Abstract
In addition to well established trophic functions, neurotrophins acutely affect neurotransmitter secretion from the presynaptic nerve terminal, influence synaptic development, and may serve as selective retrograde messengers that regulate synaptic efficacy. The crucial question related to the mechanisms of neurotrophin-mediated signaling is whether acute effects of neurotrophins are spatially restricted to the activated synapses. Here we have used a local perfusion technique for local delivery of neurotrophin-3 (NT-3) to various regions of developing Xenopus embryo neurons in culture. Within minutes after a focal exposure of a soma or a small (≈30 μm in length) axonal segment to NT-3, we observed an increase in the spontaneous neurotransmitter secretion from the presynaptic nerve terminals located ≈300–400 μm away from the site of NT-3 application. Secretory activity along the axonal shaft was not affected. Our findings suggest that the NT-3-mediated signal may rapidly travel through neuronal cytoplasm over unexpectedly long distances and modulate neurotransmitter release specifically at the presynaptic nerve terminals.
The neurotrophin hypothesis holds that proliferation, survival, and differentiation of various neuronal populations are determined by the competition between neurons for a limited amount of trophic factors produced primarily by target tissues (1–3). In addition to these classic long-lasting trophic activities, neurotrophins mediate neurotransmitter secretion from the presynaptic neurons, both in cell culture (4) and in hippocampal slices (5). Neurotrophin synthesis in the central nervous system is rapidly regulated by neuronal activity (6, 7), and neurotrophin release from the postsynaptic targets is activity dependent (8). Taken together, these results support a positive feedback model, in which presynaptic activity enhances neurotrophin synthesis and release from the postsynaptic cells, leading to potentiation of synaptic efficacy (9–12). Thus, neurotrophins may serve as selective retrograde messengers involved in the processes of synaptic maturation and synaptic competition (13).
In the past, neurotrophin effects were thought to be primarily mediated by long-range retrograde signaling to the soma, where changes in gene transcription are induced (14, 15). The signaling requires autophosphorylation of specific tyrosine residues on neurotrophin receptors (Trks), followed by receptor endocytosis and retrograde transport to the cell body (15). However, there are neurotrophin-mediated effects that occur on the time scale of minutes; these do not require protein synthesis or signaling to the cell body (4, 11, 16). Although the signal transduction pathways involved in such acute effects have yet to be firmly established, it is generally believed that the neurotrophin-mediated acute effects are spatially restricted to the site of neurotrophin secretion (11, 17).
Xenopus nerve-muscle coculture is a well established model for synaptic plasticity. In these developing neuromuscular synapses, spontaneous synaptic currents (SSCs) and impulse-evoked currents are rapidly potentiated by neurotrophic factors neurotrophin-3 (NT-3) brain-derived neurotrophic factor (BDNF), NT-4, or ciliary neurotrophic factor (CNTF) (4, 18, 19). On removal of NT-3 from the culture medium, the frequency of SSCs returns to control value, suggesting that NT-3-mediated signaling cascade does not induce a permanent alteration in the secretory machinery (4). In this paper, we have investigated whether NT-3-mediated signal may propagate within isolated Xenopus neurons in culture. To mimic a local release of NT-3 by a postsynaptic target or by neighboring cells, we used a local perfusion technique to deliver NT-3 to either a small axonal segment or to the soma. We detected rapid potentiation of neurotransmitter release from the distant presynaptic terminals, suggesting spreading of NT-3-mediated signal over long distances (≈300–400 μm). This propagation of NT-3-mediated signal appears to be mediated by a cytoplasmic factor. Our results suggest that local exposure of the cell body or the proximal axon to neurotrophic factors may rapidly modulate neurotransmitter secretion from the distant presynaptic nerve terminals.
MATERIALS AND METHODS
Cell Culture.
Cultured Xenopus spinal cord neurons were prepared according to previously reported methods (20, 21). The cultures were used for experiments after 1-day incubation at 20°C. Human recombinant NT-3 was generously provided by Regeneron Pharmaceuticals (Tarrytown, NY).
Electrophysiology.
Gigaohm-seal whole-cell recording methods followed those described previously (4, 22). The data were analyzed with the scan program, kindly provided by J. Dempster, Strathclyde University, Glasgow, U.K. To determine significant differences between averages, unpaired t-tests assuming unequal variance were performed.
Micromanipulation.
Manipulation of Xenopus myocytes followed the procedures described previously (23, 24). Briefly, myocytes were gently detached from the surface of the Petri dish by heat-polished micropipettes attached to a hydraulic micromanipulator (Newport, Irvine, CA). The cells were transferred into the vicinity of the axon, allowed to reattach to the glass surface, and manipulated into the contact with the neuron.
Local Perfusion of the Axon and Soma.
Local perfusion of the axon was performed according to previously reported methods (25). Briefly, two pipettes were placed opposite each other at a distance of 20 to 40 μm from the neuronal surface. The first pipette, with tip opening of ≈4 μm, served for continuous delivery of solution and was filled with culture medium containing NT-3 alone or NT-3 and α-bungarotoxin (Molecular Probes). The second pipette with tip opening of ≈10 μm was used for removal of the superfusion solution from the culture medium, thereby reducing the affected area to ≈30 μm.
Local Perfusion of the Presynaptic Nerve Terminal.
