Abstract
Introduction
Breast cancer at a young age is associated with an unfavorable prognosis. Very young patients with breast cancer therefore are advised to undergo adjuvant chemotherapy irrespective of tumor stage or grade. However, chemotherapy alone may not be adequate in young patients with hormone receptor-positive breast cancer. Therefore, we studied the effect of adjuvant chemotherapy in young patients with breast cancer in relation to hormone receptor status.
Methods
Paraffin-embedded tumor material was collected from 480 early-stage breast cancer patients younger than 41 years who participated in one of four European Organization for Research and Treatment of Cancer trials. Using immunohistochemistry on the whole series of tumors, we assessed estrogen receptor (ER) status and progesterone receptor (PgR) status in a standardized way. Endpoints in this study were overall survival (OS) and distant metastasis-free survival (DMFS). The median follow-up period was 7.3 years.
Results
Overall, patients with ER-positive tumors had better OS rates (hazard ratio [HR] 0.63; P = 0.02) compared with those with ER-negative tumors. However, in the subgroup of patients who received chemotherapy, no significant difference in OS (HR 0.87; P = 0.63) and DMFS (HR 1.36; P = 0.23) was found between patients with ER-positive tumors or those with ER-negative tumors. These differences were similar for PgR status.
Conclusion
Young patients with hormone receptor-positive tumors benefit less from adjuvant systemic chemotherapy than patients with hormone receptor-negative tumors. These results confirm that chemotherapy alone cannot be considered optimal adjuvant systemic treatment in breast cancer patients 40 years old or younger with hormone receptor-positive tumors.
Introduction
Breast cancer in premenopausal women is associated with worse outcome compared with postmenopausal patients [1]. Approximately 7% of women diagnosed with breast cancer are younger than 40 years old [2]. Very young women (that is, younger than 35 years old), especially, are at a high risk of developing distant metastases. Therefore, they are recommended to receive adjuvant systemic chemotherapy regardless of tumor stage or grade [3]. In addition, high local regional recurrence rates after breast-conserving therapy have been reported in young premenopausal patients with breast cancer [4]. Although it is clear that young age is an independent prognosticator of adverse outcome in breast cancer, controversies regarding the optimal treatment in this population exist.
Adjuvant systemic chemotherapy in premenopausal patients has been shown to improve survival [1], but controversy about the role of chemotherapy in patients with hormone receptor-positive tumors still exists. Aebi and colleagues [5] clearly showed that the endocrine effects of chemotherapy alone might not be sufficient for very young patients with breast cancer. In this study, it was shown that estrogen receptor (ER)-positive tumors in patients younger than 35 years and treated with cyclophosphamide, methotrexate, and 5-fluorouracil (CMF) had a significantly worse disease-free survival compared with ER-negative patients.
To detect whether we could confirm these data by finding similar results, we studied the efficacy of chemotherapy in young patients with breast cancer according to ER status and progesterone receptor (PgR) status and selected patients 40 years old or younger at the time of primary diagnosis from four European Organization for Research and Treatment of Cancer (EORTC) trials that were conducted by the EORTC Breast Cancer Group and Radiotherapy Group.
Materials and methods
Data were collected from four EORTC trials. In total, 9,938 patients participated in these trials; 934 of these patients were 40 years old or younger at the time of diagnosis. The trial designs are summarized below:
EORTC trial 10801 (1980 to 1986, median follow-up 13.4 years) was conducted to assess the safety of breast-conserving treatment. In this trial, patients were randomly assigned between breast-conserving surgery combined with radiotherapy and radical mastectomy. Six cycles of adjuvant chemotherapy with cyclophosphamide 100 mg/m2 given orally on days 1 to 14, methotrexate 40 mg/m2 given intravenously on days 1 and 8, and 5-fluorouracil 600 mg/m2 (CMF) given intravenously on days 1 and 8 were indicated for all patients under the age of 55 with positive nodes. A total of 902 patients were randomly assigned [6].
EORTC trial 10854 (1986 to 1991, median follow-up 10.8 years) studied the question of whether one course of perioperative chemotherapy given directly after surgery yields better results in terms of treatment outcome than surgery alone. Perioperative chemotherapy consisted of a single course of doxorubicin 50 mg/m2, 5-fluorouracil 600 mg/m2, and cyclophosphamide 600 mg/m2 (FAC) administered intravenously within 36 hours after surgery. For axillary lymph node-positive premenopausal patients in the perioperative chemotherapy group, adjuvant chemotherapy consisting of five cycles of CMF was recommended. For node-positive patients younger than 50 years who did not receive perioperative chemotherapy, one conventional course of FAC followed by five cycles of CMF after surgery was recommended. Postmenopausal patients were recommended to receive tamoxifen. A total of 2,795 patients were included [7].
EORTC trial 10902 (1991 to 1999, median follow-up 6.1 years) was set up to determine the value of preoperative chemotherapy. Patients were randomly assigned to receive four cycles of chemotherapy either before or after surgery. Chemotherapy consisted of four cycles of 5-fluorouracil 600 mg/m2, epirubicin 60 mg/m2, and cyclophosphamide 600 mg/m2 (FEC) administered intravenously at 3-weekly intervals. In the preoperative chemotherapy group, surgical therapy followed within 4 weeks of the fourth course of chemotherapy. In the postoperative chemotherapy group, the first cycle was given within 36 hours after surgery. Patients not younger than 50 years received tamoxifen for 2 years. A total of 698 patients were randomly assigned [8].
