Abstract
Using data from over 1,000 male and female twins participating in the Minnesota Twin Family Study, the authors examined developmental change, gender differences, and genetic and environmental contributions to the symptom levels of four externalizing disorders (adult antisocial behavior, alcohol dependence, nicotine dependence, and drug dependence) from ages 17 to 24. Both men and women increased in symptoms for each externalizing disorder, with men increasing at a greater rate than women, such that a modest gender gap at age 17 widened to a large one at age 24. Additionally, a mean-level gender difference on a latent Externalizing factor could account for the mean-level gender differences for the individual disorders. Biometric analyses revealed increasing genetic variation and heritability for men but a trend toward decreasing genetic variation and increasing environmental effects for women. Results illustrate the importance of gender and developmental context for symptom expression and the utility of structural models to integrate general trends and disorder-specific characteristics.
Keywords: externalizing disorders, gender differences, heritability substance use disorders, antisocial behavior
Antisocial behavior and substance use disorders, also referred to collectively as externalizing disorders, constitute major public health and safety problems that exact a costly toll on societal resources (Cohen, Miller, & Rossman, 1994; Miller, Cohen, & Wiersema, 1996; Rice, 1999; Room, Babor, & Rehm, 2005). Epidemiological research on externalizing disorders has identified three notable patterns. First, antisocial behavior and substance use disorders are characterized by high rates of comorbidity—that is, co-occurrence at greater than chance levels (Kessler et al., 1994; Newman et al., 1996). Second, men exhibit higher prevalence rates of externalizing disorders than women (Kessler et al., 1994; Newman et al., 1996). Third, the prevalence of externalizing disorders increases throughout late adolescence and peaks in early adulthood, followed by a steady decline through the remainder of the life span (Bachman, Wadsworth, O’Malley, Johnston, & Schulenberg, 1997; Chassin, Flora, & King, 2004; Chen & Kandel, 1995; Harford, Grant, Yi, & Chen, 2005; Harpur & Hare, 1994; Jackson, Sher, & Wood, 2000; Moffitt, 1993; Moffitt, Caspi, Rutter, & Silva, 2001; Sher & Gotham, 1999). In this investigation, we examine the utility of the externalizing (EXT) spectrum model to provide a theoretical framework to organize these fundamental observations regarding externalizing disorders.
There is a long precedent to conceptualizing disruptive, aggressive, and rule-breaking behaviors as indicators of a common disposition to act out, most widely recognized as the externalizing dimension of child psychopathology (Achenbach & Edelbrock, 1984). Jessor and Jessor (1977; Jessor, Donovan, & Costa, 1991) extended this model of a general disposition to engage in nonnormative behavior to adolescence by incorporating developmentally appropriate indicators, such as substance use and precocious sexual behavior. Others researchers have proposed etiological models that posit the interplay among various genetic, biological, environmental, and developmental factors to account for the co-occurrence among different forms of disinhibitory behavior (Fowles & Kochanksa, 2000; Gorenstein & Newman, 1980; Patterson & Newman, 1993). More recently, Krueger et al. (2002; Krueger, Markon, Patrick, Benning, & Kramer, 2006; Krueger, Markon, Patrick, & Iacono, 2005) proposed an EXT spectrum model to account for the high rates of comorbidity among disorders defined by the Diagnostic and Statistical Manual of Mental Disorders (3rd ed., rev; DSM–III–R; American Psychiatric Association, 1987), including conduct disorder (CD), antisocial personality disorder (ASPD), alcohol dependence, nicotine dependence, and illicit drug dependence. The EXT spectrum model posits that a general liability to all these disorders underlies their comorbidity, such that a person who is high on this general liability is more likely to express multiple disorders.
Several investigations using confirmatory factor analysis to model the comorbidity among externalizing disorders in large epidemiological samples have reported results consistent with the EXT spectrum model (Krueger, 1999; Krueger, Caspi, Moffitt, & Silva, 1998; Vollebergh et al., 2001). Furthermore, twin studies have estimated the heritability of this general EXT liability at roughly .80—that is, the comorbidity among the disorders is primarily a function of common genetic risk factors (Kendler, Prescott, Myers, & Neale, 2003; Krueger et al., 2002; Young, Stallings, Corley, Krauter, & Hewitt, 2000). Additionally, a recent twin-family study extended this finding by demonstrating that familial resemblance between parents and offspring on externalizing disorders could be accounted for by the transmission of the general EXT vulnerability rather than the transmission of disorder-specific vulnerabilities (Hicks, Krueger, Iacono, McGue, & Patrick, 2004). Finally, statistical models have been used to investigate the continuous versus categorical nature of the expression and co-occurrence of externalizing disorders, with a model that conceptualized individual disorders as a normally distributed liability continuum providing the best fit to the data (Krueger et al., 2005; Markon & Krueger, 2005).
Gender Differences
To date, no study has tested the utility of the EXT spectrum model to account for the large gender difference in the prevalence rates of externalizing disorders, with an average male-to-female ratio of 2.5:1 (Kessler et al., 1994; Newman et al., 1996). This gender gap is evident in childhood precursors of externalizing disorders such as CD, oppositional defiant disorder, and attention-deficit/hyperactivity disorder (King, Iacono, & McGue, 2004; Lynskey & Fergusson, 1995; Moffitt et al., 2001); narrows during adolescence, in particular around the onset of menarche (Moffitt et al., 2001; Windle, 1990); then increases throughout late adolescence and early adulthood as the prevalence of antisocial behavior and substance use disorders reaches its zenith (Bachman et al., 1997; Chassin et al., 2004; Chen & Kandel, 1995; Harford et al., 2005; Harpur & Hare, 1994; Jackson et al., 2000; Moffitt, 1993; Moffitt et al., 2001; Sher & Gotham, 1999).
A tenable hypothesis is that a gender difference in mean levels of the general EXT liability underlies the gender difference in mean levels (i.e., prevalence rates) of individual externalizing disorders. If verified, this hypothesis would suggest that the emergence of gender differences is due to risk factors operating at the general vulnerability level rather than the individual syndrome level and so would encourage a shift from examining gender differences at the disorder level to examining them at the general factor level. This hypothesis can be tested with newer confirmatory factor models that incorporate the means of the individual disorders into the model, which then provides the capability to estimate means on the latent EXT liability (Muthén & Muthén, 2003). More generally, the analytic framework of factorial invariance in the context of multiple-groups confirmatory factor analysis can be used to test hypotheses regarding the degree of generalizability of a construct across groups—for example, across men and women (Meredith, 1993). The increasingly restrictive (i.e., parsimonious) levels of factorial invariance then have certain psychological interpretations or inferences (see the Method section) that can assist in theory building used to organize key epidemiological observations regarding externalizing disorders. In addition to gender differences, these models can also be used to examine the normative increases in symptoms of externalizing disorders from late adolescence to early adulthood.
Developmental Change
The transition from late adolescence to early adulthood has traditionally been referred to as a period of “storm and stress” characterized by major demographic changes and relatively high levels of psychological distress (Hall, 1904). More recent conceptualizations of emerging adulthood now recognize individual differences in this transitory period, in particular that many young people navigate this period with relatively little difficulty (Arnett, 1999). That being said, the transition into adulthood continues to entail many closely spaced, important life tasks, such as leaving the family of origin, obtaining higher education or job training, and beginning a career and a family, as well as ongoing psychological processes of identity formation and consolidation (Arnett, 2000; Erikson, 1963).
Perhaps as a consequence of this potentially turbulent period, there is also a precipitous increase in the prevalence of both internalizing and externalizing disorders that begins in mid-adolescence and peaks in early adulthood (Kessler et al., 1994; Kessler, Chiu, Demler, & Walters, 2005; Newman et al., 1996). It is notable that gender moderates this developmental increase in psychopathology. For example, a large gender gap in the prevalence rates of major depression emerges in mid-adolescence, primarily as a result of a dramatic spike in the prevalence rate for women (Hankin et al., 1998). Alternatively, the large gender gap in alcohol dependence emerges in late adolescence, primarily because of large symptom increases in men (Newman et al., 1996). Given these changes and their association with gender, delineating the developmental changes that occur during the transition from late adolescence to early adulthood has the potential to be especially useful in understanding the etiological mechanisms underlying externalizing disorders.
A multifaceted analytic approach is necessary to gain a full understanding of developmental change and stability. One approach is to examine mean-level or normative change, which refers to change at the population level. Another approach is to explore individual-level change, which refers to the number of individuals who exhibit a reliable and clinically significant (i.e., too large to be attributed to measurement error or regression to the mean) change in the frequency of a given behavior over time. Another approach is to examine relative stability, which refers to the stability of the rank ordering of individuals across time. Last, there is change in variances, which indexes the change in the magnitude of individual differences for a particular behavioral phenotype over time.
Each index of change provides additional information about the developmental processes associated with a given phenotype. Taking alcohol use as an example, we might find that for a given cohort of individuals from late adolescence to early adulthood, the average number of drinks consumed a week increases (mean-level change), a large number of individuals exhibit a significant increase in their weekly consumption of alcohol (individual-level change), individuals who tended to drink the most as teenagers continue to drink the most as young adults (rank-order stability), and individuals exhibit a greater diversity in their pattern (i.e., quantity and frequency) of weekly consumption of alcohol (variance change). One gap in the literature regarding the natural history of externalizing disorders is a comprehensive treatment of the developmental trends exhibited by the general EXT liability. That is, there remains a need to estimate the magnitude of mean-level change, variance change, and rank-order stability for the general EXT liability as well as any moderating effects resulting from gender.
Behavior Genetics
Behavior genetic designs can also contribute to our understanding of developmental processes by delineating the underlying etiological structure of phenotypes—that is, the contribution of genes and environments to individual differences. By examining the genetic and environmental architecture of a phenotype, we can gain an important understanding of its developmental dynamics. For example, if the variance increases, is this due to greater genetic or environmental variability over time? How stable are the genetic and environmental effects across time, and what are the genetic and environmental contributions to stability in the phenotype?
