Abstract
Sickle trait, the heterozygous state of normal hemoglobin A (HbA) and sickle hemoglobin S (HbS), confers protection against malaria in Africa. AS children infected with Plasmodium falciparum are less likely than AA children to suffer the symptoms or severe manifestations of malaria, and they often carry lower parasite densities than AA children. The mechanisms by which sickle trait might confer such malaria protection remain unclear. We have compared the cytoadherence properties of parasitized AS and AA erythrocytes, because it is by these properties that parasitized erythrocytes can sequester in postcapillary microvessels of critical tissues such as the brain and cause the life-threatening complications of malaria. Our results show that the binding of parasitized AS erythrocytes to microvascular endothelial cells and blood monocytes is significantly reduced relative to the binding of parasitized AA erythrocytes. Reduced binding correlates with the altered display of P. falciparum erythrocyte membrane protein-1 (PfEMP-1), the parasite's major cytoadherence ligand and virulence factor on the erythrocyte surface. These findings identify a mechanism of protection for HbS that has features in common with that of hemoglobin C (HbC). Coinherited hemoglobin polymorphisms and naturally acquired antibodies to PfEMP-1 may influence the degree of malaria protection in AS children by further weakening cytoadherence interactions.
Keywords: disease severity, malaria, PfEMP-1, hemoglobin S, hemoglobin C
Evolutionary pressure from Plasmodium falciparum malaria on human populations has selected various erythrocyte polymorphisms that protect against severe complications and death from the disease. One important example is the mutation of sickle hemoglobin (HbS), a glutamate-to-valine substitution in the sixth position of the β-globin chain (1, 2). Molecular-genetic evidence suggests that this mutation has been selected independently at least five times in Africa, Arabia, and India. Although the homozygous SS (sickle cell disease) state is often fatal to young children, high frequencies (in some regions >20%) of the HbS gene are maintained by the protection that the benign heterozygous AS (sickle trait) state can confer to children against life-threatening P. falciparum malaria. Epidemiological evidence for this protection is convincing, yet the mechanisms by which HbS exerts protection remain unclear. Studies have shown that AS children with parasitemia are 50–90% less likely to progress to mild or severe malaria (3–6), and AS children who do develop severe malaria are less likely to die from the disease (7). Although sickle trait does not seem to protect children against infection by P. falciparum parasites, AS children generally have lower parasite densities than AA children during episodes of asymptomatic infection and symptomatic malaria (1, 3, 6, 8, 9).
Investigators have proposed several mechanisms by which AS erythrocytes might impede the ability of parasites to multiply to high density in vivo and thereby protect against malaria. Luzzatto et al. (10) reported a sickling rate of parasitized AS erythrocytes that was significantly greater than that of nonparasitized AS erythrocytes and suggested that enhanced sickling of parasitized AS erythrocytes in vivo promotes their increased removal from the blood by the spleen. Friedman (11) found that parasite development was impaired in AS erythrocytes under reduced oxygen conditions and proposed that the sequestration of parasitized AS erythrocytes in the low-oxygen environment of postcapillary venules could result in parasite death in situ. Pasvol et al. (12) reported both impaired invasion and development of parasites in AS erythrocytes under conditions of reduced oxygen tension and also suggested that these abnormalities could lead to reductions in parasite density in vivo. Under standard culture conditions in vitro, normal parasite development in AA and AS erythrocytes has been reported (12–14). At least some AS erythrocytes seem to support efficient invasion, development, and replication of parasites in vivo because relatively high parasitemias (>10,000/μl whole blood) are found in naturally infected AS children (3, 5, 6, 15, 16) and in experimentally infected nonimmune AS adults (17).
Another mutation in the sixth position of the β-globin chain is that of hemoglobin C (HbC; glutamate-to-lysine), which also protects against malaria (6, 15, 18, 19). Our recent studies have indicated reduced cytoadherence of parasitized HbC erythrocytes as a mechanism of malaria protection (20). This effect would reduce the inflammation associated with sequestration in the pathogenesis of mild and severe disease and also would reduce rosetting, which is associated with cerebral malaria (21, 22). P. falciparum erythrocyte membrane protein-1 (PfEMP-1) expressed on knob-like protrusions at the surface of parasitized erythrocytes (23–27) promotes their adherence to microvascular endothelial cells, thereby enabling parasites to avoid clearance from the bloodstream by the spleen. Although specific receptors and interactions can vary, PfEMP-1 typically attaches to CD36 (28–30), the main host cytoadherence receptor on the surface of endothelial cells and blood monocytes. PfEMP-1 attachment to ICAM-1 is believed to be a major mediator of parasitized erythrocyte adherence to cerebral microvessels, which generally do not express CD36, although CD36-mediated interactions with other cells in these vessels are thought to contribute to pathogenesis through processes of monocyte and platelet recruitment, cytokine release, and fibrin deposition, aggravating inflammation. The sequestration of large numbers of parasitized erythrocytes in the systemic and cerebral microvascular beds also contributes to the pathogenesis of severe malaria by inducing an increased production of cytokines and other inflammatory mediators from endothelial cells and blood monocytes (31, 32). PfEMP-1 also binds complement receptor 1 on noninfected erythrocytes to form rosettes, which are believed to impair microcirculatory flow and to contribute to the ischemic complications of malaria (33, 34).
In light of the influence of HbC on the binding properties of parasitized erythrocytes, we have performed experiments to test whether HbS also might alter the display of PfEMP-1 and reduce the cytoadherence of P. falciparum-infected erythrocytes as a mechanism of malaria protection.
Results
Parasitized AS and SS Erythrocytes Show Reduced Adherence to Human Microvascular Endothelial Cells (HMVECs).