EORTC trial 22881 (1989 to 1996, median follow-up 5.1 years) was conducted to study the effect of an additional dose of 16 Gy radiation to the tumor bed among early-stage breast cancer patients who received 50 Gy radiotherapy after lumpectomy. Patients with a microscopically incomplete resection were assigned to receive boost doses of 10 or 26 Gy. Premenopausal patients with axillary lymph node involvement received six cycles of adjuvant CMF, and all postmenopausal patients received tamoxifen 20 mg per day during at least 2 years. A total of 5,569 patients were enrolled [9].
In all trials, if adjuvant chemotherapy was indicated, patients received either CMF or an anthracyclin-based regimen (FAC or FEC). Adjuvant hormonal therapy for premenopausal hormone receptor-positive patients was not yet recommended at the time these trials were conducted. In the two oldest trials, tamoxifen administration was not even recorded. This explains the high number of patients for whom no information was found on tamoxifen use. In the trials in which tamoxifen use was recorded, less than 5% of patients 41 years old or younger received tamoxifen. Therefore, we have to assume that only a very small fraction of the patient population in this study received tamoxifen.
Hormone receptor staining
Paraffin-embedded tumor material was collected from 480 patients 40 years old or younger. Tumors were histologically graded using hematoxylin and eosin slides as described previously [10]. Immunohistochemical staining for ER and PgR status was performed using a tissue microarray [11-14]. Three core biopsies were taken from each tumor block and inserted into a donor block. Immunohistochemical staining for ER was performed using the monoclonal antibody DAKO-ER, 1D5 (DakoCytomation Denmark A/S, Glostrup, Denmark), for PgR using the monoclonal antibody mPRI (TRANSBIO, Paris, France). Immunohistochemical staining was scored using a semiquantative system based on the percentage of positive nuclei. After the percentage of positive nuclei in three core biopsies was counted, the mean value was taken. For both ER and PgR, tumors with greater than 10% of the tumor cells showing nuclear staining were considered positive.
Statistical analysis
Analyses were performed for distant metastasis-free survival (DMFS) and overall survival (OS). DMFS was defined as the interval from time of randomization to time of distant metastasis or death, whichever came first. OS was defined as time from randomization to death from any cause. Survival curves were estimated using the Kaplan-Meier method [15]. Differences in survival were analyzed using Cox proportional hazard models [16]. The statistical analyses were performed using SPSS software (SPSS Inc., Chicago, IL, USA). A direct comparison of patients who received chemotherapy versus those who did not receive chemotherapy was not feasible. (This would have introduced a selection bias in this retrospective analysis as the vast majority of patients receiving chemotherapy had positive axillary lymph nodes.) Therefore, conclusions in this explorative analysis were based on indirect comparisons.
Results
Participants
Paraffin-embedded tumor specimens were collected from 480 patients 40 years old or younger at the time of diagnosis (Table 1). For 12 patients, ER status could not be scored, and for 16 patients, PgR status could not be scored. Positive ER status and positive PgR status were found in 288 and 223 patients, respectively. Two hundred patients received prolonged adjuvant systemic chemotherapy, whereas 279 patients did not receive adjuvant systemic chemotherapy. Of the patients not receiving chemotherapy, 94% were node-negative; 85% of patients who did receive chemotherapy were node-positive. Characteristics related to adjuvant systemic chemotherapy treatment are listed in Table 2. At the time of the analysis, the median follow-up was 7.3 years, 106 (22%) patients had died, and 155 (32%) patients developed a distant recurrence or died. The distribution of events stratified by ER status is listed in Table 3.
Table 1.
Characteristics of 480 breast cancer patients 40 years old or younger
| Characteristic | Number of patients (percentage) Total = 480 | |
| Clinical tumor size | T1 | 185 (39) |
| T2/3 | 293 (61) | |
| Missing data | 2 (0) | |
| Histological tumor size | T1 | 292 (61) |
| T2/3 | 151 (31) | |
| Missing data | 37 (8) | |
| Histological nodal status | Negative | 288 (60) |
| Positive | 188 (39) | |
| Missing data | 4 (1) | |
| Surgery | Breast-conserving surgery | 393 (82) |
| Mastectomy | 86 (18) | |
| Missing data | 1 (0) | |
| Adjuvant chemotherapy | No | 279 (58) |
| Yes | 200 (42) | |
| Missing data | 1 (0) | |
| Tamoxifena | No | 273 (57) |
| Yes | 9 (2)b | |
| Missing data | 198 (41) | |
| Histological grade | I | 70 (15) |
| II | 145 (30) | |
| III | 255 (53) | |
| Missing data | 10 (2) | |
| Estrogen receptor | Positive | 288 (60) |
| Negative | 180 (38) | |
| Missing data | 12 (3) | |
| Progesterone receptor | Positive | 223 (46) |
| Negative | 241 (50) | |
| Missing data | 16 (3) |
aDuring the period of time in which these trials were conducted, tamoxifen was not routinely given to premenopausal patients with estrogen receptor (ER)-positive tumors. bAll patients who received tamoxifen had ER-positive tumors. T, tumor size.
