Abstract
Several members of the ATP-binding cassette (ABC) transporter superfamily, including P-glycoprotein and the half-transporter ABCG2, can confer multidrug resistance to cancer cells in culture by functioning as ATP-dependent efflux pumps. ABCG2 variants harboring a mutation at arginine 482 have been cloned from several drug-resistant cell lines, and these variants differ in their substrate transport phenotype. In this study, we changed the wild-type arginine 482 in human ABCG2 to each one of the 19 other standard amino acids and expressed each one transiently in HeLa cells. Using the 5D3 antibody that recognizes a cell surface epitope of ABCG2, we observed that all the mutants were expressed at the cell surface. However, the mutant ABCG2 proteins differed markedly in transport activity. All of the variants were capable of transporting one or more of the substrates used in this study, with the exception of the R482K mutant, which is completely devoid of transport ability. Six of the mutants (R482G, R482H, R482K, R482P, R482T, and R482Y) and the wild-type protein (R482wt) were selected for studies of basal and stimulated ATPase activity and photoaffinity labeling with the substrate analog [125I]iodoarylazidoprazosin. Whereas these seven ABCG2 variants differed markedly in ATPase activity, all were able to specifically bind the substrate analog [125I]iodoarylazidoprazosin. These data suggest that residue 482 plays an important role in substrate transport and ATP turnover, but that the nature of this amino acid may not be important for substrate recognition and binding.
Keywords: ABCG2, BCRP, ABC transporter, P-glycoprotein, [125I]iodoarylazidoprazosin
Mammalian ABC (ATP-binding cassette) transporter proteins are important for a variety of cell processes, such as the export of toxic substances out of the cell. In addition, several human ABC transporters have been shown to play important roles in diseases. For instance, mutations in the cystic fibrosis transmembrane conductance regulator (CFTR) cause cystic fibrosis (Riordan et al. 1989; Riordan 1993; Devidas and Guggino 1997); overexpression of multidrug resistance proteins, such as P-glycoprotein (P-gp), can confer drug resistance to cancer cells in culture (Gottesman et al. 2002); and mutations in the half-transporters ABCG5 and ABCG8 lead to sitosterolemia, a cholesterol accumulation disorder (Graf et al. 2002). P-gp is one of the best characterized mammalian ABC transporters and therefore has become one of the paradigms for understanding other ABC transporters.
In addition to P-gp, ABCG2 is another ABC transporter capable of extruding anti-cancer drugs from cells (Ejendal and Hrycyna 2002; Mao and Unadkat 2005; Krishnamurthy and Schuetz 2006). ABCG2, also called BCRP, MXR1, and ABCP, was first identified in placenta (Allikmets and Dean 1998) and soon thereafter cloned independently from breast and colon cancer cell lines selected in cytotoxic drugs such as doxorubicin and mitoxantrone (Doyle et al. 1998; Miyake et al. 1999; Robey et al. 2001). ABCG2, comprised of 655 amino acids, is classified as a half-transporter and is thought to function as a homodimer or higher-order oligomer (Kage et al. 2002; Litman et al. 2002; Xu et al. 2004; Bhatia et al. 2005). Expression of ABCG2 has been detected in the placenta, the ovary, the kidney, breast epithelial cells, the small intestine, the blood brain barrier, and hematopoietic stem cells (Allikmets and Dean 1998; Jonker et al. 2000; Maliepaard et al. 2001; Taipalensuu et al. 2001; Zhou et al. 2001; Cisternino et al. 2004; Krishnamurthy and Schuetz 2006). Although the physiological role of ABCG2 is not known precisely, its expression pattern suggests that its functions may include protecting tissues such as the fetus from toxic compounds and playing a role in red blood cells and stem cell biology (Maliepaard et al. 2001; Mao and Unadkat 2005; Krishnamurthy and Schuetz 2006). ABCG2 has been shown to transport a wide variety of substrates such as antibiotics, sterols and steroid hormones, folates, HIV protease inhibitors, porphyrins, and cytotoxic drugs (Honjo et al. 2001; Chen et al. 2003; Imai et al. 2003; Mitomo et al. 2003; Gupta et al. 2004; Janvilisri et al. 2005; Shafran et al. 2005; Krishnamurthy and Schuetz 2006). Taken together, the substrate specificity and expression pattern of ABCG2 suggest that expression of this transporter may compromise the treatment of, and play a role in, numerous disorders, as well as alter the bioavailability of therapeutic agents.
Examination of the transport phenotypes of several drug-resistant cell lines overexpressing ABCG2 revealed that, although they all overexpress ABCG2, they differ in substrate specificity (Honjo et al. 2001). These transport phenotypes were attributed to a point mutation in the ABCG2 gene, causing a mutation of arginine 482 to either glycine or threonine, and these observations were confirmed by studies of transfected cells (Honjo et al. 2001). Recently, another ABCG2 variant was found to have a mutation of arginine to methionine at position 482 in a doxorubicin-resistant CD4+ T-cell line (Wang et al. 2003). Furthermore, drug-resistant murine fibroblast cells selected in doxorubicin were also found to overexpress ABCG2 with mutations of R482 to either methionine or serine (Allen et al. 2002). The ABCG2 mutants from these drug-selected cell lines all have a broader substrate transport phenotype than the wild-type ABCG2 protein; thus the mutations were named gain-of-function mutations (Honjo et al. 2001). However, several recent reports have shown that the wild-type protein, but not any of these mutants, is capable of transporting potential physiological substrates such as folates and therapeutic agents such as antibiotics and methotrexate and other antifolates (Chen et al. 2003; Ishikawa et al. 2003; Janvilisri et al. 2005; Shafran et al. 2005). Therefore, the mutations of R482 should be considered more appropriately as change-of-function rather than gain-of-function mutations. Since amino acid 482 is predicted to be located in the beginning of the third transmembrane segment, or at the membrane/cytoplasm interface, it is not surprising that this residue influences substrate transport. In P-gp, residues located in transmembrane domains 4, 5, 6, 9, 10, 11, and 12 are known to be important for substrate binding and transport (Greenberger 1993; Morris et al. 1994; Hafkemeyer et al. 1998; Loo and Clarke 1999, 2002).
In two recent papers, the substrate transport and the catalytic and drug resistance phenotypes of several R482 ABCG2 mutants were studied (Miwa et al. 2003; Özvegy-Laczka et al. 2005a). In the present study, our goal was to analyze all of the possible R482X variants, where X represents any one of the standard amino acids, in order to gain further understanding of the function of the ABCG2 transporter protein. To this end, we constructed all 20 human ABCG2 variants, expressed them transiently in HeLa cells using a vaccinia virus expression system, and examined them for expression and function. Based on these initial studies, we selected several variants for further analysis of basal and prazosin-stimulated ATPase activity and substrate binding using the substrate analog [125I]iodoarylazidoprazosin ([125I]IAAP).
Our data show that the nature of the amino acid side chain greatly influences the function of the ABCG2 protein. Similar to the variants that occurred as a result of drug selection, the majority of the mutants were capable of transporting rhodamine 123, Bodipy FL prazosin and mitoxantrone. However, some of the mutants are deficient in transport, primarily of rhodamine 123 and Bodipy FL prazosin. When analyzed for catalytic activity, the mutants deficient in transport were also deficient in prazosin-stimulated ATPase activity. One mutant, R482K, did not transport any of the substrates tested, and the addition of prazosin had little effect on its ATPase activity. In contrast, all the variants analyzed here, including R482K, specifically bound the substrate analog [125I]IAAP. Taken together, residue 482 in ABCG2 plays an important role in the activity of the transporter but may not be essential for the interaction between ABCG2 and its substrates.
