the highly insoluble VVLGAAIV sequence corresponding to the 291–298 segment of the murine H-2K protein (Brown et al. 1974; Narita et al. 1988), were selected for this solute–solvent interaction study.
Results
Dissociation of peptide chains bound to resins
Before investigating possible rules related to dissociating model insoluble peptide sequences free in solution, we first decided to determine whether the Lewis acidity (AN) and Lewis basicity (DN) strength of the solvent system would affect the solvation capacity of peptide chains when bound to a resin structure. The 4-(oxymethyl)-phenylacetamidomethyl (PAM) resin (Barany and Merrifield 1980), a polystyrene-type polymer commonly used in peptide synthesis was selected for the assembly of DAEFRHDSGYDVHHQKLVFFAEDVGSQKGAIIGLMVGGVVIA-COO- and DAEFRHDSGYDVHHQKLVFFA-COO- sequences corresponding to the (1–42) Aβ-amyloid peptide and its minor (1–21) fragment (peptide-resins 1 and 2, respectively). Table 1 displays the measured swelling degree of these two peptide resins in 24 single and mixed solvents, together with their AN, DN, and (AN + DN) values.
Table 1.
Solvent parameters and swelling degrees of peptide-resinsa
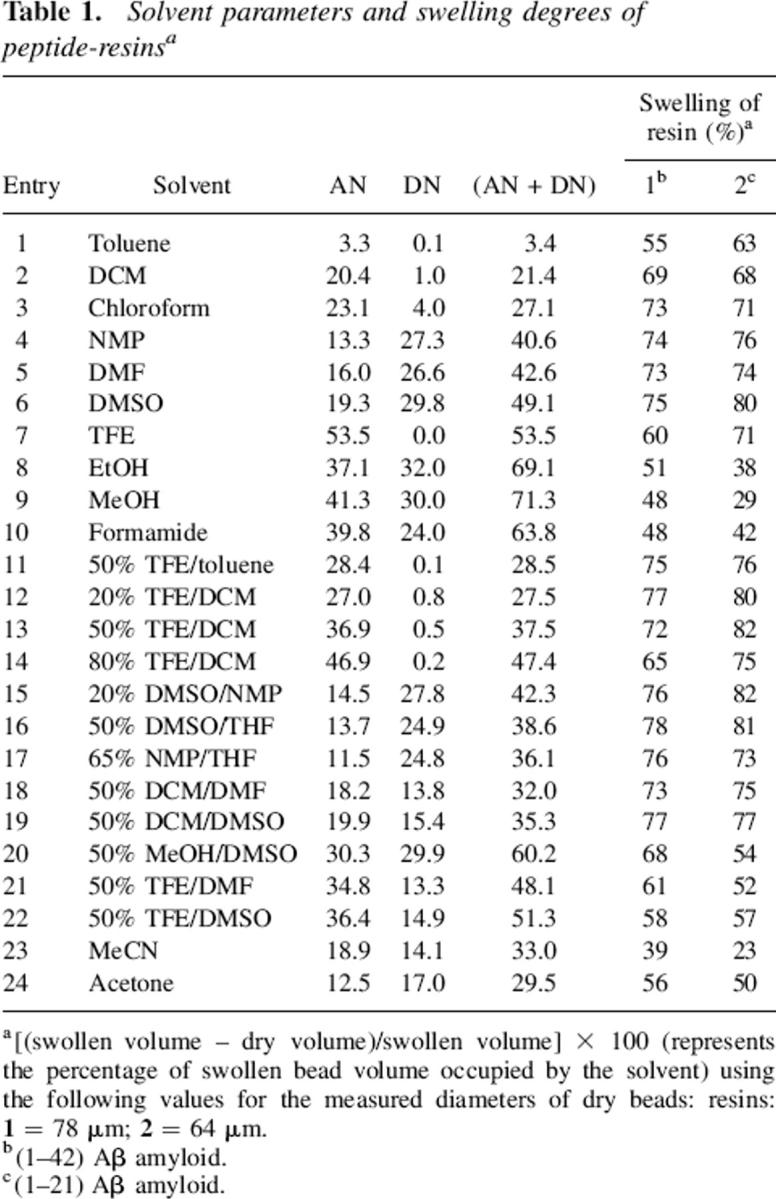
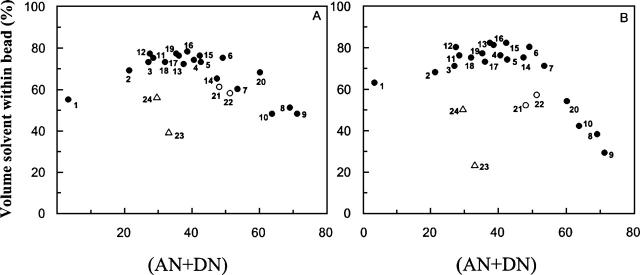
In accordance with the findings of previous studies (Cilli et al. 1996; Malavolta et al. 2002; Malavolta and Nakaie 2004), both peptide-resins presented a maximum solvation region located around solvents with (AN + DN) polarities of ∼40 (Fig. 1). Since it is well known that each polymer achieves its maximum swelling in solvents of similar polarity (Barton 1975), these results again attest to the sensitivity of the amphoteric (AN + DN) solvent parameter in scaling polarity.
Figure 1.
Swelling degree of resins (1–42) β-amyloid-resin (A) and (1–21) β-amyloid-resin (B), as a function of solvent polarity (AN + DN) values.
The presupposition that a self-neutralization effect occurs in heterogeneous mixed solvents composed of highly electrophilic solvents, such as TFE or hexafluoroisopropanol (HFIP), and strong nucleophilic solvents, such as DMSO or DMF, was confirmed. The swelling of resin beads in heterogeneous TFE/DMF and TFE/DMSO solutions (solvents 21 and 22, respectively) was less extensive than what had been expected based on their polarities (Fig. 1).
The same effect was observed in single solvents presenting values of Lewis acidity and Lewis basicity that were proximate. The MeCN and acetone (solvents 23 and 24, respectively), which present virtually equivalent AN and DN values (Table 1), revealed a pronounced lack of capacity to solvate either peptide-resin (Fig. 1). These findings may be relevant in some circumstances, since MeCN is often applied in chemical and biochemical methodologies. Therefore, when optimized solvation of polymeric materials is essential, as is the case in column chromatography experiments, MeCN should be used with care.
The present findings indicate that the use of the AN and DN concepts, including the (AN + DN) term, is unique and considerably more advantageous than other solvent properties. For instance, the self-neutralizing effect occurring with mixed or single solvents could be solely explained by these terms and not by any other existing single-component solvent parameter such as the dielectric constant, Hildebrand's δ solubility constant (Hildebrand 1949), and Dimroth-Reichardt's ET(30) term (Dimroth et al. 1963).