A substantial body of research supports the heritability of externalizing disorders (Ball & Collier, 2002; Heath, 1995; Kendler, Heath, Neale, Kessler, & Eaves, 1992; Kendler, Neale, Sullivan, Corey, & Gardner, 1999; Lynskey et al., 2002; McGue, Elkins, & Iacono, 2000; Prescott & Kendler, 1999; Rhee & Waldman, 2002; True et al., 1999; Tsuang et al., 1996). Developmental theories that take a behavioral genetic perspective generally posit that the heritability of phenotypes tends to increase, whereas shared environmental effects decrease with age (Scarr & McCartney, 1983). This shift is believed to be due to active and evocative gene–environment correlational processes, such that heritability increases as individuals take a greater role in selecting and shaping their environments, which tend to be congruent with their genotype (Scarr & McCartney, 1983). Some empirical evidence supports this model, in particular for the general EXT liability. For example, investigators have reported a large shared environmental contribution to the common variance among childhood externalizing disorders (CD, oppositional defiant disorder, attention-deficit/hyperactivity disorder; Burt, Krueger, McGue, & Iacono, 2001) and adolescent problem behavior (rule breaking, smoking, alcohol and drug use, early sexual behavior; McGue, Iacono, & Krueger, 2006), whereas the heritability of the common variance among adult externalizing disorders has been estimated to be much higher, with little or no shared environmental contribution (Kendler et al., 2003; Krueger et al., 2002; McGue et al., 2006).
Behavior genetic designs can also help inform gender differences in externalizing disorders. That is, if the genetic and environmental architecture for a given externalizing disorder differs across gender, it suggests differential causal mechanisms for men and women. Most behavior genetic studies of externalizing disorders have failed to detect significant gender differences in heritability (e.g., Kendler et al., 2003; Krueger et al., 2002; McGue et al., 2006). However, there are some exceptions. For example, the heritability of alcohol dependence is consistently found to be roughly .50 in men, with little or no shared environmental contribution. Results for women, however, are less consistent, with treatment-seeking samples evincing significant shared environmental contributions and low heritability (McGue, Pickens, & Svikis, 1992; Prescott et al., 2005), whereas population-based samples yield results similar to those for men (Heath et al., 1997; Kendler et al., 1992). Although the reasons for this discrepancy are not fully understood, it raises the possibility that the emergence of alcohol dependence in women is more contingent on environmental factors than is the case for men.
Goals of the Current Study
For the present study, we used a sample of over 1,000 twins to examine gender differences and developmental change from late adolescence to early adulthood on four externalizing disorders: adult antisocial behavior, alcohol dependence, nicotine dependence, and drug dependence. We had several aims, including the following:
Estimate the magnitude of mean-level, individual-level, and rank-order stability for the four externalizing disorders from ages 17 to 24.
Use multiple-groups confirmatory factor analysis to test whether a mean-level gender difference on the latent EXT liability can account for mean-level gender differences on the specific disorders.
Estimate the genetic and environmental contributions to individual differences in externalizing disorders at ages 17 and 24, and test for any differences in heritability across time and gender.
Method
Participants
Participants were members of the 626 male and female twin pairs (men: monozygotic [MZ], n = 188, dizygotic [DZ], n = 101; women: MZ, n = 223, DZ, n = 114) that constitute the older cohort of the Minnesota Twin Family Study (MTFS), a longitudinal–epidemiological study investigating the development of substance dependence and related disorders. A comprehensive description of the goals, design, and sample characteristics of the MTFS can be found in previous reports (Iacono, Carlson, Taylor, Elkins, & McGue, 1999; Iacono, Malone, & McGue, 2003). Public birth records were used to identify twin births in Minnesota from January 1, 1972, to December 31, 1979, with over 90% of twin families located for each birth year. Families were recruited into the study the year the twins turned 17 years old, with 17% of families declining participation. Comparisons between participating and nonparticipating families on demographic, socioeconomic, and mental health measures showed that participating families were slightly better educated (approximately 0.3 more years of education) but that the groups did not differ in self-reported mental health. The twins were then provided the opportunity to participate in follow-up assessments at ages 20 and 24. Data were available for 1,014 twins for all analyses that required complete data at both the age 17 intake assessment (M = 17.5 years, SD = 0.5) and the age 24 follow-up assessment (M = 24.6 years, SD = 1.0). This listwise sample consisted of 465 twin pairs (230 male and 235 female pairs) and 84 unmatched twins (34 men and 50 women). Participants with complete data at both the intake and the follow-up assessments did not differ significantly from participants with incomplete data in terms of mean number of symptoms at age 17 for any of the disorders of interest (Cohen’s d = −.01 to −.14). Consistent with the demographics of Minnesota during the years the twins were born, 98% of the participants were Caucasian. All participants gave informed assent or consent, as appropriate for the age of their assessment, and all study protocols were reviewed by an internal review board.
Assessment
At the age 17 intake assessment, lifetime symptoms of adult antisocial behavior and substance use disorders were assessed in person at our Minneapolis laboratories via structured clinical interviews administered by trained interviewers who held either a bachelor’s or a master’s degree in psychology. For the age 24 assessment, participants reported on symptoms that were present since their last assessment (either the age 20 follow-up or the age 17 intake assessment). For the current analyses, symptoms at age 24 refer to all symptoms reported over the 7-year period since the age 17 intake assessment (i.e., symptoms were combined for the age 20 and age 24 follow-up assessments). This strategy provided a measure of participants’ maximal number of externalizing symptoms through early adulthood, as the vast majority of participants should have reached or passed their period of peak symptom expression by age 24. In addition to the 1,014 participants with complete data at each time point, a subsample (n = 167) completed an abbreviated telephone assessment at age 24 that covered the substance use disorders but not adult antisocial behavior. Compared with the listwise sample, this subsample exhibited only minimal differences in mean number of externalizing disorder symptoms at age 17 (d = −.03 to −.17) but did exhibit, on average, slightly fewer substance use disorder symptoms at age 24 (d = −.18 to −.34).
Symptoms of DSM–III–R (American Psychiatric Association, 1987) nicotine, alcohol, and drug dependence were assessed with the Substance Abuse Module of the Composite International Diagnostic Interview (Robins, Baber, & Cottler, 1987). The drug assessment covered amphetamines, cannabis, cocaine, hallucinogens, inhalants, opioids, PCP, and sedatives. The drug class for which a participant reported the most symptoms was used as his or her number of drug dependence symptoms. As is the convention for the DSM–III–R, abuse symptoms were included in the symptom tally for the alcohol and drug dependence symptom count variables. DSM–III–R symptoms of adult antisocial behavior (i.e., the adult criteria for ASPD) were assessed with a structured interview designed by MTFS staff that is comparable to the Structured Clinical Interview for Axis II Personality Disorders (First, Gibbon, Spitzer, & Williams, 1997). For our analyses, only the adult symptoms of ASPD (present at age 15 or older) were used, as the childhood symptoms (i.e., CD) are not developmentally appropriate at age 24 and it was necessary to maintain a common metric across time to assess any normative changes.
All interview data were reviewed (with reference to audio tapes of the interview when necessary) in a case conference consisting of at least two clinical psychology graduate students with extensive training in descriptive psychopathology and differential diagnosis. Consensus between the diagnosticians regarding the presence or absence of symptoms was reached prior to symptom assignment. The consensus process yielded diagnostic kappa reliabilities of .95 for adult antisocial behavior and of more than .91 for each substance disorder. We used symptom counts rather than dichotomous diagnoses as this is more consistent with our dimensional conceptualization of externalizing psychopathology and to increase statistical power, as the diagnostic prevalence of certain disorders was not high (although it was comparable to that of other epidemiological samples; cf. Kessler et al., 1994; Newman et al., 1996), given that the MTFS is a community rather clinically referred sample.
Data Analysis
Gender differences and developmental change for individual disorders
We conducted three separate levels of analysis (cf. Roberts, Caspi, & Moffitt, 2001) to assess development change on the four externalizing disorders: mean-level change, individual-level change, and rank-order stability. Mean-level change refers to the magnitude of change in the average number of symptoms over time for a given population. In addition to mean-level change over time, we also examined mean-level gender differences at ages 17 and 24. Mean-level analyses were conducted with a two-factor repeated measures analysis of variance with time as the within-subject factor and sex as the between-subjects factor. Linear mixed models fitted in SAS PROC MIXED were used to adjust all statistics for the nonindependence of the twin observations.
Individual-level change refers to the number of persons who exhibit a clinically significant change in the number of their symptoms that cannot be accounted for solely by measurement error or regression to the mean (i.e., as a result of chance). Individual-level change is typically assessed with the reliable change index (RCI; Christensen & Mendoza, 1986; Jacobson & Traux, 1991). The RCI is the difference in an individual’s number of symptoms from Time 1 to Time 2 (X2 − X1) divided by the standard error of the difference between the two scores (Sdiff). The Sdiff is calculated via the standard error of measurement of the symptom count variables at Time 1 and Time 2 (Sdiff = √ [(SEMT1)2 + (SEMT2)2]) and represents the distribution of change scores that would be expected if no actual change had occurred. If we assume that change scores follow a normal distribution, persons with RCI values of ± 1.96 are considered to have experienced reliable or clinically significant change—that is, the change score has a significance value less than .05. Therefore, if individual-level change were solely due to measurement error or regression to the mean, only 5.0% of the sample would exhibit RCI scores greater than 1.96 (2.5%) or less than −1.96 (2.5%).
Unfortunately, because of the ordinal nature and positive skew of the distributions of symptom count variables in community samples, the RCI is a problematic index of individual-level change. That is, RCI values for symptom counts lack sensitivity (because of a limited number of possible values) and are biased to detect increases rather than decreases in symptoms (because of a floor effect). Therefore, we attempted to link RCI values to a clinically meaningful benchmark. For the substance use disorders, the necessary RCI value indicative of reliable change was equivalent to a change in two symptoms: one less than necessary for a diagnosis and indicative of problematic, if not diagnosable, behavior. For consistency across externalizing disorders, a change of two symptoms was also necessary to be considered a reliable and clinically significant change for adult antisocial behavior.
Our final index of developmental change was rank-order stability, which refers to the consistency of the relative ordering of individuals in a population over time. This measure provides an index of the extent to which persons maintain their relative position in a group over time. Rank-order stability was assessed via the 7-year test–retest correlation coefficients for the four symptom count variables. Significance levels were adjusted with linear mixed models in SAS to account for the nonindependence of the twin observations.
EXT spectrum model of gender differences and developmental change
We tested a series of increasingly restrictive (i.e., nested) models of factorial invariance to better conceptualize (a) the developmental course of the four externalizing disorders from late adolescence to early adulthood and (b) the nature of any observed gender differences. If the more parsimonious models are able to provide an adequate fit to the data, then certain psychological interpretations can be made regarding the nature of gender differences and longitudinal change and stability of externalizing disorders.
Configural invariance is the first level of factorial invariance and stipulates that the same factor structure must be present in men and women and across time. Previous reports have demonstrated that, for men and women, a one-factor model can best account for the covariance among the multiple externalizing disorders (Kendler et al., 2003; Krueger, 1999; Krueger et al., 1998, 2002, 2005; Vollebergh et al., 2001; Young et al., 2000). Therefore, configural invariance was used to examine the nature of rank-order stability of the externalizing disorders. That is, can stability in each disorder be accounted for by stability in the general EXT liability alone, or are disorder-specific effects needed to account for the stability of certain disorders? Also, is the nature of developmental stability (both general and specific) the same across genders?
