Abstract
There is convincing evidence that, in humans, discrete sleep stages are important for daytime brain function, but whether any particular sleep stage has functional significance for the rest of the body is not known. Deep non-rapid eye movement (NREM) sleep, also known as slow-wave sleep (SWS), is thought to be the most “restorative” sleep stage, but beneficial effects of SWS for physical well being have not been demonstrated. The initiation of SWS coincides with hormonal changes that affect glucose regulation, suggesting that SWS may be important for normal glucose tolerance. If this were so, selective suppression of SWS should adversely affect glucose homeostasis and increase the risk of type 2 diabetes. Here we show that, in young healthy adults, all-night selective suppression of SWS, without any change in total sleep time, results in marked decreases in insulin sensitivity without adequate compensatory increase in insulin release, leading to reduced glucose tolerance and increased diabetes risk. SWS suppression reduced delta spectral power, the dominant EEG frequency range in SWS, and left other EEG frequency bands unchanged. Importantly, the magnitude of the decrease in insulin sensitivity was strongly correlated with the magnitude of the reduction in SWS. These findings demonstrate a clear role for SWS in the maintenance of normal glucose homeostasis. Furthermore, our data suggest that reduced sleep quality with low levels of SWS, as occurs in aging and in many obese individuals, may contribute to increase the risk of type 2 diabetes.
Keywords: aging, sleep quality, sleep disordered breathing, delta waves, insulin resistance
Human sleep is composed of rapid-eye-movement (REM) sleep and stages 1, 2, 3, and 4 of non-REM (NREM) sleep. The deeper stages of NREM sleep, i.e., stages 3 and 4, also known as slow-wave sleep (SWS), are thought to be the most “restorative.” There is indeed evidence that SWS plays a role in waking neurobehavioral function (1), particularly in memory consolidation (2, 3), but whether SWS is also important for peripheral physiological function is not known. The initiation of SWS is temporally associated with transient metabolic, hormonal, and neurophysiologic changes, all of which could potentially affect glucose homeostasis. These include decreased brain glucose utilization, stimulation of growth hormone release, inhibition of corticotropic activity, decreased sympathetic nervous activity, and increased vagal tone. We therefore hypothesized that SWS plays a role in glucose regulation and that suppression of SWS may adversely affect glucose homeostasis.
To test this hypothesis, we developed an experimental model in young healthy lean individuals that was designed to selectively suppress SWS and assessed the impact of this intervention on glucose homeostasis. The EEG was continuously monitored, and SWS was suppressed by delivering acoustic stimuli of varying frequencies and intensities. The intervention was designed to substitute deep NREM sleep, i.e., stages 3 and 4, with shallow NREM sleep, i.e., stage 2, without awakening the subject, changing total sleep duration or the amount of REM sleep. Nine healthy young volunteers were each tested under two experimental conditions in randomized order: (i) after 2 consecutive nights of undisturbed “baseline” sleep and (ii) after 3 consecutive nights of “experimental suppression of SWS”. During each night, the depth or intensity of SWS was quantified by delta spectral power, i.e., the dominant EEG frequency range (0.5–4 Hz) in SWS. Glucose regulation was assessed by i.v. glucose tolerance testing (ivGTT) at the end of each experimental condition. Glucose tolerance was quantified by the rate of decline in glucose levels after i.v. glucose injection. We simultaneously evaluated insulin sensitivity (S.I.) and insulin secretion [the “acute insulin response to glucose” (AIRg)] by using minimal model analysis of blood glucose and insulin levels measured during the ivGTT (4).
Results
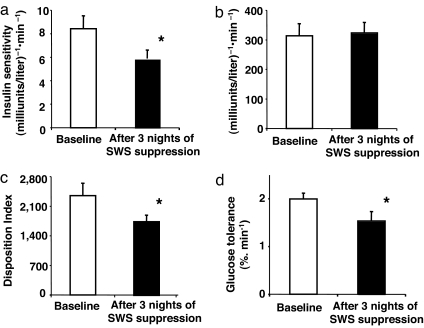
After 3 nights of experimental suppression of SWS, S.I. was decreased by ≈25% (Fig. 1a), reaching the level reported for populations at high risk for diabetes (4). The magnitude of the change in S.I. was comparable with that associated with a difference in weight of 8–13 kg (5). The decrease in S.I. was remarkably consistent, occurring in all but one subject. Under normal circumstances, when S.I. decreases, the insulin response (AIRg) should increase reciprocally such that the disposition index (DI) (DI = S.I. × AIRg) remains constant and glucose tolerance is maintained (6). However, after SWS suppression, the decrease in S.I. was not compensated by an increase in insulin release, because AIRg remained virtually unchanged (Fig. 1b). Inadequate beta cell compensation for a given decrease in S.I. results in a fall in DI, which is a validated marker of diabetes risk (7–10). Indeed, the DI was ≈20% lower after SWS suppression (Fig. 1c). Consistent with an increased diabetes risk, glucose tolerance was reduced by ≈23% (Fig. 1d) to within the range reported in older adults with impaired glucose tolerance (11).
