The discovery of neural stem cells (NSCs) in the adult mammalian central nervous system (CNS) has dramatically changed our view on the regenerative capacity of this organ (1, 2). We now realize that the adult brain, including humans, retains the ability to replenish its cellular constituents, neurons and glia, although the extent is very limited compared with lower vertebrates (2). Cell turnover driven by NSCs has been implicated in higher brain functions such as learning and memory (3). Adult NSCs have also been shown to participate in neuronal cell replacement after injury, raising the possibility of stem cell-based therapy for neurological disorders (4). Despite extensive studies in the past decade, one fundamental question remains unanswered: What is the identity of NSCs? In 1999, two groups reported apparently contradictory results: Johansson et al. (5) provided evidence that ependymal cells, which constitute a ciliated single-cell-thick epithelial layer lining the lateral ventricle (LV), retain the characteristics of NSCs (Fig. 1A). In contrast, Doetsch et al. (6) identified glial fibrillary acidic protein (GFAP)-positive astrocyte-like cells, which reside in a region beneath the ependymal layer called the subependymal layer or subventricular zone (SVZ), as NSCs. Although these two results were not necessarily mutually exclusive, they sparked a debate that has been ongoing ever since. For better understanding of the basic biology of NSCs and their future successful use for therapy, reconciliation of this long-debated issue is awaited. The work of Coskun et al. (7) in this issue of PNAS addressed this question by using new tools and approaches.
Fig. 1.
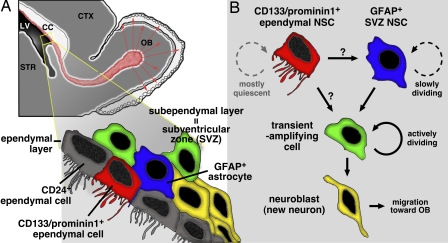
Cytoarchitecture of the ventricular wall of the adult rodent brain. The topological relationship between the ependymal layer and SVZ (A) and cell types known to reside in these regions (B) are schematically depicted (modified from a figure kindly provided by Yi Sun and Volkan Coskun of ref. 7). CC, corpus callosum; CTX, cerebral cortex; OB, olfactory bulb; STR, striatum.
New Evidence for Ependymal Stem Cells
In their new study, Coskun et al. (7) first show that the cell-surface molecule CD133/prominin1, which has increasingly been recognized as a common marker for various stem cell populations in adult tissues/organs (8), is specifically expressed in a subpopulation of ependymal cells. Importantly, they did not detect CD133 expression in GFAP+ cells in the SVZ. They also show that CD133+ ependymal cells are mostly quiescent, but can divide in situ under the condition in which actively dividing cells in the adjacent SVZ are killed off. Next, they used in vitro culture to demonstrate that CD133+ cells exhibit the canonical features of NSCs, i.e., self-renewal and multipotency. Interestingly, the stem cell activity resided in a CD133+/CD24− subfraction, but not in CD133+/CD24+ or CD133−/CD24+ cell fraction, the major cell population in the ependymal layer. To examine the capacity of CD133+ cells as stem cells in vivo, Coskun et al. took two strategies: transplantation of CD133+ cells into the brain and fate mapping of CD133+ cells in situ by transfection-mediated selective expression of Cre recombinase in the brains of ROSA26-lacZ reporter mice. In both experiments, CD133+ cells generated progenies that were expected to originate from NSCs. From these results, Coskun et al. conclude that CD133+ ependymal cells represent a quiescent NSC population in the adult brain.
Two Adjacent Stem Cell Populations?
This study by Coskun et al. (7) has added a new spin, rather than the ultimate answer, to the issue of ependymal and subependymal origins of NSCs. Identification of NSCs has been a difficult task for several reasons. First, NSCs are very rare in the adult brain, and no single molecular marker, including CD133 and GFAP, can unambiguously define them in situ. Second, because stem cells are a functional entity, the proof of their identity needs demonstration of their properties as stem cells (self-renewal and multipotency). Unlike other organs/tissues such as the hematopoietic system, however, no reliable in vivo assay of the stemness of NSCs at the single-cell level is available at present. Therefore, identification of NSCs has heavily relied on in vitro culture (5–7). An inherent problem of culture experiments is that a subtle difference in conditions may result in successful growth of one type of stem cells, but not others. In fact, a widely used method called neurosphere culture supports clonal growth of SVZ-derived NSCs, but not those from another well known NSC site, the hippocampal dentate gyrus (DG) (9, 10). Many other lines of evidence, however, support the idea that NSCs reside in the DG (2). Because negative data obtained under certain experimental conditions do not necessarily disprove the positive results obtained in other conditions, it seems reasonable to consider that cells with the characteristics of NSCs reside in both the ependymal layer and SVZ, but their properties are somehow distinct.