Taken as a whole, these results seem to be of value for any methods that depend heavily upon the groundbreaking approach (initiated >40 yr ago) of inducing chemical reactions in insoluble polymeric supports (Merrifield 1963). In keeping with the rapidly increasing trend in resin-dependent methodologies, it was possible to verify the launching of several types of solid supports (Kempe and Barany 1996; Meldal 1997; Kates et al. 1998; Labadie 1998; Lebl 1998) and spectroscopic strategies to investigate solvation of the peptide polymer complex. Among these efforts, nuclear magnetic resonance (Deber et al. 1989; Warrass et al. 2000; Furrer et al. 2001; Valente et al. 2005), CD (Pillai and Mutter 1981), infrared spectroscopy (Hendrix et al. 1990; Henkel and Bayer 1998; Yan 1998), and electron paramagnetic resonance (Chesnut and Hower 1971; Regen 1974; Vaino et al. 2000) have been successfully applied. In this regard, this laboratory has broken new ground in the use of an amino acid-type paramagnetic probe (Nakaie et al. 1981; Marchetto et al. 1993), and valuable findings have been obtained using this approach (Cilli et al. 1997; 1999; Ribeiro et al. 2001; Oliveira et al. 2002; Marchetto et al. 2005).
Dissociation of peptide chains in solution
Influence of the electrophilicity and nucleophilicity of the solvent system and the water effect
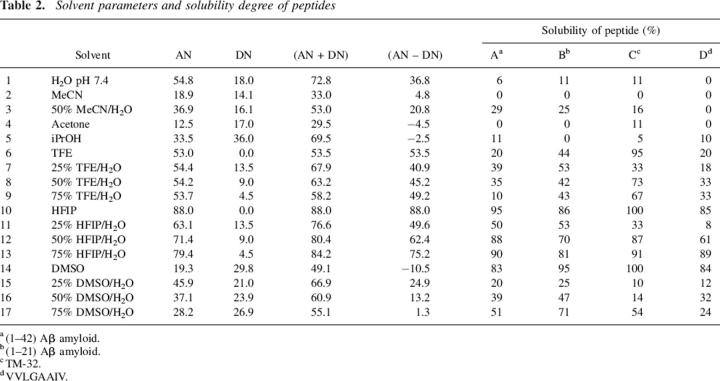
Table 2 displays the solubility data for the following insoluble peptides in 17 solvent systems: (A) (1–42) Aβ-amyloid peptide, (B) the minor (1–21) sequence which contains the reported aggregated (17–21) segment (Serpell 2000; Abe et al. 2002), (C) the VAEIYLGNLAGAKLILASGLPFWAITIANNFD-NH2 sequence corresponding to the (66–97) transmembrane segment of the bradykinin B2 receptor (henceforth referred to as TM-32), and (D) the strongly aggregated 291–298 fragment of the H-2K murine protein (VVLGAAIV). Different from previous solvation studies of peptide resins, in this peptide solubility analysis, most of the solvent systems investigated in solution involved the addition of varying amounts of water, a strong electrophilic-type solvent. In addition, attention was given to solvents with high electrophilicity (TFE and HFIP) or nucleophilicity (DMSO).
Table 2.
Solvent parameters and solubility degree of peptides
To initiate the interpretation of the solubility data shown in Table 2, Figure 2 was constructed, correlating the solubility of each of the four peptides with the various proportions of water incorporated into these organic solvents. Taking into account their amino acid sequences, the hydrophobicity indices of these peptides were also calculated according to a previous report (Meek and Rossetti 1981) and were used for further correlation with solubility data. The hydrophobicity indices of peptides A–D were 99.5, 50.2, 130.7, and 37.5, respectively.
Figure 2.
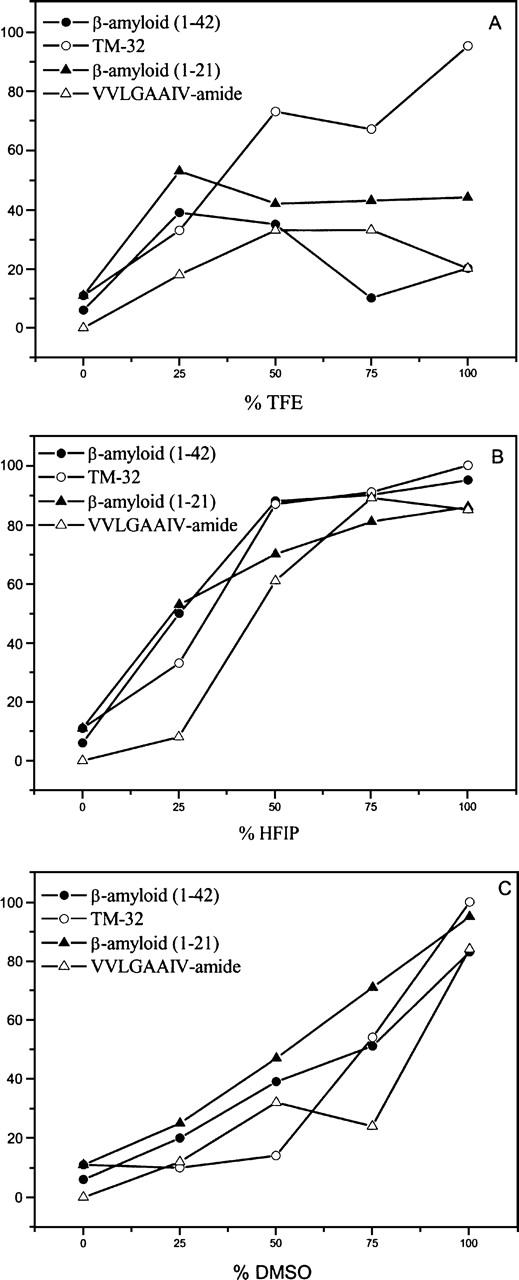
Solubility degree of (1–42) β-amyloid, (1–21) β-amyloid, TM-32, and VVLGAAIV-amide peptides as a function of percentage of solvents TFE (A), HFIP (B), and DMSO (C) in water.
Examination of the results displayed in Table 2 and Figure 2 reveals no evidence of a direct relationship between polarity of the medium and peptide solubility and hydrophobicity. For instance, DMSO presents an inverse dependence, in which the addition of water (increased polarity) induced a decrease in solubility, whereas the addition of water had the opposite effect on HFIP (increasing both solubility and polarity). No clear correlation was found in TFE/water mixtures. The negative effect that the addition of water had on DMSO might be explained by the fact that the two form a heterogeneous solution composed of a highly electrophilic solvent (water) and a highly nucleophilic solvent (DMSO). In addition, the least hydrophobic VVLGAAIV peptide (D) was clearly the most insoluble among those peptides examined, including for instance, the TM-32 (peptide C), which presents the highest hydrophobicity index. All of these findings seem to be in accordance with preliminarily reports (Malavolta and Nakaie 2004), and indicate that complex factors other than polarity and hydrophobicity affect the final degree of solubility of highly insoluble sequences.
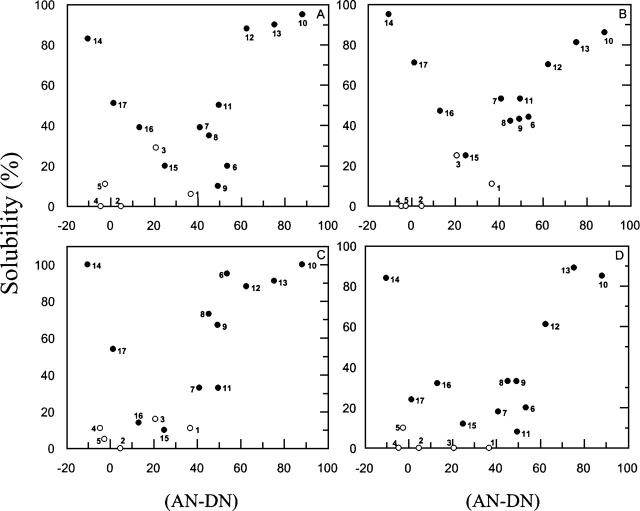
The next attempt involved looking for correlations between the solubility data for each of these peptides and the various electrophilicity/nucleophilicity values of solvent systems, as represented by the (AN–DN) term. The results are displayed in Figure 3, and, notably, the maximum dissociation of aggregated peptides, regardless of sequence, was systematically reached in parallel with the attainment of the peak value for the (AN–DN) term, which ranged from approximately +80 to −20 with the solvents herein tested. In this plot, a region comprising the solvents that induced the lowest peptide dissociation and approximately coinciding with the location of water (solvent 1, i.e., with an [AN–DN] value of ∼40) was observed.