Fig. 1.
S.I., AIRg, DI, and glucose tolerance at baseline and after 3 nights of SWS suppression. The data are means ± SEM (n = 9 subjects). The asterisks indicate significant differences (paired t test): S.I. (P = 0.009) (a); AIRg (P = 0.73) (b); DI (P = 0.02) (c); and glucose tolerance (P = 0.03) (d).
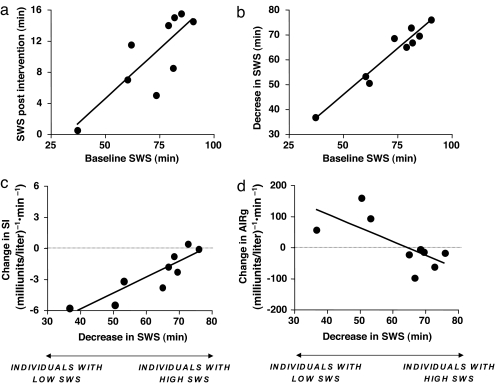
We explored the associations between the changes in S.I. and AIRg and the changes in SWS (Fig. 2). Individuals with high SWS at baseline maintained higher levels of SWS after intervention (Fig. 2a), despite experiencing larger decreases in SWS (Fig. 2b). In contrast, individuals with low SWS at baseline were suppressed to extremely low SWS levels by the intervention. Remarkably, the magnitudes of the changes in both S.I. (Fig. 2c) and AIRg (Fig. 2d) after 3 nights of SWS suppression were correlated with the magnitude of the change in the amount of SWS but beta cell responsiveness (i.e., AIRg) did not fully compensate for the decrease in S.I. Individuals with low SWS at baseline had greater decreases in S.I. with some compensatory increases in AIRg. However, only one subject had an increase in AIRg sufficient to compensate for the decrease in S.I. Individuals with high SWS at baseline suffered smaller decreases in S.I. with no compensatory increase in insulin release. Thus, the DI decreased in eight of the nine subjects.
Fig. 2.
Relationships between the changes in SWS and changes in S.I. and acute insulin response to glucose. (a) SWS at baseline and SWS after intervention (r = 0.81, P = 0.009). (b) SWS at baseline and decrease in SWS after 3 nights of SWS suppression (r = 0.97, P = 0.0001). (c) Decrease in SWS and change in S.I. after 3 nights of SWS suppression (r = 0.89, P = 0.001). (d) Decrease in SWS and change in AIRg after 3 nights of SWS suppression (r = 0.70, P = 0.03).
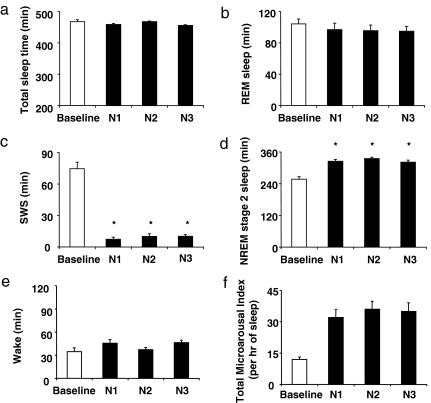
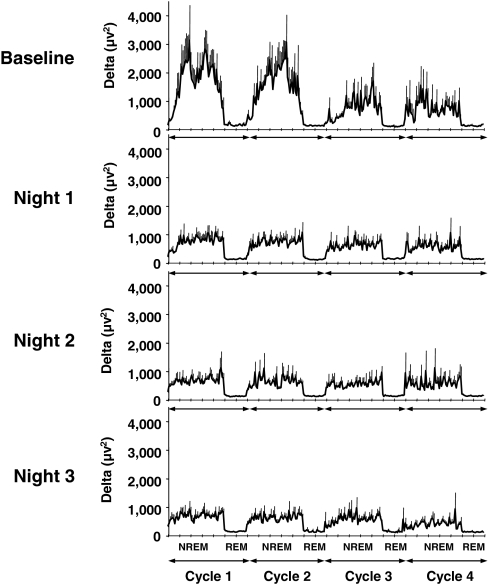
The rapid and substantial decreases in S.I. DI, and glucose tolerance suggest that neuronal activity during SWS may be an important determinant of glucose homeostasis, independently of sleep duration. Indeed, our intervention selectively suppressed SWS. First, the amount of SWS was decreased by nearly 90% (88 ± 3%, mean ± SEM across nights; P < 0.0001; Fig. 3c) without any change in total sleep time (Fig. 3a) or in the durations of REM sleep (Fig. 3b) and stage 1 of NREM sleep. This decrease in SWS is similar to that which occurs over the course of 4 decades of normal aging, because normal young adults spend 80–100 min per night in SWS, whereas individuals >60 years of age generally have <20 min of SWS (12, 13). As expected, the amount of stage 2 was increased (Fig. 3d), confirming that NREM sleep was shallower in each intervention night. Second, there was no change in wake time (Fig. 3e) because nearly all acoustic stimuli resulted in microarousals (Fig. 3f) and not full awakenings. Importantly, the magnitude of the decrease in S.I. was not correlated with measures of sleep fragmentation, including the total number of microarousals on the third night of intervention (r = 0.31, P = 0.42), the mean microarousal index across the 3 nights of intervention (r = 0.34, P = 0.37), or the increase in number of microarousals from baseline to the third night of intervention (r = 0.36, P = 0.34). The weak and nonsignificant correlations that were detected (r values between 0.31 and 0.36) were almost entirely attributable to the contribution of one subject who had exceptionally high levels of delta power [mean delta power in NREM: 3,685 vs. 814 ± 280 μV2 (mean ± SD) in the remaining eight subjects; P < 0.001 for outlier value by Grubbs test] and therefore needed an exceptionally high number of microarousals to suppress SWS [445 vs. 257 ± 57 (mean ± SD) in the remaining eight subjects; Grubbs test: P < 0.01]. Without inclusion of this subject, the correlations were ≈0 (r values from 0.007 to 0.082; P > 0.85). Thus, the alterations of glucose