For the “shielding” of the presynaptic nerve terminal from the bath-applied NT-3, the pipette with a tip opening of ≈10–20 μm was filled with culture medium and placed at a distance of about 30–50 μm from the synapse. Positive pressure was applied to the pipette for 1–2 min before bath application of NT-3.
Microscopy.
An Olympus IX 50 inverted microscope was used for fluorescence microscopy (Olympus, New Hyde Park, NY). Images were acquired with a charge-coupled device camera (ImagePoint, Photometrics, Tucson, AZ) driven by IPLab (Signal Analytics, Vienna, VA) imaging software and background subtracted.
Immunocytochemistry.
The neurons were fixed for 10 min with 4% paraformaldehyde in PBS (pH 7.4), permeabilized with 0.1% Triton X-100, and blocked in a solution of 5% bovine serum albumin in PBS for 1 hr at room temperature. Primary antibody (1:200) was applied at 4°C overnight and a secondary antibody (1:200) for 1 hr at room temperature. The primary antibodies used were: (i) a rabbit polyclonal antibody to TrkC (cat no. sc-117, Santa Cruz Biotechnology) and (ii) a chicken polyclonal IgY (cat no. 06-675, Upstate Biotechnology, Lake Placid, NY), which is raised against the extracellular domain of human TrkC. Accordingly, in this case the permeabilization step was omitted. For blocking experiments (Fig. 2D), we used the blocking peptide (cat no. sc-117 P, Santa Cruz Biotechnology) that corresponds to the epitope for TrkC antibody.
RESULTS
Acute Effect of NT-3 on Spontaneous Acetylcholine Secretion from Growing Neurons.
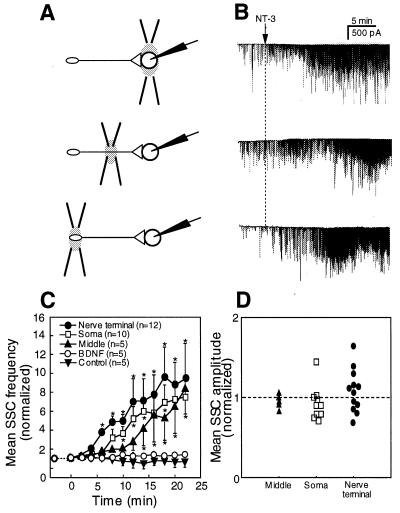
Experiments were performed on nerve-muscle cocultures from Xenopus embryos 1 day after cell culture preparation. An isolated Xenopus myocyte was detached from the substrate by using a micropipette and manipulated into contact with either the growth cone region of the axon, the middle of the axonal shaft, or the soma (Fig. 1A). Recordings of the membrane currents in the myocyte using a whole-cell voltage clamp technique revealed fast inward currents (SSCs). The individual SSCs were caused by the fusion of acetylcholine (ACh)-containing vesicles with the neuronal plasmalemma (23).
In developing Xenopus neuromuscular synapses, spontaneous ACh secretion from the presynaptic nerve terminal is known to be potentiated by acute exposure to NT-3 (4). To investigate whether ACh release from other neuronal segments is affected by NT-3, we recorded membrane currents from the myocytes manipulated into contact with the growth cone region, the middle axonal segment and the soma of the neuron. Fig. 1B shows examples of SSC recordings from a myocyte at the spontaneously formed (“performed”) neuromuscular synapse and from myocytes manipulated into contact with various neuronal regions. In agreement with previously published data (4), bath application of NT-3 (50 ng/ml) resulted in a rapid (within 10 min) increase in the frequency of SSCs at the performed neuromuscular synapses (Fig. 1, B and C). Similarly, SSC frequency in recordings from the growth cone region also increased within minutes after NT-3 application. In contrast, spontaneous ACh secretion at the middle of the axon and at the soma was not affected by NT-3 treatment (Fig. 1, B and C). In all four recording configurations, no changes were observed in the mean amplitude of the SSCs (Fig. 1D), suggesting that the observed increase in SSC frequency reflects a higher frequency of exocytotic events in the neuron, rather than an increase in the sensitivity of the postsynaptic ACh receptors (4, 18).
TrkC Receptors Are Present Along the Axonal Plasmalemma and at the Soma and Are Functionally Active.
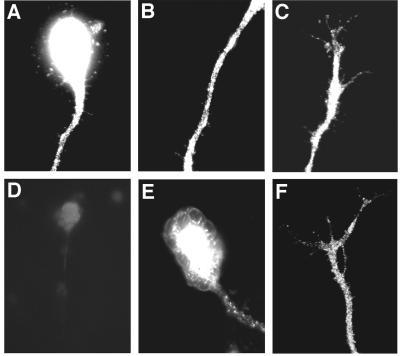
Acute effects of neurotrophins on spontaneous neurotransmitter secretion from the nerve terminal are mediated by the Trk family of tyrosine kinase receptors (4, 26). NT-3 predominantly activates TrkC receptors. The lack of secretory response to NT-3 treatment at the soma and along the axonal shaft may reflect preferential localization of TrkC receptors to the growth cone area. To determine the distribution of TrkC receptors along growing Xenopus neurites, we stained the neuronal cultures with two different polyclonal antibodies for TrkC. Immunoreactivity for the TrkC was detected at the soma, along the axon, and at the growth cone area (Fig. 2, A–F).