Table 2.
Patient and tumor characteristics specified by adjuvant chemotherapya
| Characteristic | Number of patients (percentage) | |
| No adjuvant chemotherapy Number = 279 | Adjuvant chemotherapy Number = 200 | |
| ER-positive | 161 (58) | 126 (63) |
| Anthracycline-based | 66 | |
| CMF | 60 | |
| ER-negative | 110 (39) | 70 (35) |
| Anthracycline-based | 48 | |
| CMF | 22 | |
| PgR-positive | 135 (48) | 88 (44) |
| PgR-negative | 135 (48) | 105 (53) |
| T1 | 187 (67) | 105 (53) |
| T2/T3 | 76 (27) | 75 (38) |
| Node-negative | 259 (93) | 29 (15) |
| Node-positive | 18 (6) | 170 (85) |
| Breast-conserving surgery | 247 (89) | 146 (73) |
| Mastectomy | 32 (11) | 53 (27) |
aMissing data not shown. CMF, cyclophosphamide, methotrexate, and 5-fluorouracil; ER, estrogen receptor; PgR, progesterone receptor; T, tumor size.
Table 3.
Distribution of events according to ER status and chemotherapya
| Number of events (percentage) | ||
| No adjuvant chemotherapy | Adjuvant chemotherapy | |
| Deaths (number of events = 106) | ||
| ER-positive | 19 (18) | 35 (33) |
| ER-negative | 29 (27) | 19 (18) |
| Distant metastasis or death (number of events = 155) | ||
| ER-positive | 37 (24) | 54 (35) |
| ER-negative | 38 (25) | 21 (14) |
aMissing data not shown. ER, estrogen receptor.
Overall results
Estrogen receptor status
Overall, patients with ER-positive tumors had better OS rates compared with ER-negative patients (hazard ratio [HR] 0.63, 95% confidence interval (CI) 0.43 to 0.93; P = 0.02) (Figure 1). Survival rates at 7 years were 82% for the ER-positive group and 77% for the ER-negative group. DMFS rates were 70% for the ER-positive group and 66% for the ER-negative group (HR 0.90, 95% CI 0.65 to 1.24; P = 0.51) (Figure 2).
Figure 1.
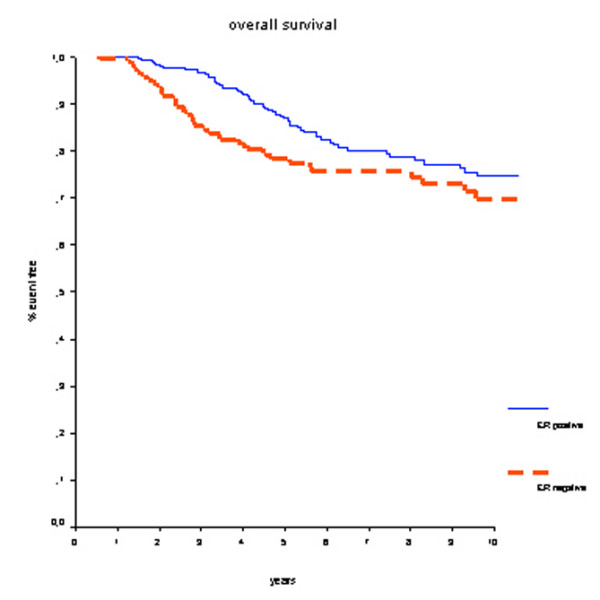
Overall survival for all patients. Estrogen receptor (ER)-positive patients have a better prognosis.
Figure 2.
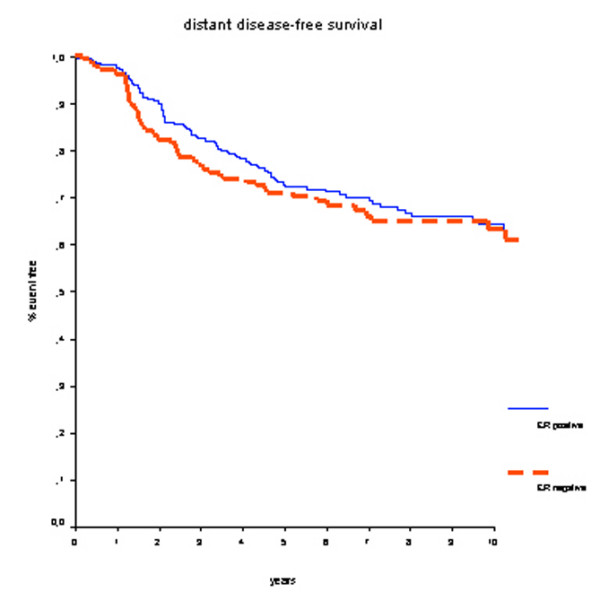
Distant metastasis-free survival for all patients. There is no difference in prognosis according to estrogen receptor (ER) status.
