Abstract
Lens α-crystallin is an oligomeric protein with a molecular mass of 500–1000 kDa and a polydispersed assembly. It consists of two types of subunits, αA and αB, each with a molecular mass of 20 kDa. The subunits also form homo-oligomers in some other tissues and in vitro. Their quaternary structures, which are dynamic and characterized by subunit exchange, have been studied by many techniques, including fluorescence resonance energy transfer (FRET) and mass spectrometry analysis. The proposed mechanism of subunit exchange has been either by dissociation/association of monomeric subunits or by rapid equilibrium between oligomers and suboligomers. To explore the nature of subunit exchange further, we performed additional FRET measurements and analyses using a fluorescent dye-labeled W9F αA-crystallin as the acceptor probe and Trp in other crystallins (wild-type and R116C αA, wild-type and R120G αB, wild-type and Q155* βB2) as the donor probe and calculated the transfer efficiency, Förster distance, and average distance between two probes. The results indicate only slight decreased efficiency and increased distance between two probes for the R116C αA and R120G αB mutations despite conformational changes.
Keywords: crystallin, FRET, subunit exchange, protein–protein interaction, fluorescence, congenital cataract crystallin mutants
Human lens crystallins consist of three major classes: α-, β-, and γ-crystallin. α-Crystallin is expressed in both epithelial and fiber cells, whereas β- and γ-crystallin are expressed only in the fiber cells. These crystallins function both as structural proteins and as refractive index gradients and are believed to interact to maintain lens transparency. α-Crystallin consists of two 20-kDa subunits, αA and αB, forming either a hetero- or a homopolymer with a molecular mass of 500–1000 kDa. The ratio of αA to αB is 3:1 in mature human lenses and may vary in other mammalian species. The three-dimensional structures of the hetero- and homopolymers are not known. The reported quaternary structure is a sphere with a cavity (Haley et al. 1998), with a dynamic structure exhibiting subunit exchange first shown by van den Oetelaar et al. (1990). α-Crystallin was found to have additional chaperone-like activity protecting other proteins from denaturation and aggregation (Horwitz 1992).
The subunit exchange has been observed by various methods, including isoelectric focusing (van den Oetelaar et al. 1990; Sun and Liang 1998), fluorescence resonance energy transfer (FRET) (Bova et al. 1997, 2000; Sun et al. 1998; Cobb and Petrash 2000), and mass spectrometry analysis (Sobott et al. 2002; Aquilina et al. 2005). FRET measurements require two probes, a donor in the first protein and an acceptor in the second protein. The energy transfer occurs when the two proteins interact and the two probes are sufficiently close to one another as defined by Förster distance (Lakowicz 1983). One earlier study indicated that FRET occurred between α-crystallins but not between α- and β- or γ-crystallin, and this was interpreted as the result of subunit exchange in α-crystallins (Bova et al. 1997). Subsequent studies indicated that the FRET decreases with protein modifications or mutations (Cobb and Petrash 2000; Bera and Abraham 2002; Liang and Fu 2002), possibly because of conformational changes that retard the subunit exchange. The mechanism of subunit exchange suggested earlier involved dissociation and association of monomeric subunits (Vanhoudt et al. 1998, 2000; Bova et al. 2000). Recently, mass spectrometry analysis suggested that small heat shock protein complexes are in rapid dissociation/association equilibria with suboligomers and that the rate of dissociation of oligomers dictates the dynamics of subunit exchange (Sobott et al. 2002; Aquilina et al. 2005). Mass spectrometry analysis study has some advantages in that it does not require labeling and provides real-time analysis of the transient species and the relative populations during subunit exchange. However, it does not yield some information, such as the distance of probes in multimeric assembly. We have used two-hybrid system assays to screen protein–protein interactions among crystallins and observed strong interactions between α-crystallins (αA–αA, αB–αB, and αA–αB), especially between αA- and αB-crystallins (Fu and Liang 2002b, 2003). However, our understanding of the mechanism of the interactions revealed by the two-hybrid system is not complete; we still do not know whether the fused αA- or αB-crystallin can undergo subunit exchange.
To understand the subunit exchange further, we have determined some FRET parameters, such as transfer efficiency, Förster distance, and distance between two probes, using W9F αA-crystallin labeled with IAEDANS (5-((((2-iodoacetyl)amino)ethyl)amino) naphthalene-1-sulfonic acid) as the acceptor probe and Trp in other crystallins as the donor probe. We have chosen αA-, αB-, and βB2-crystallin and their corresponding congenital cataract mutants R116C αA, R120G αB, and Q155* βB2 as models. The results confirmed the presence of exchange between αA- and αB-crystallin but not between αA- and βB2-crystallin. The results also indicated a slight decrease in the exchange efficiency of the mutant crystallins and some difference in FRET parameters between αA-crystallin and the R116C mutant and practically no difference between αB-crystallin and the R120G mutant.
