Abstract
The relative contributions to changes in visible and near UV circular dichroism spectra of hemoglobin of heme ligation and tertiary and quaternary conformational transitions were separated by exploiting the slowing down of structural relaxations for proteins encapsulated in wet, nanoporous silica gels. Spectral signatures, previously assumed to be characteristic of T and R quaternary states, were demonstrated to be specific to different tertiary conformations. The results support the view that ligation and allosteric effectors can modulate the structural and functional properties of hemoglobin by regulating the equilibrium between the same tertiary species within both quaternary states.
Keywords: allosteric effectors, conformational transitions, protein immobilization, heme proteins
Oxygen binding to hemoglobin (Hb) in solution exhibits a cooperative behavior. The Monod-Wyman-Changeux (MWC) model (Monod et al. 1965) for multisubunit cooperative proteins has provided a general explanation for this behavior, postulating an equilibrium between a low affinity quaternary T state and a high affinity quaternary R state. This equilibrium is affected by the level of ligation of the tetramer and by allosteric effectors (Antonini and Brunori 1971; Edelstein 1975; Imai 1982; Kister et al. 1987). However, this simplified view has been challenged by the observation that the allosteric effectors 2,3-diphosphoglycerate (DPG), inositol hexaphosphate (IHP), bezafibrate, and L35 not only shift the quaternary equilibrium but also affect the tertiary-linked functional and structural properties (Coletta et al. 1995, 1999a,b; Imai et al. 2002; Tsuneshige et al. 2002; Yonetani et al. 2002; Samuni et al. 2006; Yokoyama et al. 2006). The complexity in ligand binding is also reflected in the relaxation kinetics following laser flash photolysis of the carbon monoxide–Hb complex. At least five relaxations can be singled out in the nanosecond–millisecond time window (Jones et al. 1992; Goldbeck et al. 1996; Henry et al. 1997). Each of them has been associated with a molecular event, involving the ligand binding to the R and T state, and tertiary and quaternary conformational transitions.
Encapsulation of proteins in hydrated silica gel is a method allowing the immobilization of biological macromolecules with retention of their functional properties (Ellerby et al. 1992; Gill and Ballesteros 2000; Jin and Brennan 2002; Bettati et al. 2004). This approach was successfully applied to Hb, yielding a decrease in the rate of structural relaxations by several orders of magnitude, thus allowing the equilibrium and kinetic characterization of isolated conformational states (Shibayama and Saigo 1995, 2001; Bettati and Mozzarelli 1997; Das et al. 1999; Juszczak and Friedman 1999; Shibayama 1999; Khan et al. 2000; Bruno et al. 2001). R state Hb gels exhibit a noncooperative, high affinity oxygen binding (Shibayama and Saigo 1995), whereas T state Hb gels in the absence or presence of allosteric effectors exhibit a noncooperative oxygen binding with a p50 of about 26 and 130 torr, at 15°C, respectively (Viappiani et al. 2004). These values are close to the binding affinity of the first oxygen to T state Hb in solution in the absence and presence of allosteric effectors, respectively (Poyart et al. 1978; Imai 1982). Remarkably, the functional properties of T state Hb gels in the presence of allosteric effectors are virtually identical to those of T state Hb in the crystalline state (Mozzarelli et al. 1997), suggesting that this T state Hb possesses the same conformation as Hb in the crystal (Bruno et al. 2001). Furthermore, CO rebinding kinetics to R state Hb gels show only the geminate rebinding and the bimolecular binding to the R state (Khan et al. 2000; Viappiani et al. 2004), because the quaternary transition to the T state is virtually blocked by the gel matrix (Shibayama and Saigo 1995, 1999). Similarly, T state Hb gels, in the presence of allosteric effectors, exhibit no geminate rebinding and a slow bimolecular CO rebinding to the T quaternary state. Surprisingly, CO rebinding kinetics to T state Hb gels in the absence of allosteric effectors exhibit geminate rebinding and a complex bimolecular phase that can be accounted for by a linear combination of R and T rebinding curves (Viappiani et al. 2004).
Results from equilibrium and kinetics studies of Hb gels are in keeping with the tertiary two-state (TTS) allosteric model for Hb (Henry et al. 2002; Viappiani et al. 2004). The TTS model extends the MWC model to account for the action of allosteric effectors on tertiary conformations (Coletta et al. 1995, 1999a,b; Imai et al. 2002; Tsuneshige et al. 2002; Yonetani et al. 2002; Yokoyama et al. 2006), and functional studies carried out in solution, in silica gel, and in the crystal. The TTS model preserves the fundamental postulate of MWC that oxygen binding within a single quaternary state is noncooperative, in accordance with the experimental observations (Shibayama and Saigo 1995, 2001; Bettati and Mozzarelli 1997; Bruno et al. 2001), but assumes that both T and R quaternary states are populated by two tertiary states, t and r, endowed, respectively, with a low and a high oxygen affinity and ligand-binding rates that are independent of the overall quaternary state of the tetramer. The distribution of the unliganded t and r and the liganded tx and rx species is controlled by the quaternary state and by heterotropic allosteric effectors.