regulation observed after SWS suppression are unlikely to be related to a decrease in sleep continuity. Lastly, delta power was markedly and similarly reduced in each experimental night compared with baseline (Fig. 4), whereas spectral EEG power in other frequency bands including theta, alpha, and sigma was unaffected [see supporting information (SI) Figs. 5–7]. The largest reductions were achieved, as expected, during the first two NREM cycles when delta power was reduced overall by ≈44–55% (P < 0.001) (Fig. 4). The delta power after SWS suppression was directly proportional to the baseline amount of delta power (r = 0.917, P = 0.0005). Furthermore, in the individuals who experienced a decrease in S.I. the magnitude of the decrease was also strongly correlated with the mean NREM absolute delta power (in the first 3 h of sleep) after intervention (n = 8; r = 0.81, P = 0.01]. The individuals who had the largest decrements of S.I. had the lowest delta power after intervention.
Fig. 3.
Sleep architecture during SWS suppression on night 1 (N1), night 2 (N2), and night 3 (N3) vs. the baseline night (B1). The data are means ± SEM (n = 9 subjects). The asterisks indicate significant differences (ANOVA): total sleep time (P = 0.14 for N1, N2, and N3 vs. baseline) (a); REM sleep (P = 0.29 for N1, N2, and N3 vs. baseline) (b); SWS (P = 0.0001 for N1, N2, and N3 vs. baseline) (c); stage 2 of NREM sleep (P = 0.0001 for N1, N2, and N3 vs. baseline) (d); wake time (P = 0.12 for N1, N2, and N3 vs. baseline) (e); and total microarousal index (P = 0.0002 for N1, N2, and N3 vs. baseline) (f).
Fig. 4.
Profiles of delta power (μV2) for the first four NREM–REM sleep cycles (NREM1, NREM2, NREM3, and NREM4). The data are means ± SEM. (a) Baseline night (B1). (b) First night of SWS suppression (N1). (c) Second night of SWS suppression (N2). (d) Third night of SWS suppression (N3). In all experimental nights, as compared with baseline, the amount of delta power was reduced by ≈44–48% for NREM1 (P < 0.002, ANOVA), by ≈50–55% for NREM2 (P < 0.001, ANOVA), by ≈16–30% for NREM3 (P, not significant), and by ≈8–17% for NREM4 (P, not significant).
We explored possible mechanisms that could explain decreased S.I. after selective SWS suppression. Insulin resistance can rapidly develop when circulating levels of cortisol are elevated (14). To determine whether our experimental intervention resulted in stimulation of the corticotropic axis, we measured plasma cortisol levels during the 24-hour period preceding the ivGTT. Mean plasma cortisol profiles at baseline and after SWS suppression were essentially identical. Neither daytime (8.5 ± 0.5 μg/dl at baseline vs. 8.2 ± 0.5 μg/dl after SWS suppression, P = 0.46) nor nighttime (6.7 ± 0.6 μg/dl at baseline vs. 6.7 ± 0.4 μg/dl after SWS suppression, P = 0.92) cortisol levels were elevated after SWS suppression. Thus the observed decrease in S.I. after SWS suppression cannot be attributed to increased cortisol concentrations. The lack of effect of SWS suppression on nocturnal corticotropic activity further indicates that our intervention did not result in stimulation of this important arousal system. Insulin resistance can also occur secondary to increased sympathetic nervous activity. We therefore evaluated changes in the autonomic nervous system by using spectral analysis of HRV of daytime ECG recordings. We used the spectral power in high-frequency band (HF) in normalized units (HFn) as a marker of vagal activity, and the spectral power in low-frequency band (LF) in normalized units (LFn) band as a marker of sympathetic activity. After 3 nights of SWS suppression as compared with baseline, HFn was reduced by ≈15%, LFn was increased by ≈11%, and the sympathovagal balance (as assessed by the ratio of LF to HF) was ≈14% higher (Table 1). It is likely that this elevation in cardiac sympathovagal balance reflects a generalized shift toward higher sympathetic activity at multiple peripheral levels, as occurs in aging (15).