Results
Expression of ABCG2 protein variants
HeLa cells were coinfected with the vTF7-3 vaccinia virus and transfected with each of the different ABCG2 cDNAs independently, and the cells were harvested after 16–24 h incubation at 32°C. To investigate whether the mutant proteins were expressed on the cell surface, we labeled the cells with the conformation-sensitive monoclonal antibody 5D3 that recognizes a cell surface epitope of ABCG2 (Zhou et al. 2001; Özvegy-Laczka et al. 2005b). All the mutant proteins were expressed at the cell surface, albeit to varying degrees (Fig. 1A). While these observed differences could be due to different levels of expression at the surface, they may also be due to different conformations of the transporter variants, which would affect the accessibility of the 5D3 antibody epitope. Cell lysates were prepared and total expression of all of the R482X variants was verified by immunoblot analysis using the monoclonal antibody BXP-21 (Novus Biologicals) (Fig. 1B). All of the variants were expressed and appear as multiple bands on the blot, which is due to differential glycosylation of ABCG2 in our expression system (Diop and Hrycyna 2005).
Figure 1.
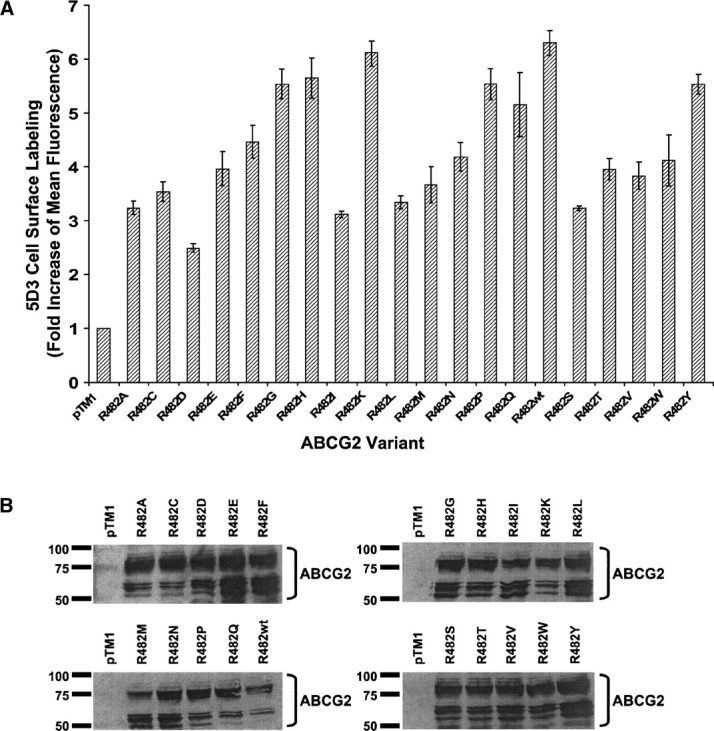
Expression and cell surface localization of R482X ABCG2 variants expressed in HeLa cells. (A) Cells were stained with the monoclonal antibody 5D3 (2 μg/200,000 cells) that recognizes a cell surface epitope of ABCG2. As the negative control, cells were transfected with the empty vector pTM1. The protein–antibody complex was visualized with a FITC-labeled secondary antibody (2.5 μg/200,000 cells) and the cells were analyzed for fluorescence by flow cytometry. Each bar represents the fold increase compared with the negative control cells transfected with pTM1, and X denotes any of the 20 standard amino acids. (B) Immunoblot analysis of cell lysates (20 μg protein), using the monoclonal antibody BXP-21 as described in Materials and Methods.
Analysis of substrate transport properties of wild-type and mutant ABCG2 proteins
We and numerous others have shown that the amino acid at position 482 affects the substrate phenotype of ABCG2 (Honjo et al. 2001; Özvegy et al. 2002; Chen et al. 2003; Ishikawa et al. 2003; Mitomo et al. 2003; Miwa et al. 2003; Alqawi et al. 2004; Özvegy-Laczka et al. 2005a). By analyzing the function of all of the mutant proteins, we aimed to further determine how the different properties of the amino acid at position 482 affect the function of the transporter. Of these 20 possible variants, three (R482F, R482L, R482P) have not previously been described. Out of these previously described variants, some have been analyzed for substrate transport, catalytic activity, and drug resistance, and some only for drug resistance (Miwa et al. 2003; Özvegy-Laczka et al. 2005a).
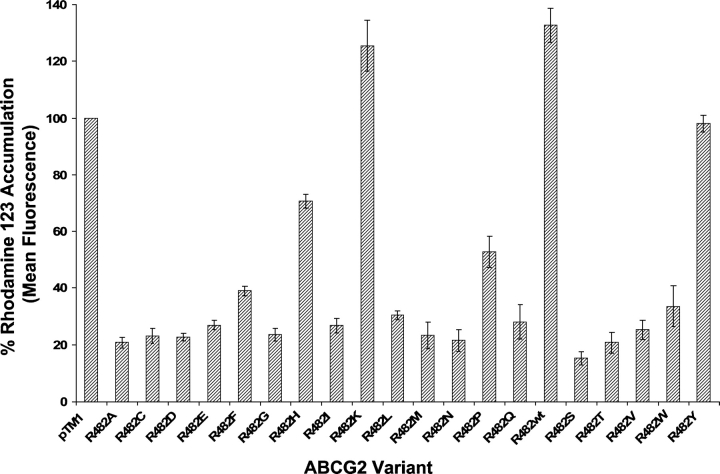
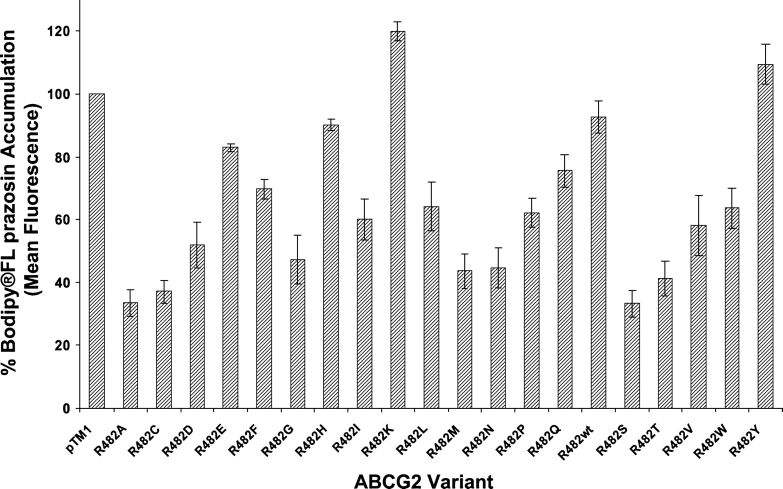
As depicted in Figure 2 and as shown previously (Honjo et al. 2001), wild-type ABCG2 (R482wt) does not transport rhodamine 123. Like R482wt, the R482K mutant was also incapable of rhodamine 123 transport, and R482H, which also contains a basic side chain at position 482, was also impaired in rhodamine 123 transport. However, most of the R482 variants were capable of rhodamine 123 efflux to varying degrees. ABCG2 variants with amino acids containing side chains with a ring structure have a reduced ability to transport rhodamine 123, compared with those with a small amino acid at this position, such as the R482G variant. The negative effect of the ring structure is minimized for the more hydrophobic amino acids such as phenylalanine and tryptophan. Transport of the fluorescent compound Bodipy FL prazosin followed a similar pattern to that observed for rhodamine 123, where the variants R482wt, R482K, R482H, and R482Y show the least transport (Fig. 3). All the variants of ABCG2, except the R482K variant, were able to efflux the substrate mitoxantrone at comparable levels (data not shown).
