Abstract
Elevation of cytosolic free Ca2+ concentration ([Ca2+]i) in excitable cells often acts as a negative feedback signal on firing of action potentials and the associated voltage-gated Ca2+ influx. Increased [Ca2+]i stimulates Ca2+-sensitive K+ channels (IK-Ca), and this, in turn, hyperpolarizes the cell and inhibits Ca2+ influx. However, in some cells expressing IK-Ca the elevation in [Ca2+]i by depletion of intracellular stores facilitates voltage-gated Ca2+ influx. This phenomenon was studied in hypothalamic GT1 neuronal cells during store depletion caused by activation of gonadotropin-releasing hormone (GnRH) receptors and inhibition of endoplasmic reticulum (Ca2+)ATPase with thapsigargin. GnRH induced a rapid spike increase in [Ca2+]i accompanied by transient hyperpolarization, followed by a sustained [Ca2+]i plateau during which the depolarized cells fired with higher frequency. The transient hyperpolarization was caused by the initial spike in [Ca2+]i and was mediated by apamin-sensitive IK-Ca channels, which also were operative during the subsequent depolarization phase. Agonist-induced depolarization and increased firing were independent of [Ca2+]i and were not mediated by inhibition of K+ current, but by facilitation of a voltage-insensitive, Ca2+-conducting inward current. Store depletion by thapsigargin also activated this inward depolarizing current and increased the firing frequency. Thus, the pattern of firing in GT1 neurons is regulated coordinately by apamin-sensitive SK current and store depletion-activated Ca2+ current. This dual control of pacemaker activity facilitates voltage-gated Ca2+ influx at elevated [Ca2+]i levels, but also protects cells from Ca2+ overload. This process may also provide a general mechanism for the integration of voltage-gated Ca2+ influx into receptor-controlled Ca2+ mobilization.
Agonist-induced activation of Ca2+-mobilizing receptors and the subsequent release of Ca2+ from the endoplasmic reticulum (ER) regulate the function of many excitable and nonexcitable cell types (1). The inositol 1,4,5-trisphosphate generated by receptor activation opens Ca2+-permeable channels in the ER membrane and releases sequestered Ca2+, thereby increasing cytosolic Ca2+ concentration ([Ca2+]i) and depleting Ca2+ stores. To maintain normal cell function during sustained receptor activation, the ER store must be replenished from the vast supply of extracellular Ca2+. In nonexcitable cells, reduction of ER Ca2+ levels triggers capacitative Ca2+ influx across the plasma membrane via store-operated channels (2). In excitable neuronal and endocrine cells, activation of Ca2+-mobilizing receptors frequently is associated with an increase in action potential (AP)-driven Ca2+ entry through voltage-gated Ca2+ channels (3). In pituitary lactotrophs, for example, spontaneous AP firing is transiently abolished by activation of Ca2+-mobilizing receptors, followed by a sustained increase in firing frequency and enhanced voltage-gated Ca2+ influx (4). However, the manner in which store depletion by Ca2+-mobilizing receptors triggers an increase in membrane excitability has not been determined. In addition, excitable cells express Ca2+-controlled potassium channels (IK-Ca) that hyperpolarize the cell and abolish AP firing and Ca2+ spiking (5). Because the sustained rise in [Ca2+]i release should activate these channels, it is not clear how stimulation of Ca2+-mobilizing receptors overcomes the IK-Ca-mediated attenuation of excitability to facilitate voltage-gated Ca2+ influx.
In this study, the effects of receptor- and non-receptor-mediated store depletion on membrane excitability were examined in immortalized hypothalamic GT1–7 neurons (GT1 cells). In addition to gonadotropin-releasing hormone (GnRH) receptors, these well differentiated neuronal cells express a variety of plasma-membrane channels that endow them with the ability to fire spontaneous APs and generate Ca2+ transients (6, 7). These include tetrodotoxin-sensitive Na+ channels, low-voltage-activated Ca2+ channels, dihydropyridine-sensitive Ca2+ channels, γ-aminobutyric acid type A channels, inward-rectifying K+ channels (Kir), several types of outward K+ channels, and the BK subtype of IK-Ca channels (8–10). Activation of Ca2+-mobilizing GnRH receptors in these cells causes a biphasic rise in [Ca2+]i (7, 11), with a sustained plateau response that coincides with an increase in spike frequency (7). Here we show that GT1 cells also express apamin-sensitive IK-Ca channels (SK channels) that are activated by increased Ca2+ release from GnRH-sensitive ER Ca2+ pools. These SK channels, rather than BK channels, influence membrane excitability in agonist-stimulated GT1 neurons. In addition, depletion of the ER Ca2+ pool by receptor- or non-receptor-mediated pathways stimulates a Ca2+-permeable depolarizing channel that overcomes SK channel activation to increase membrane excitability and [Ca2+]i. Thus, store depletion activates both hyperpolarizing SK channels and depolarizing Ca2+-conducting channels that act in synchrony to control membrane potential (Vm) and replenish the ER Ca2+ stores after activation of Ca2+-mobilizing receptors.