Progesterone receptor status
PgR status yielded similar results: patients with progesterone-positive tumors had better OS (HR 0.59, 95% CI 0.40 to 0.88; P = 0.01). However, for DMFS this difference was not of statistical significance (HR 0.78, 95% CI 0.57 to 1.01; P = 0.14).
Patients who did not receive prolonged adjuvant chemotherapy
Estrogen receptor status
In patients who did not receive adjuvant systemic chemotherapy, positive ER status was associated with better OS (HR 0.41, 95% CI 0.23 to 0.74; P < 0.01) (Figure 3). Survival rates at 7 years were 90% for the ER-positive group and 77% for the ER-negative group. Also, DMFS rates at 7 years were significantly better for ER-positive patients: 80% versus 64% (HR 0.59, 95% CI 0.37 to 0.92; P = 0.02) (Figure 4).
Figure 3.
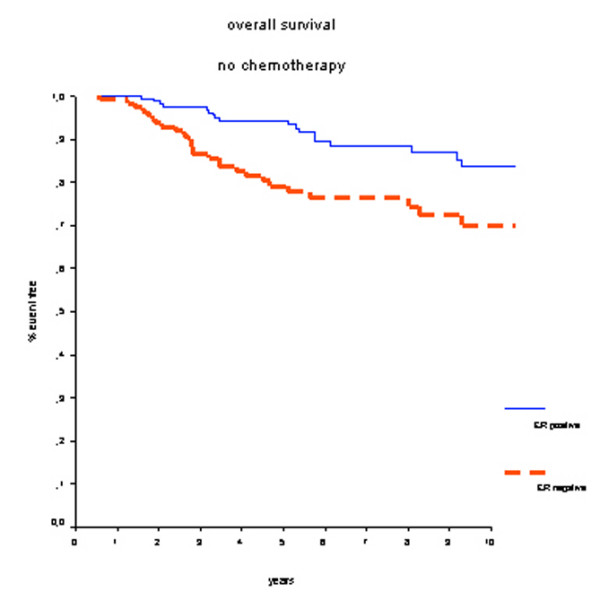
Overall survival in patients who did not receive adjuvant chemotherapy. Estrogen receptor (ER)-positive patients have a better prognosis.
Figure 4.
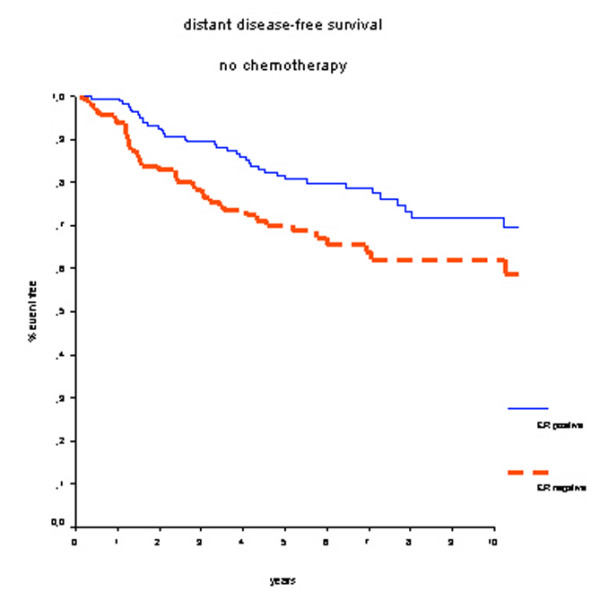
Distant metastasis-free survival in patients who did not receive adjuvant chemotherapy. Estrogen receptor (ER)-positive patients have a better prognosis.
Progesterone receptor status
In patients who did not receive adjuvant systemic chemotherapy, positive PgR status was associated with better OS (HR 0.44, 95% CI 0.24 to 0.80; P < 0.01). Survival rates at 7 years were 88% for the PgR-positive group and 75% for PgR-negative group. DMFS rates at 7 years were 79% for PgR-positive patients and 67% for PgR-negative patients (HR 0.66, 95% CI 0.42 to 1.04; P = 0.07).
Patients who received prolonged adjuvant systemic chemotherapy
Estrogen receptor status
In the group of 200 patients who did receive adjuvant systemic chemotherapy, treatment outcome was not significantly different between ER-positive and ER-negative breast cancer patients. Survival rates at 7 years were 70% for the ER-positive group and 75% for the ER-negative group (HR 0.87, 95% CI 0.50 to 1.52; P = 0.63) (Figure 5), and DMFS rates were 59% for the ER-positive group and 70% for the ER-negative group (HR 1.36, 95% CI 0.82 to 2.26; P = 0.23) (Figure 6). No further subgroup analyses specified by type of chemotherapy were performed since these groups would have had insufficient numbers and events.
Figure 5.