Metric invariance is the next level of factorial invariance and stipulates that the factor loadings be the same across men and women and across time. The interpretation of metric invariance is that the pattern of correlations (i.e., pattern of comorbidity) among the four externalizing disorders is the same across genders and across time.
Strong invariance is the next level of factorial invariance and includes the additional constraint that the intercepts (parameters corresponding to the means) of the four externalizing disorders be the same across genders but allows the means of the general EXT liability to differ across genders. If strong invariance is present (i.e., the more restrictive model provides an adequate fit to the data), the substantive interpretation is that the gender differences in the mean number of symptoms of the individual disorders can be attributed to a mean-level gender difference in the general EXT liability.
Strict invariance is the most stringent form of factorial invariance and adds the final constraint that the residual variances be the same across groups. For strict invariance to be present, each externalizing disorder must exhibit comparable variability across genders, so that the portion of variance not accounted for by the general EXT liability (i.e., the residual variance) is the same for men and women.
Model fitting was conducted in Mplus 3 (Muthén & Muthén, 2003) via a maximum-likelihood estimator with robust standard errors, which adjusts model fit for the nonnormal distributions of the symptom count variables and adjusts the standard errors of the parameter estimates for the nonindependence of the twin observations. This maximum-likelihood estimator requires complete data for all cases. The fit of models was evaluated with an adjusted chi-square fit statistic (Muthén & Muthén, 2003), the root-mean-square error of approximation (RMSEA; Browne & Cudeck, 1993), and the Bayesian information criterion (BIC = χ2 − df ln N, where N = number of twin pairs plus number of singletons; Raftery, 1995). The adjusted chi-square provides an overall estimate of model fit for nonnormal data. The RMSEA provides an estimate of discrepancy in model fit per degree of freedom and is also an index of overall model fit. RMSEA values less than .08 indicate an adequate fit to the data, and values less than .05 indicate a very good fit to the data. The BIC balances overall model fit and model complexity and favors the model that incorporates fewer parameters given two models that yield an equivalent overall fit to the data (i.e., chi-square value). In comparison of models, BIC differences of 0–2 are considered weak evidence in support of the model with the lower BIC value, differences of 2–6 are considered positive evidence, differences of 6–10 are considered strong evidence, and differences over 10 are considered very strong evidence (Raftery, 1995). Because models of factorial invariance are increasingly restrictive, we sought to identify the most parsimonious model (i.e., lowest BIC value) that still maintained a good overall fit to the data (i.e., a low RMSEA value).
Biometric analysis
We used a classical twin design and standard biometric modeling to examine the genetic and environmental contributions to change and stability in symptoms of externalizing disorders. Twin methodology assumes that the variance of an observed phenotype can be decomposed into additive genetic (A, i.e., the genetic effect due to the summation of genes across loci), shared environmental (C), and nonshared environmental (E) variance components. Shared environmental variance is due to environmental effects that contribute to twin similarity, whereas non-shared environmental variance is due to environmental effects that contribute to differences between members of a twin pair. Non-shared environmental variance also includes variance due to measurement error. Because MZ twins share 100% of additive genetic effects, whereas DZ twins share only 50%, and because shared environmental effects are assumed to affect the two types of twins equally, the genetic and environmental variance components can be estimated through comparison of the similarity of MZ and DZ twins. That is, greater MZ relative to DZ twin similarity is indicative of genetic effects, whereas comparable MZ and DZ similarity is indicative of shared environmental effects. Differences between MZ twins are indicative of nonshared environmental effects. For the current analyses, we sought only to estimate the main effects of genes and environments on the observed phenotypes, so we make the additional standard assumptions of no gene–environment correlations or interactions and no assortative mating.
Longitudinal biometric models were fitted for each externalizing disorder symptom count variable as well as for the latent EXT liability. For each externalizing disorder, a standard Cholesky decomposition (Figure 1) was used to parse its phenotypic variance into separate additive genetic, shared environmental, and nonshared environmental components at ages 17 and 24. Stability in the genetic and environmental variance components was estimated via correlations between the additive genetic (rA), shared environmental (rC), and nonshared environmental (rE) variance components at ages 17 and 24. This model allowed us to estimate the genetic and environmental contributions at each time point, test whether these effects differed across time, and estimate their longitudinal stability. For the latent EXT liability, we fitted a two-factor biometric model in which genetic and environmental contributions to the common latent factor variance were estimated at ages 17 and 24 (Figure 2). Model parameters were initially estimated separately for men and women, then were constrained to be equal to test for any significant differences in the relative genetic and environmental contributions to the phenotypic variance (i.e., differences in heritability).
Figure 1.
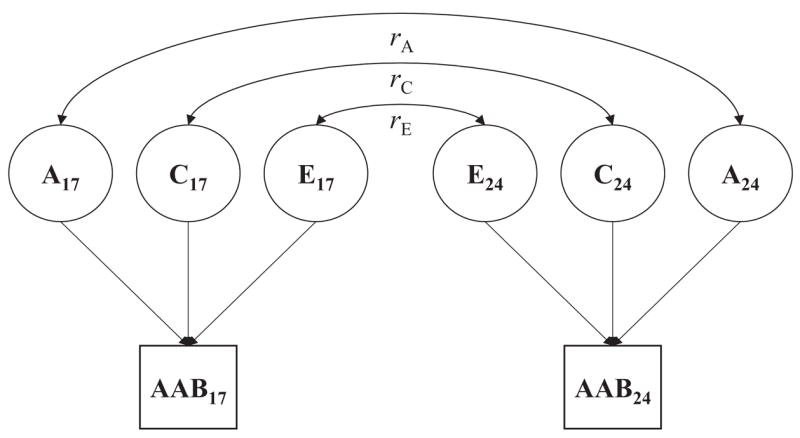
Longitudinal biometric model fitted separately for the symptom count of each externalizing disorder. Longitudinal stability is modeled in terms of the stability of additive genetic (rA), shared environmental (rC), and nonshared environmental effects (rE). Subscripts refer to age of assessment in years. A = additive genetic variance component; C = shared environmental variance component; E = nonshared environmental variance component; AAB = adult antisocial behavior.
Figure 2.
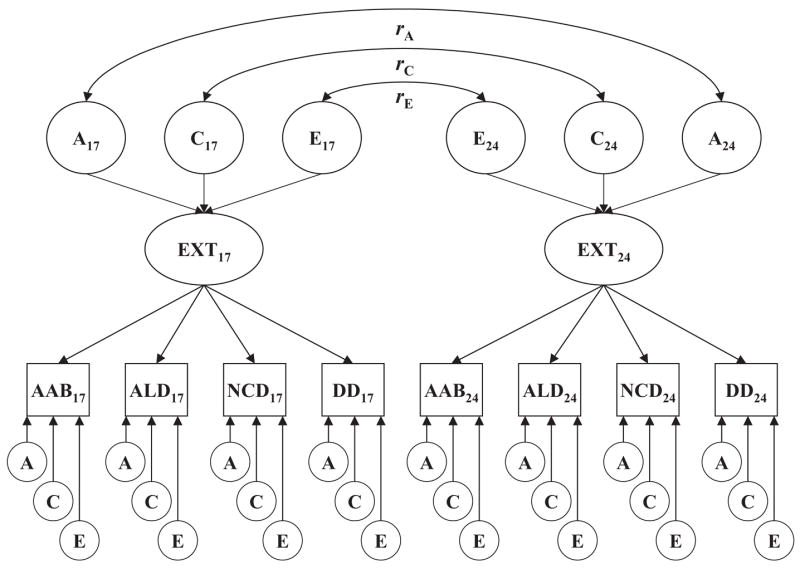
Longitudinal biometric factor model fitted simultaneously to the symptom counts of the four externalizing disorders at ages 17 and 24. Longitudinal stability is modeled in terms of the stability of additive genetic (rA), shared environmental (rC), and nonshared environmental effects (rE) at the factor level. Subscripts refer to assessment age in years. A = additive genetic variance component; C = shared environmental variance component; E = nonshared environmental variance component; EXT = latent Externalizing factor; AAB = adult antisocial behavior; ALD = alcohol dependence; NCD = nicotine dependence; DD = drug dependence.
Model fitting analyses were performed with the computer program Mx (Neale et al., 2002). Models were fitted to the raw data via full information maximum-likelihood estimation, which allows for missing data and adjusts parameter estimates to account for the reduced precision resulting from incomplete data. All symptom count variables were log(x + 1) transformed to better approximate normality, as the fit indexes provided by Mx do not adjust for the nonnormality of the distributions of the symptom count variables. Because we primarily sought simply to provide parameter estimates for the full ACE longitudinal biometric models fitted separately for men and women (as opposed to testing a series of nested models), we do not report fit statistics for each model, but these can be provided by Brian M. Hicks on request.
Results
Gender Differences at Ages 17 and 24
Table 1 provides the prevalence rates and the means and standard deviations for the symptom count variables of the four externalizing disorders separately for men and women at ages 17 and 24.1 At age 17, compared with women, men exhibited more symptoms of adult antisocial behavior, t(547) = 5.10, p < .001, but roughly the same number of symptoms of alcohol dependence, t(547) = 1.94, p = .053; nicotine dependence, t(547) = 0.01, p > .10; and drug dependence, t(547) = 0.69, p > .10. At age 24, compared with women, men exhibited substantially more symptoms of adult antisocial behavior, t(547) = 8.03, p < .001; alcohol dependence, t(547) = 8.18, p < .001; and drug dependence, t(547) = 2.82, p = .005, but only slightly more symptoms of nicotine dependence, t(547) = 2.24, p = .025.
Table 1.