MATERIALS AND METHODS
Chemicals.
Stock solutions of apamin, charybdotoxin (Research Biochemicals, Natick, MA), and GnRH (Peptides International) were prepared in double-distilled water. Stock solutions of thapsigargin and nifedipine (Research Biochemicals) were dissolved in dimethyl sulfoxide (DMSO). The maximum final concentration of DMSO was 0.1%, which did not alter Vm or ionic currents.
Cell Culture.
All experiments were performed on the GT1–7 subtype of immortalized GnRH neurons (GT1 cells), cultured as described previously (11). Morphological differentiation was optimized by culturing the cells in medium containing B-27 serum-free supplement (GIBCO) for 3–5 days before experimental recordings.
125I-Labeled Apamin Binding.
Binding studies were performed on intact GT1 cells at 22°C in medium 199 with 25 mM Hepes, pH 7.4. The cells were washed twice with binding medium and then incubated for 90 min with 10 pM 125I-labeled apamin (Amersham) and increasing concentrations of unlabeled apamin. After incubation to equilibrium the cells were washed three times with ice-cold PBS/0.1% BSA, solubilized in 1 M NaOH containing 0.1% SDS, and analyzed for bound radioactivity in a γ-spectrometer.
Simultaneous Measurement of [Ca2+]i and Membrane Current or Vm.
GT1 cells were cultured on coverslips and incubated for 30 min at 37°C in phenol red-free medium 199 containing Hanks’ salts/20 mM sodium bicarbonate/20 mM Hepes/0.5 μM indo-1 acetoxymethyl ester (Molecular Probes). The cells then were washed twice with recording solution containing 120 mM NaCl/4.7 mM KCl/2.6 mM CaCl2/2 mM MgCl2/0.7 mM MgSO4/10 mM Hepes/10 mM glucose (pH adjusted to 7.4 with NaOH) and mounted on the stage of an inverted epifluorescence Nikon microscope. A photon-counter system (Nikon) was used to simultaneously measure the intensity of light emitted at 405 nm and 480 nm after excitation at 340 nm, with correction for background intensity at each emission wavelength. Perforated-patch, current-clamp, and voltage-clamp recordings were performed with an Axopatch 200 B patch-clamp amplifier (Axon Instruments) as described previously (12). Patch pipette tips (3–5 MΩ) were immersed briefly in a solution containing 70 mM KCl/70 mM potassium aspartate/1 mM MgCl2/10 mM Hepes (pH adjusted to 7.2 with KOH) and then back-filled with the same solution containing amphotericin B (240 μg/ml). Before seal formation, liquid junction potentials were canceled. An average series resistance of 19 ± 1 MΩ (n = 130) was reached after GΩ seal (seal resistance > 5 GΩ) formation. The data were digitized at 4 kHz by using a PC equipped with the pclamp 7 software package in conjunction with a Digidata 1200 A/D converter (Axon Instruments). [Ca2+]i was calibrated in vivo according to Kao (13), resulting in values for Rmin, Rmax, Sf,480/Sb,480, and Kd of 0.4719, 3.634, 3.187, and 230 nM, respectively. All Vm values were corrected for a liquid junction potential error of 10 mV (14). The bath contained <500 μl of saline and was perfused continuously at a rate of 2 ml/min by using a gravity-driven superfusion system. In some experiments, CaCl2 was omitted from the recording medium to give nominally Ca2+-free medium (Ca2+-deficient medium, free Ca2+ of about 10 μM). A solid Ag/AgCl reference electrode was connected to the bath via a 3-M KCl agar bridge.
[Ca2+]i-Clamp Experiments.
[Ca2+]i was clamped at ≈20 nM by including EGTA in the pipette solution, and Vm was monitored by conventional whole-cell recordings in the current-clamp mode (Fig. 1C, ref. 15). The bath contained normal recording solution and the pipette contained 70 mM KCl/70 mM potassium aspartate/1 mM MgCl2/10 mM Hepes/10 mM EGTA/0.3 mM Tris-GTP/4 mM Mg-ATP/14 mM phosphocreatine/50 units/ml creatine phosphokinase (pH adjusted to 7.2 with KOH). An average series resistance of 11 ± 1 MΩ (n = 5) was reached after GΩ seal (seal resistance >10 GΩ) formation, and current-clamp recordings were acquired as described above.