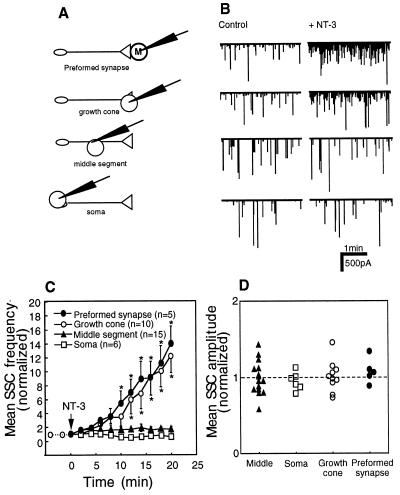
To test for the functional activity of Trk receptors along the axon and/or at the soma, we used a pair of perfusion micropipettes to apply NT-3 locally to the presynaptic terminal, to the middle axonal segment, or to the soma of a neuron with a synaptic contact at a distance of 300–400 μm from the soma (Fig. 3A). We simultaneously recorded SSCs from the myocytes in the preformed synapses. The size of the affected area (≈30 μm in a typical experiment) was made visible by using a food-color dye in the perfusion medium. Within minutes after local NT-3 application, we observed a marked increase in the SSC frequency in recordings from the postsynaptic myocyte (Fig. 3). This increase suggests functional activation of Trks at the proximal axonal regions and anterograde spread of the NT-3-mediated signal toward the presynaptic nerve terminal. For a period of 15–20 min after the onset of NT-3 application, the average SSC frequency was 6.8 ± 1.1 (mean ± SEM, n = 10, NT-3 application to the soma), 4.5 ± 1.1 (n = 5, NT-3 application to the middle axonal segment) and 7.7 ± 1.8 (n = 12, NT-3 application to the presynaptic terminal) times higher than the corresponding initial frequency. These values were not significantly different from that recorded from the preformed synapses after bath application of NT-3 to the culture medium (Fig. 1). In none of the three experimental configurations was the average amplitude of SSCs affected (Fig. 3D), suggesting presynaptic mechanism of NT-3 action. In agreement with previously published data (26), local application of BDNF to the cell body had no effect on the SSC frequency recorded at the distal synapses (Fig. 3C). Thus, the observed anterograde spreading of neurotrophin-mediated signal is specific to NT-3.
Figure 3.
Local exposure of different axonal segments to NT-3 rapidly potentiates ACh secretion from the distant presynaptic nerve terminals. (A) Schematic representation of experimental approach. Neurons with an axon ≈300–400 μm in length and synaptic contact with a myocyte were chosen for experiments. Two glass micropipettes positioned in the vicinity of the neuron were used for local perfusion of a specific site with a culture medium containing 200 ng/ml NT-3. SSCs in the postsynaptic myocyte were recorded by using the whole-cell patch-clamp method. (B) Examples of current traces recorded from the postsynaptic myocytes. Arrow marks the onset of local perfusion of the neuron with the NT-3-containing culture medium. Gradual increase in the SSC frequency with time is evident in all three recording configurations. (C) Changes in the SSC frequency with time after the onset of local perfusion. Each data point represents the mean ± SEM of 5–12 experiments. No change in the SSC frequency was detected after perfusion of the cell body with the fresh culture medium (control) or with BDNF-containing culture medium (two lower lines). ∗, P < 0.05. (D) Mean SSC amplitudes for a period of 15–20 min after the onset of local NT-3-application normalized to the mean SSC amplitude for a 5-min period before NT-3 application.
Figure 1.
Acute effect of NT-3 application on SSC frequency at different axonal segments. (A) Schematic diagram of recording configurations. Whole-cell patch-clamp recordings were performed from the myocytes (M) at the spontaneous formed (“preformed”) synapses and from myocytes manipulated into contact with the growth cone region, the middle axonal segment, and the soma of isolated neurons 1 day after cell culture preparation. The neurons chosen for manipulation experiments were free of contact with other cells and had a single axon ≈300–400 μm in length. (B) Membrane currents recorded from the myocytes for a 3-min period before NT-3 application (“control”) and for a period of 15–18 min after the onset of NT-3 (50 ng/ml) treatment. Downward spikes are inward currents reflecting spontaneous ACh secretion from the neuron. (C) Changes in the SSC frequency with time after the bath application of NT-3 (marked by arrow). The mean SSC frequency was calculated for 2-min intervals and normalized to the mean SSC frequency for a 5-min period before NT-3 application. Each data point represents the mean ± SEM of 5–15 experiments. ∗, significantly different from control values (P < 0.05). (D) Changes in the mean SSC amplitude after bath application of NT-3. In each recording, the mean SSC amplitude was determined 20–25 min after NT-3 application and normalized to the mean SSC amplitude before NT-3 application.
“Leakage” of NT-3 from the Perfusion Site into the Dish Medium Does Not Contribute to the Potentiation of ACh Release at the Nerve Terminal.