Figure 3.
Solubility degree of (1–42) β-amyloid (A), (1–21) β-amyloid (B), TM-32 (C), and VVLGAAIV-amide (D) peptides as a function of solvent (AN–DN) values.
The data displayed in Figure 3 are also illustrative of the previously discussed lack of chain dissociation forces attributable for the single solvents MeCN, acetone, and isopropanol (solvents 2, 4, and 5, respectively). Similar to the solvents presenting poor solvation in the swelling study of peptide resins, these single solvents, characterized by having similar AN and DN numbers (Table 2), were also unable to disrupt strongly aggregated peptide chains in solution.
Taken together, these results highlight the fact that the solubility effect induced by the addition of electrophilic water (AN = 54.8) to other strong polar organic solvents, such as DMSO, HFIP, and TFE, is highly dependent upon the type and amount of organic solvent to be cosolvated. Nevertheless, the basic rule for dissociation of insoluble and intractable sequences seems to lie in the absolute value of the (AN–DN) term for each solvent system, as depicted in Figure 3.
Of note, the differentiated behavior of HFIP, if compared with its electrophilic partner TFE, should be credited to its higher potential for participating not only in hydrogen bonding (greater electrophilicity) but also in hydrophobic interactions with solute molecules, favored by the presence of two –CF3 groups in its structure (Cheong et al. 2002; Hirota-Nakaoka et al. 2003; Vieira et al. 2003) These results indicate that, as expected, an appropriate association between hydrogen bonding and the van der Waals forces of the solvent is important for disruption of strongly aggregated sequences.
Influence of the pH of the medium
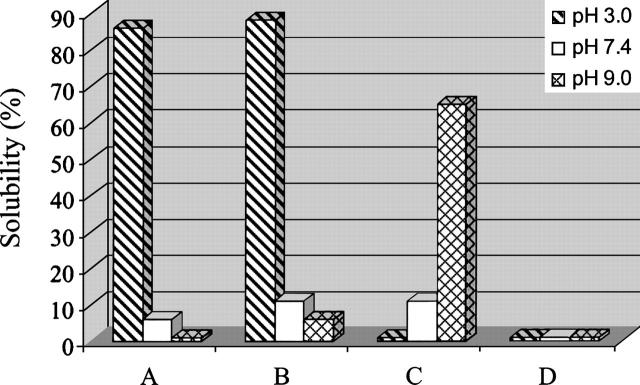
The histogram shown in Figure 4 reveals that, interestingly, the solubility of some peptides was dependent upon the pH of the medium. With the exception of the more highly aggregated VVLGAAIV sequence (peptide D), which did not dissolve at any pH, the other two classes of peptides (Aβ amyloid and transmembrane TM-32) revealed prominent sequence-dependency on the pH of the medium. Due to the increase in the net positive charge of their structures induced by progressive protonation of basic groups (His, Arg, Lys, and the N-terminal amino function) and acidic groups (Asp and Glu), as the pH decreased from 9 to 3, the solubility of (1–42) and (1–21) Aβ-amyloid peptides (A and B, respectively) increased sharply (from almost zero to near 90%). These dramatic and surprising changes in solubility, even in water solution, are quite similar to those achieved in strong dissociating organic solvents such as HFIP or DMSO (Table 2). Therefore, these data indicate a significant pH effect occurring with amyloid-type segments, but in physiological conditions (pH 7.4), both peptides present very low solubility (Fig. 4).
Figure 4.
Solubility degree of (1–42) β-amyloid (A), (1–21) β-amyloid (B), TM-32 (C), and VVLGAAIV-amide (D) peptides as a function of the pH of the medium.
In contrast, the behavior of the hydrophobic, long-receptor fragment TM-32 (peptide C) toward the same variation in the pH (from 9–3) was the opposite, presenting a pronounced decrease in solubility at lower pH. By examining the primary structure of this 34-mer peptide, it can be observed that, different from amyloid-type peptides, it does not contain basic groups in its sequence. Due to this characteristic, the change in pH from 3 to 9 induces the appearance of three additional negative groups, thus increasing the solubility of this sequence in parallel with increases in the pH (to ∼60% at pH 9). At physiological pH levels or lower (pH 3), the solubility of the sequence dropped significantly (to ∼10% and 0%, respectively). This strong dependence of these two important classes of scarcely soluble peptides on changes in the pH of the medium leave it open to speculation whether there is some delicate pH-dependent chain dissociation balance, eliciting some physiological response from the organism.
Correlation with CD spectroscopy data regarding secondary peptide structures
The pH effect
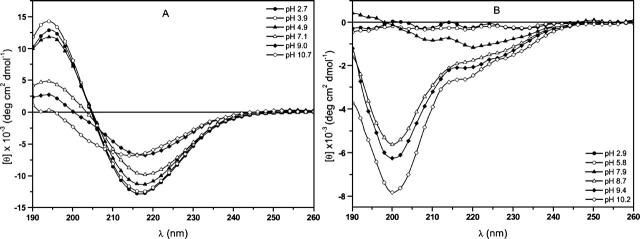
Based upon the results found in the previous section (Fig. 4), the (1–42) Aβ-amyloid, TM-32 and VVLGAAIV sequences were selected as models for CD spectroscopy aimed at initiating a conformation-solubility correlation study. First, the pH effect was determined, and the CD curves shown in Figure 5A depict a pronounced variation in the conformation property of the Aβ-amyloid peptide as a function of the pH of the media. At pH values higher than ∼5 (lower solubility, Fig. 4), the structures were undefined and were more disordered than the β-sheet conformation (Perczel et al. 1992) observed at lower pH (greater solubility).
Figure 5.
CD spectra of (1–42) β-amyloid (A) and TM-32 (B) peptides as a function of the pH of the medium.
In contrast to the Aβ-amyloid peptide, a different pH-solubility correlation was revealed in the CD curves for the transmembrane TM-32 segment (Fig. 5B). In this peptide, improved solubility was accompanied by disordered secondary structure, characterized by a minimum of ∼200 nm in the curves (at pH values higher than 8), whereas a complex combination of extended structures was observed at lower pH values (poorer solubility). Finally, the short and strongly aggregated VVLGAAIV sequence presented the expected constancy in CD curves (disordered structures; data not shown), since its solubility did not vary with the pH of the media (complete lack of solubility, Fig. 4). Taken together, these CD findings, together with the pH effect observed, indicate that a better understanding of the structural characteristics of these aggregated peptides would be of great value for evaluating their solubility properties. However, the correlation between these properties is extremely complex, demonstrating that, in addition to the secondary structure factor, other factors, such as the total amount of ionic groups in each sequence, which is dependent of the pH of the medium, might affect this correlation, as discussed in the previous section.