Figure 2.
Functional analysis of rhodamine 123 accumulation in HeLa cells transiently expressing wild-type ABCG2 and all 19 ABCG2 mutants. Cells were incubated with 0.5 μg/mL rhodamine 123 for 30 min at 37°C. After incubation, cells were harvested by centrifugation and resuspended and incubated in drug-free medium for an additional 30 min at 37°C, followed by analysis of fluorescence by flow cytometry. The percent of accumulation was calculated by dividing the mean fluorescence values obtained for all the variants by the fluorescence value of the pTM1 vector and multiplying by 100.
Figure 3.
Functional analysis of Bodipy FL prazosin accumulation in HeLa cells transiently expressing wild-type ABCG2 and all 19 ABCG2 mutants. Cells were incubated with 0.5 μM Bodipy FL prazosin for 30 min at 37°C and kept on ice until flow cytometric analysis. The percent of accumulation was calculated by dividing the mean fluorescence values obtained for all the variants by the mean fluorescence value of the pTM1 vector and multiplying by 100.
Analysis of the substrate binding properties of wild-type and six mutant ABCG2 proteins
In order to further investigate the effects of the R482X mutations, we studied the drug-binding ability of a selection of ABCG2 mutants (R482G, R482H, R482K, R482P, R482T, and R482Y) and the wild-type ABCG2 (R482wt). Based on the transport data described above (Figs. 2, 3), we studied in further detail variants that differ substantially from wild-type ABCG2. We selected mutants R482H, R482P, and R482Y because they are partially deficient in rhodamine 123 transport, whereas mitoxantrone transport is intact. R482K was chosen because it is completely devoid of any transport, and the R482G and R482T mutants were chosen because these two mutants have been used in many different studies and would therefore be of broader interest. Of these six ABCG2 mutants plus the wild-type protein, biochemical analyses of R482H and R482P have not been described previously.
HeLa cells were transfected/infected with the different mutant constructs and crude membrane extracts were prepared from each batch of transfected cells. The membrane extracts were subjected to photoaffinity cross-linking with the prazosin analog [125I]IAAP, immunoprecipitation, SDS-PAGE, and autoradiography as described in Materials and Methods. As shown in Figure 4, all ABCG2 variants examined were labeled efficiently by [125I]IAAP, and the labeling was competed specifically with 10 μM prazosin. Furthermore, we quantified the autoradiogram using a PhosphorImager and compared the intensity of the two bands, i.e., with and without prazosin (data not shown). We found that the percent decrease of [125I]IAAP labeling in the presence of prazosin was similar for the seven variants of ABCG2 studied here. Bodipy FL prazosin was also capable of competing for IAAP labeling even though it was not transported in many cases (data not shown; Fig. 3).
Figure 4.
Specific [125I]IAAP photoaffinity labeling of crude membranes derived from HeLa cells expressing wild-type ABCG2 (R482wt) and the ABCG2 variants R482G, R482H, R482K, R482P, R482T, and R482Y. Crude membrane extracts (140 μg) derived from HeLa cells were labeled with [125I]IAAP alone (−) or in the presence of 10 μM prazosin (+) as described in Materials and Methods. After labeling at room temperature for 10 min and UV cross-linking (365 nm) for 20 min on ice, the samples were subjected to immunoprecipitation using 4 μg BXP-21 antibody. The samples were then separated by SDS-PAGE (7.5%), and the gels were dried and exposed to autoradiography film for 4–7 d at −80°C. The positions of ABCG2 are denoted by brackets.
Analysis of the catalytic properties of wild-type and six of the ABCG2 mutants
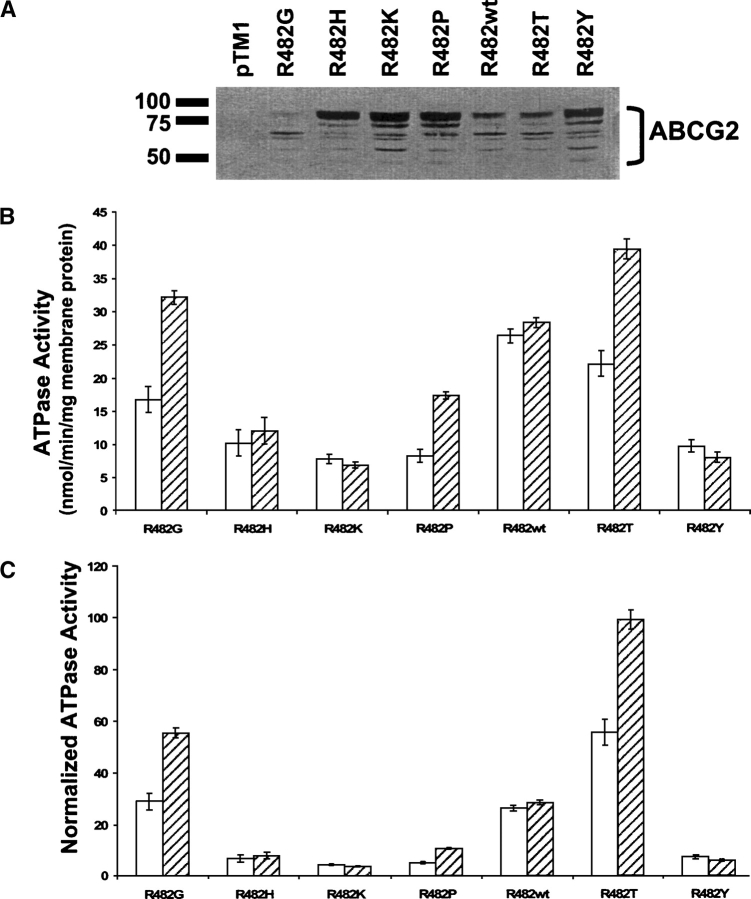
To evaluate the catalytic properties of the wild-type and mutant ABCG2 proteins, we used ABCG2 transiently expressed in HeLa cells and analyzed crude membrane extracts for basal and prazosin-stimulated ATPase hydrolysis. We analyzed expression of ABCG2 in the membranes using the monoclonal antibody BXP-21 (Fig. 5A), which shows that the R482G, R482wt, and R482T membranes used here express less ABCG2, compared with the membranes expressing the R482H, R482K, R482P, and R482Y variants. These differences are likely due to the fact that the membranes were prepared on two different days. As shown in Figure 5B, these seven variants of ABCG2 show very different ATP hydrolytic properties. The R482G, R482P, and R482T variants are stimulated 1.9-, 2.1-, and 1.8-fold, respectively, by the addition of 20 μM prazosin. In contrast, the R482H, R482K, R482Y, and R482wt variants are not markedly affected by the addition of 20 μM prazosin. In addition to changes in fold stimulation, the seven variants of ABCG2 examined here differ substantially in the basal ATP activities. We found that in comparison with the mutants, the R482wt protein has the highest basal ATPase activity, which may be attributed to the presence of unknown endogenous substrate(s) in the membranes. In Figure 5C, we show the ATPase activities normalized to their expression level. The expression level of each ABCG2 variant was quantitated from the immunoblot analysis in Figure 5A, using R482wt as the baseline.