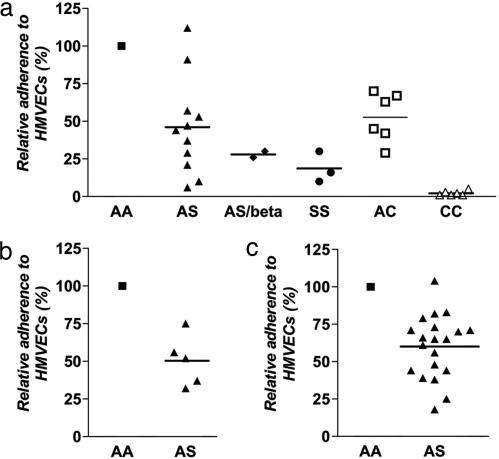
To evaluate the influence of HbS on the binding properties of parasitized erythrocytes in host microvessels, we tested P. falciparum-infected AA, AS, and SS erythrocytes for their adherence to HMVECs expressing the major host adherence receptor CD36. Relative to parasitized AA erythrocytes, parasitized AS erythrocytes showed a 54% reduction in adherence to HMVECs in a semistatic binding assay (mean ± SEM; 46% ± 9.7%, P = 0.0003, n = 11, one sample t test of the mean; GraphPad) (Fig. 1a). Parasitized SS erythrocytes and AS/β-thalassemic erythrocytes (containing levels of HbS intermediate between AS and SS erythrocytes) showed even greater (>70%) reductions (Fig. 1a). In parallel assays, parasitized AC and CC erythrocytes showed relative reductions of 47% and 98%, respectively (mean ± SEM; 53% ± 6.7%, P = 0.0009, n = 6 for AC; 2% ± 0.6%, P < 0.0001, n = 6 for CC) (Fig. 1a).
Fig. 1.
Adherence of parasitized HbS and HbC erythrocytes to HMVECs. (a) Adherence of parasitized erythrocytes to HMVECs under semistatic conditions. Results from AS, SS, AC, and CC erythrocytes were normalized to those from AA erythrocytes run in parallel. Mean percentages are indicated by horizontal bars. Results were obtained from three parasite lines (3D7, FVO, and 7G8) and multiple blood donors (17AA, 9AS, 1AS/β-thalassemia, 3SS, 2AC, and 2CC) (not all combinations tested). The mean (± SEM) number of parasitized AA erythrocytes per HMVEC was 3.7 ± 0.5. (b) Adherence of P. falciparum 7G8-infected erythrocytes to HMVECs under physiologic flow conditions. Results from three AS erythrocyte donors were normalized to those from five AA erythrocyte donors run in parallel and are expressed as mean percentages (not all combinations tested). The mean (± SEM) number of parasitized AA erythrocytes at 5% parasitemia was 179 ± 48/mm2. (c) Adherence of erythrocytes infected with naturally occurring P. falciparum isolates to HMVECs under semistatic conditions. Results from four AS erythrocyte donors were normalized to those from four AA erythrocyte donors run in parallel and are expressed as mean percentages. Data are from 13 parasite isolates (not all combinations tested). The mean (± SEM) number of parasitized AA erythrocytes per HMVEC was 3.7 ± 0.5.
To confirm reduced cytoadherence of parasitized AS erythrocytes under physiological flow conditions, we investigated the adherence of these cells to HMVECs in a flow-chamber assay. Relative to AA erythrocytes infected with 7G8 P. falciparum parasites, 7G8-infected AS erythrocytes showed a 50% reduction in adherence to HMVECs (mean ± SEM; 50% ± 7.6%, P = 0.003, n = 5) (Fig. 1b). We previously found that the adherence of 7G8-infected AC and CC erythrocytes to HMVECs was reduced relative to that of 7G8-infected AA cells by 30% and 98%, respectively, under physiological flow conditions (20).
We also investigated whether HbS influenced the cytoadherence of naturally occurring P. falciparum isolates. Ring-stage parasites were obtained from Malian children with malaria, cultured in vitro to trophozoites, purified by a magnetic column, and inoculated into AA and AS erythrocytes. After allowing time for the parasites to reinvade the fresh erythrocytes and mature to trophozoites, we compared these samples for their adherence to HMVECs. This procedure enabled direct comparisons between AA and AS erythrocytes infected with the same parasite isolate. Relative to parasitized AA erythrocytes, the parasitized AS erythrocytes showed a 40% reduction in adherence to HMVECs (mean ± SEM; 60% ± 4.7%, P < 0.0001, n = 20) (Fig. 1c).
Parasitized AS and SS Erythrocytes Show Reduced Interaction with Blood Monocytes.
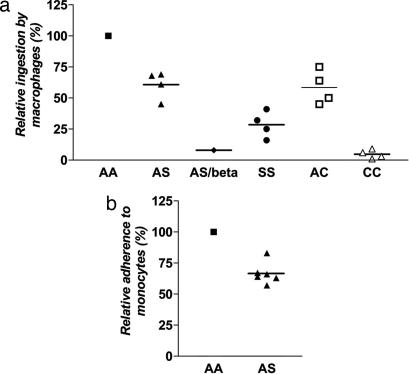
Widespread monocyte activation during acute P. falciparum malaria produces elevated levels of TNF and other cytokines implicated in the pathogenesis of mild and severe disease (35). We therefore investigated whether parasitized HbS erythrocytes also showed impaired interactions with monocytes. In the first of these experiments, we allowed parasitized erythrocytes to interact with blood monocyte-derived macrophages (5 days in culture) and then counted the number of parasitized erythrocytes that were phagocytosed as a measure of initial cytoadherence. Large numbers of trophozoite-infected erythrocytes (with surface PfEMP-1) interacted with macrophages, as evidenced by their phagocytosis. However, control experiments with ring-infected erythrocytes (without PfEMP-1 on their surface yet) (23) showed no ingestion, as expected (data not shown). Relative to trophozoite-infected AA erythrocytes, trophozoite-infected AS and SS erythrocytes were ingested 39% and 71% less effectively by macrophages, respectively (mean ± SEM; 61% ± 5.5%, P = 0.006, n = 4 for AS; 29% ± 5.3%, P = 0.0009, n = 4 for SS) (Fig. 2a). Trophozoite-infected AC and CC erythrocytes also showed significant reductions (42% and 94%) in phagocytosis (mean ± SEM; 58% ± 6.8%, P = 0.009, n = 4 for AC; 6% ± 1.7%, P = 0.0003, n = 4 for CC) (Fig. 2a). Consistent with a central role of PfEMP-1-mediated interactions, phagocytosis of each of the trophozoite-infected erythrocyte samples was almost completely blocked by the monoclonal anti-CD36 antibody OKM5 (data not shown).