Organic solvent effect
In this section, CD studies of the three model insoluble peptides were extended to examining the influence of organic solvents. The highly electrophilic and secondary structure-inducing TFE (Vieira et al. 2003) and the weakly dissociating MeCN (Sen et al. 2003) were selected, and the results obtained are collectively displayed in Figure 6.
Figure 6.
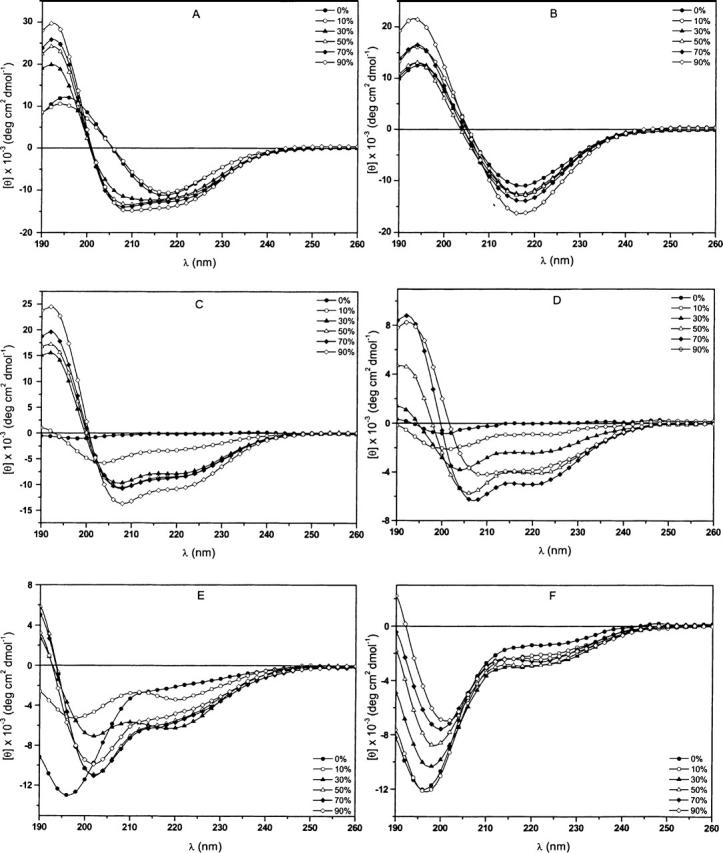
CD spectra of (1–42) β-amyloid (A,B), TM-32 (C,D) and VVLGAAIV amide (E,F) peptides as a function of the percentage of TFE (A,C,D) and MeCN (B,D,F) in water.
Aqueous TFE solutions
For the (1–42) Aβ-amyloid peptide, the CD curves obtained in TFE solutions (Fig. 6A) indicate pronounced structural variation as the amount of this fluorinated alcohol increased. Above 30% TFE, at which greater solubility was measured (Table 2), typical α-helical conformations appeared with characteristic minima of 208 nm and 222 nm and a maximum of 192 nm (Fasman 1996). Otherwise, at lower TFE concentrations (from 0%–10%), at which the peptide presents low solubility, the CD curves are representative of typical β-sheet conformations.
The highly hydrophobic TM-32 peptide also displayed the appearance of α-helical structures as the amount of TFE in the solution was increased (Fig. 6C). This result is in accordance with the findings of earlier reports regarding membrane proteins (Corbin et al. 1998; Opella et al. 1999), as well as with those of our own preliminarily studies (Oliveira et al. 1997; Grijalba et al. 2000), in which an analog of TM-32 was examined in polyfluorinated solvents. Since the solubility of this sequence increased under this condition (greater amount of TFE, Table 2), we can infer that there is a direct relationship between the degree of solubility and α-helical content, as was observed for the Aβ-amyloid peptide. Finally, for the octapeptide VVLGAAIV, the addition of TFE induced a progressive but unclear variation in its secondary structure (Fig. 6E). This process varied from a typical disordered structure (at 0% TFE) to a mixture of folded structures when the degree of solubility reached a maximum of 33% (Table 2).
Aqueous MeCN solutions
We have previously emphasized the fact that, in contrast to TFE, MeCN is characterized by lower solute solvation strength. This is primarily due to the self-neutralizing effect of its balance of electrophilic and nucleophilic properties (equivalent AN and DN values). The CD results acquired for MeCN are in agreement with this presupposition, and a strong tendency to induce more ordered structures was observed for the three peptides in this solvent. However, a significant difference was observed in comparison with findings in the TFE solutions. For instance, Figure 6B shows a typical β-sheet structure for the Aβ-amyloid segment in MeCN, without significant variation in the CD curves, despite a slight increase in its solubility, which reached 29% in 50% MeCN aqueous solution (Table 2).
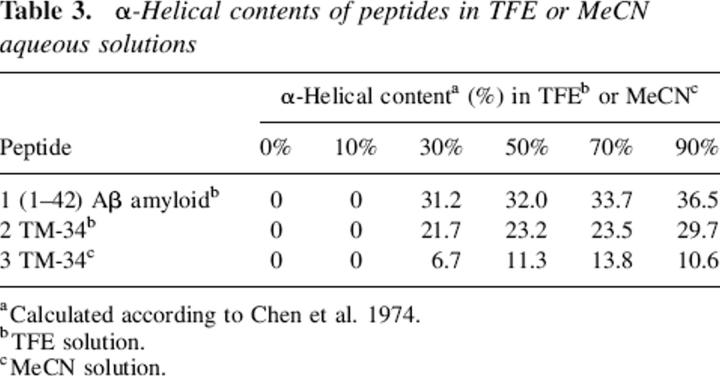
Contrariwise, the TM-32 sequence presented increased folding of its secondary structures as the amount of MeCN increased, acquiring surprisingly α-helical conformation at MeCN concentrations >30% (Fig. 6D). The solubility of this peptide increased slightly (up to 16% in 50% MeCN), thereby again indicating a direct relationship between α-helical content and degree of solubility. To facilitate the analysis of the degree of solubility versus the degree of α-helical conformation, Table 3 summarizes the values of the α-helix contents estimated according to a previous report (Chen et al. 1974) of all the peptides in TFE and MeCN, estimated using the current CD approach. It can be seen that α-helix values ranging from 0% up to ∼40% were observed. However, taken as a whole, these values did not present rigorous correlations with the corresponding solubility data. For instance, in TFE solutions, the TM-32 sequence presented greater solubility than did the other peptides evaluated (Table 2). However, the TM-32 values of α-helical content were lower than those calculated for the (1–42) Aβ-amyloid segment. Finally, the VVLGAAIV sequence, which presented no variation in solubility (completely insoluble), displayed the expected constancy in the CD curves, characterized by disordered structures with a minimum of ∼198 nm, as the MeCN increased (Fig. 6F).
Table 3.
α-Helical contents of peptides in TFE or MeCN aqueous solutions
Discussion
The main objectives of the present study were (1) to design an experimental approach which would demonstrate that using appropriate solutions that present electrophilic and nucleophilic properties, represented herein by Gutman's AN and DN solvent terms, is probably a more accurate strategy for interpreting various types of solute–solvent interactions; (2) to propose a more consistent explanation for the solvent-dependent dissociation effect of strong aggregated peptide chains, in solution (with emphasis to the role of water in the mixture) or attached to a polymeric network; (3) to initiate a study aimed at examining possible correlations between the conformational properties and the degree of solubility of individual peptides.