Mean-Level Gender Differences, Longitudinal Change, and Rank-Order Stability of Externalizing Disorder Symptoms From Age 17 to 24
| Cross-sectional differences
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Age 17
|
Age 24
|
|||||||||
| Symptom
|
Symptom
|
Longitudinal change
|
||||||||
| Disorder | Prevalence rate (%) | M | SD | Gender d score | Prevalence rate (%) | M | SD | Gender d score | Change over time d score | Gender × Time η2 |
| Adult antisocial behavior | ||||||||||
| Men | 5.7 | 0.80 | 1.24 | .37* | 5.5 | 1.23 | 1.24 | .60* | .35* | .01* |
| Women | 1.8 | 0.40 | 0.92 | 1.9 | 0.62 | 0.75 | .26* | |||
| Alcohol dependence | ||||||||||
| Men | 10.2 | 0.60 | 1.36 | .14 | 27.9 | 1.70 | 1.99 | .59* | .65* | .06* |
| Women | 6.4 | 0.42 | 1.21 | 9.7 | 0.65 | 1.52 | .17* | |||
| Nicotine dependence | ||||||||||
| Men | 12.8 | 0.66 | 1.56 | −.01 | 35.3 | 1.78 | 2.29 | .18 | .57* | .01* |
| Women | 14.1 | 0.67 | 1.64 | 26.4 | 1.39 | 2.09 | .38* | |||
| Drug dependence | ||||||||||
| Men | 3.8 | 0.28 | 1.15 | .06 | 11.9 | 0.79 | 1.86 | .22* | .33* | .01* |
| Women | 3.4 | 0.22 | 0.94 | 7.2 | 0.42 | 1.42 | .17* | |||
Note. Cohen’s ds were calculated as (M1 − M2)/(pooled SD) for the raw symptom count variables. The eta-square values refer to the percentage of variance accounted for by the Gender × Time interaction. At both age 17 and age 24, values for the symptom count variables ranged from 0 to 7 for adult antisocial behavior and nicotine dependence and 0 to 9 for alcohol and drug dependence.
p < .01.
Mean-Level Change, Individual-Level Change, and Rank-Order Stability From Ages 17 to 24
Because significant gender differences were detected at each time point, we examined mean-level changes in symptoms of externalizing disorders from ages 17 to 24 separately for men and women using linear mixed models analogous to repeated measures analyses of variance. Men exhibited large increases in symptoms of adult antisocial behavior, t(723) = 7.30, p < .001; alcohol dependence, t(723) = 11.61, p < .001; nicotine dependence, t(723) = 10.18, p < .001; and drug dependence, t(723) = 5.74, p < .001. Women also exhibited moderate to large increases in symptoms of adult antisocial behavior, t(754) = 4.99, p < .001; alcohol dependence, t(754) = 3.05, p < .002; nicotine dependence, t(754) = 8.29, p = .001; and drug dependence, t(754) = 3.08, p = .002.
We also tested whether the slopes of the increases in mean number of symptoms were significantly different for men and women using the Gender × Time interaction term in a model that included the full sample. For each disorder, the Gender × Time interaction was significant at p < .01, indicating that the mean-level increase in symptoms of each externalizing disorder was significantly greater for men than women. However, only the interaction term for alcohol dependence accounted for more than 1% of the variance.
Table 2 provides results of analyses examining individual-level change. Men exhibited significant individual-level change for each externalizing disorder. That is, more men exhibited a reliable and clinically significant increase or decrease in their number of symptoms than would be expected as a result of chance alone. Women showed significant individual-level change for each substance use disorder but not for adult antisocial behavior. Men displayed more individual-level change than women for each externalizing disorder, although the amount of individual-level change for nicotine dependence was fairly comparable for men and women. Both men and women were more likely to increase rather than decrease in their number of symptoms. Finally, although significant individual-level change was detected for each disorder, the majority of men and women remained stable in terms of their number of symptoms.
Table 2.
Percentages of Participants Who Exhibited Significant Individual-Level Change in Externalizing Disorder Symptoms From Age 17 to 24
| Individual-level change
|
Sex differences
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Disorder | n | Decrease | Stable | Increase | χ2(2, N =) | p | χ2(2, N =) | p |
| Adult antisocial behavior | ||||||||
| Men | 494 | 6.1 | 78.1 | 15.8 | 63.2 | <.001 | 56.4 | <.001 |
| Women | 520 | 3.1 | 93.8 | 3.1 | 0.7 | >.10 | ||
| Alcohol dependence | ||||||||
| Men | 538 | 3.5 | 64.9 | 31.6 | 165.1 | <.001 | 80.5 | <.001 |
| Women | 646 | 5.9 | 83.4 | 10.7 | 46.5 | <.001 | ||
| Nicotine dependence | ||||||||
| Men | 538 | 2.2 | 69.7 | 28.1 | 136.0 | <.001 | 9.0 | <.05 |
| Women | 646 | 4.0 | 74.5 | 21.5 | 115.7 | <.001 | ||
| Drug dependence | ||||||||
| Men | 538 | 2.0 | 84.4 | 13.6 | 44.6 | <.001 | 15.9 | <.001 |
| Women | 646 | 2.3 | 91.0 | 6.7 | 12.8 | <.01 | ||
Note. For the substance use disorders, the reliable change index, which corresponds to a change of two symptoms, was used to determine the percentage of individuals who increased, decreased, or remained stable. For adult antisocial behavior, a change of two symptoms was also necessary to be considered significant individual-level change. The chi-square test for individual-level change tested whether the observed distribution in the sample differed from the expected distribution (2.5% decrease, 95.0% stable, 2.5% increase) if change were completely random. The chi-square test for sex differences tested whether the distribution of persons who changed or remained stable was the same for men and women.
Table 3 provides the correlations among the symptom count variables at ages 17 and 24 separately for men and women. Within a given assessment, the externalizing disorders were moderately intercorrelated for both men and women, with a mean intercorrelation of .52 at age 17 and .40 at age 24. This indicates that a common EXT liability factor was present at both ages, although there appeared to be a slight decline in the magnitude of overlapping variance among the disorders at the older age, which suggests greater differentiation of the disorders over time. Finally, for both men and women, each disorder evinced moderate rank-order stability, as indexed by the 7-year test–retest correlation (rs = .30 to .56, all ps < .001).
Table 3.
Phenotypic Correlations Among the Externalizing Disorders at Age 17 and 24
| Disorder | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
|---|---|---|---|---|---|---|---|---|
| Age 17 | ||||||||
| 1. Adult antisocial behavior | — | .65 | .48 | .55 | .40 | .30 | .36 | .29 |
| 2. Alcohol dependence | .60 | — | .50 | .64 | .39 | .38 | .37 | .20 |
| 3. Nicotine dependence | .47 | .49 | — | .54 | .28 | .28 | .56 | .27 |
| 4. Drug dependence | .38 | .45 | .45 | — | .22 | .32 | .28 | .30 |
| Age 24 | ||||||||
| 5. Adult antisocial behavior | .44 | .38 | .34 | .28 | — | .42 | .32 | .44 |
| 6. Alcohol dependence | .32 | .41 | .17 | .13 | .36 | — | .40 | .48 |
| 7. Nicotine dependence | .38 | .35 | .49 | .22 | .39 | .38 | — | .36 |
| 8. Drug dependence | .44 | .40 | .35 | .38 | .42 | .39 | .42 | — |
Note. Correlations for men are below the diagonal; correlations for women are above the diagonal. Correlations in boldface are the test-retest stability coefficients. All correlations are significant at p < .01.
EXT Spectrum Model of Gender Differences and Developmental Change and Stability
Next, we tested a series of confirmatory factor models to simultaneously account for the comorbidity, gender differences, and longitudinal stability in symptoms of the four externalizing disorders. The general model included an EXT factor at ages 17 and 24 (Model 1; see Table 4 for fit statistics), with all parameters allowed to vary across gender. The EXT factor represents the common variance or general liability to the four externalizing disorders at each age and can be conceptualized as accounting for the comorbidity among the disorders. The correlation between the EXT factors at the two ages reflects the rank-order stability of the general EXT liability over the 7-year period.
Table 4.
Indexes of Fit for Models of Factorial Invariance Across Gender and Time (Age 17 and 24) for Externalizing Disorders
| Model and constraints | χ2 | df | RMSEA | BIC |
|---|---|---|---|---|
| Model 1 | ||||
| Configural invariance: one-factor model at age 17 and 24 for men and women | 147.56 | 38 | .075 | −92.15 |
| Model 2 | ||||
| Test specific effects on stability of externalizing disorders: allow nicotine dependence residuals to correlate across time | 88.21 | 36 | .053 | −138.88 |
| Model 3 | ||||
| Metric invariance across time: factor loadings equal at ages 17 and 24 | 122.18 | 42 | .061 | −142.76 |
| Model 4 | ||||
| Metric invariance across time and gender: factor loadings equal for men and women and at ages 17 and 24 | 120.91 | 46 | .057 | −169.26 |
| Model 5 | ||||
| Strong factorial invariance: intercepts equal across gender, factor loadings equal across gender and time | 157.02 | 52 | .063 | −171.00 |
| Model 6 | ||||
| Strict factorial invariance: residual variances and intercepts equal across gender, factor loadings equal across gender and time | 243.85 | 60 | .078 | −134.64 |
| Model 7 | ||||
| Strong factorial invariance and equate stability coefficient of EXT factor across gender | 161.29 | 53 | .063 | −173.04 |
Note. RMSEA = root-mean-square error of approximation; BIC = Bayesian information criterion; EXT = latent Externalizing factor.
Table 4 contains the fit statistics for the models of factorial invariance. First, we tested whether rank-order stability on the EXT factor could account for the rank-order stability in the symptoms of four externalizing disorders by allowing the residual variance in each symptom count variable to correlate across time. Allowing a correlation between the residuals of nicotine dependence at ages 17 and 24 improved the fit of the model, as indicated by a lower BIC value (Model 2). Allowing residuals of the other symptom count variables to correlate across time did not result in a lower BIC value. Therefore, the EXT factor could account for the rank-order stability in symptoms of adult antisocial behavior, alcohol dependence, and drug dependence, but specific factors independent of the EXT factor were necessary to account for the rank-order stability in symptoms of nicotine dependence. That is, nicotine dependence was more stable than would be expected given a person’s level of the EXT factor alone.
Second, we tested whether the pattern of correlations among the symptom count variables was the same at ages 17 and 24 by constraining the factor loadings of the EXT factors to be the same across time (Model 3). Conceptually, this is analogous to testing whether the pattern of comorbidity among the four externalizing disorders was the same at ages 17 and 24. This model yielded a lower BIC value, indicating that the pattern of comorbidity was comparable at ages 17 and 24.
Third, we tested whether the pattern of correlations among the symptom count variables was the same for men and women by constraining the factor loadings of the EXT factor to be the same across genders (Model 4; the correlation between the residuals of nicotine dependence was also equated across genders). Conceptually, this model is analogous to testing whether the pattern of comorbidity among the four externalizing disorders was the same for men and women. This model also yielded a lower BIC value, indicating that the pattern of comorbidity among the four externalizing disorders was the same for men and women.
Fourth, we tested whether the gender differences in mean number of symptoms could be accounted for by a mean difference in the EXT factor by constraining the intercepts of the four externalizing disorders to be the same across men and women and allowing the mean of the EXT factor to differ across genders (Model 5). Although the decrease in BIC was modest, it favored the more parsimonious model while maintaining a good overall fit to the data, thus indicating that the mean-level differences in the specific disorders could be accounted for by a gender difference in the general EXT liability.