The presence of different tertiary conformations within the T and R quaternary states, both in the unliganded and liganded forms, was suggested by different methods, primarily X-ray crystallography (Liddington et al. 1992; Paoli et al. 1996; Perutz et al. 1998; Shibayama et al. 2002; Kavanaugh et al. 2005; Safo and Abraham 2005; Yokoyama et al. 2006). X-ray investigation of R state Hb bound to allosteric effectors has recently shed some light on the tertiary structure of unliganded (Yokoyama et al. 2006) and liganded R state Hb (Shibayama et al. 2002). Structure-function relationships for Hb encapsulated in silica gel were obtained by visible and UV-resonance Raman spectroscopy (Das et al. 1999; Juszczak and Friedman 1999; Samuni et al. 2004, 2006). It was found that the unliganded T state Hb gels in the absence or presence of allosteric effectors are spectroscopically indistinguishable, in agreement with the prediction of the TTS model that only the t species populates the unliganded T state. Upon binding of CO, T state Hb gels undergo different spectral changes, depending on the absence or presence of allosteric effectors. Results suggest that the presence of allosteric effectors limits the tertiary relaxations triggered by ligation within a T quaternary structure, stabilizing tx with respect to rx. This is fully consistent with the TTS model prediction and the experimental observation that, in the presence of negative allosteric effectors, all liganded subunits of a T state tetramer remain in the t tertiary conformation (Viappiani et al. 2004). In the absence of allosteric effectors, instead, a substantial fraction of molecules switches from t to rx upon ligation. On a time scale of days, at 15°C, the resonance Raman spectra and the CO rebinding properties of liganded T state Hb gels, either in the absence or presence of allosteric effectors, evolve toward those characteristic of the R state Hb (Samuni et al. 2004; Viappiani et al. 2004).
In the present study, we have investigated the structural properties of unliganded and liganded Hb gels, in the absence and presence of allosteric effectors, by exploiting circular dichroism (CD) spectroscopy. This technique measures the optical activity associated with electronic transitions of chromophores located in a nonsymmetrical environment. Both aromatic side chains and porphyrins are symmetric per se, but exhibit optical activity when located in an asymmetric environment within the protein matrix. Changes in the local environment as a consequence of a conformational transition may lead to a variation in optical activity. Therefore, the signal can be used as a conformational marker for monitoring function-structure relationships. In Hb, almost all regions of the near UV-visible spectrum are dominated by the optical activity associated with the electronic transitions of the heme and the spectral changes that follow ligation as a result of the iron ion-spin change. The decoupling of this large contribution from smaller ones arising from local and global conformational changes, affecting either the heme itself or the aromatic residues, would be very useful in the identification of tertiary and quaternary conformational markers. So far, this problem was tackled by using mutant, hybrid, or chemically modified Hb that remains in the same quaternary state regardless of the ligation state. For example, the Zn heme of αZnβFe and αFeβZn hybrids does not bind oxygen, and consequently, the tetramer remains in a half-saturated T state even in the presence of CO (Miyazaki et al. 1999). From these studies, a spectral region around 260 nm was shown to depend almost exclusively on the electronic state of the heme (Zentz et al. 1994), whereas the 280–290 nm region was shown to contain contributions from Tyrα42, Trpβ37, Tyrα140, and Tyrβ145 (Li et al. 2000a, b; Jin et al. 2004). These residues are located at the α1β2 interface and, therefore, might be sensitive to the quaternary state of the tetramer (Perutz et al. 1974b; Plese and Amma 1977). Differences between the spectrum of tetrameric Hb and the spectrum reconstructed from isolated α and β chains (Ueda et al. 1969; Li et al. 2000a) further confirm the diagnostic relevance of this region as a marker for intersubunit interactions. However, doubts have arisen about the actual quaternary state of metal hybrid Hb (Simolo et al. 1985). Moreover, the Zn heme could induce local changes at the heme pocket with respect to native Hb (Sudhakar et al. 1998). We have exploited silica gel encapsulation as an alternative and powerful strategy to selectively isolate the different tertiary and quaternary conformations of Hb.