Figure 5.
Basal and drug-stimulated ATPase activity of wild-type ABCG2 (R482wt) and ABCG2 variants R482G, R482H, R482K, R482P, R482T, and R482Y. (A) Immunoblot analysis using 10 μg of total membrane protein separated using 10% SDS-PAGE. (B) Vanadate-sensitive ATPase hydrolysis of ABCG2 was measured as free phosphate release in crude membrane extracts (5–10 μg) from transiently transfected HeLa cells. The assays were performed both in the absence (open bars) and the presence (hatched bars) of 20 μM prazosin, resulting in basal and drug-stimulated activities, respectively. The activities from at least three independent experiments done in triplicates are shown (mean ± SD). (C) ATPase activity normalized to protein expression, using R482wt as the baseline.
Discussion
The nature of the amino acid residue at position 482 affects the function of the ABCG2 protein, and several recent studies suggest that residue 482 is involved in determining the substrate specificity of the transporter (Honjo et al. 2001; Özvegy et al. 2002; Chen et al. 2003; Ishikawa et al. 2003; Mitomo et al. 2003; Miwa et al. 2003; Alqawi et al. 2004; Özvegy-Laczka et al. 2005a). Although this mutation may affect the expression level of ABCG2 to some degree (Özvegy-Laczka et al. 2005a; present study), the variations seen in drug resistance and substrate transport appear to be primarily due to inherent differences in how these ABCG2 variants transport substrates (Miwa et al. 2003; Özvegy-Laczka et al. 2005a). Miwa et al. (2003) analyzed the drug resistance phenotype of 15 of the R482 mutants transfected into murine fibroblast PA317 cells and concluded that most of the mutants confer increased resistance to mitoxantrone compared with the wild-type protein, whereas the resistance to SN-38 is not affected. These data suggest that mitoxantrone and SN-38 interact differently with the transporter. Özvegy-Laczka et al. (2005a) selected eight of these 15 R482 variants and the R482I mutant to further study substrate transport and ATPase activity in insect cells. Most of these mutants could actively extrude mitoxantrone and the dye Hoechst 33342. However, the R482K mutant is devoid of substrate transport and shows inhibition of ATPase activity in the presence of substrate.
The ABCG2-mediated transport of rhodamine 123 and Bodipy FL prazosin varies greatly between the mutants described here (Figs. 2, 3), whereas wild-type ABCG2 (R482) and all but one of the ABCG2 mutants (R482K) are able to transport mitoxantrone (data not shown). In general, small side chains at this position result in proteins with wider substrate specificities. The R482K mutant is completely devoid of any transport, even though lysine is generally considered a conservative substitution for arginine. With a pKa between 6 and 6.5, histidine can be uncharged or positively charged, depending upon its local environment. Our data demonstrate that the R482H mutant is able to efflux mitoxantrone to the same extent as the wild-type protein (data not shown), although it is somewhat deficient in rhodamine 123 and Bodipy FL prazosin transport. Although it is tempting to draw conclusions about specificity based on the charge of the substrates, we are unable to determine the exact charge state of the substrate when it interacts with the transporter in the membrane. Our results demonstrate that it is difficult to predict both the function of the 20 variants of ABCG2 based on the amino acid moiety at position 482 and whether a particular substrate will be transported.
We have previously shown that the prazosin substrate analog [125I]iodoarylazidoprazosin ([125I]IAAP) can be photoaffinity cross-linked to R482wt, R482G, and R482T variants of ABCG2 when overexpressed in MCF-7/FLV1000, S1-M1-80, and MCF-7/AdVp3000 cells, respectively (Ejendal and Hrycyna 2005). Alqawi and colleagues have also shown that ABCG2, overexpressed in MCF-7/AdVp1000 cells (R482T) and MCF-7/Mito (R482wt), binds directly and specifically to a photoactive drug analog of rhodamine 123, [125I]Iodoarylazidorhodamine123 ([125I]IAARh123) (Alqawi et al. 2004). Here, we show [125I]IAAP labeling of ABCG2 expressed in transiently transfected/infected HeLa cells. As shown in Figure 4, all of the seven selected mutants were labeled efficiently by [125I]IAAP, and the labeling can be competed off by the addition of 10 μM prazosin. Importantly, the R482K mutant, which is unable to transport any of the substrates tested here, including Bodipy FL prazosin, and the R482 wild-type protein, which only transports Bodipy FL prazosin to a low extent, are labeled with [125I]IAAP. This finding is consistent with that observed by Alqawi et al. (2004), where they found that, even though wild-type ABCG2 does not efflux rhodamine 123, the protein is labeled with [125I]IAARh123. Thus, mutations in residue 482 do not appear to interfere directly with substrate binding, but they may affect other factors important for substrate translocation. Previously, we have shown that, out of seven agents tested, only prazosin and GF120918 can compete for [125I]IAAP labeling of R482G, R482wt, and R482T and that known substrates such as rhodamine 123 and mitoxantrone do not (Ejendal and Hrycyna 2005). These results suggest that, in ABCG2, prazosin has a distinct binding site from rhodamine 123 and mitoxantrone. In contrast, transport of rhodamine 123, Bodipy FL prazosin, and mitoxantrone can be inhibited by unlabeled prazosin (data not shown), indicating that these binding sites are at least partially overlapping.
Substrate labeling and transport were performed using the prazosin analogs [125I]IAAP and Bodipy FL prazosin, respectively, whereas drug-stimulated ATPase activity assays were performed with prazosin. The prazosin-stimulated ATPase activities correlated well with Bodipy FL prazosin transport, but we observed no correlation of the transport and ATPase data with the binding of [125I]IAAP. Furthermore, both prazosin and Bodipy FL prazosin can compete with [125I]IAAP binding, and prazosin can compete with Bodipy FL prazosin transport (data not shown), indicating that these three compounds are recognized similarly by ABCG2. Thus, the differences observed between transport, ATP hydrolysis, and binding are not likely due to the different prazosin analogs used here.
The basal ATPase activities varied from ∼7.8 to 26.3 nmol Pi/min/mg, which may be attributed to inherent differences in how these mutants interact with endogenous substrates, or alternatively due to variations in expression levels. It has previously been suggested for the R482Y variant that unknown endogenous substrates already fully stimulate the basal activity, which cannot be further stimulated by added exogenous substrates like prazosin (Özvegy-Laczka et al. 2005a). This hypothesis may also explain the differences in the ATPase activity seen for R482H and R482K mutants and the wild-type R482 protein. Moreover, several variants (R482H, R482K, R482wt, and R482Y) showed no prazosin-stimulated ATPase activity. This observation suggests that these mutants are impaired in ATP turnover, although none of these mutants have mutations in the nucleotide binding domain.