Fifth, we tested whether the proportion of variance in the symptom count variables that was not accounted for by the EXT factor was the same for men and women by constraining the residual variances to be the same across genders (Model 6). This strict invariance model failed to provide a better fit to the data. The poor fit of the strict invariance model was primarily due to men exhibiting greater variance in symptoms of externalizing disorders relative to women.
Finally, for the strong invariance model (Model 5), we further constrained the stability coefficient of the EXT factor to be the same across men and women (Model 7). Again, although the decrease in the BIC was modest, it favored the more parsimonious model while maintaining a good overall fit to the data, thus indicating that men and women exhibited comparable rank-order stability in terms of their standing on the EXT factor from ages 17 to 24.
Figure 3 depicts parameter estimates for the final model with the lowest BIC value. The gender difference in mean levels of the EXT factor was relatively modest at age 17 (d = .21, 95% confidence interval [CI] = 0.05–0.38) but was large at age 24 (d = .73, 95% CI = 0.54–0.93). To estimate mean-level developmental change on the EXT factor, we fitted longitudinal factor models separately for men and women. Men exhibited a large (d = .80, 95% CI = 0.66–0.94) and women a moderate (d = .42, 95% CI = 0.31–0.53) increase in mean levels of the EXT factor from ages 17 to 24. Also, as already discussed, the rank-order stability coefficient for the EXT factor was high and comparable for men (r = .76, p < .001) and women (r = .66, p < .001).
Figure 3.
Standardized parameters from the best fitting strong invariance model. The latent EXT variables represent the general vulnerability factors common among the four disorders at ages 17 and 24. The factor loadings on the EXT variables have been equated across time and gender. The standardized factor loadings differ at ages 17 and 24 because of different variances in the disorders at the two ages. The standardized loadings reported in the figure are the means for men and women. The line connecting the latent EXT variables provides the rank-order stability coefficient. The residual correlation linking nicotine dependence at ages 17 and 24 represents unique effects independent of the EXT liability that contribute to the stability of nicotine dependence. The Cohen’s d values in the figure refer to the effective sizes for the mean-level difference between men and women on the EXT liability at the two ages (i.e., a positive d value indicates that men exhibited greater mean levels of the EXT liability). All factor loadings are significant at p < .01. Subscripts refer to assessment age in years. EXT = latent Externalizing factor; AAB = adult antisocial behavior; ALD = alcohol dependence; NCD = nicotine dependence; DD = drug dependence.
Genetic and Environmental Contributions to Variance Change and Stability
Parameter estimates of the biometric analyses for the symptom count variables and latent EXT factor are presented separately by gender in Tables 5 and 6. Raw variance components are presented in Table 5. To facilitate comparisons across time and gender, we set the phenotypic variance of men’s disorders at age 17 to have a value of 100.0, with the age 24 and all women’s phenotypic variance components scaled to that reference group. Several trends are evident in the results presented in Table 5. First, for both men and women, the phenotypic variance increased with age for each substance use disorder but declined for adult antisocial behavior. A second major trend was that men exhibited greater variance than women for each disorder at age 17 (with the exception of nicotine dependence), and this difference increased at age 24. For men, increases in the phenotypic variance were due to increases in the additive genetic and nonshared environmental variance, with both nearly doubling for each substance use disorder. For adult antisocial behavior, in both men and women, there was a slight decline in the additive genetic and nonshared environment variance from ages 17 to 24. Additionally, for men, shared environmental variance declined for each disorder from ages 17 to 24.
Table 5.
Variance Component Estimates for Longitudinal Models to Individual Externalizing Disorders and Latent Externalizing Factor
| Age 17 variance components
|
Age 24 variance components
|
Correlations
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Externalizing phenotype | A | C | E | P | A | C | E | P | rA | rC | rE |
| Adult antisocial behavior | |||||||||||
| Men | 49.6 | 0.0 | 50.4 | 100.0 | 39.6 | 0.0 | 43.9 | 83.0 | .90 | .00 | .01 |
| Women | 30.2 | 0.7 | 33.9 | 64.9 | 18.6 | 4.2 | 26.7 | 49.6 | .94 | −1.00 | .17 |
| Alcohol dependence | |||||||||||
| Men | 36.8 | 19.6 | 43.6 | 100.0 | 75.4 | 0.1 | 82.9 | 158.5 | .88 | −1.00 | .17 |
| Women | 55.3 | 0.3 | 23.7 | 79.4 | 8.1 | 23.1 | 67.1 | 98.4 | 1.00 | 1.00 | .30 |
| Nicotine dependence | |||||||||||
| Men | 38.3 | 32.4 | 29.3 | 100.0 | 88.9 | 22.6 | 64.3 | 175.8 | .47 | 1.00 | .38 |
| Women | 59.1 | 0.6 | 46.5 | 106.2 | 61.2 | 16.5 | 78.8 | 156.3 | .81 | 1.00 | .40 |
| Drug dependence | |||||||||||
| Men | 55.6 | 0.0 | 44.4 | 100.0 | 134.6 | 0.0 | 88.6 | 222.9 | .54 | .00 | .23 |
| Women | 7.0 | 21.0 | 46.3 | 74.3 | 40.9 | 23.2 | 67.6 | 131.7 | .89 | .45 | .27 |
| Externalizing factor | |||||||||||
| Men | 44.3 | 32.2 | 23.4 | 100.0 | 108.7 | 17.9 | 26.8 | 153.4 | .91 | 1.00 | .67 |
| Women | 74.4 | 0.4 | 20.1 | 94.9 | 47.1 | 32.6 | 34.6 | 114.3 | 1.00 | 1.00 | .73 |
Note. Variance estimates have been scaled so that the total phenotypic variance for all male externalizing phenotypes at age 17 is 100. A = additive genetic component of variance; C = shared environmental component of variance; E = nonshared environmental component of variance; P = total phenotypic variance; rA = additive genetic correlation between age 17 and age 24 symptoms; rC = shared environmental correlation between age 17 and age 24 symptoms; rE = nonshared environmental correlation between age 17 and age 24 symptoms.
Table 6.
Standardized Variance Component Estimates and 95% Confidence Intervals
| Age and component | AAB | ALD | NCD | DD | EXT |
|---|---|---|---|---|---|
| Men | |||||
| Age 17 | |||||
| a2 | .50 (.19–.59) | .37 (.08–.63)a | .38 (.14–.65) | .55 (.26–.64)a | .44 (.13–.81) |
| c2 | .00 (.00–.27) | .20 (.00–.44) | .33 (.07–.53) | .00 (.00–.25) | .32 (.00–.60) |
| e2 | .50 (.41–.62) | .43 (.35–.55) | .29 (.23–.37) | .45 (.36–.56) | .23 (.16–.34) |
| Age 24 | |||||
| a2 | .47 (.14–.57) | .48 (.18–.57)a | .51 (.28–.67) | .60 (.39–.68) | .71 (.41–.88) |
| c2 | .00 (.00–.31) | .00 (.00–.26) | .13 (.00–.33) | .00 (.00–.19) | .12 (.00–.39) |
| e2 | .53 (.43–.64) | .52 (.43–.64) | .37 (.29–.46) | .40 (.32–.49) | .17 (.10–.27) |
|
| |||||
| Women | |||||
| Age 17 | |||||
| a2 | .47 (.23–.57) | .70 (.54–.76)a,b | .56 (.25–.64) | .09 (.00–.45)a | .78 (.54–.85)b |
| c2 | .01 (.00–.21) | .00 (.00–.15) | .01 (.00–.27) | .28 (.00–.43) | .00 (.00–.23) |
| e2 | .52 (.43–.63) | .30 (.24–.37) | .44 (.36–.53) | .62 (.51–.73) | .22 (.15–.30) |
| Age 24 | |||||
| a2 | .38 (.12–.55) | .08 (.01–.26)a,b | .39 (.07–.59) | .31 (.00–.58) | .41 (.16–.71)b |
| c2 | .08 (.00–.31) | .22 (.07–.35) | .11 (.00–.38) | .18 (.00–.43) | .29 (.02–.53) |
| e2 | .54 (.44–.65) | .68 (.59–.79) | .50 (.41–.62) | .51 (.41–.64) | .30 (.21–.42) |
|
| |||||
| Combined | |||||
| Age 17 | |||||
| a2 | .49 (.32–.56) | .63 (.41–.69) | .46 (.26–.66) | .38 (.13–.55) | .70 (.46–.83) |
| c2 | .00 (.00–.15) | .01 (.00–.21) | .16 (.00–.34) | .10 (.00–.30) | .08 (.00–.29) |
| e2 | .51 (.44–.58) | .36 (.31–.42) | .38 (.32–.44) | .52 (.45–.61) | .22 (.17–.27) |
| Age 24 | |||||
| a2 | .47 (.23–.54) | .25 (.05–.46) | .49 (.29–.61) | .54 (.36–.62) | .66 (.37–.83) |
| c2 | .00 (.00–.22) | .14 (.00–.33) | .08 (.00–.25) | .03 (.00–.18) | .11 (.00–.37) |
| e2 | .53 (.46–.60) | .61 (.53–.69) | .43 (.37–.50) | .43 (.37–.51) | |
Note. The combined model constrains the parameter estimates to be equal across men and women. AAB = adult antisocial behavior; ALD = alcohol dependence; NCD = nicotine dependence; DD = drug dependence; EXT = latent Externalizing factor; a2 = proportion of variation due to additive genetic effects; c2 = proportion of variation due to shared environmental effects; e2 = proportion of variation due to nonshared environmental effects. Confidence intervals appear in parentheses.
There was a significant gender difference in the heritability estimate at the given assessment.
The heritability estimate was significantly different across the two assessments.
Women displayed a less consistent pattern of developmental change. For nicotine dependence, there was a slight increase in the additive genetic variance and a large increase in the nonshared environmental variance, whereas drug dependence exhibited a large increase in additive genetic variance and a moderate increase in nonshared environmental variance. For alcohol dependence, additive genetic variance declined substantially from ages 17 to 24, whereas there were large increases in the shared and nonshared environmental variance. For women, the shared environmental variance increased for each disorder from ages 17 to 24.
For the latent EXT factor, men exhibited a large increase in additive genetic variance from ages 17 to 24 and a decline in shared environmental variance. Nonshared environmental variance remained stable. For women, there was a decline in the additive genetic variance and a substantial increase in the shared and nonshared environmental variance from ages 17 to 24. Therefore, variance increases in the latent EXT factor were primarily due to additive genetic effects in men and environmental effects in women.