In the first, short topic addressed in this report, solvation results for model peptide- resins in various single or mixed solvents provided definitive evidence that the maximum swelling of a peptide-resin occurs in a solvent system possessing a polarity equal to that of its structure. In this case, the validity of the previously proposed (AN + DN) summation term for use as a novel polarity scale was also confirmed. In addition, it was demonstrated that mixed or single solvents presenting a balance between AN values and DN values do not properly solvate solutes, in general due to a molecular self-neutralization effect. This was the case for the mixed solvent TFE/DMSO and for the single solvent MeCN, neither of which were able to solvate peptide-resins, as would have been expected based on their polarities. If any other existing polarity parameter is used, this explanation does not apply to these unexpected results.
The relevance of applying the concept of solvent AN and DN values was also clearly demonstrated in the explanation of the dissociation process of the four model insoluble peptides when free in solution. The lack of solubility capacity observed with the single solvents MeCN, acetone, and isopropanol supports the assumption that an internal self-neutralization effect occurs in the molecules of these solvents. In this respect, the often reported β-structure-inducing property of MeCN (Sen et al. 2003) would, due to its weakness in disrupting chain–chain interactions, lead the sequence to this specific folded structure.
The poor solubilization power of the highly electrophilic water indicates that the peptide dissociation process should be interpreted with caution. The enhanced solubility observed for all aggregated sequences in the highly electrophilic organic solvents (HFIP and TFE) or highly nucleophilic organic solvent (DMSO) proved how crucial is the participation of the van der Waals forces. Figure 3 provides an unusual view of the dependence of solubility on the solvent properties, taking into account the (AN–DN) term. Solubility improved in parallel with increases in (AN–DN) values, which ranged from +80 (HFIP) to −20 (DMSO). In this aspect, one might explain the weakness of water to dissociate strong aggregated peptides, since the (AN–DN) value of water is 40, exactly the region in which solvents present more difficulty in disrupting aggregated structures. Therefore, the addition of water to these organic solvents should be interpreted in view of the (AN–DN) values of the solution. From our perspective, these findings complement successfully previous efforts to identify more consistent rules for interpretation of the effect that solvents have on insoluble peptides.
A very clear sequence-dependence effect between solubility and pH of the medium was demonstrated (Fig. 4). The solubility results achieved at pH 3, pH 7.4, and pH 9 revealed that both amyloid-type sequences are much more soluble at low pH, whereas the transmembrane receptor segment showed an inverse dependence (higher solubility at high pH). These results can be explained by the approximate net charge of each type of sequence. However, the marked difference in this solubility dependence on pH was surprising, and the solubility values were equal to the highest values achieved with the best dissociation organic solvents such as HFIP or DMSO (Table 2). The short and weakly ionized (VVLGAAIV) segment was the only peptide segment in which the secondary structure was insensitive to the pH of the media.
The CD spectroscopy examining the effect of pH and of the addition of secondary structure-inducing organic solvents was carried out with the objective of developing an approach to investigating the degree and type of correlations existing between the conformation properties and solubility properties of insoluble peptides. The CD curves displayed in Figure 5 depict the pH effect for the model insoluble peptides, and indicate that these two peptide properties parallel each other. However, under favorable solubilization conditions (Fig. 4), the type of secondary structure acquired by each peptide is sequence-dependent, typical β-sheet structures appearing in the Aβ amyloid sequence (low pH) and disordered conformations appearing in the TM-32 sequence (high pH). The VVLGAAIV segment, although completely insoluble, presented a disordered conformation similar to that presented by the TM-32 segment. These initial results indicate that there is a direct correlation between solubility and conformation for all of the peptides tested when the pH of solution was varied. However, no clear relationship was established between solubility and the type of secondary structure.
A direct relationship was also identified in CD curves for these peptides when in the presence of the structuring solvents TFE and MeCN (Table 2; Fig. 6). However, the type of secondary structure acquired for peptides in each solvent is clearly sequence-dependent. In TFE, Aβ amyloid and TM-32 presented α-helix conformations, and TM-32 also presented such conformations in MeCN. Therefore, as a preliminary conclusion, we can state that the occurrence of conformational changes is always accompanied by variation in solubility. However, despite the results presented herein, the exact correlation between the type of secondary structure and the degree of solubility remains unclear. As demonstrated in the previous section, the knowledge of the electrophilicity/nucleophilicity properties of solvents facilitated the selection of the appropriate milieu for dissolution of each insoluble peptide. However, in proposing a complete and rigorous interpretation of the relationship between solubility and peptide secondary structure, other factors must be taken into consideration.
In conclusion, the physicochemical findings described herein are relevant to interpreting the dissociation effect of insoluble and intractable peptide fragments. The importance of these results cannot be overemphasized, since many chemical or biochemical interactions occurring between solute and solvent molecules have not as yet been definitively elucidated. To date, no clear rules have been proffered for the way in which solvent systems are selected for a given experiment, and this process continues to be conducted in a random fashion. From our perspective, the results of the present study reveal some consistent guidelines for addressing this problem, primarily based on the Lewis acid-base concepts in the presence or absence of water. Owing to the extremely complex process comprising solute–solvent interaction, which can obviously be extended to many other macromolecules, a considerable amount of further research is warranted. Future studies will necessarily involve not only other types of solute and solvent molecules but also the use of complementary experimental approaches. The use of alternative spectroscopy methods could further clarify the influence of other factors governing the mechanisms involved in the solute–solvent association/dissociation phenomenon.
Materials and methods
Materials
All amino acid derivatives were purchased from Bachem. Solvents and reagents from Fluka or Sigma-Aldrich were of analytical grade, and were taken from recently opened containers, without further purification.
Peptide synthesis and purification
The peptides were synthesized manually accordingly to the standard tert-butyloxycarbonyl (Boc) solid-phase peptide synthesis protocol (Barany and Merrifield 1980). The amino acids were coupled using 2-(1H-benzotriazol-1-yl)-1,1,3,3-tetramethyluronium tetrafluoroborate (TBTU) in the presence of 1-hydroxybenzotriazole (HOBt) and diisopropylethylamine (DIEA) using 20% DMSO/N-methylpirrolidinone (NMP) as a solvent system. To check the purity of the synthesized peptide sequence attached to the resin, cleavage reactions were carried out with anhydrous HF. Preparative HPLC was used for purification of peptides, and their homogeneities were determined through analytical HPLC, as well as LC/MS (electrospray) mass spectrometry (Micromass) and amino acid analysis (Biochrom 20 Plus, Amersham Biosciences). The following peptides were synthesized and purified for solubility studies.
DAEFRHDSGYDVHHQKLVFFAEDVGSQKGAIIGLMVGGVVIA-COO-: (1–42) Aβ-amyloid peptide
This peptide was synthesized in 0.2 mmol scale applying the Boc strategy. A 0.6 mmol/g 4-(oxymethyl)-phenylacetamidomethyl-resin (Barany and Merrifield 1980) was used, and after HF cleavage, this peptide was purified using a reverse-phase C4 preparative HPLC column at 70°C. Solvent A: 0.1% TFA/H2O and solvent B: 90% MeCN/0.1% TFA/H2O with a gradient from 15% to 45% of B in 90 min., at a flow rate of 10 mL/min was used. The detection was at λ = 220 nm.