Table 1.
Measurements of sympathovagal balance
| Parameter | Baseline | After three nights of SWS suppression | P |
|---|---|---|---|
| HF, ms2 | 3,305 ± 829 | 2,750 ± 839 | 0.14 |
| LF, ms2 | 4,489 ± 1031 | 4,989 ± 1,272 | 0.56 |
| HFn, % | 42.4 ± 4.5 | 36.4 ± 4.8 | 0.04 |
| LFn, % | 57.6 ± 4.5 | 63.6 ± 4.8 | 0.04 |
| LF/HF | 1.6 ± 0.3 | 2.2 ± 0.5 | 0.03 |
Discussion
Previous studies of experimental SWS suppression have focused on subjective sleepiness and measures of cognitive performance and have suggested that SWS may be important for waking neurobehavioral function (1, 16). In the present study, we were able to induce a selective and profound reduction in SWS and to observe a clear adverse impact on daytime glucose tolerance with a clear increase in a well validated marker of diabetes risk. Remarkably, the changes in the two main determinants of glucose tolerance, S.I. and insulin secretion (AIRg) were correlated with the changes in SWS after our intervention. These findings therefore provide strong evidence for a restorative role of SWS for metabolic function.
SWS suppression resulted in lower S.I. without compensatory increase in insulin release. It is possible that, under more chronic conditions, insulin secretion might increase to a level sufficient to compensate for the decrease in S.I. However, evidence to the contrary has been obtained in four independent prospective epidemiologic studies showing that poor sleep quality is associated with an increase in the risk of incident type 2 diabetes (17–20). We observed an elevation in sympathovagal balance, which could be involved both in the decrease in S.I. and in the lack of appropriate compensatory increase in AIRg. Indeed, overactivity of the sympathetic nervous system results in insulin resistance (21), and pancreatic insulin release is inhibited by increased sympathetic vs. parasympathetic tone (22). Individuals with low SWS at baseline had the lowest amounts of SWS after our intervention and experienced the largest decrements in S.I. (Fig. 2c). Because the amount of SWS as well as the amount of delta power are stable individual traits that are highly heritable (23–26), our findings suggest that there may be a genetic predisposition to develop diabetes when SWS deteriorates.
Chronic shallow non-REM sleep, decreased S.I., and elevated diabetes risk are typical of aging (12, 13, 27, 28). Our findings raise the question of whether age-related changes in sleep quality contribute to the development of these metabolic alterations. This issue is worthy of further investigation. Low levels of SWS are also frequently observed in obese individuals. Indeed, obesity is a major risk factor for sleep-disordered breathing (SDB) (29), an increasingly common condition characterized by repetitive respiratory disturbances and sleep fragmentation by microarousals resulting in low amounts of SWS and delta power (30). Even in the absence of SDB, obese individuals have reduced sleep quality with low amounts of SWS (31, 32). Thus, low SWS may increase the severity of insulin resistance in obesity.
There is an alarming rise in the prevalence of type 2 diabetes that is generally attributed to the epidemic of obesity and the aging of the population (33). As the burden of diabetes on public health continues to rise, so does the need to understand its pathogenesis. We had shown previously that restricting sleep duration in healthy young adults results in decreased glucose tolerance (34, 35). The current data further indicate that not only reduced sleep duration but also reduced sleep quality may play a role in diabetes risk. Our laboratory findings are consistent with a body of epidemiologic evidence linking short or poor sleep and increased incidence of type 2 diabetes (17–20, 36–38). Taken together, the current evidence suggests that strategies to improve sleep duration and quality should be considered as a potential intervention to prevent or delay the development of type 2 diabetes in at-risk populations.
Materials and Methods
Participants.