Results and Discussion
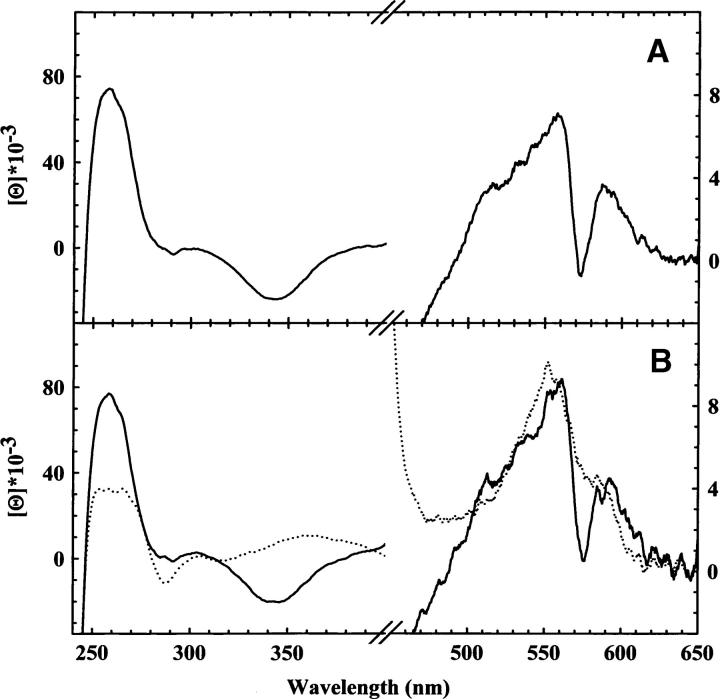
The CD spectrum of R state HbCO gels (Fig. 1A) closely corresponds to that obtained for HbCO in solution (Fig. 1B) if taking into account that the peak intensity ratio can change due to scattering from the nonperfect optical quality of the gel surface. This indicates that the encapsulation of liganded Hb in silica gels does not detectably perturb the protein structure, in agreement with previous resonance Raman studies (Samuni et al. 2004). The CD spectrum of deoxyHb, encapsulated in silica gels in the absence of allosteric effectors, exhibits both positive and negative peaks (Fig. 2B), superimposable to those of T state deoxyHb in solution (Fig. 1B). Moreover, the CD spectra of deoxyHb gels in the absence and presence of allosteric effectors are almost indistinguishable (Fig. 2A, B), indicating that the tertiary conformation of unliganded Hb is not affected by the binding of allosteric effectors. This conclusion is in agreement with resonance Raman spectra of T state deoxyHb gels recorded in the low-frequency visible region (Samuni et al. 2004), showing that the band at 214 cm−1, associated with the Fe-His bond stretching and diagnostic of the degree of proximal strain, is identical for T state Hb gels in the absence and presence of allosteric effectors. These findings indicate that unliganded T state Hb is populated by a well-defined tertiary structure, which is considered to be the same species crystallographically resolved in crystals of T state Hb (Liddington et al. 1992; Paoli et al. 1996).
Figure 1.
(A) CD spectra of R state HbCO (solid line) gels in 100 mM HEPES, 1 mM EDTA (pH 7.0). (B) CD spectra of Hb in a solution containing 100 mM HEPES, 1 mM EDTA, 0.2 mM sodium dithionite (pH 7.0), in the presence (solid line) and absence (dotted line) of CO.
Figure 2.
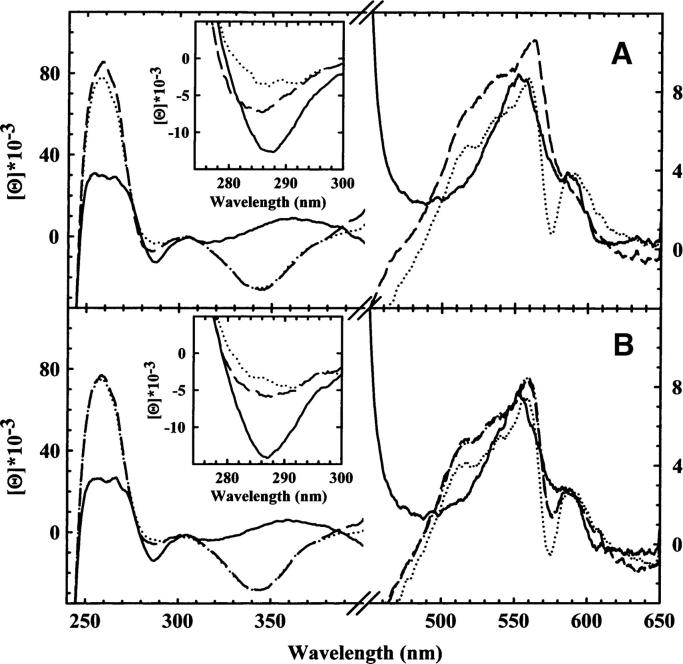
(A) CD spectra of Hb gels in 100 mM HEPES, 10 mM IHP, 1 mM EDTA (pH 7.0), 4°C, in the deoxy form (solid line), within 20 min upon CO addition (broken line), at 4°C, and after 140 h (dotted line) at 15°C. (B) CD spectra of Hb gels in 100 mM HEPES, 1 mM EDTA (pH 7.0), 4°C, in the deoxy form (solid line), within 20 min upon CO addition (broken line) at 4°C, and after 140 h (dotted line) at 15°C. The spectrum reconstructed from T state deoxyHb gels and R state HbCO gels (broken/dotted line) corresponds to 60% of r tertiary state and 40% of t tertiary state. The quality of the fit is such that the observed and fitted spectrum are virtually indistinguishable.