During the transport cycle, P-gp displays different affinities for its substrates and alternates between high-affinity and low-affinity binding sites (Ramachandra et al. 1998; Martin et al. 2000; Sauna and Ambudkar 2000; Higgins and Linton 2004). Based on these findings, it is possible that the transport-deficient ABCG2 variant R482K, which specifically binds [125I]IAAP, is unable to undergo these necessary conformational changes. Thus, substrate can be bound but not released and therefore not effectively transported. The R482K mutation may also play an analogous role to the allosteric effect mediated by the P-gp inhibitor cis(Z)-flupentixol, which allows P-gp to bind substrates but not transport them (Maki et al. 2003). Furthermore, we noted that the transport-incompetent variants of ABCG2 are generally better recognized by the 5D3 antibody (Fig. 1A). It has previously been shown that 5D3 can detect conformational changes in ABCG2 (Özvegy-Laczka et al. 2005b). In conclusion, it is believed that ABC transporters, including ABCG2, change conformations and affinities for substrates throughout the transport cycle. We hypothesize that a mutation in residue 482 may affect the ability of ABCG2 to change between conformations that would prevent substrate translocation and substrate-stimulated ATPase activity. These observations may also be interesting from a structural point of view. The main difficulties with structure determinations of ABC transporters are the presence of hydrophobic regions and flexible loops that prevent protein–protein contact and successful crystal packing. Among the 20 R482X mutants analyzed, R482K may show to be an interesting ABCG2 variant to study structurally since finding a transporter locked in a rigid conformation may aid in three-dimensional structure solving.
Protein sequence alignment shows that arginine 482 is not conserved in the ABCG subfamily, with the exception for mouse ABCG2 and mouse ABCG3 (Lorkowski 2002). However, a proline residue at position 480 (P480) is conserved in the ABCG subfamily. In addition to the analysis of the full-length proteins, we aligned the transmembrane domains (TMD) of ABCG1, ABCG2, ABCG4, ABCG5, and ABCG8 using ClustalW (http://www.ebi.ac.uk/clustalw/) and observed 19%–24% sequence identity while aligning the TMDs alone, compared with 24%–25% sequence identity for the full-length protein (data not shown). Furthermore, alignment of human ABCG2 with each of the TMDs of human P-gp and MRP1, the Escherichia coli BtuC, and the TMD of E. coli MsbA also indicate residue R482 is not conserved (data not shown). In fact, the sequence identity of the transmembrane domain of ABCG2 compared with those of BtuC and MsbA, proteins for which crystal structures have been determined (Chang and Roth 2001; Locher et al. 2002; Chang 2003; Reyes et al. 2006), is <10%. Therefore, based on sequence analysis alone, it is complicated to draw conclusions about the role this specific arginine residue may play in determining conformational changes, substrate interactions, and transport function of ABCG2.
Taken together, amino acid residue 482 in the ABCG2 protein plays an important role for the function of the protein, but the exact nature of the side chain is not a crucial determinant for the interaction of ABCG2 with the substrate analog [125I]IAAP. We also found that the 482 residue is not crucial for trafficking of ABCG2 to the plasma membrane, since all of the R482X mutants were expressed at the cell surface. Since all of the mutants that are deficient in transport and ATPase function are still able to bind the drug, residue 482 may not be involved directly in substrate binding but, rather, may play an important role in the intramolecular cross-talk that conveys the signal from the transmembrane domain to the ABC or may be involved in promoting conformational changes. Understanding how ABCG2 functions, how it adopts different conformations, and how the signal is transmitted from the transmembrane domain to the ATP-binding domain to elicit ATP hydrolysis could potentially contribute to the development of better inhibitors and modulators for ABCG2.
Materials and methods
Reagents
Rhodamine 123, prazosin, mitoxantrone, ATP, sodium orthovanadate, oubain, and EGTA were obtained from Sigma-Aldrich, and Bodipy FL prazosin was bought from Molecular Probes. AEBSF, DTT, and aprotinin were purchased from Fisher Scientific, and micrococcal nuclease was purchased from Worthington. Recombinant vaccinia virus (vTF7-3) and the pTM1 plasmid were gifts from Dr. Steven Broyles (Purdue University), and Dr. Bernard Moss (NIH), respectively.
Construction of ABCG2 mutants
The ABCG2 cDNA was cloned into the NcoI and XhoI sites of the pTM1 plasmid, where expression is under the control of the T7 promoter (Hrycyna et al. 1998). Coinfection with the vaccinia virus (vTF7-3) causes overexpression of genes regulated by this promoter. Sequence overlap extension PCR was performed using the outer primers binding immediately upstream of the internal PstI site in the ABCG2 gene (5′-CACTGTGAGGCCTATAATAAC-3′) and immediately downstream from the XhoI site (5′-TCGTCGACTTAATTAATTAGG-3′). Twenty inner primer pairs, forward and reverse primers, designed to change the amino acid at position 482 were based on the following sequences: 5′-TTTATTACCCATGXXXATGTTACCAAG-3′ and 5′-CTTGGTAACATXXXCATGGGTAATAAA-3′, respectively, where XXX indicates where they differ to introduce any of the twenty amino acids. The plasmid constructs were sequenced to verify the desired sequence. When cloning ABCG2 into the pTM1 plasmid, the serine residue at position 2 was changed to alanine; thus all our constructs carry the S2A mutation. To ensure that this substitution does not affect the function or the surface expression of the ABCG2 protein, we mutated the alanine back to serine in the R482G variant of ABCG2 and performed flow cytometric analysis to test for both function and expression; the two constructs were indistinguishable (data not shown).
Cell culture and vaccinia virus mediated transient transfection
All cells were cultured at 37°C with 5% CO2. HeLa cells (cervical epitheloid carcinoma) were maintained in DMEM (Cambrex) supplemented with 10% fetal bovine serum (FBS) (Cambrex), 2 mM L-glutamine (Cellgro), 50 units/mL penicillin, and 50 μg/mL streptomycin (Cellgro). For transient expression of ABCG2, HeLa cells were cotransfected/infected with vaccinia virus (vTF7-3) as described previously (Hrycyna et al. 1998). HeLa cells were generously provided by Dr. Michael Gottesman (NCI/NIH).
SDS-PAGE and immunoblot analysis
Cells were harvested and lysed by three freeze-thaw cycles in lysis buffer (10 mM Tris at pH 8.0, 10 mM MgSO4, 1 mM DTT, 2 mM CaCl2, 1% Triton X-100, and protease inhibitors), and samples were mixed with SDS-sample buffer. Cell lysates (20 μg protein) or crude membrane extracts (10 μg protein) were loaded on 7.5% SDS-PAGE gels and separated by electrophoresis, followed by blotting to pure Protran nitrocellulose membranes (Schleicher & Schuell BioScience). The nitrocellulose membranes were blocked in 20% nonfat dry milk dissolved in PBS with 0.05% (v/v) Tween 20 (PBST), and all antibodies were diluted in 5% (w/v) nonfat dry milk/PBST. For detection of ABCG2 protein, the membrane was incubated in a 1:2000 dilution of the monoclonal antibody BXP-21 (Novus Biologicals) (Maliepaard et al. 2001) followed by incubation in a 1:4000 dilution of horseradish peroxidase-labeled goat anti-mouse antibody (Caltag). The immunocomplex was visualized with Supersignal West Pico Chemiluminescence Reagent (Pierce).