Results
Recombinant R116C αA- and R120G αB-crystallin
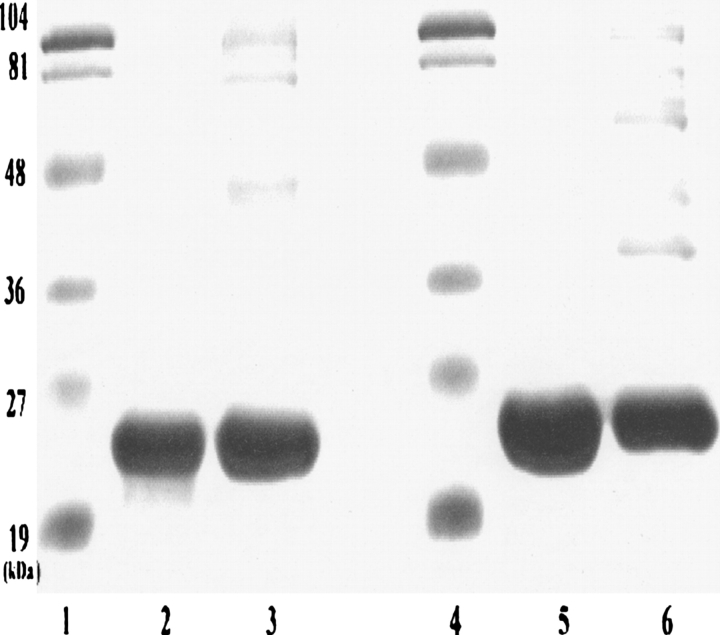
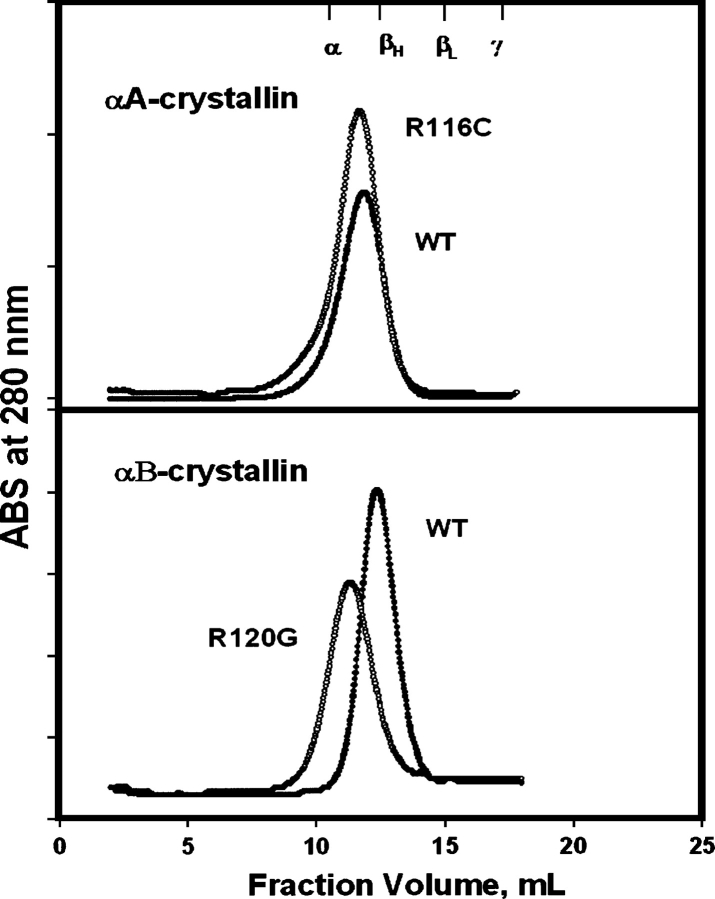
The preparations of R116C αA- and R120G αB-crystallin were checked for purity with SDS-PAGE (Fig. 1) and for molecular size with FPLC size-exclusion chromatography (Fig. 2). The molecular size appeared to increase slightly in R116C αA-crystallin and appreciably in R120G αB-crystallin relative to the wild-type protein.
Figure 1.
SDS-PAGE of R116C αA- and R120G αB-crystallin. (Lanes 1,4) Markers, (lanes 2,5) R116C αA- and R120G αB-crystallin, (lanes 3,6) wild-type αA- and αB-crystallin.
Figure 2.
FPLC size exclusion chromatography of R116C αA- (top) and R120G αB-crystallin (bottom) on Superose 6 column: protein concentrations at 0.5–1.0 mg/mL and flow rate at 0.5 mL/min. The markers indicated in the upper X-axis are from the profile of calf lens soluble proteins (α-, βH-, βL-, and γ-crystallin).
Fluorescence and CD
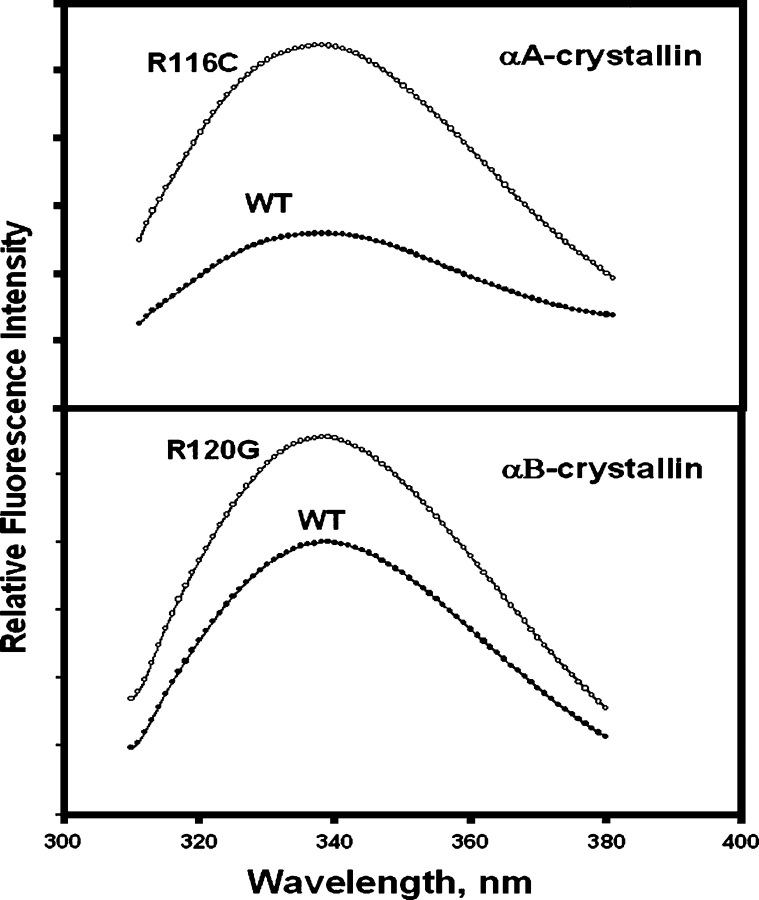
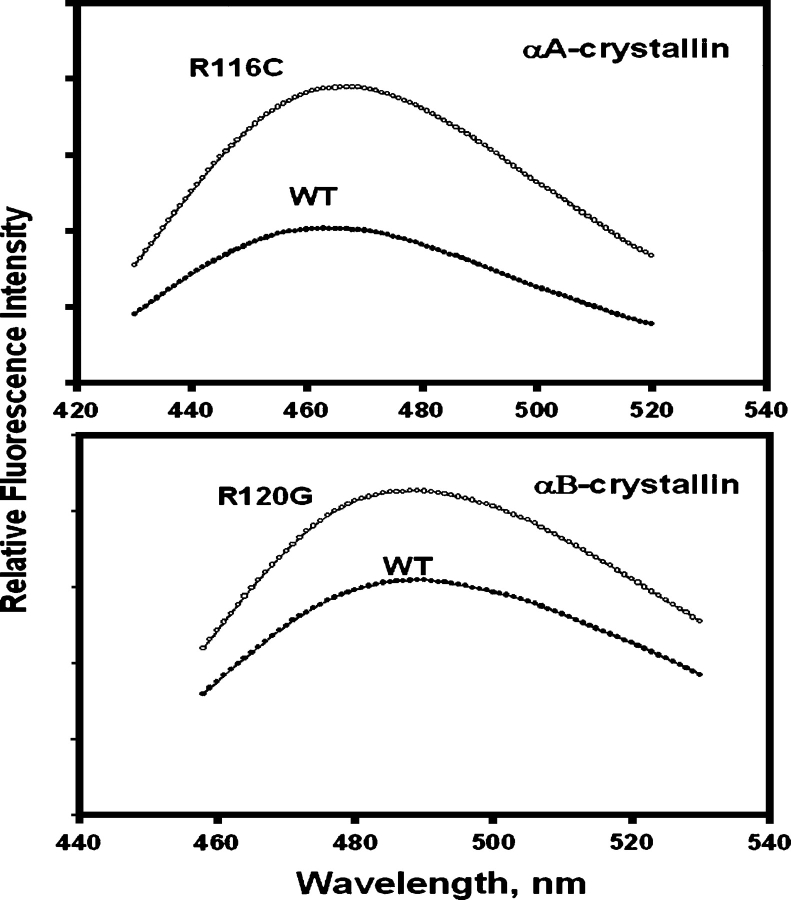
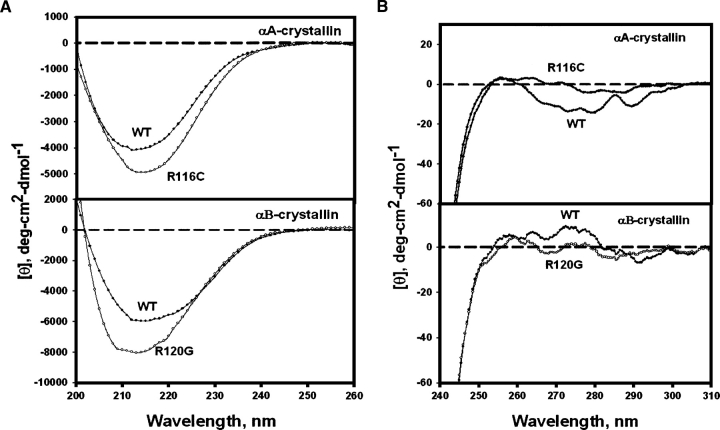
The fluorescence intensities of both Trp (Fig. 3) and Bis-ANS (4,4′-dianilino-1,1′-binaphthyl-5,5′-disulfonic acid) (Fig. 4) were increased by mutations, but the extent of increase was greater for the R116C mutation in αA than for the R120G mutation in αB. CD (circular dichroism) signals also changed: The near-UV region decreased (Fig. 5A) and the far-UV region increased (Fig. 5B). The changes in the fluorescence