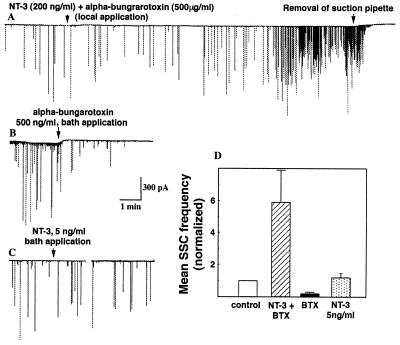
The validity of the local perfusion technique is based on the assumption that very little NT-3 escapes from the superfused region into the dish medium. The “leakage” of NT-3 from the perfusion site may lead to the gradual elevation of the local concentration of NT-3 in the vicinity of the preformed synapse and to the potentiation of ACh release because of the direct effect of NT-3 on the presynaptic membrane. To estimate the concentration of NT-3 in the vicinity of the synapse, we locally applied NT-3 (200 ng/ml) together with α-bungarotoxin (500 μg/ml) to the middle axonal segment, while simultaneously recording SSCs at the preformed synapse. α-bungarotoxin is a potent blocker of ACh channels (27). If a significant amount of the superfusion medium escapes from the locally perfused region, α-bungarotoxin would be expected to block ACh channels in the myocyte and thus inhibit SSCs. However, local perfusion of the middle axonal segment with a culture medium containing both NT-3 and α-bungarotoxin resulted in the potentiation of ACh secretion at the preformed synapse (Fig. 4A). For a period of 15–18 min after the onset of NT-3 and α-bungarotoxin application, the average SSC frequency was 5.9 ± 2.0 (mean ± SEM, n = 7) times higher than the initial frequency (Fig. 4D); the average amplitude of SSCs normalized to the control value was 0.92 ± 0.23 (n = 7).
As expected, bath application of α-bungarotoxin (500 ng/ml) resulted in the rapid reduction in the average frequency of SSCs at the preformed synapses (Fig. 4B). Therefore, as a very conservative estimate, the local concentration of α-bungarotoxin near the synapse during superfusion of the middle axon is <500 ng/ml, which is 1,000-fold lower than the concentration of α-bungarotoxin in the perfusion pipette (500 μg/ml). Similarly, we estimate the local concentration of NT-3 near the preformed synapse to be <200 pg/ml. In agreement with previously reported data (4), we found that bath application of NT-3 in the concentration 25-fold higher than the above estimate (5 ng/ml) has no statistically significant effect on the SSC frequency at the preformed synapse (Fig. 4D). Therefore, we conclude that “leakage” of NT-3 from the superfusion medium does not contribute to the observed potentiation of ACh secretion at the preformed synapses.
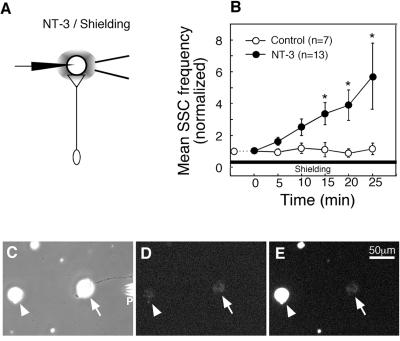
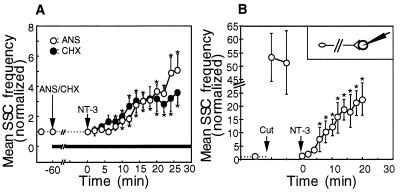
As an alternative to the local perfusion method described above, we attempted to activate Trks preferentially at the soma and along proximal axon by concurrent bath application of NT-3 and local perfusion of the preformed synapse with a fresh culture medium (Fig. 5A). We expected that the perfusion of the synapse with a fresh culture medium will prevent NT-3 from reaching the nerve terminal and thus will “shield” this area from the NT-3 action. The size of the shielded area was estimated by using a food-color dye in the perfusion pipette and in a typical experiment was about 100 μm in diameter. We simultaneously recorded SSCs from the myocytes in the superfused synapses. Bath application of NT-3 (50 ng/ml) resulted in a significant and characteristically rapid (within 5–10 min) increase in the SSC frequency in the shielded synapses (Fig. 5B).
To estimate the degree of synapse protection from the molecules present in the extracellular medium, we performed a series of control video microscopy experiments. In these experiments, we added the fluorescent dye acridine orange to the culture medium while continuously perfusing the synapse with fresh medium. As expected, the myocytes and the fragments of broken cells located at a distance >100 μm from the superfused region were rapidly stained with acridine orange (Fig. 5, C–E). However, we observed no staining of the myocyte in the shielded region (Fig. 5E). Quantitative analysis of the fluorescent images indicated that the signal from the myocyte outside the protected region overloaded the CCD sensor of the eight-bit camera that we used. No changes in the intensity of the fluorescence staining were observed for the myocytes in the shielded region. Therefore, as a conservative estimate, the concentration of acridine orange in the shielded region is at least 256-fold lower than that outside this region. Similar results were obtained by using another fluorescent dye, FM1-43 (data not shown). Both acridine orange and FM1-43 have molecular weights smaller, and consequently diffusion coefficient higher, than that of NT-3. Therefore, the protection of a superfused synapse from extracellularly added acridine orange and FM1-43 molecules that we observe provides a high-stringency test for the shielding against NT-3.
Potentiation of Neurotransmitter Release Does Not Require Protein Synthesis.