Fig. 2.
Adherence of parasitized HbS and HbC erythrocytes to blood monocytes. (a) Phagocytosis of trophozoite-infected erythrocytes by blood monocyte-derived macrophages. Results from AS, AS/β-thal (AS/beta), SS, AC, and CC erythrocytes were normalized to those from AA erythrocytes run in parallel. Mean percentages are indicated by horizontal bars. Results were obtained from two parasite lines (3D7 and FVO) and multiple blood donors (7AA, 4AS, 1AS/β-thal, 4SS, 4AC, and 4CC) (not all combinations tested). (b) Adherence of trophozoite-infected erythrocytes to blood monocytes. Results from AS erythrocytes were normalized to those from AA erythrocytes run in parallel. Tests included two parasite lines (3D7 and 7G8) and multiple blood donors (3AA and 3AS) (not all combinations tested). The mean (± SEM) number of parasitized AA erythrocytes per monocyte was 1.6 ± 0.2.
We also compared the adherence of trophozoite-infected AA and AS erythrocytes to blood monocytes that had been cultured for only 48 h. At this time, blood monocytes firmly adhered to chamber slides, but did not yet differentiate into phagocytes. Relative to parasitized AA erythrocytes, parasitized AS erythrocytes showed a 33% reduction in adherence to monocytes (mean ± SEM; 67% ± 3.6%, P = 0.0002, n = 6) (Fig. 2b).
Parasitized AS and SS Erythrocytes Express Reduced Surface Levels of PfEMP-1.
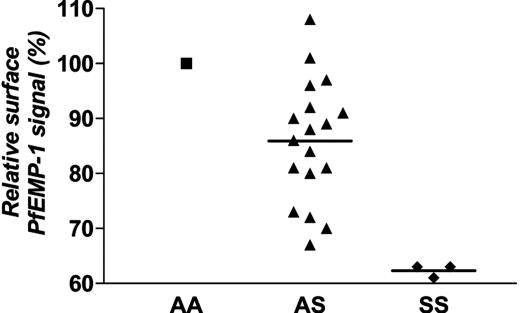
Because reductions of adherence to HMVECs and monocytes are associated with altered PfEMP-1 display on parasitized HbC erythrocytes (Fig. 2) (20), we evaluated PfEMP-1 display on parasitized HbS erythrocytes. Using PfEMP-1-specific antisera in flow-cytometric assays, we found an average 14% reduction of PfEMP-1 signal on unfixed parasitized AS erythrocytes relative to parasitized AA erythrocytes (mean ± SEM; 86% ± 2.6%, P < 0.0001, n = 18) (Fig. 3). Parasitized SS erythrocytes showed even greater reductions (38%) in PfEMP-1 signal (mean ± SEM; 62% ± 0.7%, P = 0.0003, n = 3) (Fig. 3). The relative reductions previously reported for PfEMP-1 signal on parasitized AC and CC erythrocytes were 15% and 70%, respectively (20).
Fig. 3.
PfEMP-1 expression levels on the surface of unfixed parasitized erythrocytes. Flow-cytometry results from AS and SS erythrocytes were normalized to those from parasitized AA erythrocytes run in parallel. Mean percentages are indicated by horizontal bars. Assays included three parasite lines (3D7.41, FVO, and MCR+) and multiple blood donors (9AA, 18AS, and 3SS) (not all combinations tested).
Parasitized AS and SS Erythrocytes Show Abnormal Cell-Surface Distributions of PfEMP-1.
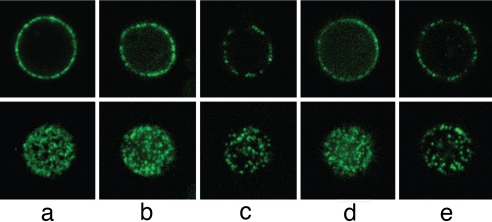
The reduced PfEMP-1 signal on parasitized AS and SS erythrocytes indicated that these cells might show abnormal PfEMP-1 distributions, as do parasitized AC and CC erythrocytes (20). To investigate this possibility, we recorded midplane confocal sections and upper surface maximum projection-stacked series of unfixed parasitized erythrocytes that had been incubated with antiserum specific for PfEMP-1. Parasitized AA erythrocytes exhibited a typically homogeneous distribution of PfEMP-1 signal (Fig. 4a). The images from parasitized AS and SS erythrocytes were more heterogeneous, with some cells showing AA-like patterns (Fig. 4 b and d) and others showing dimmer and uneven distributions of PfEMP-1 (Fig. 4 c and e).
Fig. 4.