and CD spectra indicated that the mutations changed both tertiary and secondary structures. Bis-ANS is a hydrophobic probe; an increase in hydrophobicity may result from partial unfolding. A similar trend in spectroscopic changes was observed for the other congenital cataract crystallin mutants, Q155* βB2- and T5P γC-crystallin (Fu and Liang 2002a; Liu and Liang 2005).
Figure 3.
Trp fluorescence of R11C αA- (top) and R120G αB-crystallin (bottom): excitation wavelength at 295 nm and protein concentration at 0.1 mg/mL in 50 mM phosphate buffer (pH 7.6).
Figure 4.
Bis-ANS fluorescence of R11C αA- (top) and R120G αB-crystallin (bottom): excitation at 336 nm, protein concentration at 0.1 mg/mL, and Bis-ANS at 5 × 10−5 M in 50 mM phosphate buffer (pH 7.6).
Figure 5.
Far-UV (A) and near-UV (B) CD spectra of R11C αA- and R120G αB-crystallin: protein concentration at 0.2–0.5 mg/mL in 50 mM phosphate buffer (pH 7.6), and cell path-length of 1 mm for far-UV region and 10 mm for near-UV region.
FRET
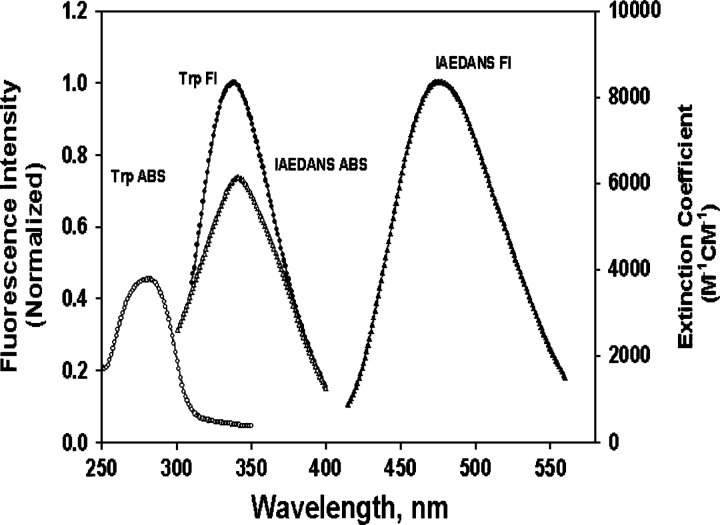
The Trp fluorescence and IAEDANS absorption spectra of αB-crystallin and IAEDANS-W9FαA (I-W9FαA) overlap to a great extent (Fig. 6), facilitating the energy transfer. All other crystallins show the similar spectral properties.
Figure 6.
Absorption and fluorescence spectra of αB-crystallin and IAEDANS-labeled W9F αA-crystallin: (•) Trp fluorescence (λex = 295 nm); (○) Trp absorption; (▴) IAEDANS fluorescence (λex = 336 nm); (△) IAEDANS absorption.