Considering a rapid (within minutes) onset of synaptic potentiation after local application of NT-3 to the proximal axon and to the soma, it appears unlikely that either induction of gene transcription or new protein synthesis is involved in the acute effect of NT-3 on spontaneous neurotransmitter secretion. To test this prediction, we pretreated cell cultures with a protein synthesis inhibitors anisomycin (40 μM) or cycloheximide (10 μM) for 1 h before bath application of NT-3. Similar to control experiments (Fig. 1), bath application of NT-3 to the neuronal cultures pretreated with anisomycin or with cycloheximide rapidly potentiated SSC frequency at preformed neuromuscular synapses (Fig. 6A).
To directly test the idea that the NT-3-induced potentiation of ACh release from the presynaptic terminal does not require signaling to the soma, we transected the axonal shaft near the soma with a sharp micropipette. ACh secretion at the preformed neuromuscular synapse was continuously recorded for a period of 5–10 min before transection and for 30 min after transection. Immediately after the transection, we observed about 50-fold increase in the SSC frequency as a result of Ca2+ influx at the injured site. Within 10 min, the level of ACh secretion returned to the baseline level, and NT-3 (50 ng/ml) was added to the culture medium. Exposure of the distal axonal fragments (which were now physically separated from the soma) to NT-3 resulted in the increase in the SSC frequency similar to that found in intact neurons (Fig. 6B). Thus, neither protein synthesis nor signaling to the soma is required for the potentiation of neurotransmitter release at the presynaptic nerve terminal in response to NT-3 application.
DISCUSSION
Experimental evidence from a number of neuronal systems indicates that activity-dependent changes in synaptic strength, such as long-term potentiation and long-term depression, can spread over considerable distances from activated synapse (28–31). Neurotrophins secreted from postsynaptic targets may contribute to the activity-dependent remodeling of synaptic connections (10). An important question related to the mechanisms and functional significance of neurotrophin-mediated signaling is whether acute effects of neurotrophins are spatially restricted to the activated synapses. Can activation of TrkC receptors at the proximal axon and/or at the soma because of the local NT-3 release in the vicinity of these segments affect neurotransmitter secretion from the distant nerve terminals? To mimic neurotrophin release near the soma or along the axon, we used two versions of a local perfusion technique to apply NT-3 to neuronal regions other than presynaptic terminal (Figs. 3 and 5). In both cases, we observed characteristically rapid (within 5–10 min of NT-3 application) potentiation of neurotransmitter release at the synapses formed by the NT-3-exposed neuron. These results suggest a rapid propagation of the NT-3-mediated signal from the proximal axon and the soma to the nerve terminal. Interestingly, it has been reported previously that the action of NT-4 in Xenopus neurons is restricted to <60 μm from the site of NT-4 application (17). Therefore, TrkC-mediated cascade of intracellular effector molecules elicited by NT-3 application is likely to be different from that induced by activation of TrkB receptors. This idea is consistent with reported differences in the mechanisms of NT-3 and BDNF action on growth cone motility in Xenopus embryonic neurons (32, 33).
Figure 5.
Bath application of NT-3 potentiates neurotransmitter secretion at the synapses protected from direct exposure to NT-3. (A) Schematic representation of experimental approach. ACh secretion from the presynaptic nerve terminal was continuously monitored by whole-cell patch-clamp recordings from the innervated myocyte in spontaneously formed synapses. After establishing a baseline level of synaptic activity, NT-3 was applied to the bath (final concentration 50 ng/ml). The synaptic area was protected from exposure to NT-3 by continuous perfusion of the synaptic contact with a fresh culture medium through a glass micropipette. (B) Changes in the mean SSC frequency with time after the onset NT-3 treatment. Data points represent mean ± SEM of experiments in which NT-3 (filled circles, n = 13) or culture medium without NT-3 (open circles, n = 7) was added to the bath. ∗, P < 0.05. (C–E) Illustration of the degree of protection of the synaptic area from the acridine orange molecules applied to the bath. The pipette (P) was used for continuous perfusion of the synapse with a fresh culture medium (see A). Phase contrast (C) and fluorescent images before (D) and 5 min after (E) application of acridine orange (30 μM). Notice the bright staining of the myocyte (arrowhead) located ≈150 μm away from the protected region. No staining of the myocyte in the shielded region (arrows in C–E) was observed. Similar results were obtained in six different experiments.
There is considerable evidence for the rapid internalization of Trk receptors on activation (34) and retrograde transport of the putative signaling vesicles to the soma (14, 35–37). The exact nature of the retrograde signal carrier, however, remains controversial (38, 39). There are also precedents for the rapid anterograde signaling mediated by neurotrophins (11, 26) and anterograde transport of neurotrophins themselves (40, 41). In our experiments, NT-3-mediated signal may be transmitted from the site of NT-3 application to the presynaptic terminal through the extracellular medium, by lateral diffusion in the axonal plasmalemma or through the axonal cytoplasm. The results of our experiments strongly suggest that the signal spreads through the axonal cytoplasm. Indeed, the synaptic transmission was potentiated after bath application of NT-3 under conditions in which the nerve terminal was protected from exposure to the molecules present in the culture medium (Fig. 5). The simple estimate also excludes mechanisms that are based on the lateral diffusion of the putative messenger in the axonal plasma membrane. Based on these considerations, we speculate that the NT-3-mediated signal propagates through axonal cytoplasm. The signaling appears to be very fast: the onset of synaptic potentiation after perfusion of the soma or the middle axonal segment is delayed by only ≈5 min in comparison to the local perfusion of the synaptic terminal (Fig. 3). Assuming that the signal travels by diffusion in the axonal cytoplasm, the diffusion coefficient of the putative messenger molecule is expected to be ≈L2/t ≈ 300 μm2/s, which is comparable to the diffusion coefficient of small cytoplasmic molecules such as ACh or cAMP (42). Thus, the distances of a few hundred μm appear to be within reach of diffusional transport. However, if the signal is indeed transmitted by diffusion in the axonal cytoplasm, the size of the putative cytoplasmic messenger is unlikely to exceed a few hundred daltons. Alternatively, the signal may travel from the site of NT-3 application to the distal nerve terminal by anterograde axonal transport. Because the rate of a fast axonal transport in Xenopus neurons is ≈3 μm/sec (43), the vesicles may be transported anterogradely to a distance of 300 μm from the site of internalization within 2 min.