DAEFRHDSGYDVHHQKLVFFA-COO-: (1–21) Aβ amyloid peptide
This sequence was synthesized in 1.0 mmol scale in the Boc-Ala-Merrifield resin and purified in the same manner as the previous sequence.
VAEIYLGNLAGAKLILASGLPFWAITIANNFD-CONH2: (66–97) segment of the transmembrane B2 receptor of bradykinin (TM-32)
This peptide was synthesized in 0.2 mmol scale, starting from 0.6 mmol/g methylbenzhydrylamine-resin (MBHAR) in order to obtain the Cα-carboxamide terminal. After HF cleavage, this peptide was purified using a reverse-phase C18 preparative HPLC column. Purification conditions: solvent A, 0.1% TFA/H2O; solvent B, 90% MeCN/0.1% TFA/H2O. Gradient from 40% to 70% of B in 90 min, at a flow rate of 10 mL/min was used. The detection was at λ = 220 nm.
VVLGAAIV-CONH2
This peptide was synthesized in 0.4 mmol scales starting from a 0.6 mmol/g MBHAR. After HF cleavage, this peptide was purified in preparative HPLC using a C18 column. Purification conditions: solvent A, 0.1% TFA/H2O; solvent B, 60% MeCN/0.1% TFA/H2O with a gradient of 20% to 50% of B in 90 min at a flow rate of 10 mL/min. The detection was at λ = 220 nm.
Swelling measuring of resin beads
Swelling measuring of resin beads was performed as described previously (Sarin et al. 1980; Marchetto et al. 1992; Tam and Lu 1995). Before use in peptide synthesis and/or in microscopic measurement of bead sizes, most resin batches were sized by sifting in porous metal sieves to lower the standard deviations of resin diameters to about 4%. Briefly, 150–200 dry and swollen beads of each resin, allowed to solvate overnight, were spread over a microscope slide and measured directly with an Olympus, model SZ11 microscope coupled with an Image-Pro Plus, 3.0.01.00 version software. Since the sizes in a sample of beads are not normally but log-normally distributed, the central value and the distribution of the particle diameters were estimated by the more accurate geometric mean values and geometric standard deviations. The resins were measured with their amino groups in the deprotonated form, obtained by 3 × 5 min TEA/DCM/DMF (1:4.5:4.5, v/v/v) washings followed by 5 × 2 min DCM/DMF (1:1, v/v) and 5 × 2 min DCM washings. Resins were dried in vacuum using an Abderhalden-type apparatus with MeOH reflux.
Solubility determination of peptides
The solubility of each peptide was determined by dissolving 10 mg of pre-purified peptide in 1 mL (ca. 2–10 mM) of each of the solvents. The solution was centrifuged for 1 h at 14,000 rpm, and the supernatant and the precipitate were lyophilized until constant weight was attained. Solubility data are expressed as percentages.
CD spectroscopy
The analyses were performed at 25°C using a Jasco-810 spectropolarimeter. All spectra were recorded using a 1-mm path-length rectangular quartz cell, 0.5-nm bandwidth, 50 nm/min scan speed, 8-sec response time, four accumulations, within a wavelength range of 190 to 260 nm.
Acknowledgments
This study received financial support in the form of grants from FAPESP, CNPq, and CAPES. L.M. and J.H.C. are fellows of FAPESP and CNPq, respectively. C.R.N. is the recipient of research fellowships from CNPq and FADA.
Footnotes
Reprint requests to: Clovis R. Nakaie, Department of Biophysics, Universidade Federal de São Paulo, Rua 3 de Maio 100, CEP 04044-020, São Paulo, SP, Brazil; e-mail: clovis@biofis.epm.br; fax: 55-11-5575-9617.
Article and publication are at http://www.proteinscience.org/cgi/doi/10.1110/ps.051956206.
References
- Abe H., Kawasaki K., Nakanishi H. 2002. pH-dependent aggregate forms and conformation of Alzheimer amyloid β-peptide (12–24) J. Biochem. 132 863–874. [DOI] [PubMed] [Google Scholar]
- Barany G. and Merrifield R.B. In The peptides: Analysis, synthesis and biology (eds. Gross E. and Meienhofer J.) . pp. 1–284. 1980. Academic Press, New York vol. 2. [Google Scholar]
- Barton A.F.M. 1975. Solubility parameters Chem. Rev. 75 731–753. [Google Scholar]
- Brown J.L., Kato K., Silver J., Nathenso S.G. 1974. Notable diversity in peptide composition of murine h-2k and h-2d alloantigens Biochemistry 13 3174–3178. [DOI] [PubMed] [Google Scholar]
- Caldwell G.W., Ritchie D.M., Masucci J.A., Hageman W., Yan Z. 2001. The new pre-preclinical paradigm: Compound optimization in early and late phase drug discovery Curr. Top. Med. Chem. 1 353–366. [DOI] [PubMed] [Google Scholar]
- Chen S. and Wetzel R. 2001. Solubilization and disaggregation of polyglutamine peptides Protein Sci. 10 887–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y.H., Yang J.T., Chau K.H. 1974. Determination of the helix and β form of proteins in aqueous solution by circular dichroism Biochemistry 13 3350–3359. [DOI] [PubMed] [Google Scholar]
- Cheong W.J., Keum Y., Ko J.H. 2002. The hydroxyl group-solvent and carbonyl group-solvent specific interactions for some selected solutes including positional isomers in acetonitrile/water mixed solvents monitored by HPLC Bull. Korean Chem. Soc. 23 65–70. [Google Scholar]
- Chesnut D.B. and Hower J.F. 1971. Spin-label investigation of ion-exchange resins J. Phys. Chem. 75 907–912. [Google Scholar]
- Chi E.Y., Krishnan S., Randolph T.W., Carpenter J.F. 2003. Physical stability of proteins in aqueous solution: Mechanism and driving forces in nonnative protein aggregation Pharm. Res. 20 1325–1336. [DOI] [PubMed] [Google Scholar]
- Cilli E.M., Oliveira E., Marchetto R., Nakaie C.R. 1996. Correlation between solvation of peptide-resins and solvent properties J. Org. Chem. 61 8992–9000. [DOI] [PubMed] [Google Scholar]
- Cilli E.M., Marchetto R., Schreier S., Nakaie C.R. 1997. Use of spin label EPR spectra to monitor peptide chain agreggation inside resin beads Tetrahedron Lett. 38 517–520. [Google Scholar]
- Cilli E.M., Marchetto R., Schreier S., Nakaie C.R. 1999. Correlation between the mobility of spin labeled peptide chains and resin solvation: An approach to optimize the synthesis of aggregating sequences J. Org. Chem. 64 9118–9123. [Google Scholar]
- Cleland J.L., Powell M.F., Shire S.J. 1993. The development of stable protein formulations—A close look at protein aggregation, deamidation and oxidation Crit. Rev. Ther. Drug Carrier Syst. 10 307–377. [PubMed] [Google Scholar]
- Corbin J., Methot N., Wang H.H., Baezinger J.E., Blanton M.P. 1998. Secondary structure analysis of individual transmembrane segments of the nicotinic acetylcholine receptor by circular dichroism and Fourier transform infrared spectroscopy J. Biol. Chem. 273 771–777. [DOI] [PubMed] [Google Scholar]
- Cruz M., Tusell J.M., Grillo-Bosch D., Albericio F., Serratosa J., Rabanal F., Giralt E. 2004. Inhibition of β-amyloid toxicity by short peptides containing N-methyl amino acids J. Pept. Res. 63 324–328. [DOI] [PubMed] [Google Scholar]
- Deber C.M., Lutek M.K., Heimer E.P., Felix A.M. 1989. Conformational origin of a difficult coupling in a human growth hormone releasing factor analog Pept. Res. 2 184–188. [PubMed] [Google Scholar]
- De-Felice F.G., Vieira M.N.N., Saraiva L.M., Figueroa-Villar J.D., Garcia-Abreu J., Liu R., Chang L., Klein W.L., Ferreira S.T. 2004. Targeting the neurotoxic species in Alzheimer's disease: Inhibitors of Aβ oligomerization FASEB J. 18 1366–1372. [DOI] [PubMed] [Google Scholar]
- Dimroth K., Bohlman F., Reichardt C., Siepmann T. 1963. Uber pyridinium-n-phenol-betaine und ihre verwendung zur charakterisierung der polaritat von losungsmitteln Liebigs Ann. Chem. 661 1–37. [Google Scholar]
- Fasman G.D. In Circular dichroism and the conformational analysis of biomolecules . 1996. Plenum Press, New York.