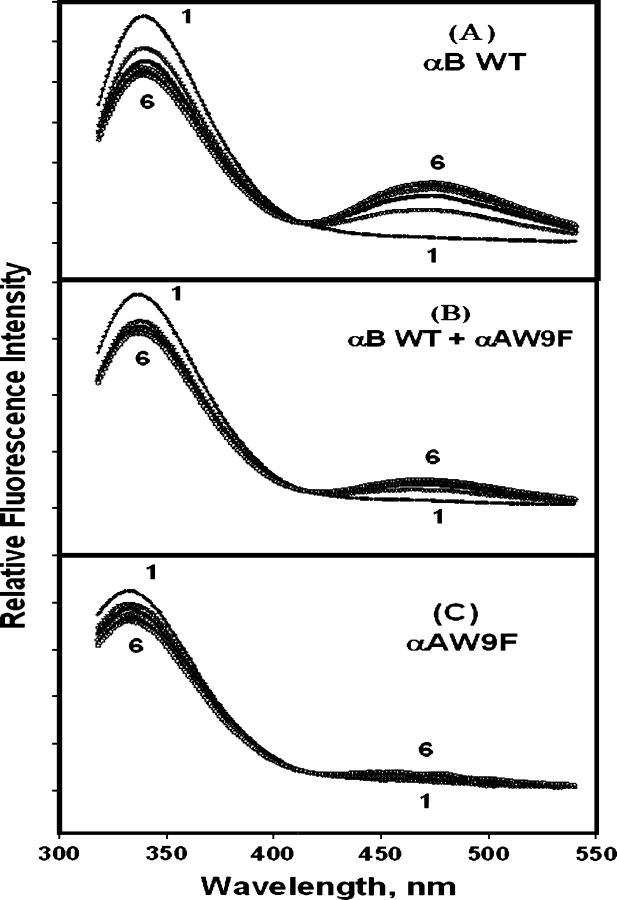
To demonstrate the occurrence of energy transfer, we obtained the fluorescence emission spectra (λex = 295 nm) of the donor crystallins and the acceptor I-W9FαA. Initially, we chose three donors: αB wild-type, (αB wild-type + nonlabeled-αAW9F), and nonlabeled-αAW9F. The Trp emission intensity decreased with a concomitant increase of IAEDANS fluorescence for (αBWT + I-αAW9F) (Fig. 7A), indicating that energy transfer and subunit exchange occur in the system. The presence of nonlabeled αAW9F decreased the extent of the energy transfer because of competition with αB-crystallin (Fig. 7B). There was no energy transfer in (I-αAW9F + αAW9F) because of the absence of a Trp residue in W9F αA (Fig. 7C).
Figure 7.
Time-dependent emission spectra (λem = 295 nm) of the FRET system of IAEDANS-αAW9F with crystallin donors: (A) αB wild-type, (B) αB wild-type + αAW9F, (C) αAW9F. The sample solutions were kept at 37°C, and measurements were made each hour from 0 to 5 h (curves 1–6).
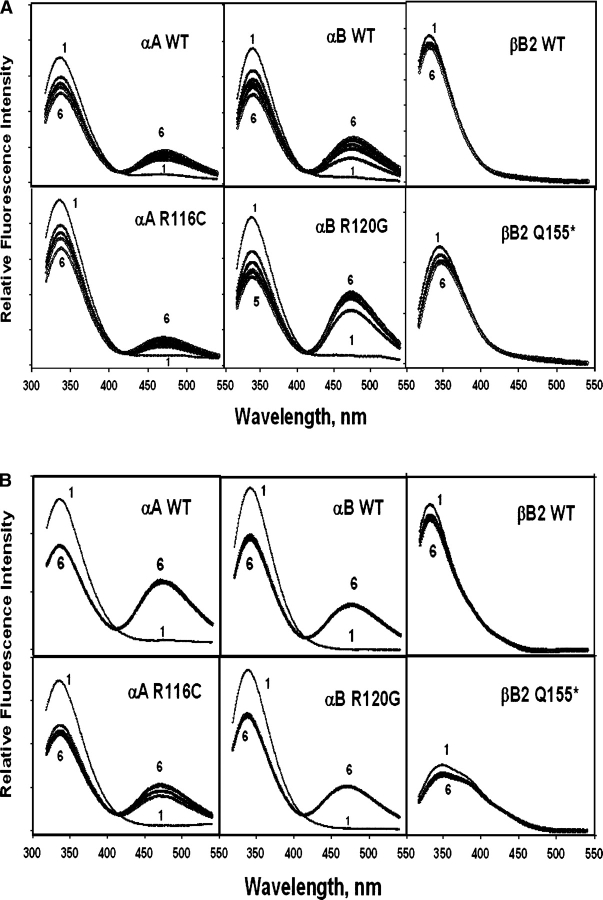
Subsequently, FRET spectra were obtained for the following donors: αA (wild-type and R116C), αB (wild-type and R120G), and βB2 (wild-type and Q155*). The results showed an energy transfer for αA and αB but not for βB2 (Fig. 8A,B). The increased rate of subunit exchange at the elevated temperature is obvious. Complete exchange took 4–5 h at 37°C but <30 min at 45°C. The extent of the energy transfers for the mutants was slightly decreased at 37°C but considerably decreased with R116C αA at 45°C. These fluorescence data for αA- and αB-crystallin were used to calculate transfer efficiency and other FRET parameters (see below).
Figure 8.
Time-dependent emission spectra (λem = 295 nm) of the FRET system of IAEDANS-αAW9F with crystallin donors: wild-type and R116C αA-crystallin, wild-type and R120G αB-crystallin, and wild-type and Q155* βB2-crystallin. The sample solutions of equal amounts of donor and acceptor proteins (0.1 mg/mL) were kept at either 37°C (A) or 45°C (B), and measurements were made each hour from 0 to 5 h (curves 1–6).
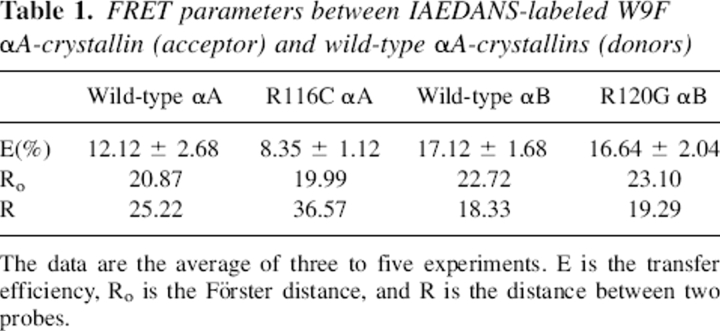
Quantum yields were obtained according to Equation 6 (see Materials and Methods) with free Trp as the reference (ϕR = 0.20): wild-type αA (0.071), R116C αA (0.055), wild-type αB (0.117), and R120G αB (0.128). The values are close to those reported for crystallins (Liang and Chakrabarti 1982; Liang et al. 1985) and were used in calculating the overlap integral Js. The transfer efficiency E was calculated with Equation 2. Unexpectedly, the transfer efficiency for αA–αB was higher than that for αA–αA. For both αA–αB and αA–αA, mutations slightly decreased the efficiencies. The values of Ro and R (Table 1) were obtained with Equations 3 and 1, respectively. The values of Ro and R are expressed in angstroms (Å). The Ro values are well within the Förster distance (∼50 Å); some reported Ro values between protein Trp (donor) and labeled dyes (acceptor) are 22 Å for Trp-IAEDANS, 40 Å for Trp-DPH, and 80 Å for Trp-Pyrene (Le Doan et al. 1983; Wu and Brand 1994; Narahara et al. 2000). The distance R between Trp residues and labeled dye IAEDANS was shorter in αA–αB than in αA–αA.