Neurotransmitters appear early in developing embryos and may have important functions in the development of the nervous system (10, 44). Neurotransmitter release is generally believed to be localized to the nerve terminal of the presynaptic neuron or to the growth cone region of isolated growing axons (45, 46). However, using myocytes as the sensitive detectors of ACh release, neurotransmitter secretion could be detected not only at the growth cone region of Xenopus spinal cord neurons but along the axon as well (47, 48). This finding is not surprising in view of the reports on constitutive membrane recycling along the entire developing processes of cultured neurons (49–51).
An increase in the spontaneous synaptic activity induced by neurotrophins may have an important role in maturation of the initial contacts between the presynaptic neuron and the target cell (10). In this study, we tested whether NT-3 has a stimulatory effect on the spontaneous ACh release at the middle segments and at the soma. We have shown here that in developing Xenopus neurons, potentiation of spontaneous neurotransmitter secretion by NT-3 is limited to the presynaptic nerve terminal and to the growth cone region. Therefore, middle axonal segment and the cell body are expected to be less competent for establishing functional presynaptic contacts with postsynaptic targets. The molecular mechanisms that limit the potentiating effect of NT-3 on neurotransmitter secretion to synapses remain to be established. It should be noted that the identity of the vesicles responsible for neurotransmitter secretion along the developing axons and their relation to genuine synaptic vesicles is not known. The electrophysiological and pharmacological properties of neurotransmitter secretion at the nerve terminal and along the axons of Xenopus neurons are somewhat different (our unpublished data), suggesting different molecular composition of the ACh-releasing vesicles at the different axonal segments. Therefore, ACh-containing vesicles at the middle axonal segment and at the soma may lack molecular components conferring sensitivity to neurotrophins.
Our results indicate that there is an extensive and rapid propagation of the NT-3-mediated signal within single neuronal cells. Although the phenomena that we observed in cell culture may be an exaggeration of those occurring in vivo, the developing Xenopus neurons in culture provide a simple model to study the basic mechanisms of neurotrophin-mediated signaling within neurons. Further investigation is necessary to determine the molecular basis of the signaling machinery and the mechanisms of signal propagation in more complex neuronal cells with divergent synaptic outputs.
Figure 2.
TrkC immunoreactivity is detected at different neuronal segments. (A–C) Representative examples of neurons stained with antibodies to TrkC (Santa Cruz Biotechnology). The immunofluorescence signal is evident at the soma (A), along the axon (A and B); and at the growth cone (C). (D) Control experiment demonstrating specificity of staining. Preincubation of primary antibodies with the blocking peptide largely abolished the immunofluorescence signal. (E–F) Neurons were stained with antibodies to TrkC (Upstate Biotechnology); this antibody was raised against the extracellular domain of TrkC receptor. The immunofluorescence signal can be detected at the cell body and along the axon (E) and at the distal axon (F).
Figure 4.
“Leakage” of NT-3 from the superfused region does not contribute to the potentiation of ACh secretion at the preformed synapses. (A) Membrane currents recorded from the myocyte in the preformed synapse. NT-3 (200 ng/ml) together with α-bungarotoxin (500 μg/ml) was locally applied to the middle axonal segment as in Fig. 3A. The start of local perfusion is marked by the arrow. The postsynaptic myocyte was ≈400 μm away from the site of drug application. Withdrawal of the pipette used for the removal of the superfused solution (arrow) resulted in the accumulation of α-bungarotoxin in the dish medium and the inhibition of SSC at the preformed synapse. (B) Membrane currents recorded from an innervated myocyte in the preformed synapse. Bath application of α-bungarotoxin (500 ng/ml, marked by arrow) resulted in the decrease in the frequency of SSCs. (C) Membrane currents recorded from the innervated myocyte before bath application of NT-3 application (5 ng/ml, marked by arrow) and for a period of 15–18 min after NT-3 application (right trace). (D) Quantitative analysis of the data. In each experiment, the mean SSC frequency for a period of 15–18 min after the drug application was normalized to the mean SSC frequency before drug application. Each data point represents the mean ± SEM of seven to nine experiments.
Figure 6.