Distribution of PfEMP-1 signal on the surface of unfixed parasitized AA, AS, and SS erythrocytes. Confocal cross-sections through the midplane (Upper) or maximum projections of z stacks through the upper cell surface (Lower) of trophozoite-infected erythrocytes probed with a polyclonal antiserum against PfEMP-1 (FVO line). (a) PfEMP-1 fluorescence patterns typical of a parasitized AA erythrocyte. (b) Fluorescence patterns from a parasitized AS erythrocyte, similar to those of parasitized AA erythrocytes. (c) Irregular, patchy PfEMP-1 distribution on a parasitized AS erythrocyte. (d) Fluorescence patterns from a parasitized SS erythrocyte, similar to those of parasitized AA erythrocytes. (e) Irregular, patchy fluorescence signals from abnormally displayed PfEMP-1 on an SS erythrocyte.
Parasitized AS and SS Erythrocytes Show Abnormal Knob Morphologies and Distributions.
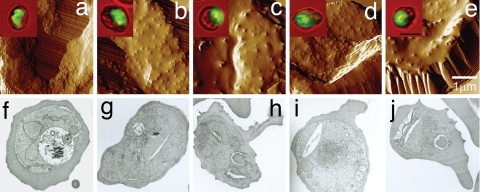
PfEMP-1 molecules are concentrated at the surface knobs of parasitized erythrocytes, where they serve as contact points with HMVECs (27, 36) and monocytes (29). The amount and distribution of PfEMP-1 can be abnormal when knob assembly is altered (20, 27, 37). To further examine whether abnormal display of PfEMP-1 on parasitized AS and SS erythrocytes is associated with abnormal knob morphology and distribution, we observed the surface of these cells by atomic force microscopy (AFM) and transmission electron microscopy (TEM). In experiments with AS erythrocytes parasitized with laboratory-adapted P. falciparum lines, we identified subpopulations with fine, regularly distributed knobs similar to those of parasitized AA erythrocytes (38), whereas other subpopulations showed large, widely separated knobs reminiscent of those on AC and CC erythrocytes (Fig. 5 a–c) (20, 38). Similar findings were obtained with SS erythrocytes (Fig. 5 d and e). TEM images of parasitized AS and SS erythrocytes obtained directly from Malian children and cultured for 24 h to the trophozoite stage also showed cells with normal knob morphologies similar to parasitized AA erythrocytes (Fig. 5 f, g, and i) and cells with abnormal knob morphologies (Fig. 5 h and j). Using AFM, we observed abnormal knobs on subsets of parasitized AS and SS erythrocytes (but not AA erythrocytes) obtained directly from Malian children with malaria (data not shown). These findings indicate that subsets of merozoite-infected AS and SS erythrocytes mature to parasitized cells carrying abnormal knobs in vivo.
Fig. 5.
Knob morphology and distribution on the surface of parasitized AS and SS erythrocytes. (a–e) AFM images of parasitized AA (a), AS (b and c), and SS (d and e) erythrocytes in continuous culture showing normal AA-like (a, b, and d) or abnormal CC-like (c and e) appearances (38). (f–j) TEM images of parasitized AA (f), AS (g and h), and SS (i and j) erythrocytes from children with malaria showing normal AA-like (f, g, and i) or abnormal CC-like (h and j) knob appearances.
Discussion
Abundant epidemiological evidence supports the hypothesis that HbS is naturally selected because of the protection it provides against severe malaria, yet the mechanisms by which this mutation acts have not been clearly established. Although decreased erythrocyte invasion or poor growth under low-oxygen tension and enhanced removal of ring-parasitized erythrocytes might contribute to reduced parasite densities in AS and SS children, these mechanisms do not explain how the substantial parasitized erythrocyte densities that still arise in AS children are less likely to cause severe disease. In our view, a tenable hypothesis is that the HbS mutation renders parasitized erythrocytes less likely to engender pathogenic effects where they sequester in the microvasculature of critical tissues. Two PfEMP-1-dependent cytoadherence interactions that promote inflammatory states attendant to symptomatic and severe malaria are impaired with parasitized AS and SS erythrocytes. Impairment of cytoadherence also might lead to decreased numbers of sequestered parasites during episodes of malaria. Because relative reductions in cytoadherence and PfEMP-1 expression are greater for SS erythrocytes than for AS erythrocytes, this mechanism of protection is consistent with lower parasite densities reported for SS children (8). It also predicts that SS homozygosity can confer some protection against mild and severe malaria, although the high morbidity and mortality of SS children from sickle-cell anemia make this proposition a difficult possibility to test in African populations.
Naturally acquired antibodies to PfEMP-1 may enhance HbS protection by further destabilizing cytoadherence interactions. Additive or synergistic effects between abnormal PfEMP-1 display and anti-PfEMP-1 antibodies would likely participate in the effects of acquired immunity on sickle trait protection against malaria reported by Williams et al. (9). These effects would help to explain the observations of lower parasite densities in AS relative to AA children in regions where multiple infections each year are the rule and disease-controlling immunity is acquired at an early age.
Other investigators have suggested that sickle trait promotes enhanced phagocytosis of ring-infected erythrocytes by splenic macrophages. Ayi et al. (13) reported that these parasitized AS erythrocytes were ingested by macrophages more readily than their AA counterparts and proposed that enhanced phagocytosis of ring-parasitized AS erythrocytes in the spleen help to lower parasite densities in AS children. In the experiments of Ayi et al. (13), parasitized erythrocytes were opsonized with human serum-containing IgG and complement to promote macrophage ingestion. Our experiments, by contrast, were designed to probe the interaction between PfEMP-1 on trophozoite-infected erythrocytes and CD36 on blood monocytes. We did not opsonize parasitized erythrocytes with human serum but instead cultivated them in Albumax-supplemented medium devoid of human immunoglobulins and complement. Under these conditions, phagocytosis of ring-infected AS (or AC) erythrocytes was not enhanced. However, phagocytosis of trophozoite-infected AS (or AC) erythrocytes was markedly decreased relative to trophozoite-infected AA erythrocytes, consistent with different efficiencies of PfEMP-1 interactions with CD36 of monocytes. We note that opsonization of ring-infected AS erythrocytes by IgG and complement in vivo would remove these cells before their sequestration. Ring-infected AS erythrocytes that escape this phagocytic clearance mechanism presumably would be subject to the malaria-protective effects of reduced cytoadherence in the bloodstream.