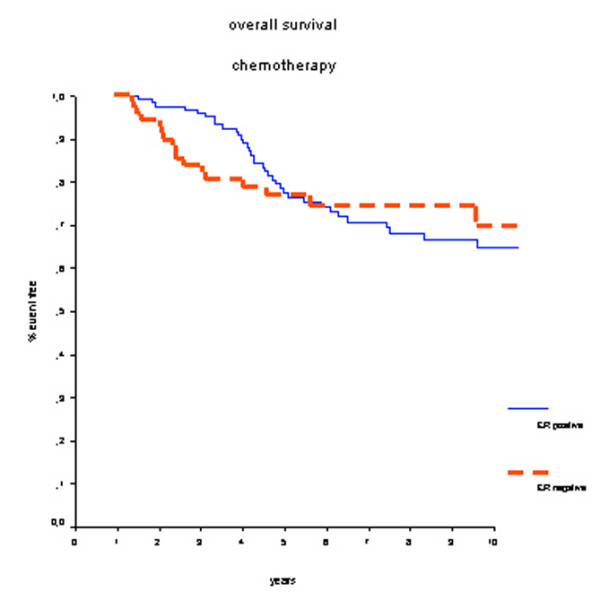
Overall survival in patients who received adjuvant chemotherapy. There is no long-term survival benefit for estrogen receptor (ER)-positive patients. The crossing lines could be explained by the initial beneficial effect of chemotherapy-induced amenorrhea.
Figure 6.
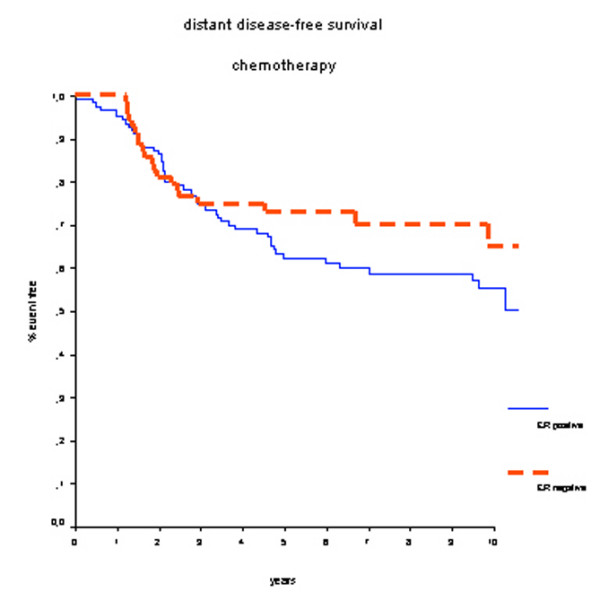
Distant metastasis-free survival in patients who received adjuvant chemotherapy. There is no statistically significant difference in prognosis according to estrogen receptor (ER) status.
Progesterone receptor status
According to PgR status, no difference in treatment outcome for patients who have received adjuvant systemic chemotherapy was found. In both the PgR-positive and PgR-negative patient groups, the survival rate at 7 years was 72% (HR 0.84, 95% CI 0.49 to 1.43; P = 0.51). Also, DMFS rates did not differ significantly between the PgR-positive group (59%) and the PgR-negative group (64%) (HR 1.02, 95% CI 0.65 to 1.60; P = 0.93).
Multivariate analysis
Multivariate Cox regression OS analyses were performed separately for ER status and PgR status. Other covariates included nodal status, tumor size, and the administration of prolonged adjuvant chemotherapy. Both ER status (relative risk [RR] 1.65) and PgR status (RR 1.56; data not shown) remained independent prognostic factors with a significant impact on OS (Table 4).
Table 4.
Multivariate Cox regression analysis of overall survival
| Characteristic | Relative risk | 95% confidence interval | P value |
| ER-negative | 1.65 | 1.09–2.50 | 0.02 |
| Node-positive | 1.70 | 0.79–3.66 | 0.17 |
| Tumor size >2 cm | 1.66 | 1.09–2.52 | 0.02 |
| Adjuvant chemotherapy | 1.02 | 0.48–2.17 | 0.96 |
ER status remains an independent prognostic factor of overall survival. ER, estrogen receptor.
Discussion
This pooled analysis of patients 40 years old or younger demonstrated that hormone receptor-positive patients experienced no survival advantage of prolonged adjuvant CMF chemotherapy compared with hormone receptor-negative patients. However, in patients who did not receive adjuvant chemotherapy, hormone receptor-positive status was associated with improved survival rates compared with hormone receptor-negative status. In overall multivariate analyses, both ER-positive status and PgR-positive status remained independent prognostic factors of OS.