In solution, CO ligation to Hb induces large CD spectral changes (Fig. 1B). The major effects are associated with the modification of the heme ligation state, which affects both the visible and the near-UV regions (Ueda et al. 1969). A residual contribution is attributed to a change in the optical activity of aromatic amino acids due to either tertiary or quaternary transitions (Perutz et al. 1974b; Plese and Amma 1977; Li et al. 2000a,b; Jin et al. 2004). A comparison between the spectra of unliganded and liganded Hb in solution (Fig. 1B) and of unliganded and liganded T state Hb gels (Fig. 2A,B) allows the separation of these contributions. Spectra of liganded T state Hb gels in the presence of allosteric effectors report on liganded subunits that are in the t tertiary conformation within the T quaternary state (Henry et al. 2002; Viappiani et al. 2004), whereas spectra of liganded T state Hb gels in the absence of allosteric effectors report on liganded subunits that are distributed between the tertiary r and t conformations, yet within the quaternary T state. In the frame of the TTS model (Henry et al. 2002), the fraction of subunits in the liganded rx conformation after CO ligation was estimated to be ∼65%, while the remaining part of liganded subunits stays in the tx conformation (Viappiani et al. 2004). In all T state Hb gels (Fig. 2A,B) the positive band at 250–260 nm and the negative one at 340–350 nm increase upon binding of CO and become similar to those observed in liganded R state Hb in gels and in solution, indicating that they are primarly associated with the ligation state. No significant further spectral changes of these bands occur within 140 h at 15°C (Fig. 2A,B). The latter conditions have been proven to afford the complete relaxation toward the R state, as indicated by the time evolution of resonance Raman spectra (Samuni et al. 2004) and of CO rebinding kinetics upon flash photolysis (Viappiani et al. 2004). A negative peak between 280 and 295 nm, which is sensitive to the microenvironment near the aromatic amino acids of the α1β2 interface (Perutz et al. 1974b; Plese and Amma 1977; Li et al. 2000a,b; Jin et al. 2004), is observed in unliganded Hb in solution and in the gel (Figs. 1B, 2A,B). The same spectral pattern was observed in CD spectra of mutant or hybrid Hbs that remain in the T state even upon ligand binding, suggesting that this band might be a conformational marker for the T quaternary state (Perutz et al. 1974a,b). Upon binding of CO to T state Hb gels, this peak is still present (Fig. 2A,B) and it fades only when Hb gels are warmed up at 15°C and the structure relaxes to the R state (Figs. 1A, 2A,B). In liganded T state gels the peak is smaller in the absence than in the presence of allosteric effectors (Fig. 2A,B). Considering that in T state Hb gels in the absence of allosteric effectors, about two-thirds of the subunits relax to the r tertiary conformation upon ligation, while all molecules stay in a t conformation in the presence of allosteric effectors (Viappiani et al. 2004), our results suggest that the 280–295 nm band is a marker for a tertiary t, rather than quaternary T conformation. This conclusion is supported by the observation that the negative peak at 280 nm appears when L35 binds to R state HbCO in solution (Chen et al. 2005), in keeping with the TTS model postulate that t and r conformations can populate all quaternary states, depending on the concentration of negative allosteric effectors. Structural changes at the intersubunit interface, preceding the quaternary subunit rearrangement, were previously observed in time-resolved CD spectroscopy experiments by Kliger and coworkers (Bjorling et al. 1996), and attributed to a metastable kinetic intermediate, possibly transforming an R2/Y quaternary structure into an R quaternary structure. Our results suggest that these transitions are purely tertiary in nature.
The visible part of CD spectra reveals the presence of a marked trough centered at 575 nm in liganded R state Hb in solution and in the gel (Fig. 1A,B). The trough is absent in deoxyHb in solution and in the gel as well as in liganded T state Hb gels in the presence of allosteric effectors (Figs. 1A, 2A). In liganded T state Hb gels in the absence of allosteric effectors (Fig. 2B), the trough is well defined but less pronounced and right-shifted with respect to R state Hb in solution and in the gel. This structural marker was not previously detected using mutant or hybrid Hbs as models of T state liganded Hb. These results suggest that this trough, scarcely influenced, if at all, by ligation is a marker for liganded r species, only partially affected by the quaternary state. Under the assumption that liganded T state Hb in the absence of allosteric effectors is populated by both r and t tertiary conformations (Henry et al. 2002; Viappiani et al. 2004), its spectrum was fitted to a linear combination of the spectra of liganded R state gels and liganded T state gels. The fitting yields the same results for both the visible and the near UV region, although the fitting of the latter (data not shown) is not qualitatively as good as for the spectrum reported in Figure 2B, possibly because of quaternary contributions. The fitting shows that 60% of the subunits of liganded T state are in the r tertiary state, in excellent agreement with the results from flash photolysis experiments (Viappiani et al. 2004). We are aware that the sensitivity of CD spectroscopy does not allow quantitative support to the validation of any model for allosteric regulation. Indeed, a detailed discussion of the existing models is beyond the scope of the present work. Data interpretation in terms of the TTS model is intended to provide what we believe to be the simplest and more general explanation of the observed results within a view that does not take into account the α/β inequivalence or the possible structural and functional heterogeneity of tertiary and quaternary conformations. Since it is known that there are tyrosine and tryptophan residues located at the α1β2 and α2β1 switch region involved in the T-to-R structural transition, it is not surprising that a conformational marker of the tertiary state falls in the 280–295 nm spectral region. On the contrary, the band centered at 575 nm is in a region where only the heme-associated electronic transitions are predicted to contribute. Hsu and Woody (1971) proposed that the major contribution to the heme optical activity arises from the coupling of the heme π→π* transition with the π→π* transition of aromatic residues located within a 12 Å radius from the heme. Therefore, the 575 nm band may be associated with a local structure of the heme pocket in the r tertiary state, with minor influence from the quaternary structure.