- Ferrao-Gonzales A.D., Robbs B.K., Moreau V.H., Ferreira A., Juliano L., Valente A.P., Almeida F.C.L., Silva J.L., Foguel D. 2005. Controlling β-amyloid oligomerization by the use of naphthalene sulfonates J. Biol. Chem. 280 34747–34754. [DOI] [PubMed] [Google Scholar]
- Fields G.B. and Noble R.L. 1990. Solid-phase peptide-synthesis utilizing 9-fluorenylmethoxycarbonyl amino-acids Int. J. Pept. Protein Res. 35 161–214. [DOI] [PubMed] [Google Scholar]
- Fowler S.B., Poon S., Muff R., Chiti F., Dobson C.M., Zurdo Z. 2005. Rational design of aggregation resistant bioactive peptides: Reengineering human calcitonin Proc. Natl. Acad. Sci. 102 10105–10110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furrer J., Piotto M., Bourdonneau M., Limal D., Guichard G., Elbayed K., Raya J., Briand J.P., Bianco A. 2001. Evidence of secondary structure by high-resolution magic angle spinning NMR spectroscopy of a bioactive peptide bound to different solid supports J. Am. Chem. Soc. 123 4130–4138. [DOI] [PubMed] [Google Scholar]
- Gordon D.J. and Meredith S.C. 2003. Probing the role of backbone hydrogen bonding in β-amyloid fibrils with inhibitor peptides containing ester bonds at alternate positions Biochemistry 42 475–485. [DOI] [PubMed] [Google Scholar]
- Grijalba M.T., Schreier S., Oliveira E., Nakaie C.R., Miranda A., Tominaga M., Paiva A.C.M. 2000. Conformational studies of a detergent-bound transmembrane segement of the rat bradykinin receptor In Peptides for the new millennium (eds. Fields G.B.et al.) . pp. 385–386. Kluwer Academic Publishers, New York.
- Gutmann V. 1976. Empirical parameters for donor and acceptor properties of solvents Electrochim. Acta 21 661–670. [Google Scholar]
- Gutmann V. In The donor–acceptor approach to molecular interactions . 1978. Plenum, New York.
- Hendrix J.C., Halverson K.J., Jarret J.T., Lansbury Jr. P.T. 1990. A novel solvent system for solid-phase synthesis of protected peptides—The disaggregation of resin-bound antiparallel β-sheet J. Org. Chem. 55 4517–4518. [Google Scholar]
- Henkel B. and Bayer E. 1998. Monitoring of solid phase peptide synthesis by FT-IR spectroscopy J. Pept. Sci. 4 461–470. [DOI] [PubMed] [Google Scholar]
- Hildebrand J.H. 1949. A critique of the theory of solubility of non-electrolytes Chem. Rev. 44 37–45. [DOI] [PubMed] [Google Scholar]
- Hirota-Nakaoka N., Hasegawa K., Naiki H., Goto Y. 2003. Dissolution of β2-microglobulin amyloid fibrils by dimethylsulfoxide J. Biochem. 134 159–164. [DOI] [PubMed] [Google Scholar]
- Kates S.A., McGuiness B.F., Blackburn C., Griffin G.W., Solé N.A., Barany G., Albericio F. 1998. High-load polyethylene glycol-polystyrene (PEG-PS) graft supports for solid-phase synthesis Biopolymers 47 365–380. [DOI] [PubMed] [Google Scholar]
- Kempe M. and Barany G. 1996. Clear: A novel family of highly cross-linked polymeric supports for solid-phase peptide synthesis J. Am. Chem. Soc. 118 7083–7093. [Google Scholar]
- Koo E.H., Lansbury Jr. P.T., Kelly J.W. 1999. Amyloid diseases: Abnormal protein aggregation in neurodegeneration Proc. Natl. Acad. Sci. 96 9989–9990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labadie J.W. 1998. Polymeric supports for solid phase synthesis Curr. Opin. Chem. Biol. 2 346–352. [DOI] [PubMed] [Google Scholar]
- Lansbury Jr. P.T. 1992. In pursuit of the molecular-structure of amyloid plaque—New technology provides unexpected and critical information Biochemistry 31 6865–6870. [DOI] [PubMed] [Google Scholar]
- Lebl M. 1998. Solid-phase synthesis on planar supports Biopolymers 47 397–404. [Google Scholar]
- Lynn D.G. and Meredith S.C. 2000. Model peptides and the physicochemical approach to β-amyloids J. Struct. Biol. 130 153–173. [DOI] [PubMed] [Google Scholar]
- Malavolta L. and Nakaie C.R. 2004. Peptide dissociation in solution or bound to a polymer: Comparative solvent effect Tetrahedron 60 9417–9424. [Google Scholar]
- Malavolta L., Oliveira E., Cilli E.M., Nakaie C.R. 2002. Solvation of polymers as model for solvent effect investigation: Proposition of a novel polarity scale Tetrahedron 58 4383–4394. [Google Scholar]
- Marchetto R., Etchegaray A., Nakaie C.R. 1992. Kinetics of synthesis and swelling of highly substituted benzhydrylamine-resins: Implications for peptide synthesis and perspectives for use as anion exchanger resin J. Braz. Chem. Soc. 3 30–37. [Google Scholar]
- Marchetto R., Schreier S., Nakaie C.R. 1993. A novel spin-labeled aminoacid derivative for use in peptide synthesis: (9-fluorenylmethyloxycarbonyl) 2,2,6,6-tetramethylpiperidine-N-oxyl-4-amino-4-carboxylic acid J. Am. Chem. Soc. 115 11042–11043. [Google Scholar]
- Marchetto R., Cilli E.M., Jubilut G.N., Schreier S., Nakaie C.R. 2005. Determination of site-site distance and site concentration within polymer beads: A combined swelling-electron paramagnetic resonance study J. Org. Chem. 70 4561–4568. [DOI] [PubMed] [Google Scholar]
- McEarchen A.E., Shelton E.R., Obernolte R., Bach C., Zuppan P., Fujisaki J., Aldrich R.W., Jarnagin K. 1991. Expression cloning of a rat b2-bradykinin receptor Proc. Natl. Acad. Sci. 88 7724–7728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meek J.L. and Rossetti Z.L. 1981. Factors affecting retention and resolution of peptides in high-performance liquid-chromatography J. Chromatogr. 211 15–28. [Google Scholar]
- Meldal M. 1997. Solid phase peptide synthesis Methods Enzymol. 289 83–103.9353719 [Google Scholar]
- Merrifield R.B. 1963. Solid phase peptide synthesis. Synthesis of a tetrapeptide J. Am. Chem. Soc. 85 2149–2154. [Google Scholar]