Nine healthy volunteers (age 20–31 years; five men and four women) participated in the study. All participants were lean (body mass index, 19–24 kg/m2), and average weight did not change over the study period (64.2 kg at baseline condition vs. 64.3 kg in SWS suppression condition; P = 0.95). All had normal findings on clinical examination, normal routine laboratory tests results, normal ECG, and no history of psychiatric, endocrine, cardiac, or sleep disorders. All participants had normal results on validated questionnaires, including the Pittsburgh sleep quality index, Berlin questionnaire, Epworth sleepiness scale, Center for Epidemiologic Studies (CES) and Beck depression scales, and functional outcome of sleep questionnaire (39–41). All had an oral glucose tolerance test to verify normal glucose tolerance at baseline and an overnight screening sleep study to confirm that they were free of sleep disorders. They did not smoke or take any medications. All participants had regular nocturnal time in bed of 7.5 to 8.5 h. We excluded shift workers and persons who had traveled across time zones <4 weeks before the study. We studied women in the early follicular phase.
The Institutional Review Board of the University of Chicago approved the protocol and all participants gave written informed consent.
Experimental Protocol.
Each subject was tested under two conditions in randomized order and separated at least by 4 weeks (i) after 2 consecutive nights of undisturbed “baseline” sleep (nights B1 and B2) and (ii) after 3 consecutive nights of experimental suppression of SWS (nights N1, N2, and N3). During the week preceding each study, we asked participants to maintain a standardized schedule of bedtimes and mealtimes in accordance with their usual habits. We instructed the subjects not to deviate from this schedule by >30 min and asked them to wear a wrist activity monitor (Actiwatch, MiniMitter Inc.) to verify the compliance of the subjects with scheduled bedtimes. Naps were not permitted.
In the laboratory, time in bed was 2300 to 0730 hours, and sleep was recorded on each night. On the days after nights B1, B2, N2, and N3, participants remained in the laboratory and had sedentary activities. An investigator was present continuously to monitor wakefulness. On the mornings after nights B2 and N3, we performed a frequently sampled ivGTT after an overnight fast and resting ECG recordings. We collected blood samples at 20-min intervals during the 24-h period preceding the ivGTT for measurement of cortisol levels. Participants ate carbohydrate-rich (65%) meals at 0900, 1400, and 1900 hours during the blood-sampling period.
Procedures and Assessment.
We performed sleep recordings by using a digital EEG acquisition system (Neurofax EEG- 1100A, Nihon Kohden). Surface electrodes were used to record EEG signals [two central (C3–A2 and C4–A1) and two occipital (O1–A2 and O2–A1)], bilateral electrooculograms (EOGs), and submental electromyogram (EMGs). Screening recordings included oronasal airflow signal by thermocouples, respiratory effort signal by thoracic and abdominal piezoelectric belts, leg movements by tibial EMG, and arterial oxygen saturation by pulse oximetry in addition to EEG, EOG, and EMG signals. This screening night also helped the volunteers to become familiar with the recording equipment and the study environment. Sleep recordings were visually scored at 30-s intervals as the stages wake, REM, or 1, 2, 3, and 4 (i.e., NREM sleep) after standard criteria (42) by an experienced rater who was blind to the age and sex of the participants and the study condition. Respiratory events, periodic limb movements, and microarousals were scored according to established criteria (43–45). Total microarousal index was defined as the number of microarousals per hour of sleep. During the acquisition, the EEG signals were filtered (0.3–35 Hz) and sampled at 200 Hz with a 16-bit resolution. After removal of artifacts by visual inspection, a fast Fourier transform was computed on EEG signals by using a Hanning window on consecutive 2-s intervals, resulting in a frequency resolution of 0.5 Hz. Power spectra of 15 consecutive 2-s intervals were averaged and matched with sleep scores. Intervals with artifacts were considered as missing data to preserve sleep continuity in the analysis. We performed a spectral analysis of the sleep EEG (PRANA software; PhiTools) on the central EEG lead (C4-A1) and estimated spectral power in the delta (0.5–4 Hz), theta (4.5–8.0 Hz), alpha (8.5–12 Hz), and sigma (12.5–15 Hz) frequency bands. To account for individual differences in the duration of NREM/REM cycles, each individual NREM period was subdivided into 50 equal time intervals (i.e., time bins) and each REM period was subdivided into 20 time bins. NREM/REM cycles were defined according to the criteria of Feinberg and Floyd (46).
SWS was suppressed by delivering acoustic tones of varying frequency (500–2,000 Hz) and intensity to speakers placed on each side of the bed. The acoustic stimulus was sent whenever at least two delta waves (≤4 Hz, >75 μV), determined by visual inspection, appeared in a 15-s recording interval. Beginning from the lowest intensity (40 dB), the sound was increased in steps of 10 dB if no microarousal response occurred. If there was no response after delivery of the maximum tone (110 dB) at any frequency, recordings of sounds simulating “knocks on a door” were delivered or the name of the subject was spoken over an intercom by the experimenter. If there was still no response, the experimenter entered the room and gently shook the shoulder of the subject until a response occurred. This procedure prevented the subjects from entering NREM stage 3 sleep. Full awakenings were carefully avoided.