Table 1.
FRET parameters between IAEDANS-labeled W9F αA-crystallin (acceptor) and wild-type αA-crystallins (donors)
Discussion
Although short-range ordering of crystallins is sufficient to explain lens transparency, many techniques have been used to detect the specific interactions between crystallins both in vitro and in vivo. FRET has been used extensively to characterize the interaction properties of lens crystallins, especially α-crystallin (Bova et al. 1997, 2000; Sun et al. 1998; Cobb and Petrash 2000). One study reported strong FRET between αA-crystallins themselves but no interaction between αA- and β- or γ-crystallins, presumably because the β- or γ-crystallin was unable to participate in subunit exchange (Bova et al. 1997). A mass spectrometry study also showed that αA- and αB-crystallins readily underwent subunit exchange (Aquilina et al. 2005). Our data on the time-dependent increase of energy transfer and increased transfer at higher temperatures are consistent with the previous observations. Two-hybrid system assays also showed strong interactions in αA–αA, αB–αB, and αA–αB systems, although the nature of the interactions is not known (Boelens et al. 1998; Liu and Welsh 1999; Fu and Liang 2002b). In the two-hybrid system, the first crystallin (bait) was fused to the vector containing a DNA-binding domain, and the second crystallin (prey) was fused to the vector containing the transcription-activation domain; the reporter gene is expressed only if the bait and prey proteins associate. It is not known whether individual fused proteins (i.e., pM-αB or pV16-αB) are in an oligomeric form. If they are not, it would be difficult to perceive subunit exchange.
The observation of greater transfer efficiency for αA–αB than for αA–αA indicates that energy transfer between αA–αB is more favorable than that between αA–αA, which may be related to the greater stability of the αA–αB hetero-oligomer than of either αA or αB alone (Sun and Liang 1998; Horwitz et al. 1999). The structure of αA–αB hetero-oligomers is more compact than that of αA homo-oligomers; the distance between two probes is shorter in the αA–αB hetero-oligomer than in αA homo-oligomers. The observation of this short distance agrees with the recent report of a chemical cross-linking study; the distance between Lys-166 in αA-crystallin and Lys-175 in αB-crystallin was found to be within 12 Å in the native assembly (Swaim et al. 2004). This short distance reflects the close proximity of the flexible C termini. Our finding of a distance of 18 Å between Cys-131 or Cys-141 in the central domain of αA-crystallin and Trp-9 or Trp-60 in the N terminus of αB-crystallin is logical. Thus, our analyses of transfer efficiency, Förster distance, and the average distance between two probes provide a clearer picture of subunit exchange than do the previous FRET studies.
The effects of congenital cataract mutations on the protein–protein interactions have also been studied by both FRET (Cobb and Petrash 2000) and the two-hybrid system (Fu and Liang 2003). Conformational changes are believed to contribute to the decreased protein–protein interactions. Our spectroscopic data, as well as those of others (Horwitz et al. 1999; Kumar et al. 1999; Perng et al. 1999; Cobb and Petrash 2000; Bera and Abraham 2002), indicate that the R116C mutation of αA- and the R120G mutation of αB-crystallin have caused conformational changes and partial unfolding of the proteins, which then affects subunit exchange. Both partial unfolding and decreased subunit exchange lead to decreased protein stability; subunit exchange enables α-crystallin to respond to or offset the destabilizing changes under various stresses. α-Crystallin was found in the high-molecular-weight aggregated state either in aging lenses or after heat treatment, and decreased subunit exchange was observed, which might have accelerated the deterioration in the structure and function of α-crystallin (Liang and Akhtar 2000; Liang and Fu 2002). On the other hand, a smaller αA1–168 truncated protein aggregate also showed a significant decrease in the rate of exchange with αB-crystallin as compared with the wild-type αA-crystallin in a recent mass spectrometry analysis (Aquilina et al. 2005), indicating that either an increased or a decreased aggregate size could contribute to the decreased exchange.
It is somewhat surprising that the partially unfolded Q155* βB2-crystallin did not show FRET with αA-crystallin, since α-crystallin is supposed to interact with the partially unfolded proteins (Horwitz 1992). Apparently, the partially unfolded proteins that are induced by mutation may not be good substrates for the αA-crystallin chaperone in vitro. The same was reported for the T5P γC-crystallin mutant (Liang 2004). It needs to be noted that, upon heating, both wild-type and mutant βB2- and γC-crystallins bind to αA-crystallin. A recent study also showed that the I4F γD-crystallin mutant did not bind to α-crystallin at 37°C but did bind at higher temperature (45°C) to form large aggregates (Liu et al. 2005). It is not clear why the two-hybrid system study shows a weak association between αA- and βB2- or γC-crystallin (Fu and Liang 2002b), but FRET cannot detect any interaction. Perhaps the former detection is performed under physiological conditions and is more sensitive.
The mechanism of subunit exchange suggested earlier involves dissociation and association of monomeric subunits (Vanhoudt et al. 1998, 2000; Bova et al. 2000), advocating the presence of a dynamic structure of α-crystallin in which monomer subunits can be removed or added. However, no monomer subunits were observed in physiological solutions because the process is far faster than the time scale of conventional detection methods. A mass spectrometry study has proposed that small heat shock protein complexes are in rapid dissociation/association equilibria with suboligomeric forms (Sobott et al. 2002) and that subunit exchange involves the C-terminal extensions (Aquilina et al. 2005). Subunit exchange may occur through participation in equilibria in which suboligomeric subunits can move out from one oligomeric assembly and move into a different oligomeric assembly. Any factors that change the equilibrium will thus affect subunit exchange. The subunit exchange in R116C and R120G mutants did not change much because the mutations did not remove the C-terminal extensions, but the conformational change is believed to alter the equilibrium that slightly reduced subunit exchange.
The observed FRET between αA- and αA- or αB-, but not between αA- and βB2- or γC-crystallin, may also indicate that the subunit exchange involves the “α-crystallin” domain (Caspers et al. 1995); this domain is the primary site for dimerization in α-crystallin (Sreelakshmi et al. 2004; Ghosh and Clark 2005). The dimers are the building blocks for the various oligomers/suboligomers participating in exchange. βB2- and γC-crystallin do not have such a domain and are thus unable to participate in equilibrium and subunit exchange with αA-crystallin.