Protein synthesis and signaling to the soma are not required for the acute potentiation of ACh release induced by NT-3. (A) Pretreatment with anisomycin (40 μM) or with cycloheximide (10 μM) for 1 h before bath application of NT-3 does not prevent rapid potentiation of ACh release at spontaneously formed synapses. Changes in the SSC frequency with time after the onset of NT-3 treatment. Each data point represents the mean ± SEM of five experiments. ∗, P < 0.01. (B) Potentiation of ACh release at the distal axonal fragments. The axon was transected in the vicinity of the cell body (Insert) and the SSC frequency was measured. Bath application of NT-3 (marked by arrow) resulted in a characteristically rapid potentiation of ACh release from the distal axonal fragments innervating muscles. Each data point is a mean ± SEM of five experiments. ∗, P < 0.01.
Acknowledgments
We are grateful to Regeneron Pharmaceuticals for generously providing NT-3. This work was supported by National Institutes of Health Grant NS 33570.
ABBREVIATIONS
- NT-3
neurotrophin 3
- BDNF
brain-derived neurotrophic factor
- SSC
spontaneous synaptic current
- ACh
acetylcholine
- Trks
neurotrophin receptors
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
References
- 1.Oppenheim R W. Annu Rev Neurosci. 1991;14:453–501. doi: 10.1146/annurev.ne.14.030191.002321. [DOI] [PubMed] [Google Scholar]
- 2.Bothwell M. Annu Rev Neurosci. 1995;18:223–253. doi: 10.1146/annurev.ne.18.030195.001255. [DOI] [PubMed] [Google Scholar]
- 3.Purves, D., Augustine, G. J., Fitzpatrick, D., Katz, L. C., LaMantia, A.-S. & McNamara, J. O. (1997) Sunderland, MA: Sinauer.
- 4.Lohof A M, Ip N Y, Poo M-m. Nature (London) 1993;363:350–353. doi: 10.1038/363350a0. [DOI] [PubMed] [Google Scholar]
- 5.Kang H, Schuman E M. Science. 1995;267:1658–1662. doi: 10.1126/science.7886457. [DOI] [PubMed] [Google Scholar]
- 6.Gall C M, Isackson P J. Science. 1989;245:758–761. doi: 10.1126/science.2549634. [DOI] [PubMed] [Google Scholar]
- 7.Lu B, Yokoyama M, Dreyfus C F, Black I B. Proc Natl Acad Sci USA. 1991;88:6289–6292. doi: 10.1073/pnas.88.14.6289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Canossa M, Griesbeck O, Berninger B, Campana G, Kolbeck R, Thoenen H. Proc Natl Acad Sci USA. 1997;94:13279–13286. doi: 10.1073/pnas.94.24.13279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lo D C. Neuron. 1995;15:979–981. doi: 10.1016/0896-6273(95)90085-3. [DOI] [PubMed] [Google Scholar]
- 10.Thoenen H. Science. 1995;270:593–598. doi: 10.1126/science.270.5236.593. [DOI] [PubMed] [Google Scholar]
- 11.Berninger B, Poo M-m. Curr Opin Neurobiol. 1996;6:324–330. doi: 10.1016/s0959-4388(96)80115-2. [DOI] [PubMed] [Google Scholar]
- 12.Bonhoeffer T. Curr Opin Neurobiol. 1996;6:119–126. doi: 10.1016/s0959-4388(96)80017-1. [DOI] [PubMed] [Google Scholar]
- 13.Bartheld C S v, Williams R, Lefcort F, Clary D O, Reichardt L F, Bothwell M. J Neurosci. 1996;16:2995–3008. doi: 10.1523/JNEUROSCI.16-09-02995.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ehlers M, Kaplan D, Price D, Koliatsos V. J Cell Biol. 1995;130:149–156. doi: 10.1083/jcb.130.1.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Segel R, Greenberg M. Annu Rev Neurosci. 1996;19:463–489. doi: 10.1146/annurev.ne.19.030196.002335. [DOI] [PubMed] [Google Scholar]
- 16.Greene L A, Kaplan D R. Curr Opin Neurobiol. 1995;5:579–587. doi: 10.1016/0959-4388(95)80062-x. [DOI] [PubMed] [Google Scholar]
- 17.Wang X-h, Berninger B, Poo M-m. J Neurosci. 1998;18:4985–4992. doi: 10.1523/JNEUROSCI.18-13-04985.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stoop R, Poo M-m. J Neurosci. 1996;16:3256–3264. doi: 10.1523/JNEUROSCI.16-10-03256.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang X-h, Poo M-m. Neuron. 1997;19:825–835. doi: 10.1016/s0896-6273(00)80964-2. [DOI] [PubMed] [Google Scholar]
- 20.Spitzer N C, Lamborghini J C. Proc Natl Acad Sci USA. 1976;73:1641–1645. doi: 10.1073/pnas.73.5.1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Anderson M J, Cohen M W, Zorychta E. J Physiol (London) 1977;268:731–758. doi: 10.1113/jphysiol.1977.sp011879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hamill O P, Marty A, Neher E, Sakmann B, Sigworth F J. Pflügers Arch. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- 23.Girod R, Popov S, Alder J, Zheng J Q, Lohof A, Poo M-m. J Neurosci. 1995;15:2826–2838. doi: 10.1523/JNEUROSCI.15-04-02826.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morimoto T, Popov S, Buckely K, Poo M-m. Neuron. 1995;15:689–696. doi: 10.1016/0896-6273(95)90156-6. [DOI] [PubMed] [Google Scholar]