In all, the results of the present work indicate that the circular dichroism bands at 280–295 and 575 nm, the former one previously considered to be a specific marker of the T quaternary conformation, are actually sensitive probes of Hb tertiary structure. These bands report on the local conformation of the interdimer interface and the heme environment, respectively. A simple way to explain their anticorrelated behavior is to assume that ligation and negative allosteric effectors control the equilibrium between the same two tertiary conformations, t and r, in any quaternary state of the tetramer, as predicted by the TTS model (Henry et al. 2002; Viappiani et al. 2004). Heterogeneity within the T quaternary state was recently investigated in CO rebinding kinetics and resonance Raman experiments on gel-encapsulated Hb (Samuni et al. 2006). The authors observed a tight coupling between the ligation and effector-dependent decrease in proximal strain/quaternary constraint and a weakening of the hinge interactions within the α1β2 interface associated with the deoxy T state, in agreement with the results of our CD study, and explained the results in terms of a distribution of T state species.
Materials and methods
Chemicals
Tetramethyl orthosilicate, potassium phosphate, HEPES, sodium dithionite, inositol hexaphosphate (IHP), and catalase were of the best commercially available quality and were used without further purification. All gases were of research-grade purity.
Preparation of samples
Human Hb A0 was purified from a nonsmoking donor as described previously (Rivetti et al. 1993), concentrated to 96 g/L in a buffer solution containing 10 mM HEPES, 1 mM EDTA (pH 7.2), and stored at −80°C. For experiments in solution, the Hb stock was diluted to a concentration of 200 μM in heme in a buffer containing 100 mM HEPES, 1 mM EDTA (pH 7.0). The Hayashi enzymatic reducing system (Hayashi et al. 1973) was added to prevent metHb accumulation. For experiments with HbCO, the dilution buffer, containing 0.2 mM sodium dithionite, was previously saturated with CO.
All samples of encapsulated Hb were made into ∼1 mm-thin layers by pouring 200 μL of the solution mixture onto 9 mm 2 × 235 mm quartz plates before gelification. The plates fit in standard 1 cm cuvettes.
DeoxyHb gels in the presence of allosteric effectors
A solution containing tetramethyl orthosilicate, water, and hydrochloric acid was sonicated for 20 min in ice. An equal volume of a buffer solution containing 10 mM potassium phosphate, 1 mM EDTA (pH 6.0) was then added. The mixture was deoxygenated by bubbling nitrogen for 40 min at 4°C. Finally, 1.5 vol of a solution containing ∼10 g/L Hb A0 in 50 mM potassium phosphate, 10 mM IHP, 1 mM EDTA, 10 mM sodium dithionite (pH 7.2) was anaerobically added to the mixture. Gelification occurred in a few minutes at 4°C. A solution containing 100 mM HEPES, 10 mM IHP, 1 mM EDTA, 30 mM sodium dithionite (pH 7.0) was layered on the gel for storage. All experiments on low affinity gels were carried out in the presence of 10 mM IHP.
DeoxyHb gels in the absence of allosteric effectors
A solution containing 10 mM HEPES, 1 mM EDTA (pH 6.2) was added to an equal volume of tetramethyl orthosilicate and vortexed for 2 min. The mixture was then deoxygenated by bubbling humidified helium for 90 min in ice. A solution containing ∼10 g/L deoxyHb A0 in 10 mM HEPES, 1 mM EDTA, 10 mM sodium dithionite (pH 6.2) was finally added. The solution mixture was left at 4°C for 30 min. Gelification occurred in about 10 min after the mixture was brought to room temperature. For storage, a solution containing 100 mM HEPES, 1 mM EDTA, 30 mM sodium dithionite (pH 7.0) was layered on the gel.
Liganded Hb gels
A Hb solution was saturated with CO prior to gelification in the presence of sodium dithionite. The gelification protocol was the same as for deoxyHb gels in the presence of allosteric effectors, except for the absence of IHP. Samples were stored in a buffer solution containing 100 mM HEPES, 1 mM EDTA (pH 7.0).
Circular dichroism measurements
Circular dichroism spectra were collected with a JASCO J-715 spectropolarimeter in the 240–650 nm range using quartz cuvettes sealed with rubber septa. The optical activity was normalized to molar ellipticity ([Θ]). The high extinction coefficient of Hb in the Soret region leads to unreliable spectroscopic information at the protein concentration and gel sample thickness used in the present work. Therefore, no discussion of the behavior of Soret bands is reported in the Results and Discussion section. The protein concentration was calculated from spectra collected with the spectropolarimeter during each experiment. This procedure allowed the quantitative comparison of different Hb gel samples, which are inevitably slightly heterogeneous in thickness. All spectra were collected at 4°C, unless otherwise stated.
Acknowledgments
This work was partially supported by the 6th European Union Framework Project “Euroblood Substitutes.” We thank the Centro Interfacoltà Misure (CIM) of the University of Parma for the use of the spectropolarimeter.