This view, however, raises several new questions. First, what is the relationship between these two stem cell populations? Although Coskun et al. (7) imply that SVZ stem cells may be downstream of ependymal stem cells, their lineage relationship is currently unknown. They may constitute independent stem cell compartments, as is the case for the skin where multiple types of stem cells coexist (11). The answer to this question must await unambiguous in vivo fate mapping studies of each cell type. Second, what is the true identity of NSCs in each stem cell compartment? Evidence has shown that only a small subpopulation (<10%) of GFAP+ cells in the SVZ and CD133+ ependymal cells exhibit the properties of NSCs in vitro (6, 7). More markers are necessary to define them in situ. Alternatively, if different populations of NSCs do coexist, no fixed set of markers may define the stemness of NSCs.
Another important question is to what extent each of these stem cell populations contributes to the production of new neurons (neurogenesis) in the adult brain. Although SVZ stem cells are slowly dividing cells, they are thought to produce more actively dividing intermediate progenitors called transient-amplifying cells, thereby serving as a founder of a large number of new neurons (6) (Fig. 1B). In contrast, ependymal cells are believed to either not divide or divide at a negligible rate in the intact brain (5, 7), so it is unlikely that they serve as a major contributor to neurogenesis. They could be, however, the founder of SVZ stem cells through a very rare cell division as Coskun et al. (7) suggest. Alternatively, they may only participate in neurogenesis under pathological conditions because ependymal cells are known to proliferate after injury (5, 7).
Neurogenic Niche and Stem Cell Niche
In adults, tissue-specific stem cells are thought to be nested in a specialized microenvironment, the so-called stem cell niche (12). In particular, the SVZ is a narrow cell dense region easily recognizable only around the anterior part of the LV and thus thought to constitute a very specialized niche for NSCs. In the adult brain, active neurogenesis occurs in only a few restricted regions, the aforementioned SVZ and DG (2). Thus, stem cell niches have been considered to be
Cell turnover driven by NSCs has been implicated in higher brain functions.
limited to these two neurogenic regions. Coskun et al. (7), however, propose that that may not be the case because the ependymal layer, where continuous cell divisions do not take place, contains NSCs. Along this idea, the occurrence of NSC niches may be more widespread than previously appreciated. In fact, previous cell culture studies have identified cells with characteristics of NSCs even outside of the ependymal layer and SVZ. These NSC-like cells have been found along the entire anteroposterior axis of the ventricular system, including the areas around the third and fourth ventricles and the central canal of the spinal cord (13). Some studies have further shown the occurrence of similar cells in the CNS parenchyma distant from the ventricle (14–16). Notably, the spinal cord contains the ependymal layer, but not an anatomically distinguishable SVZ-like structure. Nevertheless, a culture condition that does not support the growth of NSCs derived from the ependymal layer lining the LV or hippocampal DG has been shown to be sufficient for expansion of spinal cord-derived NSCs (17). Conversely, although the DG does not contain ependymal cells or a structure comparable with the SVZ, many studies successfully isolated NSCs from this unique region distant from the LV (2, 14). It could be that such NSC-like cells in normally non-neurogenic regions are not stem cells in vivo, but transform into stem cell-like cells in culture. Surprisingly, however, their frequency detected in culture from respective tissues is similar to that of authentic NSCs in the SVZ, and their properties in vitro are often indistinguishable from those of SVZ NSCs (13–17). Whether such cells share the same or similar properties in vivo with either SVZ or ependymal NSCs is currently unknown.