Our data indicate that HbS protection against severe malaria has features in common with the protective mechanism of HbC. In both cases, reduced PfEMP-1 signal on the surface of parasitized erythrocytes correlates with reductions of cytoadherence as detected by endothelial cell and monocyte binding as well as phagocytosis. The relative cytoadherence and PfEMP-1 levels of parasitized SS erythrocytes in our studies were lower than those of parasitized AS erythrocytes but higher than those of parasitized CC erythrocytes. Taken together, these findings suggest that HbS and HbC alter the erythrocyte membrane in a way that interferes with the parasite's ability to efficiently remodel the surface of its host cell. HbS and HbC erythrocyte membranes contain elevated levels of denatured hemoglobins (hemichromes), aggregated band 3 proteins, and cross-linked cytoskeletal proteins, and they are associated with aggregates of plasma proteins (immunoglobulins, complement, and albumin) (20, 39, 40). We speculate that some or all of these molecular species may impair the trafficking and/or docking of parasite proteins in infected erythrocytes, thus affecting the placement of knob components and PfEMP-1.
Our finding that parasitized AS and AC erythrocytes show similar reductions in surface PfEMP-1 expression and in adherence to HMVECs would suggest that the levels of malaria protection afforded by HbS and HbC are comparable in vivo. However, most epidemiological studies have reported that, compared with AA children, AS children carry lower parasite densities than AC children. In Ghana, where HbS and HbC can each be found at high prevalence in a single-study population, relatively lower parasite densities in AS children were associated with relatively greater protection against severe malaria (6). Parasite-induced sickling of AS erythrocytes (10), reduced invasion and impaired development of parasites in AS erythrocytes at low-oxygen tension (12), and enhanced phagocytosis of ring-parasitized AS erythrocytes (13) all might contribute to the increased removal of parasites by the spleen and reduce parasite densities in AS children. In different human population groups, it appears that additional effects such as genetic background and acquired immunity can further modify the AS and AC phenotypes. In the Dogon ethnic group of Mali, for example, HbC may provide greater protection against severe malaria than HbS (15). In the Mossi of Burkina Faso, in contrast, relative protection against severe malaria was markedly greater for HbS than for HbC (18). Although various genetic and environmental influences have yet to be definitively identified, α-thalassemia has recently been associated with negative epistatic effects on the protective capacity of HbS but not HbC (6, 16).
Materials and Methods
Erythrocytes.
Whole-blood samples were drawn into Vacutainers containing heparin, acid citrate dextrose, or EDTA. Erythrocytes were washed three times with RPMI medium 1640 and stored at 50% hematocrit at 4°C before use (within 1–3 days of blood draw). Hb types were determined by HPLC (D-10 Instrument; Bio-Rad). In all experiments, AA and mutant erythrocytes were obtained simultaneously and tested in parallel. Blood collection was approved by Institutional Review Boards of the National Institute of Allergy and Infectious Diseases and the University of Bamako. Informed consent was obtained from all subjects.
Parasite Culture.
P. falciparum lines (3D7, 7G8, FVO, and MCR+) were cultured in O+ erythrocytes at 5% hematocrit in complete medium (RPMI medium 1640 supplemented with 25 mg/ml Hepes, 2 mg/ml sodium bicarbonate, 50 μg/ml gentamicin, and either 10% heat-inactivated human AB+ serum or 0.5% Albumax II; Gibco/BRL). Knobby parasite lines (3D7, 7G8, and FVO) were maintained by gelatin flotation (41). Fresh ring-stage parasites from Malian children were cultured with 0.5% Albumax II at a lower hematocrit (1–2%) for only ≈24 h to the trophozoite stage. Parasites were cultured in 0.2 μm-vented, 25-cm2 flasks (Corning) in 5-ml complete medium in a humidified atmosphere of 5% CO2 in air. Trophozoite-infected erythrocytes were enriched to >95% purity by magnetic separation (Miltenyi Biotec), inoculated into normal AA or mutant erythrocytes, and cultured at 1–2% hematocrit. Parasitized erythrocytes were always assayed after a single cycle of invasion and growth to the trophozoite stage (≈36 h).
Endothelial Cell-Adherence Assay Under Static Conditions.
Dermal HMVECs (HMVECs-d; Cambrex) were maintained in the manufacturer's EGM2-MV medium and grown on LabTek CC2-coated eight-well chamber slides (Nalge). Trophozoites purified to >95% parasitemia by magnetic separation were adjusted to 20% parasitemia and 0.5–1% hematocrit by adding noninfected erythrocytes in binding media (BM; RPMI medium 1640, 0.5% BSA, pH 6.7). Fresh parasite isolates tended to bind more strongly to HMVECs and were therefore tested at 5% parasitemia to reduce crowding in the chamber slide and enable accurate counting. Adherent HMVECs were washed with BM and then incubated with 150 μl of the parasitize suspension for 1 h at 37°C with horizontal agitation (100 rpm) (A. Daigger, model no. 0R50 117V 2A). After parasite suspensions were removed from each well, slides were washed by dipping twice in BM (37°C), fixed in 2% glutaraldehyde at ambient temperature for 2 h, and stained in 1% Giemsa for 30 min. The number of parasitized erythrocytes bound to ≈700 HMVECs was counted from duplicate wells. For each slide, the number of parasitized mutant erythrocytes per HMVEC was normalized to counts from parasitized AA erythrocytes.
Endothelial Cell-Adherence Assay Under Flow Conditions.