To assess glucose metabolism, we performed a frequently sampled ivGTT starting at 1000 hours, blood samples (1 ml) were drawn every 5 min for 15 min, at which time 0.3 g/kg glucose was administered as an i.v. bolus. Blood samples were then taken at times 2, 3, 4, 5, 6, 8, 10, 12, 15, 19, 21, 22, 24, 26, 28, 30, 40, 50, 60, 70, 90, 100, 120, 140, 180, 210, and 240 min. At time 20 min, i.v. insulin (0.02 units/kg) was administered. Plasma glucose was assayed by the glucose oxidase method, and serum insulin was measured by chemiluminescence assays by using the Immulite Immunochemistry System (Diagnostic Products Corporation). We calculated glucose tolerance from the rate of decline of glucose values between the 5th and 19th minutes after glucose injection. To evaluate S.I. the AIRg, and the DI (i.e., the product AIRg × S.I.), we analyzed the glucose and insulin profiles by using Bergmann's minimal model (4).
The ECG was recorded by using two thoracic electrodes while the subjects were sitting in a comfortable armchair on the days after the nights B2 and N3 between 1100 and 1300 hours. Changes in cardiac autonomic activity were estimated from analyses of heart rate variability (HRV). To account for changes in HRV attributable to breathing frequency, we simultaneously measured the respiratory effort signal by thoracic belts during ECG recordings. For each subject and each study condition, we performed a spectral analysis of HRV by fast Fourier transform on a 5-min section of recording free from ectopic beats and artifacts. The average number of beats used in the analysis was 313.5 ± 18.0 at baseline and 312.7 ± 20 after suppression of SWS (P = 0.93). The average number of beats used in the analysis was 313.5 ± 18.0 at baseline and 312.7 ± 20 after suppression of SWS (P = 0.93). The respiratory rate was similar between baseline (17.2 ± 0.7) and after suppression of SWS (17.7 ± 0.7). We used the spectral power in the HF (0.15–0.40 Hz) as a marker of vagal activity and the spectral power in the LF (0.04–0.14 Hz) as a marker of sympathetic activity. To better quantify the balance of the two branches of autonomic nervous system, we calculated HF and LF in normalized units (HFn and LFn) that represent the percentage of power in each band relative to their sum, and we minimized the impact of changes in total power on the absolute values of HF and LF (milliseconds squared). We used the LF-to-HF ratio (LF/HF) as an index of cardiac sympathovagal balance.
We collected blood samples at 20-min intervals starting at 0900 hours during the 24-h period preceding the ivGTT. A sterile heparin-lock catheter was inserted into the forearm, and the line was kept patent by a slow drip (10 cc/hr) of heparinized saline (750 units/dl). During waking hours, blood samples were collected at the bedside. During sleep hours, the i.v. line was extended and fed through a light-tight port in the wall, thus allowing disturbance-free blood drawing from a next-door room. Blood samples were centrifuged immediately at 4°C, and plasma was frozen and stored at −80°C until assay. For each 24-h profile, all samples obtained from the same subject were measured in the same assay. Plasma cortisol (micrograms per deciliter) was measured by chemiluminescence assay by using the Immulite Immunochemistry System (Diagnostic Products Corporation).
Statistical Analysis.
Statistical analysis was performed by using StatView and SuperANOVA software (Abacus Concepts). We compared metabolic, hormonal, and cardiovascular measures in the two study conditions by using two-tailed paired Student's t tests. We used ANOVA for repeated measures to compare sleep variables obtained during the baseline night (B1) and during the nights with SWS suppression (N1, N2, and N3). Correlations between sleep and metabolic variables were estimated by using the Pearson coefficient (rP). Statistical significance was defined as P < 0.05. All group data were expressed as means ± SEM.
Supplementary Material
ACKNOWLEDGMENTS.
We thank T. Wardzala and W. Selman for help with the acoustic system, J. Imperial and all other nursing staff of the General Clinical Research Center for expert assistance, and the volunteers for participating in this study. This research was supported by National Institutes of Health Grants P01 AG-11412, R01 HL-086459-01, R01 HL075079, M01 RR000055, and DK-20595.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0706446105/DC1.