In conclusion, we have demonstrated subunit exchange between α-crystallins through observations of energy transfer; subunit exchange likely involves participation in equilibrium of oligomer/suboligomer assemblies. We also showed that mutations in autosomal dominant congenital cataracts slightly decrease transfer efficiencies as a result of conformational change.
Materials and methods
Cloning of lens crystallins
The recombinant crystallins wild-type αA-, αB-, and βB2-crystallin, and the W9F αA- and Q155* βB2-crystallin mutants were prepared as described previously (Sun et al. 1997, 1998; Fu and Liang 2002a, 2003; Liu and Liang 2005). R116C αA- and R120G αB-crystallin mutants were cloned using a QuikChange Mutagenesis kit (Stratagene) as described elsewhere (Sun et al. 1998). The PCR was performed with pAED4-αA or pAED4-αB plasmids (Sun et al. 1997). Two primers, the forward (TTCCCGTGAGTTCCACTGCCGCTACCGCCTGCC for R116C αA and CTCCAGGGAGTTCCACGGGAAATACCGGATCCC for R120G αB) and the reverse (GGCAGGCGGTAGCGGCAGTGGAACTCACGGGAA for R116C αA and GGGATCCGGTATTTCCCGTGGAACTCCCTGGAG for R120G αB), were custom-synthesized by Invitrogen. Protein expression and purifications were carried out as previously reported (Sun et al. 1997).
Purity of expressed proteins was checked with an SDS-PAGE slab gel (15% acrylamide) under reducing conditions according to the method of Laemmli (1970). Molecular size was determined by FPLC size exclusion chromatography on a Superose 6 column.
Purified proteins were dialyzed against 50 mM phosphate buffer (pH 7.6), and were used in the following spectroscopic measurements. Protein concentrations were determined by reading at 280 nm with the following absorbance: A0.1% = 0.724 for wild-type and 0.422 for W9F αA-crystallin; 0.696 for wild-type and R120G αB-crystallin; and 1.74 for wild-type and 1.70 for Q155* βB2-γC-crystallin, calculated using the average values of the protein resident extinction coefficients of tryptophan and tyrosine residues extracted from a large experimental data set (Mach et al. 1992).
Fluorescence and CD
Fluorescence was measured with a Shimadzu spectrofluorometer (model RF-5301PC, Shimadzu Instruments). Trp emission was scanned with an excitation wavelength of 295 nm. Bis-ANS fluorescence emission spectra were scanned between 460 and 560 nm with an excitation wavelength of 395 nm.
CD spectra were obtained with an Aviv Circular Dichroism Spectrometer (model 60 DS, Aviv Associates). Five scans were recorded and averaged and followed by a polynomial-fitting program. The CD was expressed with a unit of deg-cm2-dmol−1.
FRET
For the determination of quantum yield (Q) and coupling integral (J), the fluorescence spectra were corrected for the wavelength-dependent instrument response using rhodamine B as a reference (Lakowicz 1983; Wu and Brand 1994). In brief, emission or excitation spectrum correction function I(λem) or I(λex) was measured first for rhodamine B. The uncorrected emission or excitation spectrum of the sample was measured and divided by I(λem) or I(λex) to give the corrected emission or excitation spectrum F(λem) or F(λex).
In the present FRET study, W9F αA-crystallin was labeled with IAEDANS (λabs = 336 nm, λem = 490 nm, ɛ = 5700 M−1 cm−1) (Molecular Probes) as an acceptor and Trp in other crystallins as a donor (λabs = 280–290 nm, λem = 330–340 nm). IAEDANS is a sulfhydryl (SH)-specific fluorescent probe and has been used in studies of crystallin (Andley and Clark 1988; Putilina et al. 2003). The labeling protocols from the manufacturer were used. The degree of labeling was estimated to be 0.5 mol of IAEDANS/mol of protein subunit, using the formula: A/ɛ × M/c, where A = absorbance of dye at 336 nm, ɛ = molar extinction coefficient of the dye, M = molecular weight of protein, and c = protein concentration. αA-crystallin has one Trp, which was substituted with Phe in the W9F mutant; mutation did not change the protein conformation (Andley et al. 1996; Sun et al. 1998). The emission spectrum of Trp (320–350 nm) overlaps the absorption spectrum of IAEDNS (Fig. 6). Energy transfer produces a decrease in Trp fluorescence with a concomitant increase in IAEDANS fluorescence (see Fig. 7).
In FRET experiments, either the emission or the excitation spectrum can be measured to assess energy transfer. In an emission spectrum, the excitation is set at the wavelength of Trp donor absorption (λex = 295 nm), and any increase in emission intensity of the acceptor is measured. In an excitation spectrum, the detection is set at IAEDANS acceptor emission wavelength (λem = 490 nm), and enhancement of intensity at the wavelength of donor excitation is measured. In the present FRET study, we measured emission spectra. The three samples, crystallin donors (unlabeled crystallins) in the presence and the absence of acceptor (labeled W9F αA-crystallin) and labeled W9F αA-crystallin alone, were scanned (at 295-nm excitation wavelength) every hour at either 37° or 45°C. The Trp emission intensities decreased and the IAEDANS emission intensities increased with time. After 4–5 h (at 37°C) or within 30 min (at 45°C), the emission intensities stopped changing or energy transfer ceased. The fluorescence intensities of (crystallin donors + I-αAW9F) were subtracted from those of I-αAW9F alone because I-αAW9F also showed some fluorescence at 450–500 nm.
The FRET efficiency is related to the inverse sixth power of distance between the probes (R) as defined by the Förster equation (Lakowicz 1983; Steer and Merrill 1994; Wu and Brand 1994):
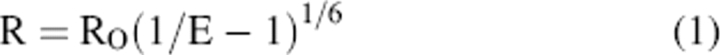 |
where Ro is the Förster distance at which the transfer efficiency is 50%. The efficiency of transfer (E) can be calculated from the equation:
where FDA and FD are the donor fluorescence intensities in the presence and the absence of energy transfer.