HMVECs-d were harvested from neonatal foreskin as described previously (42). The protocol was approved by the Conjoint Ethics Board at the University of Calgary. Parasitized erythrocyte–HMVEC interactions at 1 dyne per cm2 were studied by using a parallel plate-flow chamber (42). Parasitized erythrocytes were infused at 1% hematocrit for 7 min. The total number of adherent cells at the end of the infusion was determined by counting adherent cells in five or six microscopic fields. Results were expressed as mean percentages relative to the adherence of AA cells.
Phagocytosis of Parasitized Erythrocytes by Blood Monocyte-Derived Macrophages.
CD14+ monocytes (Cambrex) were plated onto Lab-Tek CC2-coated chamber slides at 1 × 105 per well and matured into phagocytic macrophages in RPMI medium 1640 containing 25 mM Hepes, 50 μg/ml gentamicin, and 10% FBS for 5 days at 37°C in 5% CO2. Parasite lines (3D7 and FVO) were cultured in complete medium containing Albumax II and no serum supplement. Suspensions of ring-infected erythrocytes were passed through MACS magnetic columns to remove free hemazoin, adjusted to 3% parasitemia and 1% hematocrit by adding noninfected erythrocytes, and then incubated with macrophages at a ratio of 20:1 for 4 h at 37°C in 5% CO2. The next day, suspensions of mature trophozoites were similarly adjusted to 3% parasitemia and 1% hematocrit and then incubated with macrophages. In some experiments, macrophages were pretreated for 20 min at 4°C with 10 μg/ml anti-CD36 antibody (OKM5; Ortho Diagnostics), which was then washed off before addition of parasitized erythrocytes. Slides were washed by dipping in RPMI medium 1640, fixed, and stained by using Hema 3 (Fisher Scientific), and the number of parasitized erythrocytes ingested by 200 macrophages was counted by light microscopy.
Monocyte Adherence Assay.
CD14+ monocytes were plated onto Lab-Tek CC2-coated chamber slides at 4 × 105 per well and cultured for 48 h in RPMI medium 1640 containing 25 mM Hepes, 50 μg/ml gentamicin, and 10% FBS at 37°C in 5% CO2. AA and mutant erythrocytes infected with P. falciparum trophozoites (lines 3D7 and 7G8) were purified by magnetic separation and adjusted to 10% parasitemia and 1% hematocrit. Adherent monocytes were washed with BM and incubated with 150 μl of the parasite suspension for 1 h at 37°C with gentle horizontal rotation. After parasite suspensions were removed from each well, the slides were gently washed by dipping four times in BM, dried, and stained by using Hema 3 (Fisher Scientific). Adherence was measured by counting the number of parasitized erythrocytes bound to ≈700 monocytes from duplicate wells. For each slide, the number of parasitized mutant erythrocytes per monocyte was normalized to the counts from parasitized normal AA erythrocytes.
Flow Cytometry.
Rat or mouse polyclonal antisera raised against PfEMP-1 variants expressed by the P. falciparum lines FVO, MCR+, and 3D7.41 were kindly provided by Morris Makobongo and Dror Baruch (Malaria Vaccine Development Branch, National Institute of Allergy and Infectious Diseases, Bethesda, MD). Trophozoite-infected erythrocytes (1.5 × 106; 1% parasitemia) were stained with 1:200 antiserum in FACS staining buffer (FSB; PBS, 2% FBS, and 0.1% sodium azide) for 45 min at room temperature and washed twice with FSB. Samples were then incubated with Alexa 488-conjugated anti-rat or anti-mouse IgG (Molecular Probes) and 2 μg/ml ethidium bromide (EtdBr) at room temperature for 30 min and washed twice with FSB. A FACSort instrument (Becton Dickinson) and FlowJo software version 6.2.1 were used to acquire and analyze 500,000 events from each sample. Populations of parasitized erythrocytes expressing antiserum-reactive PfEMP-1 molecules (EtdBr- and Alexa-488-positive) were gated, and their median fluorescence intensities were determined.
Surface Immunofluorescence Microscopy.
Parasitized erythrocytes (2-μl packed cell volume) were added to 20 μl of rat polyclonal antiserum (1:100 dilution, MCR+; 1:1,500 dilution, FVO) for 1 h at 25°C and washed with FSB. Bound antibody was detected with Alexa 488-conjugated anti-rat IgG. Control experiments using antiserum against the intracellular acidic terminal segment of PfEMP-1 gave no signal, confirming that the unfixed, parasitized erythrocytes were not antibody-permeable. Images were collected by using a TCS-SP2 AOBS confocal microscope (Leica) by using a ×63 oil-immersion objective NA 1.4, zoom 6. The confocal pinhole was set to 0.9 Airy units to ensure maximum resolution. Alexa 488 fluorescence was excited by using an argon laser at 488 nm. To produce a maximum projection stacked series, three-dimensional reconstructions were made by using sequential sections through the sample with a z increment of 0.12 μm. Images were deconvoluted and processed by using Leica TCS (version 2.1374), Imaris 4.1 (3D reconstructions; Bitplane), Huygens Essentials (deconvolution; SVI), and Adobe Photoshop CS (Adobe Systems).
TEM and AFM.
Parasitized erythrocytes were processed and imaged by TEM as described previously (20). For AFM, parasitized erythrocytes were prepared and imaged as described previously (38), with the exception that a custom-built closed-loop XY scanner stage (nPoint) was used on a wide-field Axiovert 200 fluorescence microscope (Carl Zeiss) to minimize scanning artifacts and thermal drift of the scanner for improved image accuracy. AFM was performed in tapping mode in air by using Nanosensors pointprobe tips (Nanosensors) with a cantilever resonance frequency of 327–397 kHz. Image-Pro Plus version 5.0 (Media Cybernetics) was used to merge bright-field and YOYO-1 fluorescent images to enable unambiguous identification of the parasite stage.