Our study has some limitations. First, the current analysis retrospectively uses heterogeneous data from different randomized trials. Second, adjuvant CMF chemotherapy to a large extent has been replaced by anthracycline-containing chemotherapeutic regimens because of higher treatment efficacy in patients with breast cancer regardless of hormone receptor or menopausal status [1]. Also, taxanes are increasingly being used, showing additional survival benefits. Therefore, different effects might have been demonstrated when newer chemotherapy regimens were used throughout the included studies. Third, the direct comparison between administration of chemotherapy versus no chemotherapy in hormone receptor-positive and hormone receptor-negative patients would have been very interesting. However, the confounding effect of axillary lymph node status would have introduced a significant selection bias because the majority of patients who received chemotherapy had positive axillary lymph nodes. Nevertheless, in multivariate analysis including axillary lymph node status, tumor size, and the administration of prolonged adjuvant chemotherapy, hormone receptor status remained an independent prognostic factor for OS. Fourth, the survival curves of the ER-positive and ER-negative group depicted in Figure 5 (overall survival in patients receiving chemotherapy) are crossing. This implies that that the proportional hazards assumption is not justified. The rapid decrease in survival benefit after a couple of years in the ER-positive group may well be explained by the chemotherapy-induced amenorrhea and the associated low estrogen levels. Unfortunately, no information on the number of patients who have become amenorrheatic could be retrieved in order to test this hypothesis. Despite these limitations, this pooled analysis of four randomized controlled trials used individual patient data with renewed pathological analysis of hormone receptor status. Because less than 5% of the study population received tamoxifen, the effect of chemotherapy alone in hormone receptor-positive patients could be well studied. By analyzing hormone receptor status centrally, we have provided standardized measurements for all tumors in the study.
Adjuvant systemic chemotherapy is a well-established treatment modality in premenopausal breast cancer. In patients 35 years old or younger, administration of chemotherapy is advocated regardless of nodal status, tumor size, or grade [3]. However, the efficacy of chemotherapy in premenopausal patients with ER-positive breast cancer has been questioned [5,17,18]. Our findings are in accordance with data from Aebi and colleagues [5], who demonstrated that young premenopausal patients with breast cancer treated with adjuvant CMF chemotherapy had a higher risk of relapse and death than older premenopausal patients, especially if their tumors were ER-positive. In addition, several neoadjuvant chemotherapy studies have demonstrated that patients with ER-negative tumors are more likely to achieve a pathological complete response than those with ER-positive tumors [19-21]. Moreover, these studies found that when patients with ER-negative tumors achieved a pathological complete response their survival was comparable with that of ER-positive patients.
To optimize adjuvant systemic treatment in premenopausal patients with breast cancer, several investigators have studied the role of ovarian suppression by luteinizing hormone-releasing hormone (LHRH) agonists. Recently, the Early Breast Cancer Overview group reported a meta-analysis of individual patient data on the use of LHRH agonists [22]. When chemotherapy alone was compared with chemotherapy in combination with an LHRH agonist, a difference between younger and older premenopausal women with hormone receptor-positive disease was found. In patients 40 years old or younger, the addition of an LHRH agonist significantly reduced the risk of recurrence and death (HR 0.74; p = 0.01). This effect was greatest in the group 35 years old or younger, whereas in the group older than 40 years, the addition of an LHRH agonist did not improve outcome. When chemotherapy alone was compared with LHRH agonist with or without tamoxifen in younger premenopausal patients with hormone receptor-positive tumors, the endocrine therapy improved outcome (mortality HR 0.82; P = 0.15). Conversely, in hormone receptor-negative patients, the same comparison significantly favored treatment with chemotherapy (62.1% increased rate of recurrence or death; P = 0.003). To date, no trial has compared an LHRH agonist against chemotherapy with tamoxifen in both arms. This relevant and important issue needs to be resolved. Although these results underline the fact that chemotherapy may be equivalent to hormonal ovarian suppression in terms of treatment outcome in hormone receptor-positive patients, these results firmly demonstrate a beneficial effect of LHRH agonists as additional therapy, especially in young patients with breast cancer.
Three important ongoing trials are specifically investigating ovarian function suppression (Suppression of Ovarian Function Trial, or SOFT), an aromatase inhibitor (Tamoxifen and EXemestane Trial, or TEXT), and the need for chemotherapy (Premenopausal Endocrine Responsive CHEmotherapy, or PERCHE) in adjuvant treatment for young patients with hormone receptor-positive breast cancer [23].
The 2005 St. Gallen Consensus Committee on adjuvant therapy for early-stage breast cancer recommended that the first consideration in treatment selection be endocrine responsiveness [24]. Three categories are identified: endocrine-responsive, endocrine-nonresponsive, and tumors of uncertain endocrine responsiveness. These categories refer to the groups of tumors that are responsive to endocrine therapies alone, chemotherapy alone, and chemotherapy and endocrine therapy combinations, respectively. The 2005 Panel viewed tamoxifen as a standard adjuvant treatment for premenopausal endocrine-responsive patients. The combination of tamoxifen with an LHRH agonist is recommended for very young patients, especially in intermediate- and high-risk groups, and for premenopausal patients of any age at high risk, especially if chemotherapy did not induce amenorrhea. The use of aromatase inhibitors in premenopausal patients is not recommended outside of clinical trials, except when tamoxifen is contraindicated, especially in node-positive disease. Chemotherapy in addition to hormone therapy is advised for endocrine-responsive patients with node-positive disease.
Conclusion
In this retrospective pooled analysis of four studies using heterogeneous chemotherapy regimens, we have demonstrated that treatment efficacy of adjuvant chemotherapy is less in young patients with hormone receptor-positive tumors compared with young patients with hormone receptor-negative tumors. Therefore, we conclude that chemotherapy alone is not a sufficient systemic treatment strategy in young patients with hormone receptor-positive breast cancer. Hormone responsiveness is the key for tailoring therapy for young patients with breast cancer.