The Förster distance (Ro) at which the transfer efficiency is 50% can be calculated by the equation:
where κ2 is the orientation factor that describes the rotational movement of the donor and acceptor probes relative to each other and is generally assumed to be equal to 2/3; ϕD is the quantum yield of donor in the absence of acceptor; n is refractive index taken as 1.4; and J is the spectral overlap integral representing the degree of overlap between the donor fluorescence spectrum and the acceptor absorption spectrum:
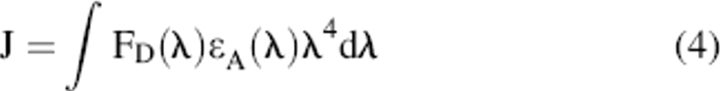 |
where FD is the corrected fluorescence spectrum of the donor in the wavelength range λ to λ + Δλ with the total intensity (area under the curve) normalized unity, and ɛA is the molar absorption coefficient (unit M−1cm−1) of the acceptor at λ (nm). J can be calculated by numerical summation from the corrected fluorescence spectrum of the donor and the absorption spectrum of the acceptor as described by the equation:
with a unit M−1cm−1 nm4. A computer program has been designed for calculating the J values.
The quantum yield for the donor Trp is estimated by the formula (Kronman et al. 1971; Liang and Chakrabarti 1982):
where A is the area under emission spectrum, ϕ is quantum yield, and O is optical density at excitation wavelength. The subscript D is for samples, and the subscript R is for reference free Trp. The ϕR value of 0.20 for free Trp is used (Kronman et al. 1971).
From obtained values of E and Ro, the average distance (R) between donor and acceptor was calculated by Equation 1.
Acknowledgments
This work was supported by a grant from the NIH (EY13968) and the Massachusetts Lions Eye Research Fund. Technical help from Michael Liang is acknowledged.
Footnotes
Reprint requests to: Jack J. Liang, Ophthalmic Research, Brigham and Women's Hospital, 221 Longwood Avenue, Boston, MA 02115, USA; e-mail: jliang@rics.bwh.harvard.edu; fax: (617) 278-0556.
Article published online ahead of print. Article and publication date are at http://www.proteinscience.org/cgi/doi/10.1110/ps.062216006.
Abbreviations: Bis-ANS, 4,4′-dianilino-1,1′-binaphthyl-5,5′-disulfonic acid; CD, circular dichroism; FRET, fluorescence resonance energy transfer; HMW, high-molecular-weight; IAEDANS, 5-((((2-iodoacetyl)amino)ethyl)amino) naphthalene-1-sulfonic acid.
References
- Andley U.P. and Clark B.A. 1988. Accessibilities of the sulfhydryl groups of native and photooxidized lens crystallins: A fluorescence lifetime and quenching study. Biochemistry 27: 810–820. [DOI] [PubMed] [Google Scholar]
- Andley U.P., Mathur S., Griest T.A., Petrash J.M. 1996. Cloning, expression, and chaperone-like activity of human αA-crystallin. J. Biol. Chem. 271: 31973–31980. [DOI] [PubMed] [Google Scholar]
- Aquilina J.A., Benesch J.L., Ding L.L., Yaron O., Horwitz J., Robinson C.V. 2005. Subunit exchange of polydisperse proteins: Mass spectrometry reveals consequences of αA-crystallin truncation. J. Biol. Chem. 280: 14485–14491. [DOI] [PubMed] [Google Scholar]
- Bera S. and Abraham E.C. 2002. The αA-crystallin R116C mutant has a higher affinity for forming heteroaggregates with αB-crystallin. Biochemistry 41: 297–305. [DOI] [PubMed] [Google Scholar]
- Boelens W.C., Croes Y., de Ruwe M., de Reu L., de Jong W.W. 1998. Negative charges in the C-terminal domain stabilize the αB-crystallin complex. J. Biol. Chem. 273: 28085–28090. [DOI] [PubMed] [Google Scholar]
- Bova M.P., Ding L.L., Horwitz J., Fung B.K. 1997. Subunit exchange of αA-crystallin. J. Biol. Chem. 272: 29511–29517. [DOI] [PubMed] [Google Scholar]
- Bova M.P., Mchaourab H.S., Han Y., Fung B.K. 2000. Subunit exchange of small heat shock proteins. Analysis of oligomer formation of αA-crystallin and Hsp27 by fluorescence resonance energy transfer and site-directed truncations. J. Biol. Chem. 275: 1035–1042. [DOI] [PubMed] [Google Scholar]
- Caspers G.J., Leunissen J.A., de Jong W.W. 1995. The expanding small heat-shock protein family, and structure predictions of the conserved “α-crystallin domain”. J. Mol. Evol. 40: 238–248. [DOI] [PubMed] [Google Scholar]
- Cobb B.A. and Petrash J.M. 2000. Structural and functional changes in the α A-crystallin R116C mutant in hereditary cataracts. Biochemistry 39: 15791–15798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu L. and Liang J.J. 2002a. Conformational change and destabilization of cataract γC-crystallin T5P mutant. FEBS Lett. 513: 213–216. [DOI] [PubMed] [Google Scholar]
- Fu L. and Liang J.J. 2002b. Detection of protein–protein interactions among lens crystallins in a mammalian two-hybrid system assay. J. Biol. Chem. 277: 4255–4260. [DOI] [PubMed] [Google Scholar]
- Fu L. and Liang J.J. 2003. Alteration of protein–protein interactions of congenital cataract crystallin mutants. Invest. Ophthalmol. Vis. Sci. 44: 1155–1159. [DOI] [PubMed] [Google Scholar]
- Ghosh J.G. and Clark J.I. 2005. Insights into the domains required for dimerization and assembly of human αB crystallin. Protein Sci. 14: 684–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haley D.A., Horwitz J., Stewart P.L. 1998. The small heat-shock protein, αB-crystallin, has a variable quaternary structure. J. Mol. Biol. 277: 27–35. [DOI] [PubMed] [Google Scholar]
- Horwitz J. 1992. α-crystallin can function as a molecular chaperone. Proc. Natl. Acad. Sci. 89: 10449–10453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz J., Bova M.P., Ding L.L., Haley D.A., Stewart P.L. 1999. Lens α-crystallin: Function and structure. Eye 13: 403–408. [DOI] [PubMed] [Google Scholar]
- Kronman M.J., Holmes L.G., Robbins F.M. 1971. Inter- and intramolecular interactions of α-lactalbumin. X. Effect of acylation of tyrosyl and lysyl side chains on molecular conformations. J. Biol. Chem. 246: 1909–1921. [PubMed] [Google Scholar]
- Kumar L.V., Ramakrishna T., Rao C.M. 1999. Structural and functional consequences of the mutation of a conserved arginine residue in αA and αB crystallins. J. Biol. Chem. 274: 24137–24141. [DOI] [PubMed] [Google Scholar]
- Laemmli U.K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227: 680–685. [DOI] [PubMed] [Google Scholar]
- Lakowicz J. In Principles of fluorescence spectroscopy . 1983. Plenum Press, New York.