- Milton R.C.L., Milton S.C.F., Adams P.A. 1990. Prediction of difficult sequences in solid-phase peptide synthesis J. Am. Chem. Soc. 112 6039–6046. [Google Scholar]
- Mutter M. and Bayer E. In The peptides: Analysis, synthesis and biology (eds. Gross E. and Meienhofer J.) . pp. 285–332. 1980. Academic Press, New York Vol. 2. [Google Scholar]
- Nakaie C.R., Goissis G., Schreier S., Paiva A.C.M. 1981. pH Dependence of ESR spectra of nitroxide containing ionizable groups Braz. J. Med. Biol. Res. 14 173–180. [PubMed] [Google Scholar]
- Narita M., Honda S., Umeyama H., Obana S. 1988. Designs of 3-dimensional structures of peptides and proteins and the synthetic route for peptides and proteins. The solubility of peptide intermediates in organic-solvents—Solubilizing potential of hexafluoro-2-propanol Bull. Chem. Soc. Jpn. 61 281–284. [Google Scholar]
- Oliveira E., Miranda A., Albericio F., Andreu D., Paiva A.C.M., Nakaie C.R., Tominaga M. 1997. Comparative evaluation of the synthesis and purification of transmembrane peptide fragments: Rat bradykinin receptor fragment 64-97 as model J. Pept. Res. 49 300–307. [DOI] [PubMed] [Google Scholar]
- Oliveira E., Cilli E.M., Miranda A., Jubilut G.N., Albericio A., Andreu A., Paiva A.C.M., Schreier S., Nakaie C.R. 2002. Monitoring the chemical assembly of a transmembrane bradykinin receptor fragment: Correlation between resin solvation, peptide chain mobility and rate of coupling Eur. J. Org. Chem. 21 3686–3694. [Google Scholar]
- Opella S.J., Marassi F.M., Gesell J.J., Valente A.P., Kim Y., Oblatt-Montal M., Montal M. 1999. Structures of the M2 channel-lining segments from nicotinic acetylcholine and NMDA receptors by NMR spectroscopy Nat. Struct. Biol. 6 374–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker A.J. 1969. Protic-dipolar aprotic solvent effects on rates of bimolecular reactions Chem. Rev. 69 1–32. [Google Scholar]
- Pawar A.P., DuBay K.F., Zurdo J., Chiti F., Vendruscolo M., Dobson C.M. 2005. Prediction of “aggregation-prone” and “aggregation-susceptible” regions in proteins associated with neurodegenerative diseases Mol. Biol. 350 379–392. [DOI] [PubMed] [Google Scholar]
- Perczel A., Park K., Fasman G.D. 1992. Deconvolution of the circular-dichroism spectra of proteins—The circular-dichroism spectra of the antiparallel beta-sheet in proteins Proteins 13 57–69. [DOI] [PubMed] [Google Scholar]
- Pillai V.N.R. and Mutter M. 1981. Conformational studies of poly(oxyethylene)-bound peptides and protein sequences Acc. Chem. Res. 14 122–130. [Google Scholar]
- Regen S.L. 1974. Influence of solvent on mobility of molecules covalently bound to polystyrene matrices J. Am. Chem. Soc. 96 5275–5276. [Google Scholar]
- Ribeiro S.C.F., Schreier S., Nakaie C.R., Cilli E.M. 2001. Effect of temperature on peptide chain aggregation: An EPR study of model peptidyl-resins Tetrahedron Lett. 42 3243–3246. [Google Scholar]
- Sarin V.K., Kent S.B.H., Merrifield R.B. 1980. Properties of swollen polymer networks—Solvation and swelling of peptide-containing resins in solid-phase peptide-synthesis J. Am. Chem. Soc. 102 5463–5470. [Google Scholar]
- Schellekens H. 2002. Bioequivalence and the immunogenicity of biopharmaceuticals Nat. Rev. Drug Discov. 1 457–462. [DOI] [PubMed] [Google Scholar]
- Sen S., Dash D., Pasha S., Brahmachari S.K. 2003. Role of histidine interruption in mitigating the pathological effects of long polyglutamine stretches in SCA1: A molecular approach Protein Sci. 12 953–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serpell L.C. 2000. Alzheimer's amyloid fibrils: Structure and assembly Biochim. Biophys. Acta 1502 16–30. [DOI] [PubMed] [Google Scholar]
- Soto C. and Frangione B. 1995. Two conformational states of amyloid β-peptide: Implications for the pathogenesis of Alzheimer's disease Neurosci. Lett. 186 115–118. [DOI] [PubMed] [Google Scholar]
- Srisailam S., Kumar T.K.S., Rajalingam D., Kathir K.M., Sheu H., Jan F., Chao P. 2003. Amyloid-like fibril formation in an all β-barrel protein J. Biol. Chem. 278 17701–17709. [DOI] [PubMed] [Google Scholar]
- Szabó Z., Jost K., Soós K., Zarándi M., Kiss J.T., Penke B. 1999. Solvent effect on aggregational properties of β-amyloid polypeptides studied by FT-IR spectroscopy J. Mol. Struct. 480 481–487. [Google Scholar]
- Tam J.P. and Lu Y.A. 1995. Coupling difficulty associated with interchain clustering and phase-transition in solid-phase peptide-synthesis J. Am. Chem. Soc. 117 12058–12063. [Google Scholar]
- Vaino A.R., Goodin D.B., Janda K.D. 2000. Investigating resins for solid phase organic synthesis: The relationship between swelling and microenvironment as probed by EPR and fluorescence spectroscopy J. Comb. Chem. 2 330–336. [DOI] [PubMed] [Google Scholar]
- Valente A.P., Almeida F.C.L., Nakaie C.R., Schreier S., Crusco E., Cilli E.M. 2005. Study of the effect of the peptide loading and solvent system in SPPS by HRMAS-NMR J. Pept. Sci. 11 556–563. [DOI] [PubMed] [Google Scholar]
- Vieira E.P., Hermel H., Möhwald H. 2003. Change and stabilization of the amyloid-β(1-40) secondary structure by fluorocompounds Biochim. Biophys. Acta 1645 6–14. [DOI] [PubMed] [Google Scholar]
- Warrass R., Wieruszeski M., Bouitillon C., Lippens G. 2000. High-resolution magic angle spinning NMR study of resin-bound polyalanine peptides J. Am. Chem. Soc. 122 1789–1795. [Google Scholar]
- Xing Y. and Higuchi K. 2002. Amyloid fibril proteins. Mech Ageing Dev. 123 1625–1636. [DOI] [PubMed] [Google Scholar]
- Yan B. 1998. Monitoring the progress and the yield of solid-phase organic reactions directly on resin supports Acc. Chem. Res. 31 621–630. [Google Scholar]