Abbreviations
CI = confidence interval; CMF = cyclophosphamide, methotrexate, and 5-fluorouracil; DMFS = distant metastasis-free survival; EORTC = European Organization for Research and Treatment of Cancer; ER = estrogen receptor; FAC = doxorubicin, 5-fluorouracil, and cyclophosphamide; FEC = 5-fluorouracil, epirubicin, and cyclophosphamide; HR = hazard ratio; LHRH = luteinizing hormone-releasing hormone; OS = overall survival; PgR = progesterone receptor; RR = relative risk.
Competing interests
JAvdH was a research fellow at the EORTC from 1999 to 2000. The other authors declare that they have no competing interests.
Authors' contributions
JAvdH was responsible for the acquisition of data and analysis and interpretation of the data and drafted the manuscript. JSDM and MJvdV were involved in drafting the manuscript and revised it critically for important intellectual content. CJHvdV revised the manuscript critically for important intellectual content and gave final approval of the version to be published. All authors read and approved the final manuscript.
Contributor Information
Jos A van der Hage, Email: j.a.van_der_hage@lumc.nl.
J Sven D Mieog, Email: j.s.d.mieog@lumc.nl.
Marc J van de Vijver, Email: m.vd.vijver@nki.nl.
Cornelis JH van de Velde, Email: c.j.h.van_de_velde@lumc.nl.
References
- Early Breast Cancer Trialists' Collaborative Group Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;365:1687–1717. doi: 10.1016/S0140-6736(05)66544-0. [DOI] [PubMed] [Google Scholar]
- Hankey BF, Miller B, Curtis R, Kosary C. Trends in breast cancer in younger women in contrast to older women. J Natl Cancer Inst Monogr. 1994;16:7–14. [PubMed] [Google Scholar]
- Goldhirsch A, Glick JH, Gelber RD, Coates AS, Senn HJ. Meeting highlights: International Consensus Panel on the Treatment of Primary Breast Cancer. Seventh International Conference on Adjuvant Therapy of Primary Breast Cancer. J Clin Oncol. 2001;19:3817–3827. doi: 10.1200/JCO.2001.19.18.3817. [DOI] [PubMed] [Google Scholar]
- Elkhuizen PH, van Slooten HJ, Clahsen PC, Hermans J, van de Velde CJH, van den Broek LC, van de Vijver MJ. High local recurrence risk after breast-conserving therapy in node-negative premenopausal breast cancer patients is greatly reduced by one course of perioperative chemotherapy: a European Organization for Research and Treatment of Cancer Breast Cancer Cooperative Group Study. J Clin Oncol. 2000;18:1075–1083. doi: 10.1200/JCO.2000.18.5.1075. [DOI] [PubMed] [Google Scholar]
- Aebi S, Gelber S, Castiglione-Gertsch M, Gelber RD, Collins J, Thurlimann B, Rudenstam CM, Lindtner J, Crivellari D, Cortes-Funes H, et al. Is chemotherapy alone adequate for young women with oestrogen-receptor-positive breast cancer? Lancet. 2000;355L:1869–1874. doi: 10.1016/S0140-6736(00)02292-3. [DOI] [PubMed] [Google Scholar]
- van Dongen JA, Voogd AC, Fentiman IS, Legrand C, Sylvester RJ, Tong D, van der Schueren E, Helle PA, van Zyl K, Bartelink H. Long-term results of a randomized trial comparing breast-conserving therapy with mastectomy: European Organization for Research and Treatment of Cancer 10801 trial. J Natl Cancer Inst. 2000;92:1143–1150. doi: 10.1093/jnci/92.14.1143. [DOI] [PubMed] [Google Scholar]
- van der Hage JA, van De Velde CJ, Julien JP, Floiras JL, Delozier T, Vandervelden C, Duchateau L. Improved survival after one course of perioperative chemotherapy in early breast cancer patients: long-term results from the European Organization for Research and Treatment of Cancer (EORTC) Trial 10854. Eur J Cancer. 2001;37:2184–2193. doi: 10.1016/S0959-8049(01)00294-5. [DOI] [PubMed] [Google Scholar]
- van der Hage JA, van de Velde CJ, Julien JP, Tubiana-Hulin M, Vandervelden C, Duchateau L. Preoperative chemotherapy in primary operable breast cancer: results from the European organization for research and treatment of cancer trial 10902. J Clin Oncol. 2001;19:4224–4237. doi: 10.1200/JCO.2001.19.22.4224. [DOI] [PubMed] [Google Scholar]
- Bartelink H, Horiot JC, Poortmans P, Struikmans H, Van den Bogaert W, Barillot I, Fourquet A, Borger J, Jager J, Hoogenraad W, et al. European Organization for Research and Treatment of Cancer Radiotherapy and Breast Cancer Groups: Recurrence rates after treatment of breast cancer with standard radiotherapy with or without additional radiation. N Engl J Med. 2001;345:1378–1387. doi: 10.1056/NEJMoa010874. [DOI] [PubMed] [Google Scholar]