- Le Doan T., Takasugi M., Aragon I., Boudet G., Montenay-Garestier T., Helene C. 1983. Excitation energy transfer from tryptophan residues of peptides and intrinsic proteins to diphenylhexatriene in phospholipid vesicles and biological membranes. Biochim. Biophys. Acta 735: 259–270. [DOI] [PubMed] [Google Scholar]
- Liang J.J. 2004. Interactions and chaperone function of αA-crystallin with T5P γC-crystallin mutant. Protein Sci. 13: 2476–2482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang J.J. and Akhtar N.J. 2000. Human lens high-molecular-weight α-crystallin aggregates. Biochem. Biophys. Res. Commun. 275: 354–359. [DOI] [PubMed] [Google Scholar]
- Liang J.N. and Chakrabarti B. 1982. Spectroscopic investigations of bovine lens crystallins. 1. Circular dichroism and intrinsic fluorescence. Biochemistry 21: 1847–1852. [DOI] [PubMed] [Google Scholar]
- Liang J.J. and Fu L. 2002. Decreased subunit exchange of heat-treated lens α A-crystallin. Biochem. Biophys. Res. Commun. 293: 7–12. [DOI] [PubMed] [Google Scholar]
- Liang J.N., Andley U.P., Chylack Jr. L.T. 1985. Spectroscopic studies on human lens crystallins. Biochim. Biophys. Acta 832: 197–203. [DOI] [PubMed] [Google Scholar]
- Liu B.F. and Liang J.J. 2005. Interaction and biophysical properties of human lens Q155* βB2-crystallin mutant. Mol. Vis. 11: 321–327. [PubMed] [Google Scholar]
- Liu C. and Welsh M.J. 1999. Identification of a site of Hsp27 binding with Hsp27 and α B-crystallin as indicated by the yeast two-hybrid system. Biochem. Biophys. Res. Commun. 255: 256–261. [DOI] [PubMed] [Google Scholar]
- Liu H., Du X., Wang M., Huang Q., Ding L., McDonald H.W., Yates III J.R., Beutler B., Horwitz J., Gong X. 2005. Crystallin γB-I4F mutant protein binds to α-crystallin and affects lens transparency. J. Biol. Chem. 280: 25071–25078. [DOI] [PubMed] [Google Scholar]
- Mach H., Middaugh C.R., Lewis R.V. 1992. Statistical determination of the average values of the extinction coefficients of tryptophan and tyrosine in native proteins. Anal. Biochem. 200: 74–80. [DOI] [PubMed] [Google Scholar]
- Narahara M., Tachibana K., Kurisu N., Kanazawa M., Miyake M. 2000. Immunohistochemical and chemical changes of β-citryl-L-glutamate in the differentiation of bovine lens epithelial cells into lens fiber cells. Biol. Pharm. Bull. 23: 704–707. [DOI] [PubMed] [Google Scholar]
- Perng M.D., Muchowski P.J., van den I.P., Wu G.J., Hutcheson A.M., Clark J.I., Quinlan R.A. 1999. The cardiomyopathy and lens cataract mutation in αB-crystallin alters its protein structure, chaperone activity, and interaction with intermediate filaments in vitro. J. Biol. Chem. 274: 33235–33243. [DOI] [PubMed] [Google Scholar]
- Putilina T., Skouri-Panet F., Prat K., Lubsen N.H., Tardieu A. 2003. Subunit exchange demonstrates a differential chaperone activity of calf α-crystallin toward β LOW- and individual γ-crystallins. J. Biol. Chem. 278: 13747–13756. [DOI] [PubMed] [Google Scholar]
- Sobott F., Benesch J.L., Vierling E., Robinson C.V. 2002. Subunit exchange of multimeric protein complexes. Real-time monitoring of subunit exchange between small heat shock proteins by using electrospray mass spectrometry. J. Biol. Chem. 277: 38921–38929. [DOI] [PubMed] [Google Scholar]
- Sreelakshmi Y., Santhoshkumar P., Bhattacharyya J., Sharma K.K. 2004. αA-crystallin interacting regions in the small heat shock protein, αB-crystallin. Biochemistry 43: 15785–15795. [DOI] [PubMed] [Google Scholar]
- Steer B.A. and Merrill A.R. 1994. The colicin E1 insertion-competent state: Detection of structural changes using fluorescence resonance energy transfer. Biochemistry 33: 1108–1115. [DOI] [PubMed] [Google Scholar]
- Sun T.X. and Liang J.J. 1998. Intermolecular exchange and stabilization of recombinant human αA- and αB-crystallin. J. Biol. Chem. 273: 286–290. [DOI] [PubMed] [Google Scholar]
- Sun T.X., Das B.K., Liang J.J. 1997. Conformational and functional differences between recombinant human lens αA- and αB-crystallin. J. Biol. Chem. 272: 6220–6225. [DOI] [PubMed] [Google Scholar]
- Sun T.X., Akhtar N.J., Liang J.J. 1998. Subunit exchange of lens α-crystallin: A fluorescence energy transfer study with the fluorescent labeled αA-crystallin mutant W9F as a probe. FEBS Lett. 430: 401–404. [DOI] [PubMed] [Google Scholar]
- Swaim C.L., Smith D.L., Smith J.B. 2004. The reaction of α-crystallin with the cross-linker3,3′-dithiobis(sulfosuccinimidyl propionate) demonstrates close proximity of the C termini of αA and αB in the native assembly. Protein Sci. 13: 2832–2835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Oetelaar P.J., van Someren P.F., Thomson J.A., Siezen R.J., Hoenders H.J. 1990. A dynamic quaternary structure of bovine α-crystallin as indicated from intermolecular exchange of subunits. Biochemistry 29: 3488–3493. [DOI] [PubMed] [Google Scholar]
- Vanhoudt J., Aerts T., Abgar S., Clauwaert J. 1998. Quaternary structure of bovine α-crystallin: Influence of temperature. Int. J. Biol. Macromol. 22: 229–237. [DOI] [PubMed] [Google Scholar]
- Vanhoudt J., Abgar S., Aerts T., Clauwaert J. 2000. Native quaternary structure of bovine α-crystallin. Biochemistry 39: 4483–4492. [DOI] [PubMed] [Google Scholar]
- Wu P. and Brand L. 1994. Resonance energy transfer: Methods and applications. Anal. Biochem. 218: 1–13. [DOI] [PubMed] [Google Scholar]