Footnotes
Reprint requests to: Stefano Bettati, Department of Biochemistry and Molecular Biology, University of Parma, Viale Usberti 23/A, 43100 Parma, Italy; e-mail: stefano.bettati@unipr.it; fax: 39-0521-905151.
Article published online ahead of print. Article and publication date are at http://www.proteinscience.org/cgi/doi/10.1110/ps.062272306.
References
- Antonini E. and Brunori M. In Hemoglobin and myoglobin in their reactions with ligands . 1971. , North-Holland, Amsterdam.
- Bettati S. and Mozzarelli A. 1997. T state hemoglobin binds oxygen noncooperatively with allosteric effects of protons, inositol hexaphosphate, and chloride. J. Biol. Chem. 272: 32050–32055. [DOI] [PubMed] [Google Scholar]
- Bettati S., Pioselli B., Campanini B., Viappiani C., Mozzarelli A. 2004. Protein-doped nanoporous silica gels. In Encyclopedia of nanoscience and nanotechnology (ed. Nalwa H.S.) . pp. 81–103. American Scientific Publishers, Stevenson Ranch, CA 9:. [Google Scholar]
- Bjorling S.C., Goldbeck R.A., Paquette S.J., Milder S.J., Kliger D.S. 1996. Allosteric intermediates in hemoglobin. 1. Nanosecond time-resolved circular dichroism spectroscopy. Biochemistry 35: 8619–8627. [DOI] [PubMed] [Google Scholar]
- Bruno S., Bonaccio M., Bettati S., Rivetti C., Viappiani C., Abbruzzetti S., Mozzarelli A. 2001. High and low oxygen affinity conformations of T state hemoglobin. Protein Sci. 10: 2401–2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q., Lalezari I., Nagel R.L., Hirsch R.E. 2005. Liganded hemoglobin structural perturbations by the allosteric effector L35. Biophys. J. 88: 2057–2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coletta M., Ascenzi P., Castagnola M., Giardina B. 1995. Functional and spectroscopic evidence for a conformational transition in ferrous liganded human hemoglobin. J. Mol. Biol. 249: 800–803. [DOI] [PubMed] [Google Scholar]
- Coletta M., Angeletti M., Ascenzi P., Bertollini A., Della Longa S., De Sanctis G., Priori A.M., Santucci R., Amiconi G. 1999a. Coupling of the oxygen-linked interaction energy for inositol hexakisphosphate and bezafibrate binding to human HbA0. J. Biol. Chem. 274: 6865–6874. [DOI] [PubMed] [Google Scholar]
- Coletta M., Angeletti M., Ascone I., Boumis G., Castellano A.C., Dell'Ariccia M., Della Longa S., De Sanctis G., Priori A.M., Santucci R. et al. 1999b. Heterotropic effectors exert more significant strain on monoligated than on unligated hemoglobin. Biophys. J. 76: 1532–1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das T.K., Khan I., Rousseau D.L., Friedman J.M. 1999. Temperature dependent quaternary relaxation in sol-gel encapsulated hemoglobin. Biospectroscopy 5: S64–S70. [DOI] [PubMed] [Google Scholar]
- Edelstein S.J. 1975. Cooperative interactions of hemoglobin. Annu. Rev. Biochem. 44: 209–232. [DOI] [PubMed] [Google Scholar]
- Ellerby L.M., Nishida C.R., Nishida F., Yamanaka S.A., Dunn B., Valentine J.S., Zink J.I. 1992. Encapsulation of proteins in transparent porous silicate glasses prepared by the sol-gel method. Science 255: 1113–1115. [DOI] [PubMed] [Google Scholar]
- Gill I. and Ballesteros A. 2000. Bioencapsulation within synthetic polymers (Part 1): Sol-gel encapsulated biologicals. Trends Biotechnol. 18: 282–296. [DOI] [PubMed] [Google Scholar]
- Goldbeck R.A., Paquette S.J., Bjorling S.C., Kliger D.S. 1996. Allosteric intermediates in hemoglobin. 2. Kinetic modeling of HbCO photolysis. Biochemistry 35: 8628–8639. [DOI] [PubMed] [Google Scholar]
- Hayashi A., Suzuki T., Shin M. 1973. An enzymic reduction system for metmyoglobin and methemoglobin, and its application to functional studies of oxygen carriers. Biochim. Biophys. Acta 310: 309–316. [DOI] [PubMed] [Google Scholar]
- Henry E.R., Jones C.M., Hofrichter J., Eaton W.A. 1997. Can a two-state MWC allosteric model explain hemoglobin kinetics? Biochemistry 36: 6511–6528. [DOI] [PubMed] [Google Scholar]
- Henry E.R., Bettati S., Hofrichter J., Eaton W.A. 2002. A tertiary two-state allosteric model for hemoglobin. Biophys. Chem. 98: 149–164. [DOI] [PubMed] [Google Scholar]
- Hsu M.C. and Woody R.W. 1971. The origin of the heme Cotton effects in myoglobin and hemoglobin. J. Am. Chem. Soc. 93: 3515–3525. [DOI] [PubMed] [Google Scholar]
- Imai K. In Allosteric effects in hemoglobin . 1982. Cambridge University Press, Cambridge, UK.