- 25.Popov S V, Brown A, Poo M-m. Science. 1993;259:244–246. doi: 10.1126/science.7678471. [DOI] [PubMed] [Google Scholar]
- 26.Stoop R, Poo M-m. Science. 1995;267:695–699. doi: 10.1126/science.7839148. [DOI] [PubMed] [Google Scholar]
- 27.Adams M E, Olivera B M. Trends Neurosci. 1994;17:151–155. doi: 10.1016/0166-2236(94)90092-2. [DOI] [PubMed] [Google Scholar]
- 28.Bonhoeffer T, Staiger V, Aertsen A. Proc Natl Acad Sci USA. 1989;86:8113–8117. doi: 10.1073/pnas.86.20.8113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schuman E M, Madison D V. Science. 1994;263:532–536. doi: 10.1126/science.8290963. [DOI] [PubMed] [Google Scholar]
- 30.Cash S, Zucker R S, Poo M-m. Science. 1996;272:998–1001. doi: 10.1126/science.272.5264.998. [DOI] [PubMed] [Google Scholar]
- 31.Fitzimonds R M, Song H-j, Poo M-m. Nature (London) 1997;388:439–448. doi: 10.1038/41267. [DOI] [PubMed] [Google Scholar]
- 32.Song H-j, Ming G-I, Poo M-m. Nature (London) 1997;388:275–279. doi: 10.1038/40864. [DOI] [PubMed] [Google Scholar]
- 33.Song H-j, Ming G-I, He Z, Lehmann M, McKerracher L, Tessier-Lavigne M, Poo M-m. Science. 1998;281:1515–1518. doi: 10.1126/science.281.5382.1515. [DOI] [PubMed] [Google Scholar]
- 34.Hosnag M, Shooter E M. EMBO J. 1987;6:1197–1202. doi: 10.1002/j.1460-2075.1987.tb02354.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Grimes M L, Zhou J, Beattie E C, Yuen E C, Hall D E, Valletta J S, Topp K S, LaVail J H, Bunnett N W, Mobley W C. J Neurosci. 1996;16:7950–7964. doi: 10.1523/JNEUROSCI.16-24-07950.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Riccio A, Pierchala B A, Ciarallo C L, Ginty D D. Science. 1997;277:1097–1100. doi: 10.1126/science.277.5329.1097. [DOI] [PubMed] [Google Scholar]
- 37.Bhattacharyya A, Watson F L, Bradlee T A, Pomeroy S L, Stiles C H, Segel R A. J Neurosci. 1997;17:7007–7016. doi: 10.1523/JNEUROSCI.17-18-07007.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Senger D L, Campenot R B. J Cell Biol. 1997;138:411–421. doi: 10.1083/jcb.138.2.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Finkbeiner S, Tavazoie S F, Maloratsky A, Jacobs K M, Harris K M, Greenberg M E. Neuron. 1997;19:1031–1047. doi: 10.1016/s0896-6273(00)80395-5. [DOI] [PubMed] [Google Scholar]
- 40.Altar C A, Cai N, Bliven T, Juhasz M, Conner J M, Acheson A L, Lindsay R M, Wiegand S J. Nature (London) 1998;389:856–860. doi: 10.1038/39885. [DOI] [PubMed] [Google Scholar]
- 41.Tonra J R, Curtis R, Wong V, Cliffer K D, Park J S, Timmes A, Nguyen T, Lindsay R M, Acheson A, DiStefano P S. J Neurosci. 1998;18:4374–4383. doi: 10.1523/JNEUROSCI.18-11-04374.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Popov S V, Poo M-m. J Neurosci. 1992;12:77–85. doi: 10.1523/JNEUROSCI.12-01-00077.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zakharenko S, Popov S V. J Cell Biol. 1998;143:1077–1086. doi: 10.1083/jcb.143.4.1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lo Y J, Lin Y C, Sanes D H, Poo M M. Science. 1991;254:1019–1022. doi: 10.1126/science.1658939. [DOI] [PubMed] [Google Scholar]
- 45.Chow I, Poo M-m. J Neurosci. 1985;5:1076–1082. doi: 10.1523/JNEUROSCI.05-04-01076.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liou J-C, Fu W-M. J Neurosci. 1997;17:2459–2468. doi: 10.1523/JNEUROSCI.17-07-02459.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Evers J, Lasek M, Sun Y, Xie Z, Poo M-m. J Neurosci. 1989;9:1523–1539. doi: 10.1523/JNEUROSCI.09-05-01523.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Antonov, I., Chang, S., Zakharenko, S. & Popov, S. V. (1999) Neuroscience, in press. [DOI] [PubMed]
- 49.Matteoli M, Takei K, Perin M S, Sudhof T C, De Camilli P. J Cell Biol. 1992;117:849–861. doi: 10.1083/jcb.117.4.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kraszewski K, Mundigl O, Daniell L, Verderio C, Matteoli M, De Camilli P. J Neurosci. 1995;15:4328–4342. doi: 10.1523/JNEUROSCI.15-06-04328.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dai Z, Peng H B. Mol Cell Neurosci. 1996;7:443–452. doi: 10.1006/mcne.1996.0032. [DOI] [PubMed] [Google Scholar]