ACKNOWLEDGMENTS.
We thank Dror Baruch, Blaise Dackouo, Issa Diallo, Matthew Ferber, Ivo Francischetti, Robert Gwadz, Karen Hayton, Randy Hirsch, Matthew Hsieh, Juraj Kabat, Abdoulaye Katile, Beth Link, Morris Makobongo, Michael Nardi, Griffin Rodgers, Aboubacar Sadou, Dick Sakai, Todd Smith, Karim Traore, and Carole Tremonti for their efforts in support of this work. This work was supported by the Intramural Research Program of the National Institutes of Health/National Institute of Allergy and Infectious Diseases.
Footnotes
The authors declare no conflict of interest.
References
- 1.Allison AC. Protection afforded by sickle-cell trait against subtertian malareal infection. Br Med J. 1954;4857:290–294. doi: 10.1136/bmj.1.4857.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allison AC. Polymorphism and Natural Selection in Human Populations. Cold Spring Harb Symp Quant Biol. 1964;29:137–149. doi: 10.1101/sqb.1964.029.01.018. [DOI] [PubMed] [Google Scholar]
- 3.Williams TN, et al. Sickle cell trait and the risk of Plasmodium falciparum malaria and other childhood diseases. J Infect Dis. 2005;192:178–186. doi: 10.1086/430744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hill AV, et al. Common west African HLA antigens are associated with protection from severe malaria. Nature. 1991;352:595–600. doi: 10.1038/352595a0. [DOI] [PubMed] [Google Scholar]
- 5.Aidoo M, et al. Protective effects of the sickle cell gene against malaria morbidity and mortality. Lancet. 2002;359:1311–1312. doi: 10.1016/S0140-6736(02)08273-9. [DOI] [PubMed] [Google Scholar]
- 6.May J, et al. Hemoglobin variants and disease manifestations in severe falciparum malaria. J Am Med Assoc. 2007;297:2220–2226. doi: 10.1001/jama.297.20.2220. [DOI] [PubMed] [Google Scholar]
- 7.Olumese PE, et al. The clinical manifestations of cerebral malaria among Nigerian children with the sickle cell trait. Ann Trop Paediatr. 1997;17:141–145. doi: 10.1080/02724936.1997.11747877. [DOI] [PubMed] [Google Scholar]
- 8.Mockenhaupt FP, et al. Limited influence of haemoglobin variants on Plasmodium falciparum msp1 and msp2 alleles in symptomatic malaria. Trans R Soc Trop Med Hyg. 2004;98:302–310. doi: 10.1016/j.trstmh.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 9.Williams TN, et al. An immune basis for malaria protection by the sickle cell trait. PLoS Med. 2005;2:e128. doi: 10.1371/journal.pmed.0020128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Luzzatto L, Nwachuku-Jarrett ES, Reddy S. Increased sickling of parasitised erythrocytes as mechanism of resistance against malaria in the sickle-cell trait. Lancet. 1970;1:319–321. doi: 10.1016/s0140-6736(70)90700-2. [DOI] [PubMed] [Google Scholar]
- 11.Friedman MJ. Erythrocytic mechanism of sickle cell resistance to malaria. Proc Natl Acad Sci USA. 1978;75:1994–1997. doi: 10.1073/pnas.75.4.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pasvol G, Weatherall DJ, Wilson RJ. Cellular mechanism for the protective effect of haemoglobin S against P. falciparum malaria. Nature. 1978;274:701–703. doi: 10.1038/274701a0. [DOI] [PubMed] [Google Scholar]
- 13.Ayi K, Turrini F, Piga A, Arese P. Enhanced phagocytosis of ring-parasitized mutant erythrocytes: A common mechanism that may explain protection against falciparum malaria in sickle trait and beta-thalassemia trait. Blood. 2004;104:3364–3371. doi: 10.1182/blood-2003-11-3820. [DOI] [PubMed] [Google Scholar]
- 14.Carlson J, Nash GB, Gabutti V, al-Yaman F, Wahlgren M. Natural protection against severe Plasmodium falciparum malaria due to impaired rosette formation. Blood. 1994;84:3909–3914. [PubMed] [Google Scholar]
- 15.Agarwal A, et al. Hemoglobin C associated with protection from severe malaria in the Dogon of Mali, a West African population with a low prevalence of hemoglobin S. Blood. 2000;96:2358–2363. [PubMed] [Google Scholar]
- 16.Williams TN, et al. Negative epistasis between the malaria-protective effects of alpha+-thalassemia and the sickle cell trait. Nat Genet. 2005;37:1253–1257. doi: 10.1038/ng1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Beutler E, Dern RJ, Flanagan CL. Effect of sickle-cell trait on resistance to malaria. Br Med J. 1955;1:1189–1191. doi: 10.1136/bmj.1.4923.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Modiano D, et al. Haemoglobin C protects against clinical Plasmodium falciparum malaria. Nature. 2001;414:305–308. doi: 10.1038/35104556. [DOI] [PubMed] [Google Scholar]
- 19.Mockenhaupt FP, et al. Hemoglobin C, resistance to severe malaria in Ghanaian children. J Infect Dis. 2004;190:1006–1009. doi: 10.1086/422847. [DOI] [PubMed] [Google Scholar]
- 20.Fairhurst RM, et al. Abnormal display of PfEMP-1 on erythrocytes carrying haemoglobin C may protect against malaria. Nature. 2005;435:1117–1121. doi: 10.1038/nature03631. [DOI] [PubMed] [Google Scholar]
- 21.Rowe A, Obeiro J, Newbold CI, Marsh K. Plasmodium falciparum rosetting is associated with malaria severity in Kenya. Infect Immun. 1995;63:2323–2326. doi: 10.1128/iai.63.6.2323-2326.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carlson J, et al. Human cerebral malaria: Association with erythrocyte rosetting and lack of anti-rosetting antibodies. Lancet. 1990;336:1457–1460. doi: 10.1016/0140-6736(90)93174-n. [DOI] [PubMed] [Google Scholar]