References
- 1.Bonnet MH. Physiol Behav. 1986;37:915–918. [PubMed] [Google Scholar]
- 2.Marshall L, Helgadottir H, Molle M, Born J. Nature. 2006;444:610–613. doi: 10.1038/nature05278. [DOI] [PubMed] [Google Scholar]
- 3.Stickgold R. Nature. 2005;437:1272–1278. doi: 10.1038/nature04286. [DOI] [PubMed] [Google Scholar]
- 4.Bergman RN. Diabetes. 1989;38:1512–1527. doi: 10.2337/diab.38.12.1512. [DOI] [PubMed] [Google Scholar]
- 5.Clausen JO, Borch-Johnsen K, Ibsen H, Bergman RN, Hougaard P, Winther K, Pedersen O. J Clin Invest. 1996;98:1195–1209. doi: 10.1172/JCI118903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bergman RN, Ader M, Huecking K, Van Citters G. Diabetes. 2002;51(Suppl 1):S212–S220. doi: 10.2337/diabetes.51.2007.s212. [DOI] [PubMed] [Google Scholar]
- 7.Palmer ND, Langefeld CD, Campbell JK, Williams AH, Saad M, Norris JM, Haffner SM, Rotter JI, Wagenknecht LE, Bergman RN, et al. Diabetes. 2006;55:911–918. doi: 10.2337/diabetes.55.04.06.db05-0813. [DOI] [PubMed] [Google Scholar]
- 8.An P, Teran-Garcia M, Rice T, Rankinen T, Weisnagel SJ, Bergman RN, Boston RC, Mandel S, Stefanovski D, Leon AS, et al. Diabetologia. 2005;48:1142–1149. doi: 10.1007/s00125-005-1769-4. [DOI] [PubMed] [Google Scholar]
- 9.Weyer C, Bogardus C, Mott DM, Pratley RE. J Clin Invest. 1999;104:787–794. doi: 10.1172/JCI7231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lyssenko V, Almgren P, Anevski D, Perfekt R, Lahti K, Nissen M, Isomaa B, Forsen B, Homstrom N, Saloranta C, et al. Diabetes. 2005;54:166–174. doi: 10.2337/diabetes.54.1.166. [DOI] [PubMed] [Google Scholar]
- 11.Prigeon RL, Kahn SE, Porte D., Jr Metabolism. 1995;44:1259–1263. doi: 10.1016/0026-0495(95)90026-8. [DOI] [PubMed] [Google Scholar]
- 12.Van Cauter E, Leproult R, Plat L. J Am Med Assoc. 2000;284:861–868. doi: 10.1001/jama.284.7.861. [DOI] [PubMed] [Google Scholar]
- 13.Carrier J, Land S, Buysse DJ, Kupfer DJ, Monk TH. Psychophysiology. 2001;38:232–242. [PubMed] [Google Scholar]
- 14.Plat L, Féry F, L'Hermite-Balériaux M, Mockel J, Van Cauter E. J Clin Endocrinol Metab. 1999;84:3082–3092. doi: 10.1210/jcem.84.9.5978. [DOI] [PubMed] [Google Scholar]
- 15.Kuchel G, Cowen T. In: Functional Neurobiology of Aging. Hof P, Mobbs C, editors. San Diego: Academic; 2001. pp. 929–939. [Google Scholar]
- 16.Ferrara M, De Gennaro L, Casagrande M, Bertini M. Psychophysiology. 2000;37:440–446. [PubMed] [Google Scholar]
- 17.Kawakami N, Takatsuka N, Shimizu H. Diabetes Care. 2004;27:282–283. doi: 10.2337/diacare.27.1.282. [DOI] [PubMed] [Google Scholar]
- 18.Nilsson PM, Roost M, Engstrom G, Hedblad B, Berglund G. Diabetes Care. 2004;27:2464–2469. doi: 10.2337/diacare.27.10.2464. [DOI] [PubMed] [Google Scholar]
- 19.Mallon L, Broman JE, Hetta J. Diabetes Care. 2005;28:2762–2767. doi: 10.2337/diacare.28.11.2762. [DOI] [PubMed] [Google Scholar]
- 20.Meisinger C, Heier M, Loewel H. Diabetologia. 2005;48:235–241. doi: 10.1007/s00125-004-1634-x. [DOI] [PubMed] [Google Scholar]
- 21.Esler M, Rumantir M, Wiesner G, Kaye D, Hastings J, Lambert G. Am J Hypertens. 2001;14:304S–309S. doi: 10.1016/s0895-7061(01)02236-1. [DOI] [PubMed] [Google Scholar]
- 22.Kahn CR. In: Principles and Practice of Endocrinology and Metabolism. Becker KL, editor. Philadelphia: Lippincott; 1995. pp. 1210–1216. [Google Scholar]
- 23.Linkowski P. J Sleep Res. 1999;8(Suppl 1):11–13. doi: 10.1046/j.1365-2869.1999.00002.x. [DOI] [PubMed] [Google Scholar]