Membrane preparation
Cells were harvested by scraping at 48 h post-transfection and pelleted by centrifugation at 300g. Crude membrane extracts were prepared as described previously (Hrycyna et al. 1998) with the only exception being that the membrane fraction was pelleted at 300,000g for 40 min at 4°C. The membrane pellet was collected, and the membranes were assayed for total protein concentration, aliquoted, frozen on dry ice, and stored at −80°C.
Flow cytometric analysis
Antibody surface staining and substrate accumulation assays were performed as described previously (Hrycyna et al. 1998), with slight modifications. For cell surface detection of ABCG2, 200,000 cells were labeled with the anti-ABCG2 antibody 5D3 (2 μg; Chemicon) (Zhou et al. 2001) for 40 min at room temperature. Control samples were incubated in parallel with the unspecific isotype-matched control antibody IgG2b (Pharmingen) (data not shown). The cells were washed and then resuspended in media with a FITC-labeled goat anti-mouse-IgG2b secondary antibody (2.5 μg; Pharmingen), and the cells were then incubated for an additional 40 min. For substrate accumulation studies, 200,000 cells were incubated with 0.5 μg/mL rhodamine 123, or 0.5 μM Bodipy FL prazosin, or 10 μM mitoxantrone and incubated for 30 min at 37°C. The cells were harvested at 300g and either kept on ice until flow cytometric analysis (Bodipy FL prazosin) or resuspended in fresh media without substrate and incubated for an additional 30 min at 37°C. The cells were harvested and cellular fluorescence was analyzed using a FACSCalibur flow cytometer (Becton Dickinson) equipped with a 488-nm argon laser and a 530-band pass filter (FL1) for rhodamine 123, Bodipy FL prazosin, and FITC labeling, and with a 633-nm laser and a 670-band pass filter (FL3) for mitoxantrone. Mean fluorescence intensity values (arbitrary units) were determined using histogram statistics in the Cell Quest program (Becton Dickinson). Each histogram is based on a sample of 10,000 cells, and all assays were performed a minimum of three independent times.
Photoaffinity labeling of ABCG2 with [125I]iodoarylazidoprazosin
[125I]Iodoarylazidoprazosin (specific activity 2200 Ci/mmol) was purchased from Perkin Elmer LifeSciences, and labeling was performed as described previously (Hrycyna et al. 1998), with a few modifications. Membrane protein (140 μg in 98 μL, 50 mM Tris-HCl at pH 7.5, 2 mM AEBSF, 1 mM DTT, 1% aprotinin) was mixed with 2 μL (1 μCi) of [125I]IAAP in the absence or the presence of 10 μM prazosin and incubated at room temperature for 10 min, followed by UV cross-linking (365 nm) for 20 min on ice. To immunoprecipitate the ABCG2 protein, 4 μL (1 mg/mL) BXP-21 antibody (Novus Biologicals) (Maliepaard et al. 2001) and RIPA buffer with inhibitors (10 mM Tris-HCl at pH 7.4, 150 mM NaCl, 0.1% SDS, 1% TritonX-100, 1% sodium deoxycholate with 2 mM AEBSF, 1% aprotinin, and 1 mM EDTA) were added to a total volume of 500 μL, and the reaction was incubated with rotation at 4°C overnight. Next, 125 μL of protein A-Sepharose slurry (20%) from Amersham Biosciences was added and the sample was incubated with rotation for 4 h at 4°C; then the samples were washed in RIPA buffer containing inhibitors to remove any unbound protein. The samples were separated by SDS-PAGE; the gels were dried with cellophane (Invitrogen Life Technologies) and exposed to single emulsion autoradiography film (Midwest Scientific) for 4–7 d at −80°C.
ATPase activity assay
Microsomal membranes were analyzed for both vanadate-sensitive basal and vanadate-sensitive drug-stimulated ATP hydrolysis by colorimetric detection of inorganic phosphate release, as described previously (Hrycyna et al. 1998). Briefly, 5–10 μg total membrane protein was incubated in 100 μL assay buffer (50 mM Tris at pH 7.5, 5 mM sodium azide, 2 mM EGTA, 1 mM oubain, 2 mM DTT, 50 mM KCl, 10 mM MgCl2) in the presence or the absence of 300 μM vanadate and 20 μM prazosin, for 30 min at 37°C. The reaction was stopped by the addition of 100 μL 5% SDS. Then, 400 μL Pi reagent (1% ammonium molybdate, 0.014% antimony potassium tartarate in 2.5 N sulfuric acid), 500 μL H2O, and 200 μL of 1% ascorbic acid solution were subsequently added to develop the color. The samples were incubated for 10 min at room temperature followed by optical density reading at 880 nm. All assays were repeated at least three times and performed in triplicates.
Acknowledgments
We thank Aarti Bhatia for critical reading of the manuscript and help with ClustalW alignments. This work was supported in part by an American Cancer Society Institutional Research Grant no. IRG-58-006-41 and the Purdue Research Foundation.
Footnotes
Reprint requests to: Christine A. Hrycyna, Department of Chemistry and Purdue Cancer Center, 560 Oval Drive, Purdue University, West Lafayette, IN 47907, USA; e-mail: hrycyna@purdue.edu; fax: (765) 494-0239.
Article and publication are at http://www.proteinscience.org/cgi/doi/10.1110/ps.051998406.
Abbreviations: ABC, ATP-binding cassette; [125I]IAAP, [125I]iodoarylazidoprazosin; P-gp, P-glycoprotein; AEBSF, 4-(2-Aminoethyl)benzenesulfonylfluoride; DTT, dithiothreitol; SDS-PAGE, sodium dodecyl sulfate polyacrylamide gel electrophoresis.