- 23.Baruch DI, et al. Cloning the P. falciparum gene encoding PfEMP1, a malarial variant antigen and adherence receptor on the surface of parasitized human erythrocytes. Cell. 1995;82:77–87. doi: 10.1016/0092-8674(95)90054-3. [DOI] [PubMed] [Google Scholar]
- 24.Smith JD, et al. Switches in expression of Plasmodium falciparum var genes correlate with changes in antigenic and cytoadherent phenotypes of infected erythrocytes. Cell. 1995;82:101–110. doi: 10.1016/0092-8674(95)90056-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Su XZ, et al. The large diverse gene family var encodes proteins involved in cytoadherence and antigenic variation of Plasmodium falciparum-infected erythrocytes. Cell. 1995;82:89–100. doi: 10.1016/0092-8674(95)90055-1. [DOI] [PubMed] [Google Scholar]
- 26.Silamut K, et al. A quantitative analysis of the microvascular sequestration of malaria parasites in the human brain. Am J Pathol. 1999;155:395–410. doi: 10.1016/S0002-9440(10)65136-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Crabb BS, et al. Targeted gene disruption shows that knobs enable malaria-infected red cells to cytoadhere under physiological shear stress. Cell. 1997;89:287–296. doi: 10.1016/s0092-8674(00)80207-x. [DOI] [PubMed] [Google Scholar]
- 28.Ockenhouse CF, Tandon NN, Magowan C, Jamieson GA, Chulay JD. Identification of a platelet membrane glycoprotein as a falciparum malaria sequestration receptor. Science. 1989;243:1469–1471. doi: 10.1126/science.2467377. [DOI] [PubMed] [Google Scholar]
- 29.Ockenhouse CF, Magowan C, Chulay JD. Activation of monocytes and platelets by monoclonal antibodies or malaria-infected erythrocytes binding to the CD36 surface receptor in vitro. J Clin Invest. 1989;84:468–475. doi: 10.1172/JCI114188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McGilvray ID, Serghides L, Kapus A, Rotstein OD, Kain KC. Nonopsonic monocyte/macrophage phagocytosis of Plasmodium falciparum-parasitized erythrocytes: A role for CD36 in malarial clearance. Blood. 2000;96:3231–3240. [PubMed] [Google Scholar]
- 31.Miller LH, Baruch DI, Marsh K, Doumbo OK. The pathogenic basis of malaria. Nature. 2002;415:673–679. doi: 10.1038/415673a. [DOI] [PubMed] [Google Scholar]
- 32.Turner GD, et al. An immunohistochemical study of the pathology of fatal malaria. Evidence for widespread endothelial activation and a potential role for intercellular adhesion molecule-1 in cerebral sequestration. Am J Pathol. 1994;145:1057–1069. [PMC free article] [PubMed] [Google Scholar]
- 33.Dondorp AM, Pongponratn E, White NJ. Reduced microcirculatory flow in severe falciparum malaria: Pathophysiology and electron-microscopic pathology. Acta Trop. 2004;89:309–317. doi: 10.1016/j.actatropica.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 34.Kaul DK, Roth EF, Jr, Nagel RL, Howard RJ, Handunnetti SM. Rosetting of Plasmodium falciparum-infected red blood cells with uninfected red blood cells enhances microvascular obstruction under flow conditions. Blood. 1991;78:812–819. [PubMed] [Google Scholar]
- 35.Grau GE, et al. Tumor necrosis factor and disease severity in children with falciparum malaria. N Engl J Med. 1989;320:1586–1591. doi: 10.1056/NEJM198906153202404. [DOI] [PubMed] [Google Scholar]
- 36.MacPherson GG, Warrell MJ, White NJ, Looareesuwan S, Warrell DA. Human cerebral malaria. A quantitative ultrastructural analysis of parasitized erythrocyte sequestration. Am J Pathol. 1985;119:385–401. [PMC free article] [PubMed] [Google Scholar]
- 37.Horrocks P, et al. PfEMP1 expression is reduced on the surface of knobless Plasmodium falciparum infected erythrocytes. J Cell Sci. 2005;118:2507–2518. doi: 10.1242/jcs.02381. [DOI] [PubMed] [Google Scholar]
- 38.Arie T, Fairhurst RM, Brittain NJ, Wellems TE, Dvorak JA. Hemoglobin C modulates the surface topography of Plasmodium falciparum-infected erythrocytes. J Struct Biol. 2005;150:163–169. doi: 10.1016/j.jsb.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 39.Brittain NJ, et al. Non-opsonising aggregates of IgG and complement in haemoglobin C erythrocytes. Br J Haematol. 2006;136:491–500. doi: 10.1111/j.1365-2141.2006.06446.x. [DOI] [PubMed] [Google Scholar]
- 40.Kannan R, Labotka R, Low PS. Isolation and characterization of the hemichrome-stabilized membrane protein aggregates from sickle erythrocytes. Major site of autologous antibody binding. J Biol Chem. 1988;263:13766–13773. [PubMed] [Google Scholar]
- 41.Pasvol G, Wilson RJ, Smalley ME, Brown J. Separation of viable schizont-infected red cells of Plasmodium falciparum from human blood. Ann Trop Med Parasitol. 1978;72:87–88. doi: 10.1080/00034983.1978.11719283. [DOI] [PubMed] [Google Scholar]
- 42.Yipp BG, et al. Synergism of multiple adhesion molecules in mediating cytoadherence of Plasmodium falciparum-infected erythrocytes to microvascular endothelial cells under flow. Blood. 2000;96:2292–2298. [PubMed] [Google Scholar]