- Bloom HJG, Richardson WW. Histological grading and prognosis in breast cancer: a study of 1409 cases of which 359 have been followed for 15 years. Br J Cancer. 1957;11:359–377. doi: 10.1038/bjc.1957.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bubendorf L, Nocito A, Moch H, Sauter G. Tissue microarray (TMA) technology: miniaturized pathology archives for high-throughput in situ studies. J Pathol. 2001;195:72–79. doi: 10.1002/path.893. [DOI] [PubMed] [Google Scholar]
- Hoos A, Urist MJ, Stojadinovic A, Mastorides S, Dudas ME, Leung DH, Kuo D, Brennan MF, Lewis JJ, Cordon-Cardo C. Validation of tissue microarrays for immunohistochemical profiling of cancer specimens using the example of human fibroblastic tumors. Am J Pathol. 2001;158:1245–1251. doi: 10.1016/S0002-9440(10)64075-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller-Holzner E, Fink V, Frede T, Marth C. Immunohistochemical determination of HER2 expression in breast cancer from core biopsy specimens: a reliable predictor of HER2 status of the whole tumor. Breast Cancer Res Treat. 2001;69:13–19. doi: 10.1023/A:1012281221647. [DOI] [PubMed] [Google Scholar]
- Kallioniemi OP, Wagner U, Kononen J, Sauter G. Tissue microarray technology for high-throughput molecular profiling of cancer. Hum Mol Genet. 2001;10:657–662. doi: 10.1093/hmg/10.7.657. [DOI] [PubMed] [Google Scholar]
- Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. doi: 10.2307/2281868. [DOI] [Google Scholar]
- Cox DR. Regression models and life-tables. J R Stat Assoc [B] 1972;34:187–220. [Google Scholar]
- Viale G, Gelber RD, Mastropasqua MG, Maiorano E, Aldrighetto S, Price KN, Castiglione-Gertsch M, Goldhirsch A. Chemo-endocrine effects of adjuvant therapies for postmenopausal node-negative breast cancer according to ER, PgR, and Her2 assessed centrally. Breast Cancer Res Treat. 2003;82S:S16–S17. [Google Scholar]
- Albain K, Barlow W, O'Malley F, Siziopikou K, Yeh I-T, Ravdin P, Lew D, Farrar W, Burton G, Ketchel S, for the Breast Cancer Intergroup of North America et al. Concurrent (CAFT) versus sequential (CAF-T) chemohormonal therapy (cyclophosphamide, doxorubicin, 5-fluorouracil, tamoxifen) versus T alone for postmenopausal, node-positive, estrogen (ER) and/or progesterone (PgR) receptor-positive breast cancer: mature outcomes and new biologic correlates on phase III intergroup trial 0100 (SWOG-8814) Breast Cancer Res Treat. 2004;88S abstract 37. [Google Scholar]
- Ring AE, Smith IE, Ashley S, Fulford LG, Lakhani SR. Oestrogen receptor status, pathological complete response and prognosis in patients receiving neoadjuvant chemotherapy for early breast cancer. Br J Cancer. 2004;91:2012–2017. doi: 10.1038/sj.bjc.6602235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colleoni M, Viale G, Zahrieh D, Pruneri G, Gentilini O, Veronesi P, Gelber RD, Curigliano G, Torrisi R, Luini A, et al. Chemotherapy is more effective in patients with breast cancer not expressing steroid hormone receptors: a study of preoperative treatment. Clin Cancer Res. 2004;10:6622–6628. doi: 10.1158/1078-0432.CCR-04-0380. [DOI] [PubMed] [Google Scholar]
- Kuerer HM, Newman LA, Smith TL, Ames FC, Hunt KK, Dhingra K, Theriault RL, Singh G, Binkley SM, Sneige N, et al. Clinical course of breast cancer patients with complete pathologic primary tumor and axillary lymph node response to doxorubicin-based neoadjuvant chemotherapy. J Clin Oncol. 1999;17:460–469. doi: 10.1200/JCO.1999.17.2.460. [DOI] [PubMed] [Google Scholar]
- LHRH-agonists in Early Breast Cancer Overview group. Cuzick J, Ambroisine L, Davidson N, Jakesz R, Kaufmann M, Regan M, Sainsbury R. Use of luteinising-hormone-releasing hormone agonists as adjuvant treatment in premenopausal patients with hormone-receptor-positive breast cancer: a meta-analysis of individual patient data from randomised adjuvant trials. Lancet. 2007;369:1711–1723. doi: 10.1016/S0140-6736(07)60778-8. [DOI] [PubMed] [Google Scholar]
- Jonat W, Pritchard KI, Sainsbury R, Klijn JG. Trends in endocrine therapy and chemotherapy for early breast cancer: a focus on the premenopausal patient. J Cancer Res Clin Oncol. 2006;132:275–286. doi: 10.1007/s00432-006-0082-z. [DOI] [PubMed] [Google Scholar]
- Goldhirsch A, Glick JH, Gelber RD, Coates AS, Thürlimann B, Senn HJ, Panel members Meeting highlights: international expert consensus on the primary therapy of early breast cancer 2005. Ann Oncol. 2005;16:1569–1583. doi: 10.1093/annonc/mdi326. [DOI] [PubMed] [Google Scholar]