References
- Allen J.D., Jackson S.C., Schinkel A.H. 2002. A mutation hot spot in the Bcrp1 (Abcg2) multidrug transporter in mouse cell lines selected for Doxorubicin resistance. Cancer Res. 62: 2294–2299. [PubMed] [Google Scholar]
- Allikmets R. and Dean M. 1998. Cloning of novel ABC transporter genes. Methods Enzymol. 292: 116–130. [DOI] [PubMed] [Google Scholar]
- Alqawi O., Bates S., Georges E. 2004. Arginine482 to threonine mutation in the breast cancer resistance protein ABCG2 inhibits rhodamine 123 transport while increasing binding. Biochem. J. 382: 711–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatia A., Schafer H.J., Hrycyna C.A. 2005. Oligomerization of the human ABC transporter ABCG2: Evaluation of the native protein and chimeric dimers. Biochemistry 44: 10893–10904. [DOI] [PubMed] [Google Scholar]
- Chang G. 2003. Structure of MsbA from Vibrio cholera: A multidrug resistance ABC transporter homolog in a closed conformation. J. Mol. Biol 330: 419–430. [DOI] [PubMed] [Google Scholar]
- Chang G. and Roth C.B. 2001. Structure of MsbA from E. coli: A homolog of the multidrug resistance ATP binding cassette (ABC) transporters. Science 293: 1793–1800. [DOI] [PubMed] [Google Scholar]
- Chen Z.S., Robey R.W., Belinsky M.G., Shchaveleva I., Ren X.Q., Sugimoto Y., Ross D.D., Bates S.E., Kruh G.D. 2003. Transport of methotrexate, methotrexate polyglutamates, and 17β-estradiol 17-(β-D-glucuronide) by ABCG2: Effects of acquired mutations at R482 on methotrexate transport. Cancer Res. 63: 4048–4054. [PubMed] [Google Scholar]
- Cisternino S., Mercier C., Bourasset F., Roux F., Scherrmann J.M. 2004. Expression, up-regulation, and transport activity of the multidrug-resistance protein Abcg2 at the mouse blood-brain barrier. Cancer Res. 64: 3296–3301. [DOI] [PubMed] [Google Scholar]
- Devidas S. and Guggino W.B. 1997. CFTR: Domains, structure, and function. J. Bioenerg. Biomembr. 29: 443–451. [DOI] [PubMed] [Google Scholar]
- Diop N.K. and Hrycyna C.A. 2005. N-linked glycosylation of the human ABC transporter ABCG2 on asparagine 596 is not essential for expression, transport activity, or trafficking to the plasma membrane. Biochemistry 44: 5420–5429. [DOI] [PubMed] [Google Scholar]
- Doyle L.A., Yang W., Abruzzo L.V., Krogmann T., Gao Y., Rishi A.K., Ross D.D. 1998. A multidrug resistance transporter from human MCF-7 breast cancer cells. Proc. Natl. Acad. Sci. 95: 15665–15670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ejendal K.F. and Hrycyna C.A. 2002. Multidrug resistance and cancer: The role of the human ABC transporter ABCG2. Curr. Protein Pept. Sci. 3: 503–511. [DOI] [PubMed] [Google Scholar]
- Ejendal K.F. and Hrycyna C.A. 2005. Differential sensitivities of the human ATP-binding cassette transporters ABCG2 and P-glycoprotein to cyclosporin A. Mol. Pharmacol. 67: 902–911. [DOI] [PubMed] [Google Scholar]
- Gottesman M.M., Fojo T., Bates S.E. 2002. Multidrug resistance in cancer: Role of ATP-dependent transporters. Nat. Rev. Cancer 2: 48–58. [DOI] [PubMed] [Google Scholar]
- Graf G.A., Li W.P., Gerard R.D., Gelissen I., White A., Cohen J.C., Hobbs H.H. 2002. Coexpression of ATP-binding cassette proteins ABCG5 and ABCG8 permits their transport to the apical surface. J. Clin. Invest. 110: 659–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberger L.M. 1993. Major photoaffinity drug labeling sites for iodoaryl azidoprazosin in P-glycoprotein are within, or immediately C-terminal to, transmembrane domain-6 and domain-12. J. Biol. Chem. 268: 11417–11425. [PubMed] [Google Scholar]
- Gupta A., Zhang Y., Unadkat J.D., Mao Q. 2004. HIV protease inhibitors are inhibitors but not substrates of the human breast cancer resistance protein (BCRP/ABCG2). J. Pharmacol. Exp. Ther. 310: 334–341. [DOI] [PubMed] [Google Scholar]
- Hafkemeyer P., Dey S., Ambudkar S.V., Hrycyna C.A., Pastan I., Gottesman M.M. 1998. Contribution to substrate specificity and transport of nonconserved residues in transmembrane domain 12 of human P-glycoprotein. Biochemistry 37: 16400–16409. [DOI] [PubMed] [Google Scholar]
- Higgins C.F. and Linton K.J. 2004. The ATP switch model for ABC transporters. Nat. Struct. Mol. Biol. 11: 918–926. [DOI] [PubMed] [Google Scholar]
- Honjo Y., Hrycyna C.A., Yan Q.W., Medina-Perez W.Y., Robey R.W., van De Laar A., Litman T., Dean M., Bates S.E. 2001. Acquired mutations in the MXR/BCRP/ABCP gene alter substrate specificity in MXR/BCRP/ABCP-overexpressing cells. Cancer Res. 61: 6635–6639. [PubMed] [Google Scholar]
- Hrycyna C.A., Ramachandra M., Pastan I., Gottesman M.M. 1998. Functional expression of human P-glycoprotein from plasmids using vaccinia virus-bacteriophage T7 RNA polymerase system. Methods Enzymol. 292: 456–473. [DOI] [PubMed] [Google Scholar]
- Imai Y., Asada S., Tsukahara S., Ishikawa E., Tsuruo T., Sugimoto Y. 2003. Breast cancer resistance protein exports sulfated estrogens but not free estrogens. Mol. Pharmacol. 64: 610–618. [DOI] [PubMed] [Google Scholar]
- Ishikawa T., Kasamatsu S., Hagiwara Y., Mitomo H., Kato R., Sumino Y. 2003. Expression and functional characterization of human ABC transporter ABCG2 variants in insect cells. Drug. Metab. Pharmacokinet. 18: 194–202. [DOI] [PubMed] [Google Scholar]
- Janvilisri T., Shahi S., Venter H., Balakrishnan L., van Veen H.W. 2005. Arginine-482 is not essential for transport of antibiotics, primary bile acids and unconjugated sterols by the human breast cancer resistance protein (ABCG2). Biochem. J. 385: 419–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonker J.W., Smit J.W., Brinkhuis R.F., Maliepaard M., Beijnen J.H., Schellens J.H., Schinkel A.H. 2000. Role of breast cancer resistance protein in the bioavailability and fetal penetration of topotecan. J. Natl. Cancer Inst. 92: 1651–1656. [DOI] [PubMed] [Google Scholar]
- Kage K., Tsukahara S., Sugiyama T., Asada S., Ishikawa E., Tsuruo T., Sugimoto Y. 2002. Dominant-negative inhibition of breast cancer resistance protein as drug efflux pump through the inhibition of S-S dependent homodimerization. Int. J. Cancer 97: 626–630. [DOI] [PubMed] [Google Scholar]
- Krishnamurthy P. and Schuetz J.D. 2006. Role of abcg2/bcrp in biology and medicine. Annu. Rev. Pharmacol. Toxicol. 46: 381–410. [DOI] [PubMed] [Google Scholar]
- Litman T., Jensen U., Hansen A., Covitz K.M., Zhan Z., Fetsch P., Abati A., Hansen P.R., Horn T., Skovsgaard T.et al. 2002. Use of peptide antibodies to probe for the mitoxantrone resistance-associated protein MXR/BCRP/ABCP/ABCG2. Biochim. Biophys. Acta 1565: 6–16. [DOI] [PubMed] [Google Scholar]
- Locher K.P., Lee A.T., Rees D.C. 2002. The E. coli BtuCD structure: A framework for ABC transporter architecture and mechanism. Science 296: 1091–1098. [DOI] [PubMed] [Google Scholar]