- Imai K., Tsuneshige A., Yonetani T. 2002. Description of hemoglobin oxygenation under universal solution conditions by a global allostery model with a single adjustable parameter. Biophys. Chem. 98: 79–91. [DOI] [PubMed] [Google Scholar]
- Jin W. and Brennan J.D. 2002. Properties and applications of proteins encapsulated within sol-gel derived materials. Anal. Chim. Acta 461: 1–36. [Google Scholar]
- Jin Y., Sakurai H., Nagai Y., Nagai M. 2004. Changes of near-UV CD spectrum of human hemoglobin upon oxygen binding: A study of mutants at α42, α140, β145 tyrosine or β37 tryptophan. Biopolymers 74: 60–63. [DOI] [PubMed] [Google Scholar]
- Jones C.M., Ansari A., Henry E.R., Christoph G.W., Hofrichter J., Eaton W.A. 1992. Speed of intersubunit communication in proteins. Biochemistry 31: 6692–6702. [DOI] [PubMed] [Google Scholar]
- Juszczak L.J. and Friedman J.M. 1999. UV resonance Raman spectra of ligand binding intermediates of sol-gel encapsulated hemoglobin. J. Biol. Chem. 274: 30357–30360. [DOI] [PubMed] [Google Scholar]
- Kavanaugh J.S., Rogers P.H., Arnone A. 2005. Crystallographic evidence for a new ensemble of ligand-induced allosteric transitions in haemoglobin: The T-to-T(high) quaternary transitions. Biochemistry 44: 6101–6121. [DOI] [PubMed] [Google Scholar]
- Khan I., Shannon C.F., Dantsker D., Friedman A.J., Perez-Gonzalez-de-Apodaca J., Friedman J.M. 2000. Sol-gel trapping of functional intermediates of hemoglobin: Geminate and bimolecular recombination studies. Biochemistry 39: 16099–16109. [DOI] [PubMed] [Google Scholar]
- Kister J., Poyart C., Edelstein S.J. 1987. An expanded two-state allosteric model for interactions of human hemoglobin A with nonsaturating concentrations of 2,3-diphosphoglycerate. J. Biol. Chem. 262: 12085–12091. [PubMed] [Google Scholar]
- Li R., Nagai Y., Nagai M. 2000a. Changes of tyrosine and tryptophan residues in human hemoglobin by oxygen binding: Near- and far-UV circular dichroism of isolated chains and recombined hemoglobin. J. Inorg. Biochem. 82: 93–101. [DOI] [PubMed] [Google Scholar]
- Li R., Nagai Y., Nagai M. 2000b. Contribution of α140Tyr and β37Trp to the near-UV CD spectra on quaternary structure transition of human hemoglobin A. Chirality 12: 216–220. [DOI] [PubMed] [Google Scholar]
- Liddington R., Derewenda Z., Dodson E., Hubbard R., Dodson G. 1992. High resolution crystal structures and comparisons of T-state deoxyhaemoglobin and two liganded T-state haemoglobins: T(α-oxy)haemoglobin and T(met)haemoglobin. J. Mol. Biol. 228: 551–579. [DOI] [PubMed] [Google Scholar]
- Miyazaki G., Morimoto H., Yun K.M., Park S.Y., Nakagawa A., Minagawa H., Shibayama N. 1999. Magnesium(II) and zinc(II)-protoporphyrin IX's stabilize the lowest oxygen affinity state of human hemoglobin even more strongly than deoxyheme. J. Mol. Biol. 292: 1121–1136. [DOI] [PubMed] [Google Scholar]
- Monod J., Wyman J., Changeux J.P. 1965. On the nature of allosteric transitions: A plausible model. J. Mol. Biol. 12: 88–118. [DOI] [PubMed] [Google Scholar]
- Mozzarelli A., Rivetti C., Rossi G.L., Eaton W.A., Henry E.R. 1997. Allosteric effectors do not alter the oxygen affinity of hemoglobin crystals. Protein Sci. 6: 484–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paoli M., Liddington R., Tame J., Wilkinson A., Dodson G. 1996. Crystal structure of T state haemoglobin with oxygen bound at all four haems. J. Mol. Biol. 256: 775–792. [DOI] [PubMed] [Google Scholar]
- Perutz M.F., Fersht A.R., Simon S.R., Roberts G.C. 1974a. Influence of globin structure on the state of the heme. II. Allosteric transitions in methemoglobin. Biochemistry 13: 2174–2186. [DOI] [PubMed] [Google Scholar]
- Perutz M.F., Ladner J.E., Simon S.R., Ho C. 1974b. Influence of globin structure on the state of the heme I. Human deoxyhemoglobin. Biochemistry 13: 2163–2173. [DOI] [PubMed] [Google Scholar]
- Perutz M.F., Wilkinson A.J., Paoli M., Dodson G. 1998. The stereochemical mechanism of the cooperative effects in hemoglobin revisited. Annu. Rev. Biophys. Biomol. Struct. 27: 1–34. [DOI] [PubMed] [Google Scholar]