Finally, the estimated genetic and environmental correlations for the symptom count variables from ages 17 to 24 indicated that the additive genetic variance was highly overlapping across the two time points, whereas the nonshared environmental variance was more distinct. The extremely variable shared environmental correlations were primarily due to the small proportion of variance accounted for by shared environmental effects at a given time point. Both the additive genetic and the environmental correlations were very high for the latent EXT factor.
Table 6 provides the standardized ACE parameter estimates and 95% CIs. For men, estimates of heritability tended to increase from ages 17 to 24, whereas estimates of the shared environmental variance component tended to decrease, although none of the estimates was significantly different across time. For women, estimates of heritability tended to decrease from ages 17 to 24, whereas estimates of the shared environmental variance component tended to increase. The one exception was drug dependence, for which the heritability estimate increased and the shared environmental variance component decreased. For women, the heritability estimate of alcohol dependence was significantly lower at age 24 compared with age 17, χ2(1) = 19.57, p < .001. Also for women, the heritability estimate of the latent EXT factor was significantly lower at age 24 compared with age 17, χ2(1) = 4.33, p < .05.
Significant gender differences in heritability estimates were also detected. At age 17, the heritability estimate of alcohol dependence was significantly greater for women compared with men, χ2(1) = 4.43, p < .05. The reverse was the case at age 24, however, in that the heritability estimate of alcohol dependence was significantly lower for women compared with men, χ2(1) = 5.74, p < .05. Finally, at age 17, the heritability estimate of drug dependence was significantly greater in men compared with women, χ2(1) = 4.03, p < .05.
Discussion
The present investigation tests several hypotheses regarding the nature of gender differences and developmental change on four externalizing disorders: adult antisocial behavior, alcohol dependence, nicotine dependence, and drug dependence. For all externalizing disorders, there was a significant increase in mean symptom levels from ages 17 to 24. Additionally, for each externalizing disorder there was a significant Gender × Time interaction, such that a modest gender difference at age 17 increased to a large gender difference at age 24. This widening gender gap was a function of men increasing in externalizing symptoms at a greater rate than women. Individual-level analyses were consistent with the mean-level analyses, such that for both men and women a significant number of individuals increased in their externalizing symptoms, with more men than women exhibiting a significant increase in symptoms. Finally, men and women evinced a comparable and moderate degree of rank-order stability for each externalizing disorder.
The latent EXT factor exhibited a similar pattern of gender differences and developmental change. Both men and women increased on the EXT factor from ages 17 to 24. Men were higher on the EXT factor at both time points, with a modest gender difference at age 17 increasing to large one at age 24. However, these mean-level differences were greater for the EXT factor than for the specific externalizing disorders. Also, the EXT factor exhibited greater rank-order stability than any of the specific externalizing disorders and could account for the rank-order stability in each disorder except nicotine dependence. Although the externalizing disorders were moderately intercorrelated at both ages, the amount of overlapping variance declined slightly with age, which suggests greater differentiation of the disorders over time. However, the level of rank-order stability and pattern of intercorrelations among the externalizing disorders did not differ across genders. This indicates that although men and women did not differ in terms of the structure of externalizing symptoms, men did experience a greater absolute number of symptoms than women. Finally, our most novel finding is that a mean-level gender difference in the latent EXT factor could account for gender differences in mean levels of the individual disorders.
Biometric analyses also revealed differential effects of genetic and environmental factors on risk for externalizing disorders across gender and time. Men exhibited a pattern consistent with behavioral genetic developmental theory—that is, increasing heritability and genetic variance with age. Also, there was some evidence of shared environmental effects on nicotine dependence, alcohol dependence, and the EXT factor at age 17, but these effects fell to near zero at age 24, with a much higher heritability estimate for the EXT factor. Women exhibited less consistent patterns. For each disorder except drug dependence, the genetic variance either remained stable or declined at age 24, with the heritability estimates declining as a consequence. The increase in the genetic variance and heritability of drug dependence was likely due to the low phenotypic variance evident at age 17. The biometry of alcohol dependence exhibited a dramatic shift, from a high heritability estimate and no shared environmental effects at age 17 to a very low heritability estimate and significant shared environmental effects at age 24. As a consequence of this shift in alcohol dependence, the heritability estimate of the EXT factor also declined substantially, with a significant shared environmental contribution at age 24. Finally, for both men and women, each externalizing phenotype except adult antisocial behavior exhibited an increase in the nonshared environmental variance indicative of the impact of a greater diversity of environmental experiences with increasing age.
Disorder-Specific Characteristics
Although there were consistent trends across the four externalizing disorders in terms of gender differences and developmental change, each disorder exhibited distinctive characteristics. Adult antisocial behavior was the only disorder whose variance decreased from ages 17 to 24. This suggests a desistance effect—that is, most men and women peaked in their antisocial behavior in late adolescence but declined in such behavior as they entered adulthood (Blumstein, Cohen, & Farrington, 1988; Moffitt, 1993). In contrast, drug dependence exhibited the greatest increases in variance from ages 17 to 24, in particular the largest increases in additive genetic variance. The low variance at age 17 was likely due to the illicit nature of these substances, which restricts access and constrains the potential effect of genetic differences for risk for dependence. However, the greater opportunities for access to illicit drugs that come with age may allow for genetic factors to emerge and so play a greater role in symptom expression.
Alcohol dependence exhibited the greatest gender differences in genetic and environmental architecture over time, with women exhibiting higher heritability than men at age 17 but lower heritability than men at age 24. This suggests that the genetic and environmental risk factors for alcohol dependence may be particularly sensitive to gender and developmental context. That is, for women in young adulthood, alcohol dependence may be more contextually driven than is the case for men. For example, after marriage, the drinking patterns of women and of their peer group tend to increase or decrease to more closely match the patterns of their male partner (Leonard & Mudar, 2003). In contrast, men’s drinking patterns remain relatively independent of their partner’s drinking habits. Attending college is another potentially important environmental effect in early adulthood that may have differential effects for men and women, as many of the social activities of college students include or emphasize heavy drinking (Bartholow, Sher, & Krull, 2003; Kahler, Wood, & Palfai, 2003; Slutske et al., 2004).
Our results for the heritability of alcohol dependence in early adulthood are somewhat inconsistent with previous population-based studies that have found moderate to high heritability and no shared environmental effects (Heath et al., 1997; Kendler et al., 1992). The reasons for this discrepancy are difficult to determine, but it may be due to the sample characteristics and length of the assessment period. Previous studies ascertained lifetime diagnoses for large cross-sectional samples with participants who spanned the full range of adulthood (20s to 80s), which introduces the possibility of cohort and retrospective bias effects. Cross-sectional samples may also obscure developmental variability regarding the impact of different genetic and environmental factors. In contrast, the female sample of the MTFS spans only a few birth years, all twins are assessed at roughly the same age, and the assessment period covers only the last 3–4 years (although this was extended to 7 years for the present analyses). The consequences of cross-sectional versus cohort samples and different assessment periods on estimates of heritability have not received a comprehensive evaluation. However, it should be noted that a heritability estimate is only valid for a particular population at a specific time point and so is subject to variation resulting from various sample, psychometric, developmental, and historical factors.
In contrast to alcohol dependence, nicotine dependence exhibited the fewest gender differences, with only modest mean-level, individual-level, variance, and biometric differences. Also, nicotine dependence was the only disorder that required effects independent of the EXT factor to account for its rank-order stability. Our model cannot determine the cause of the greater stability of nicotine dependence, but it could be due to the especially addictive properties of nicotine (Henningfield & Keenan, 1993), sociocultural factors (e.g., norms and legal restrictions regarding use and access relative to alcohol and illicit drugs), or other individual-differences variables (Pomerleau, Collins, Shiffman, & Pomerleau, 1993). Regardless, these findings provide an opportunity to gain a greater understanding of both nicotine dependence and the EXT liability by examining the similarities and differences between nicotine dependence and other disorders in the EXT spectrum. For example, what are the common risk factors among all the externalizing disorders that index the EXT liability, and what are the unique aspects of nicotine dependence that contribute to its greater persistence and weaker gender differences?
One limitation of the current study is the use of only two time points. For example, many participants likely had already reached their lifetime peak number of externalizing symptoms and begun to desist prior to age 24. Analyses that include additional time points and predictors of change will help to provide a more refined understanding of the process of escalation and desistance of externalizing symptoms. However, our aim was simply to examine the overall increase in externalizing disorder symptoms and estimate its magnitude during the developmental period of greatest change and prevalence. An additional limitation is that our sample was not ethnically or racially diverse, which limits the generalizability of our findings. Finally, we did not incorporate environmental variables (e.g., socioeconomic status, marriage, peer influence) that might moderate some findings, as this was beyond the scope of the present investigation.
Utility of EXT Spectrum Model and Mediation Analyses
It is important to discuss the utility of the EXT spectrum model to improve our understanding of externalizing disorders. In regard to gender differences, our results indicate that the focus should shift from examining the risk factors for specific disorders that might differ across gender to investigating risk factors common to multiple disorders that also exhibit reliable gender differences. In regard to developmental change and stability, our finding that the EXT factor can account for much of the rank-order stability in the disorders indicates that the common variance is key to understanding the persistence of externalizing symptoms. Finally, our finding that the effect sizes for mean-level change on the EXT factor were greater than for any of the individual disorders indicates that an increase in a general disinhibitory process is key to understanding the increase in prevalence of the disorders in early adulthood and so should be a focus of research.
Given the utility of the EXT spectrum model, the broader question that derives from this work is as follows: What are the psychological mechanisms that underlie the EXT liability? On the basis of our results, examining developmental change and gender differences is a productive strategy to help answer this question, in particular by identifying the variables that contribute to or account for these normative changes and sex differences. For example, why do men exhibit higher mean levels of EXT? Why do mean levels of EXT increase for both men and women from late adolescence to early adulthood? Why does the gender difference in EXT increase from late adolescence to early adulthood? Identifying the variables that underlie or account for these processes will help to delineate the core of EXT liability.
Previous studies have found that there is a higher prevalence of risk factors for externalizing disorders in men (e.g., Moffitt et al., 2001). Because the EXT factor represents a general vulnerability, a logical strategy is to examine risk factors common across externalizing disorders that also exhibit a reliable gender difference. For example, Moffitt et al. (2001) identified several replicable risk factors for adolescent antisocial behavior that also exhibited reliable gender differences, including neurocognitive deficits, early-emerging undercontrolled temperament, hyperactivity, deviant peer relationships, and adult personality traits associated with heightened negative affectivity (especially aggression) and weak behavioral constraint. Mediation analyses revealed that neurocognitive deficits, undercontrolled temperament, hyperactivity, and deviant peer relationships could account for approximately one half of the gender difference in adolescent antisocial behavior, whereas a separate analysis found that the gender difference in antisocial behavior was almost entirely accounted for by adult personality traits of negative affect and weak behavioral constraint.