- Loo T.W. and Clarke D.M. 1999. Identification of residues in the drug-binding domain of human P-glycoprotein. Analysis of transmembrane segment 11 by cysteine-scanning mutagenesis and inhibition by dibromobimane. J. Biol. Chem. 274: 35388–35392. [DOI] [PubMed] [Google Scholar]
- Loo T.W. and Clarke D.M. 2002. Location of the rhodamine-binding site in the human multidrug resistance P-glycoprotein. J. Biol. Chem. 277: 44332–44338. [DOI] [PubMed] [Google Scholar]
- Lorkowski S.A.C.P. 2002. ABCG subfamily of human ATP-binding cassette proteins. Pure Appl. Chem. 74: 2057–2081. [Google Scholar]
- Maki N., Hafkemeyer P., Dey S. 2003. Allosteric modulation of human P-glycoprotein. Inhibition of transport by preventing substrate translocation and dissociation. J. Biol. Chem. 278: 18132–18139. [DOI] [PubMed] [Google Scholar]
- Maliepaard M., Scheffer G.L., Faneyte I.F., van Gastelen M.A., Pijnenborg A.C., Schinkel A.H., van De Vijver M.J., Scheper R.J., Schellens J.H. 2001. Subcellular localization and distribution of the breast cancer resistance protein transporter in normal human tissues. Cancer Res. 61: 3458–3464. [PubMed] [Google Scholar]
- Mao Q. and Unadkat J.D. 2005. Role of the breast cancer resistance protein (ABCG2) in drug transport. AAPS J. 7: E118–E133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin C., Berridge G., Mistry P., Higgins C., Charlton P., Callaghan R. 2000. Drug binding sites on P-glycoprotein are altered by ATP binding prior to nucleotide hydrolysis. Biochemistry 39: 11901–11906. [DOI] [PubMed] [Google Scholar]
- Mitomo H., Kato R., Ito A., Kasamatsu S., Ikegami Y., Kii I., Kudo A., Kobatake E., Sumino Y., Ishikawa T. 2003. A functional study on polymorphism of the ATP-binding cassette transporter ABCG2: Critical role of arginine-482 in methotrexate transport. Biochem. J. 373: 767–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miwa M., Tsukahara S., Ishikawa E., Asada S., Imai Y., Sugimoto Y. 2003. Single amino acid substitutions in the transmembrane domains of breast cancer resistance protein (BCRP) alter cross resistance patterns in transfectants. Int. J. Cancer 107: 757–763. [DOI] [PubMed] [Google Scholar]
- Miyake K., Mickley L., Litman T., Zhan Z., Robey R., Cristensen B., Brangi M., Greenberger L., Dean M., Fojo T.et al. 1999. Molecular cloning of cDNAs which are highly overexpressed in mitoxantrone-resistant cells: Demonstration of homology to ABC transport genes. Cancer Res. 59: 8–13. [PubMed] [Google Scholar]
- Morris D.I., Greenberger L.M., Bruggemann E.P., Cardarelli C.O., Gottesman M.M., Pastan I., Seamon K.B. 1994. Localization of the forskolin labeling sites to both halves of P-glycoprotein: Similarity of the sites labeled by forskolin and prazosin. Mol. Pharmacol. 46: 329–337. [PubMed] [Google Scholar]
- Özvegy C., Varadi A., Sarkadi B. 2002. Characterization of drug transport, ATP hydrolysis, and nucleotide trapping by the human ABCG2 multidrug transporter. Modulation of substrate specificity by a point mutation. J. Biol. Chem. 277: 47980–47990. [DOI] [PubMed] [Google Scholar]
- Özvegy-Laczka C., Koblos G., Sarkadi B., Varadi A. 2005a. Single amino acid (482) variants of the ABCG2 multidrug transporter: Major differences in transport capacity and substrate recognition. Biochim. Biophys. Acta 1668: 53–63. [DOI] [PubMed] [Google Scholar]
- Özvegy-Laczka C., Varady G., Koblos G., Ujhelly O., Cervenak J., Schuetz J.D., Sorrentino B.P., Koomen G.J., Varadi A., Nemet K.et al. 2005b. Function-dependent conformational changes of the ABCG2 multidrug transporter modify its interaction with a monoclonal antibody on the cell surface. J. Biol. Chem. 280: 4219–4227. [DOI] [PubMed] [Google Scholar]
- Ramachandra M., Ambudkar S.V., Chen D., Hrycyna C.A., Dey S., Gottesman M.M., Pastan I. 1998. Human P-glycoprotein exhibits reduced affinity for substrates during a catalytic transition state. Biochemistry 37: 5010–5019. [DOI] [PubMed] [Google Scholar]
- Reyes C.L., Ward A., Yu J., Chang G. 2006. The structures of MsbA: Insight into ABC transporter-mediated multidrug efflux. FEBS Lett. 580: 1042–1048. [DOI] [PubMed] [Google Scholar]
- Riordan J.R. 1993. The cystic fibrosis transmembrane conductance regulator. Annu. Rev. Physiol. 55: 609–630. [DOI] [PubMed] [Google Scholar]
- Riordan J.R., Rommens J.M., Kerem B., Alon N., Rozmahel R., Grzelczak A., Aielenski J., Lok S., Plavsic N., Chou J.-l.et al. 1989. Identification of the cystic fibrosis gene: Cloning and characterization of complementary DNA. Science 245: 1066–1073. [DOI] [PubMed] [Google Scholar]
- Robey R.W., Medina-Perez W.Y., Nishiyama K., Lahusen T., Miyake K., Litman T., Senderowicz A.M., Ross D.D., Bates S.E. 2001. Overexpression of the ATP-binding cassette half-transporter, ABCG2 (Mxr/BCrp/ABCP1), in flavopiridol-resistant human breast cancer cells. Clin. Cancer Res. 7: 145–152. [PubMed] [Google Scholar]
- Sauna Z.E. and Ambudkar S.V. 2000. Evidence for a requirement for ATP hydrolysis at two distinct steps during a single turnover of the catalytic cycle of human P-glycoprotein. Proc. Natl. Acad. Sci. 97: 2515–2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafran A., Ifergan I., Bram E., Jansen G., Kathmann I., Peters G.J., Robey R.W., Bates S.E., Assaraf Y.G. 2005. ABCG2 harboring the Gly482 mutation confers high-level resistance to various hydrophilic antifolates. Cancer Res. 65: 8414–8422. [DOI] [PubMed] [Google Scholar]
- Taipalensuu J., Tornblom H., Lindberg G., Einarsson C., Sjoqvist F., Melhus H., Garberg P., Sjostrom B., Lundgren B., Artursson P. 2001. Correlation of gene expression of ten drug efflux proteins of the ATP-binding cassette transporter family in normal human jejunum and in human intestinal epithelial Caco-2 cell monolayers. J. Pharmacol. Exp. Ther. 299: 164–170. [PubMed] [Google Scholar]
- Wang X., Furukawa T., Nitanda T., Okamoto M., Sugimoto Y., Akiyama S., Baba M. 2003. Breast cancer resistance protein (BCRP/ABCG2) induces cellular resistance to HIV-1 nucleoside reverse transcriptase inhibitors. Mol. Pharmacol. 63: 65–72. [DOI] [PubMed] [Google Scholar]
- Xu J., Liu Y., Yang Y., Bates S., Zhang J.T. 2004. Characterization of oligomeric human half-ABC transporter ATP-binding cassette G2. J. Biol. Chem. 279: 19781–19789. [DOI] [PubMed] [Google Scholar]
- Zhou S., Schuetz J.D., Bunting K.D., Colapietro A.M., Sampath J., Morris J.J., Lagutina I., Grosveld G.C., Osawa M., Nakauchi H.et al. 2001. The ABC transporter Bcrp1/ABCG2 is expressed in a wide variety of stem cells and is a molecular determinant of the side-population phenotype. Nat. Med. 7: 1028–1034. [DOI] [PubMed] [Google Scholar]