- Plese C.F. and Amma E.L. 1977. Circular dichroism as a probe of the allosteric R in equilibrium T transformation in hemoglobins and modified hemoglobins. Biochem. Biophys. Res. Commun. 76: 691–697. [DOI] [PubMed] [Google Scholar]
- Poyart C.F., Bursaux E., Bohn B. 1978. An estimation of the first binding constant of O2 to human hemoglobin A. Eur. J. Biochem. 87: 75–83. [DOI] [PubMed] [Google Scholar]
- Rivetti C., Mozzarelli A., Rossi G.L., Henry E.R., Eaton W.A. 1993. Oxygen binding by single crystals of hemoglobin. Biochemistry 32: 2888–2906. [DOI] [PubMed] [Google Scholar]
- Safo M.K. and Abraham D.J. 2005. The enigma of the liganded hemoglobin end state: A novel quaternary structure of human carbonmonoxy hemoglobin. Biochemistry 44: 8347–8359. [DOI] [PubMed] [Google Scholar]
- Samuni U., Dantsker D., Juszczak L.J., Bettati S., Ronda L., Mozzarelli A., Friedman J.M. 2004. Spectroscopic and functional characterization of T state hemoglobin conformations encapsulated in silica gels. Biochemistry 43: 13674–13682. [DOI] [PubMed] [Google Scholar]
- Samuni U., Roche C.J., Dantsker D., Juszczak L., Friedman J.M. 2006. Modulation of reactivity and conformation within the T-quaternary state of human haemoglobin: The combined use of mutagenesis and sol-gel encapsulation. Biochemistry 45: 2820–2835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibayama N. 1999. Functional analysis of hemoglobin molecules locked in doubly liganded conformations. J. Mol. Biol. 285: 1383–1388. [DOI] [PubMed] [Google Scholar]
- Shibayama N. and Saigo S. 1995. Fixation of the quaternary structures of human adult haemoglobin by encapsulation in transparent porous silica gels. J. Mol. Biol. 251: 203–209. [DOI] [PubMed] [Google Scholar]
- Shibayama N. and Saigo S. 1999. Kinetics of the allosteric transition in hemoglobin within silicate sol-gels. J. Am. Chem. Soc. 121: 444–445. [Google Scholar]
- Shibayama N. and Saigo S. 2001. Direct observation of two distinct affinity conformations in the T state human deoxyhemoglobin. FEBS Lett. 492: 50–53. [DOI] [PubMed] [Google Scholar]
- Shibayama N., Miura S., Tame J.R.H., Yonetani T., Park S.-Y. 2002. Crystal structure of horse carbonmonoxyhemoglobin-bezafibrate complex at 1.55-Å resolution. A novel allosteric binding site in R-state hemoglobin. J. Biol. Chem. 277: 38791–38796. [DOI] [PubMed] [Google Scholar]
- Simolo K., Stucky G., Chen S., Bailey M., Scholes C., McLendon G. 1985. Characterization of partially ligated hemoglobins: NMR, ENDOR, CD, and stopped-flow studies of zinc-containing hemoglobin hybrids. J. Am. Chem. Soc. 107: 2865–2872. [Google Scholar]
- Sudhakar K., Laberge M., Tsuneshige A., Vanderkooi J.M. 1998. Zinc-substituted hemoglobins: α- and β-chain differences monitored by high-resolution emission spectroscopy. Biochemistry 37: 7177–7184. [DOI] [PubMed] [Google Scholar]
- Tsuneshige A., Park S., Yonetani T. 2002. Heterotropic effectors control the hemoglobin function by interacting with its T and R states—A new view on the principle of allostery. Biophys. Chem. 98: 49–63. [DOI] [PubMed] [Google Scholar]
- Ueda Y., Shiga T., Tyuma I. 1969. Circular dichroism spectra of human adult hemoglobin and its subunits. Biochem. Biophys. Res. Commun. 35: 1–5. [DOI] [PubMed] [Google Scholar]
- Viappiani C., Bettati S., Bruno S., Ronda L., Abbruzzetti S., Mozzarelli A., Eaton W.A. 2004. New insights into allosteric mechanisms from trapping unstable protein conformations in silica gels. Proc. Natl. Acad. Sci. 101: 14414–14419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoyama T., Neya S., Tsuneshige A., Yonetani T., Park S.Y., Tame J.R. 2006. R-state haemoglobin with low oxygen affinity: Crystal structures of deoxy human and carbonmonoxy horse haemoglobin bound to the effector molecule L35. J. Mol. Biol. 356: 790–801. [DOI] [PubMed] [Google Scholar]
- Yonetani T., Park S.I., Tsuneshige A., Imai K., Kanaori K. 2002. Global allostery model of hemoglobin. Modulation of O(2) affinity, cooperativity, and Bohr effect by heterotropic allosteric effectors. J. Biol. Chem. 277: 34508–34520. [DOI] [PubMed] [Google Scholar]
- Zentz C., Pin S., Alpert B. 1994. Stationary and time-resolved circular dichroism of hemoglobins. Methods Enzymol. 232: 247–266. [DOI] [PubMed] [Google Scholar]