A similar strategy could be applied to the EXT liability per se. Such analyses are useful as they provide further descriptive information and identify potential mechanisms that serve as the core processes underlying the EXT liability. Also, given the variations across externalizing disorders for gender differences and developmental change detected in the present investigation, it is also important to conceptualize the common externalizing variance that defines the EXT liability factor as a distinct construct that requires studies designed to delineate its unique properties as opposed to one of its indicators.
This strategy could also be applied to identifying mediators of mean differences in EXT across developmental periods. For example, Moffitt et al. (2001) advocated the utility of examining contexts associated with a narrowing of the gender gap in antisocial behavior, the most notable contexts being puberty, alcohol- and drug-related delinquency, and intimate partner violence. The mechanisms associated with these contexts have yet to be delineated, but Moffitt et al. (2001) speculated that these contexts provide greater exposure of women to male peer influence, yielding a reduction in the gender gap via increased symptom expression in women. One could also apply a similar strategy to identify the contextual and biological factors associated with the widening of the gender gap in EXT from late adolescence to early adulthood via the greater increase in symptom expression of men relative to women. That is, what factors allow for the greater expression of the EXT liability, and why are the effects of these factors greater for men?
In sum, our results offer further validation of the EXT spectrum model as a parsimonious theoretical framework with which to anchor our understanding of externalizing disorders. The next step is to begin to describe and delineate the underlying processes represented by the EXT factor, knowledge of which will eventually inform strategies to prevent and remedy the deleterious effects of externalizing disorders. As we have illustrated, examination of both gender differences and developmental change provide productive research strategies in this endeavor.
Acknowledgments
This research was supported in part by U.S. Public Health Service Grants AA00175, AA09367, DA05147, and MH65137. Brian M. Hicks was supported by National Institute of Mental Health Training Grant MH18869. This work was completed by Brian M. Hicks in partial fulfillment of the requirements for the degree of doctor of philosophy at the University of Minnesota under the supervision of Robert F. Krueger and Christopher J. Patrick.
Footnotes
Consistent with Criterion A of the DSM–III–R definition of ASPD, a diagnosis of adult antisocial behavior was defined as the presence of four or more adult symptoms.
References
- Achenbach TM, Edelbrock CS. Psychopathology in childhood. Annual Review of Psychology. 1984;35:227–256. doi: 10.1146/annurev.ps.35.020184.001303. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 3. Washington, DC: Author; 1987. [Google Scholar]
- Arnett JJ. Adolescent storm and stress, reconsidered. American Psychologist. 1999;54:317–326. doi: 10.1037//0003-066x.54.5.317. [DOI] [PubMed] [Google Scholar]
- Arnett JJ. Emerging adulthood: A theory of development from the late teens through the twenties. American Psychologist. 2000;55:469–480. [PubMed] [Google Scholar]
- Bachman JG, Wadsworth K, O’Malley P, Johnston L, Schulenberg J. Smoking, drinking, and drug use in young adulthood: The impacts of new freedoms and new responsibilities. Mahwah, NJ: Erlbaum; 1997. [Google Scholar]
- Ball D, Collier D. Substance misuse. In: McGuffin P, Owen MJ, Gottesman I, editors. Psychiatric genetics and genomics. Oxford, England: Oxford University Press; 2002. pp. 267–302. [Google Scholar]
- Bartholow BD, Sher KJ, Krull JL. Changes in heavy drinking over the third decade of life as a function of collegiate fraternity and sorority involvement: A prospective, multilevel analysis. Health Psychology. 2003;22:616–626. doi: 10.1037/0278-6133.22.6.616. [DOI] [PubMed] [Google Scholar]
- Blumstein A, Cohen J, Farrington DP. Criminal career research: Its value to criminology. Criminology. 1988;26:1–35. [Google Scholar]
- Browne MW, Cudeck R. Alternative ways of assessing model fit. In: Bollen KA, Long JS, editors. Testing structural equation models. Newbury Park, CA: Sage; 1993. pp. 136–162. [Google Scholar]
- Burt SA, Krueger RF, McGue M, Iacono WG. Sources of covariation among attention-deficit/hyperactivity disorder, oppositional defiant disorder, and conduct disorder: The importance of shared environment. Journal of Abnormal Psychology. 2001;110:516–525. doi: 10.1037/0021-843X.110.4.516. [DOI] [PubMed] [Google Scholar]
- Chassin L, Flora DB, King KM. Trajectories of alcohol and drug use and dependence from adolescence to adulthood: The effects of familial alcoholism and personality. Journal of Abnormal Psychology. 2004;113:483–498. doi: 10.1037/0021-843X.113.4.483. [DOI] [PubMed] [Google Scholar]
- Chen K, Kandel DB. The natural history of drug use from adolescence to the mid-thirties in a general population sample. American Journal of Public Health. 1995;85:41–47. doi: 10.2105/ajph.85.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen L, Mendoza JL. A method of assessing change in a single subject: An alteration of the RC index. Behavior Therapy. 1986;17:305–308. [Google Scholar]
- Cohen MA, Miller TR, Rossman SB. The costs and consequences of violent behavior in the United States. In: Reiss AJ Jr, Roth JA, editors. Understanding and preventing violence: Consequences and control. Washington, DC: National Academy Press; 1994. pp. 67–166. [Google Scholar]
- Erikson EH. Childhood and society. 2. New York: Norton; 1963. [Google Scholar]
- First MB, Gibbon M, Spitzer RL, Williams JBW. Structured Clinical Interviews for DSM–IV Axis II Personality Disorders (SCID-II) Arlington, VA: American Psychiatric Publishing; 1997. [Google Scholar]
- Fowles DC, Kochanksa G. Temperament as a moderator of pathways to conscience in children: The contribution of electrodermal activity. Psychophysiology. 2000;37:788–795. [PubMed] [Google Scholar]
- Gorenstein EE, Newman JP. Disinhibitory psychopathology: A new perspective and a model for research. Psychological Review. 1980;87:303–315. [PubMed] [Google Scholar]
- Hall GS. Adolescence: Its psychology and its relations to physiology, anthropology, sociology, sex, crime, religion, and education. 1–2. New York: Appleton-Century-Crofts; 1904. [Google Scholar]
- Hankin BL, Abramson LY, Moffitt TE, Silva PA, McGee R, Angell KE. Development of depression from preadolescence to young adulthood: Emerging gender differences in a 10-year longitudinal study. Journal of Abnormal Psychology. 1998;107:128–140. doi: 10.1037//0021-843x.107.1.128. [DOI] [PubMed] [Google Scholar]
- Harford TC, Grant BF, Yi H, Chen CM. Patterns of DSM–IValcohol abuse and dependence criteria among adolescents and adults: Results from the 2001 National Household Survey on Drug Abuse. Alcoholism: Clinical and Experimental Research. 2005;29:810–828. doi: 10.1097/01.alc.0000164381.67723.76. [DOI] [PubMed] [Google Scholar]
- Harpur TJ, Hare RD. Assessment of psychopathy as a function of age. Journal of Abnormal Psychology. 1994;103:604–609. doi: 10.1037//0021-843x.103.4.604. [DOI] [PubMed] [Google Scholar]
- Heath AC. Genetic influences on drinking behavior in humans. In: Kissin HBB, editor. The genetics of alcoholism. New York: Oxford University Press; 1995. pp. 82–121. [Google Scholar]
- Heath AC, Bucholz KK, Madden PA, Dinwiddie SH, Slutske WS, Statham DJ, et al. Genetic and environmental contributions to alcohol dependence risk in a national twin sample: Consistency of findings in women and men. Psychological Medicine. 1997;27:1381–1396. doi: 10.1017/s0033291797005643. [DOI] [PubMed] [Google Scholar]
- Henningfield JE, Keenan RM. Nicotine delivery kinetics and abuse liability. Journal of Consulting and Clinical Psychology. 1993;61:743–750. doi: 10.1037//0022-006x.61.5.743. [DOI] [PubMed] [Google Scholar]
- Hicks BM, Krueger RF, Iacono WG, McGue M, Patrick CJ. Family transmission and heritability of externalizing disorders: A twin-family study. Archives of General Psychiatry. 2004;61:922–928. doi: 10.1001/archpsyc.61.9.922. [DOI] [PubMed] [Google Scholar]
- Iacono WG, Carlson SR, Taylor J, Elkins IJ, McGue M. Behavioral disinhibition and the development of substance use disorders: Findings from the Minnesota Twin Family Study. Development and Psychopathology. 1999;11:869–900. doi: 10.1017/s0954579499002369. [DOI] [PubMed] [Google Scholar]
- Iacono WG, Malone SM, McGue M. Substance use disorders, externalizing psychopathology, and P300 event-related potential amplitude. International Journal of Psychophysiology. 2003;48:147–178. doi: 10.1016/s0167-8760(03)00052-7. [DOI] [PubMed] [Google Scholar]
- Jackson K, Sher KJ, Wood P. Trajectories of concurrent substance use disorders: A developmental, typological approach to comorbidity. Alcoholism: Clinical and Experimental Research. 2000;24:902–915. [PubMed] [Google Scholar]
- Jacobson NS, Truax P. Clinical significance: A statistical approach to defining meaningful change in psychotherapy research. Journal of Consulting and Clinical Psychology. 1991;59:12–19. doi: 10.1037//0022-006x.59.1.12. [DOI] [PubMed] [Google Scholar]
- Jessor R, Donovan JE, Costa FM. Beyond adolescence: Problem behavior and young adult development. Cambridge, England: Cambridge University Press; 1991. [Google Scholar]
- Jessor R, Jessor SL. Problem behavior and psychosocial development: A longitudinal study of youth. New York: Academic Press; 1977. [Google Scholar]
- Kahler CW, Wood MD, Palfai TP. Social environmental selection as a mediator of gender, ethnic, and personality effects on college student drinking. Psychology of Addictive Behaviors. 2003;17:226–234. doi: 10.1037/0893-164X.17.3.226. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Heath AC, Neale MC, Kessler RC, Eaves LJ. A population-based twin study of alcoholism in women. Journal of the American Medical Association. 1992;268:1877–1882. [PubMed] [Google Scholar]