Stem Cells in Intact Versus Injured Brains
NSCs around the LV normally give rise to neurons destined to the olfactory bulb (18) (Fig. 1A). After insults such as ischemia, neurotoxicity, and trauma, however, they produce other types of neurons as well, including striatal and cortical neurons (4). In addition to these actively dividing cells in the intact brain, normally quiescent cells can participate in injury-induced neurogenesis (19). Likewise, certain signals could mobilize otherwise dormant ependymal NSCs or parenchymal NSC-like cells after injury. Thus, multiple cell populations may have a potential to participate in repair of damaged brain. In fact, a recent elegant study of the olfactory sensory epithelium has shown a precedent example that different types of cells are responsible for neuronal cell replacement under distinct insult conditions (20). Identification of NSCs is an essential first step to distinguish the source of new neurons and glia, and the study by Coskun et al. (7) has shed new light on this important issue. More new tools and approaches are necessary to reveal what NSCs are, where they are, and what they do in intact and damaged brains. Answering these questions will ultimately contribute to both better understanding of brain functions and development of stem cell therapy for neurological diseases.
Footnotes
The authors declare no conflict of interest.
See companion article on page 1026.
References
- 1.Temple S. Nature. 2001;414:112–117. doi: 10.1038/35102174. [DOI] [PubMed] [Google Scholar]
- 2.Lie DC, Song H, Colamarino SA, Ming GL, Gage FH. Annu Rev Pharmacol Toxicol. 2004;44:399–421. doi: 10.1146/annurev.pharmtox.44.101802.121631. [DOI] [PubMed] [Google Scholar]
- 3.Leuner B, Gould E, Shors TJ. Hippocampus. 2006;16:216–224. doi: 10.1002/hipo.20153. [DOI] [PubMed] [Google Scholar]
- 4.Goldman SA. Nat Biotechnol. 2005;23:862–871. doi: 10.1038/nbt1119. [DOI] [PubMed] [Google Scholar]
- 5.Johansson CB, et al. Cell. 1999;96:25–34. doi: 10.1016/s0092-8674(00)80956-3. [DOI] [PubMed] [Google Scholar]
- 6.Doetsch F, Caillé I, Lim DA, García-Verdugo JM, Alvarez-Buylla A. Cell. 1999;97:703–716. doi: 10.1016/s0092-8674(00)80783-7. [DOI] [PubMed] [Google Scholar]
- 7.Coskun V, et al. Proc Natl Acad Sci USA. 2008;105:1026–1031. doi: 10.1073/pnas.0710000105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mizrak D, Brittan M, Alison MR. J Pathol. 2008;214:3–9. doi: 10.1002/path.2283. [DOI] [PubMed] [Google Scholar]
- 9.Seaberg RM, van der Kooy D. J Neurosci. 2002;22:1784–1793. doi: 10.1523/JNEUROSCI.22-05-01784.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bull ND, Bartlett PF. J Neurosci. 2005;25:10815–10821. doi: 10.1523/JNEUROSCI.3249-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Watt FM, Celso CL, Silva-Vargas V. Curr Opin Genet Dev. 2006;16:518–524. doi: 10.1016/j.gde.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 12.Fuchs E, Tumbar T, Guasch G. Cell. 2004;116:769–778. doi: 10.1016/s0092-8674(04)00255-7. [DOI] [PubMed] [Google Scholar]
- 13.Weiss S, et al. J Neurosci. 1996;16:7599–7609. doi: 10.1523/JNEUROSCI.16-23-07599.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Palmer TD, Markakis EA, Willhoite AR, Safar F, Gage FH. J Neurosci. 1999;19:8487–8497. doi: 10.1523/JNEUROSCI.19-19-08487.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yamamoto S, Yamamoto N, Kitamura T, Nakamura K, Nakafuku M. Exp Neurol. 2001;172:115–127. doi: 10.1006/exnr.2001.7798. [DOI] [PubMed] [Google Scholar]
- 16.Nunes MC, et al. Nat Med. 2003;9:439–447. doi: 10.1038/nm837. [DOI] [PubMed] [Google Scholar]
- 17.Martens DJ, Seaberg RM, van der Kooy D. Eur J Neurosci. 2002;16:1045–1057. doi: 10.1046/j.1460-9568.2002.02181.x. [DOI] [PubMed] [Google Scholar]
- 18.Doetsch F. Nat Neurosci. 2003;6:1127–1134. doi: 10.1038/nn1144. [DOI] [PubMed] [Google Scholar]
- 19.Nakatomi H, et al. Cell. 2002;110:429–441. doi: 10.1016/s0092-8674(02)00862-0. [DOI] [PubMed] [Google Scholar]
- 20.Leung CT, Coulombe PA, Reed RR. Nat Neurosci. 2007;10:720–726. doi: 10.1038/nn1882. [DOI] [PubMed] [Google Scholar]