- 24.Linkowski P, Kerkhofs M, Hauspie R, Susanne C, Mendlewicz J. Electroencephalogr Clin Neurophysiol. 1989;73:279–284. doi: 10.1016/0013-4694(89)90106-5. [DOI] [PubMed] [Google Scholar]
- 25.Tan X, Campbell G, Feinberg I. Clin Neurophysiol. 2001;112:1540–1552. doi: 10.1016/s1388-2457(01)00570-3. [DOI] [PubMed] [Google Scholar]
- 26.Franken P, Tafti M. Frontiers Biosci. 2003;8:381–397. doi: 10.2741/1084. [DOI] [PubMed] [Google Scholar]
- 27.Mokdad AH, Ford ES, Bowman BA, Dietz WH, Vinicor F, Bales VS, Marks JS. J Am Med Assoc. 2003;289:76–79. doi: 10.1001/jama.289.1.76. [DOI] [PubMed] [Google Scholar]
- 28.Brandle M, Zhou H, Smith BR, Marriott D, Burke R, Tabaei BP, Brown MB, Herman WH. Diabetes Care. 2003;26:2300–2304. doi: 10.2337/diacare.26.8.2300. [DOI] [PubMed] [Google Scholar]
- 29.Young T, Peppard PE, Taheri S. J Appl Physiol. 2005;99:1592–1599. doi: 10.1152/japplphysiol.00587.2005. [DOI] [PubMed] [Google Scholar]
- 30.Morisson F, Decary A, Petit D, Lavigne G, Malo J, Montplaisir J. Chest. 2001;119:45–52. doi: 10.1378/chest.119.1.45. [DOI] [PubMed] [Google Scholar]
- 31.Resta O, Foschino Barbaro MP, Bonfitto P, Giliberti T, Depalo A, Pannacciulli N, De Pergola G. J Intern Med. 2003;253:536–543. doi: 10.1046/j.1365-2796.2003.01133.x. [DOI] [PubMed] [Google Scholar]
- 32.Vgontzas AN, Tan TL, Bixler EO, Martin LF, Shubert D, Kales A. Arch Intern Med. 1994;154:1705–1711. [PubMed] [Google Scholar]
- 33.Mokdad A, Bowman B, Ford E, Vinicor F, Marks J, Koplan J. J Am Med Assoc. 2001;289:1195–1200. doi: 10.1001/jama.286.10.1195. [DOI] [PubMed] [Google Scholar]
- 34.Spiegel K, Knutson K, Leproult R, Tasali E, Van Cauter E. J Appl Physiol. 2005;99:2008–2019. doi: 10.1152/japplphysiol.00660.2005. [DOI] [PubMed] [Google Scholar]
- 35.Spiegel K, Leproult R, Van Cauter E. Lancet. 1999;354:1435–1439. doi: 10.1016/S0140-6736(99)01376-8. [DOI] [PubMed] [Google Scholar]
- 36.Knutson KL, Ryden AM, Mander BA, Van Cauter E. Arch Intern Med. 2006;166:1768–1774. doi: 10.1001/archinte.166.16.1768. [DOI] [PubMed] [Google Scholar]
- 37.Ayas NT, White DP, Al-Delaimy WK, Manson JE, Stampfer MJ, Speizer FE, Patel S, Hu FB. Diabetes Care. 2003;26:380–384. doi: 10.2337/diacare.26.2.380. [DOI] [PubMed] [Google Scholar]
- 38.Yaggi HK, Araujo AB, McKinlay JB. Diabetes Care. 2006;29:657–661. doi: 10.2337/diacare.29.03.06.dc05-0879. [DOI] [PubMed] [Google Scholar]
- 39.Johns M. Sleep. 1992;15:376–381. doi: 10.1093/sleep/15.4.376. [DOI] [PubMed] [Google Scholar]
- 40.Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. Arch Gen Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- 41.Weaver TE, Laizner AM, Evans LK, Maislin G, Chugh DK, Lyon K, Smith PL, Schwartz AR, Redline S, Pack AI, et al. Sleep. 1997;20:835–843. [PubMed] [Google Scholar]
- 42.Rechtschaffen A, Kales A. A Manual of Standardized Terminology, Techniques and Scoring System for Sleep Stages of Human Subjects. Los Angeles: Brain Inform Serv/Brain Res Inst, Univ of California; 1968. [Google Scholar]
- 43.Sleep Disorders Atlas Task Force of the American Sleep Disorders Association. Sleep. 1992;15:173–184. [PubMed] [Google Scholar]
- 44.American Academy of Sleep Medicine Task Force. Sleep. 1999;22:667–689. [PubMed] [Google Scholar]
- 45.Chesson AJ, Wise M, Davila D, Johnson S, Littner M, Anderson W, Hartse K, Rafecas J. Sleep. 1999;22:961–968. doi: 10.1093/sleep/22.7.961. [DOI] [PubMed] [Google Scholar]
- 46.Feinberg I, Floyd TC. Psychophysiology. 1979;16:283–291. doi: 10.1111/j.1469-8986.1979.tb02991.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.