- Kendler KS, Neale MC, Sullivan P, Corey LA, Gardner CO, Prescott CA. A population-based twin study in women of smoking initiation and nicotine dependence. Psychological Medicine. 1999;29:299–308. doi: 10.1017/s0033291798008022. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Prescott CA, Myers J, Neale MC. The structure of genetic and environmental risk factors for common psychiatric and substance use disorders in men and women. Archives of General Psychiatry. 2003;60:929–937. doi: 10.1001/archpsyc.60.9.929. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Chiu WT, Demler O, Walters EE. Prevalence, severity, and comorbidity of l2-month DSM–IVdisorders in the National Comorbidity Survey replication. Archives of General Psychiatry. 2005;62:617–627. doi: 10.1001/archpsyc.62.6.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, McGonagle KA, Zhao S, Nelson CB, Hughes M, Eshleman S, et al. Lifetime and 12-month prevalence of DSM–III–Rpsychiatric disorders in the United States: Results from the National Comorbidity Survey. Archives of General Psychiatry. 1994;51:8–19. doi: 10.1001/archpsyc.1994.03950010008002. [DOI] [PubMed] [Google Scholar]
- King SM, Iacono WG, McGue M. Childhood externalizing and internalizing in the prediction of early substance use. Addiction. 2004;99:1548–1559. doi: 10.1111/j.1360-0443.2004.00893.x. [DOI] [PubMed] [Google Scholar]
- Krueger RF. The structure of common mental disorders. Archives of General Psychiatry. 1999;56:921–926. doi: 10.1001/archpsyc.56.10.921. [DOI] [PubMed] [Google Scholar]
- Krueger RF, Caspi A, Moffitt TE, Silva PA. The structure and stability of common mental disorders (DSM–III–R): A longitudinal–epidemiological study. Journal of Abnormal Psychology. 1998;107:216–227. doi: 10.1037//0021-843x.107.2.216. [DOI] [PubMed] [Google Scholar]
- Krueger RF, Hicks BM, Patrick CJ, Carlson SR, Iacono WG, McGue M. Etiologic connections among substance dependence, antisocial behavior, and personality: Modeling the externalizing spectrum. Journal of Abnormal Psychology. 2002;111:411–424. [PubMed] [Google Scholar]
- Krueger RF, Markon KE, Patrick CJ, Benning SD, Kramer M. Linking antisocial behavior, substance use, and personality: An integrative quantitative model of the adult externalizing spectrum. 2006 doi: 10.1037/0021-843X.116.4.645. Manuscript submitted for publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger RF, Markon KE, Patrick CJ, Iacono WG. Externalizing psychopathology in adulthood: A dimensional-spectrum conceptualization and its implications for DSM–V. Journal of Abnormal Psychology. 2005;114:537–550. doi: 10.1037/0021-843X.114.4.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard KE, Mudar P. Peer and partner drinking and the transition to marriage: Longitudinal examination of selection and influence processes. Psychology of Addictive Behaviors. 2003;17:115–125. doi: 10.1037/0893-164x.17.2.115. [DOI] [PubMed] [Google Scholar]
- Lynskey MT, Fergusson DM. Childhood conduct problems, attention deficit behaviors, and adolescent alcohol, tobacco, and illicit drug use. Journal of Abnormal Child Psychology. 1995;23:281–302. doi: 10.1007/BF01447558. [DOI] [PubMed] [Google Scholar]
- Lynskey MT, Heath AC, Nelson EC, Bucholz KK, Madden PAF, Slutske WS. Genetic and environmental contributions to cannabis dependence in a national young adult twin sample. Psychological Medicine. 2002;32:195–207. doi: 10.1017/s0033291701005062. [DOI] [PubMed] [Google Scholar]
- Markon KE, Krueger RF. Categorical and continuous models of liability to externalizing disorders: A direct comparison in NESARC. Archives of General Psychiatry. 2005;62:1352–1359. doi: 10.1001/archpsyc.62.12.1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGue M, Elkins I, Iacono WG. Genetic and environmental influences on adolescent substance use and abuse. American Journal of Medical Genetics (Neuropsychiatric Genetics) 2000;96:671–677. doi: 10.1002/1096-8628(20001009)96:5<671::aid-ajmg14>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- McGue M, Iacono WG, Krueger RF. The association of early adolescent problem behavior and adult psychopathology: A multivariate behavioral genetic perspective. Behavior Genetics. 2006;36:591–602. doi: 10.1007/s10519-006-9061-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGue M, Pickens RW, Svikis DS. Sex and age effects on the inheritance of alcohol problems: A twin study. Journal of Abnormal Psychology. 1992;101:3–17. doi: 10.1037//0021-843x.101.1.3. [DOI] [PubMed] [Google Scholar]
- Meredith W. Measurement invariance, factor analysis, and factorial invariance. Psychometrika. 1993;58:525–543. [Google Scholar]
- Miller TR, Cohen MA, Wiersema B. Victim costs and consequences: A new look (National Institute of Justice Publication No. NCJ155282) Washington, DC: U.S. Department of Justice; 1996. [Google Scholar]
- Moffitt TE. “Life-course-persistent” and “adolescence-limited” antisocial behavior: A developmental taxonomy. Psychological Review. 1993;100:674–701. [PubMed] [Google Scholar]
- Moffitt TE, Caspi A, Rutter M, Silva PA. Sex differences in antisocial behavior: Conduct disorder, delinquency, and violence in the Dunedin Longitudinal Study. Cambridge, England: Cambridge University Press; 2001. [Google Scholar]
- Muthén LK, Muthén BO. Mplus user’s guide. 3. Los Angeles: Muthén & Muthén; 2003. [Google Scholar]
- Neale MC, Boker SM, Xie G, Maes HH. Mx: Statistical modeling (Version 6) [Computer software] Richmond, VA: Department of Psychiatry, Virginia Commonwealth University; 2002. [Google Scholar]
- Newman DL, Moffitt TE, Caspi A, Magdol L, Silva PA, Stanton WR. Psychiatric disorder in a birth cohort of young adults: Prevalence, comorbidity, clinical significance, and new case incidence from ages 11–21. Journal of Consulting and Clinical Psychology. 1996;64:552–562. [PubMed] [Google Scholar]
- Patterson CM, Newman JP. Reflectivity and learning from aversive events: Toward a psychological mechanism for the syndromes of disinhibition. Psychological Review. 1993;100:716–736. doi: 10.1037/0033-295x.100.4.716. [DOI] [PubMed] [Google Scholar]
- Pomerleau OF, Collins AC, Shiffman S, Pomerleau CS. Why some people smoke and others do not: New perspectives. Journal of Consulting and Clinical Psychology. 1993;61:723–731. doi: 10.1037//0022-006x.61.5.723. [DOI] [PubMed] [Google Scholar]
- Prescott CA, Caldwell CB, Carey G, Vogler GP, Trumbetta SL, Gottesman II. The Washington University Twin Study of Alcoholism. American Journal Medical Genetics, Part B: Neuropsychiatric Genetics. 2005;134:48–55. doi: 10.1002/ajmg.b.30124. [DOI] [PubMed] [Google Scholar]
- Prescott CA, Kendler KS. Genetic and environmental contributions to alcohol abuse and dependence in a population-based sample of male twins. American Journal of Psychiatry. 1999;156:34–40. doi: 10.1176/ajp.156.1.34. [DOI] [PubMed] [Google Scholar]
- Raftery AE. Bayesian model selection in social research. Sociological Methodology. 1995;25:111–163. [Google Scholar]
- Rhee SH, Waldman ID. Genetic and environmental influences on antisocial behavior: A meta-analysis of twin and adoption studies. Psychological Bulletin. 2002;128:490–529. [PubMed] [Google Scholar]
- Rice DP. Economic costs of substance abuse. Proceedings of the Association of American Physicians. 1999;111:119–125. doi: 10.1046/j.1525-1381.1999.09254.x. [DOI] [PubMed] [Google Scholar]
- Roberts BW, Caspi A, Moffitt TE. The kids are alright: Growth and stability in personality development from adolescence to adulthood. Journal of Personality and Social Psychology. 2001;81:670–683. [PubMed] [Google Scholar]
- Robins LN, Baber T, Cottler LB. Composite International Diagnostic Interview: Expanded Substance Abuse module. St. Louis, MO: Author; 1987. [Google Scholar]
- Room R, Babor T, Rehm J. Alcohol and public health. Lancet. 2005;365:519–530. doi: 10.1016/S0140-6736(05)17870-2. [DOI] [PubMed] [Google Scholar]
- Scarr S, McCartney K. How people make their own environments: A theory of genotype greater than environment effects. Child Development. 1983;54:424–435. doi: 10.1111/j.1467-8624.1983.tb03884.x. [DOI] [PubMed] [Google Scholar]
- Sher KJ, Gotham HJ. Pathological alcohol involvement: A developmental disorder of young adulthood. Development and Psychopathology. 1999;11:933–956. doi: 10.1017/s0954579499002394. [DOI] [PubMed] [Google Scholar]
- Slutske WS, Hunt-Carter EE, Nabors-Oberg RE, Sher KJ, Bucholz KK, Madden PAF, et al. Do college students drink more than their non-college attending peers? Evidence from a population-based longitudinal female twin study. Journal of Abnormal Psychology. 2004;113:530–540. doi: 10.1037/0021-843X.113.4.530. [DOI] [PubMed] [Google Scholar]
- True WR, Xian H, Scherrer JF, Madden PAM, Bucholz KK, Heath AC, et al. Common genetic vulnerability for nicotine dependence and alcohol dependence in men. Archives of General Psychiatry. 1999;56:655–661. doi: 10.1001/archpsyc.56.7.655. [DOI] [PubMed] [Google Scholar]
- Tsuang MT, Lyons MJ, Eisen SA, Goldberg J, True W, Lin N. Genetic influences on DSM–III–Rdrug abuse and dependence: A study of 3,372 twin pairs. American Journal of Medical Genetics (Neuropsychiatric Genetics) 1996;67:473–477. doi: 10.1002/(SICI)1096-8628(19960920)67:5<473::AID-AJMG6>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Vollebergh WAM, Iedema J, Bijl RV, de Graaf R, Smit F, Ormel J. The structure and stability of common mental disorders: The NEMESIS Study. Archives of General Psychiatry. 2001;58:597–603. doi: 10.1001/archpsyc.58.6.597. [DOI] [PubMed] [Google Scholar]
- Windle M. A longitudinal study of antisocial behaviors in early adolescence as predictors of late adolescent substance use: Gender and ethnic group differences. Journal of Abnormal Psychology. 1990;99:86–91. doi: 10.1037//0021-843x.99.1.86. [DOI] [PubMed] [Google Scholar]
- Young SE, Stallings MC, Corley RP, Krauter KS, Hewitt JK. Genetic and environmental influences on behavioral disinhibition. American Journal of Medical Genetics. 2000;96:684–695. [PubMed] [Google Scholar]