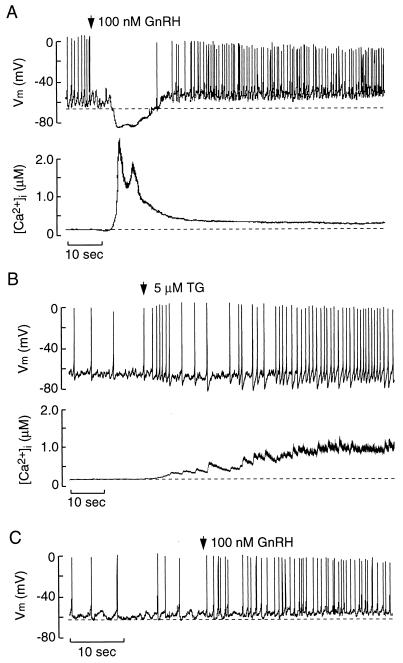
Figure 1.
Store depletion and membrane excitability in GT1 cells. (A) Simultaneous measurements of membrane potential (Vm) and [Ca2+]i in GnRH-stimulated cells. (B) Simultaneous measurements of Vm and [Ca2+]i in TG-treated cells. In A and B, perforated-patch recording techniques were used. (C) AP firing in GnRH-stimulated cells with [Ca2+]i clamped at ≈20 nM, using conventional whole-cell recording techniques. Arrows indicate the moment of application of GnRH and TG. In this and the following figures, dotted lines represent baseline potential (upper tracing) and basal [Ca2+]i (bottom tracing).
RESULTS AND DISCUSSION
Store Depletion and Vm Excitability.
Most GT1 cells exhibited spontaneous firing of APs from a baseline potential (maximum level of hyperpolarization observed; Fig. 1A Upper, dashed line) of –66 ± 1 mV (n = 48). Application of 100 nM GnRH induced transient hyperpolarization (mean duration = 14.5 ± 1.9 sec; n = 10) to −84 ± 2 mV (n = 10) and suppression of AP spiking (Fig. 1A Upper). This was followed by a sustained depolarization of 9 ± 1 mV (n = 10, P < 0.05) above the baseline potential and a concomitant increase in AP frequency from 0.6 ± 0.1 Hz to 1.5 ± 0.2 Hz (P < 0.05; n = 10). The membrane-depolarization phase and the increase in AP frequency were sustained for as long as GnRH was present. The actions of GnRH on Vm were mirrored by a biphasic increase in [Ca2+]i, with an initial spike and a sustained plateau phase (Fig. 1A Lower). During the spike phase, [Ca2+]i reached a maximum concentration of 2.2 ± 0.3 μM in 2.8 ± 0.2 sec (n = 10) and coincided with the transient Vm hyperpolarization. During the plateau phase, the [Ca2+]i reached 0.5 ± 0.1 μM (n = 10) and coincided with the sustained depolarization and increased spike frequency (Fig. 1A).
The GnRH-induced spike phase also was present in cells bathed in Ca2+-deficient medium, indicating that it is a result of Ca2+ release from ER Ca2+ stores. In contrast, the plateau phase is critically dependent on Ca2+ influx through voltage-gated and voltage-independent Ca2+ entry (7, 11). The degree of store depletion after GnRH receptor activation was examined by measuring the amplitude of the peak [Ca2+]i response to 1 μM ionomycin added 5 min after treatment with 100 nM GnRH or its vehicle in cells bathed in Ca2+-deficient medium. In GnRH-stimulated cells, the [Ca2+]i response to ionomycin was markedly reduced compared with control groups (ionomycin-induced increase in [Ca2+]i expressed as a percentage of the prestimulus [Ca2+]i; control = 436 ± 17% vs. 100 nM GnRH-treated = 117 ± 3%; P < 0.05; n = 10).
The influence of ER Ca2+ store depletion on membrane excitability in the absence of receptor stimulation was examined by using thapsigargin (TG), an inhibitor of ER(Ca2+)ATPase (16). To demonstrate that TG depletes GnRH-sensitive ER Ca2+ stores, the amplitude of the spike [Ca2+]i response to 100 nM GnRH was determined after a 15-min pretreatment with 5 μM TG or its vehicle in Ca2+-deficient medium. In TG-treated cells, GnRH was unable to increase [Ca2+]i levels (GnRH-induced spike increase in [Ca2+]i normalized to percentage pretreatment; vehicle = 307 ± 20% vs. TG treated = 100 ± 3%; P < 0.05; n = 3). These findings confirm that TG depletes GnRH-sensitive ER Ca2+ stores in GT1 cells. The maximum [Ca2+]i response to TG was highly variable, ranging from 250 nM to 1.5 μM above basal levels (Figs. 1B, 3C, and 6). Such TG-induced rises in [Ca2+]i were accompanied by a hyperpolarizing shift in the baseline potential (Fig. 1B), the magnitude of which depended on the [Ca2+]i level. As with GnRH treatment, depletion of the ER Ca2+ store by TG increased spike frequency during sustained exposure (Fig. 1B). These findings demonstrate that store depletion by receptor- and non-receptor-mediated mechanisms modulates membrane excitability of GT1 cells.
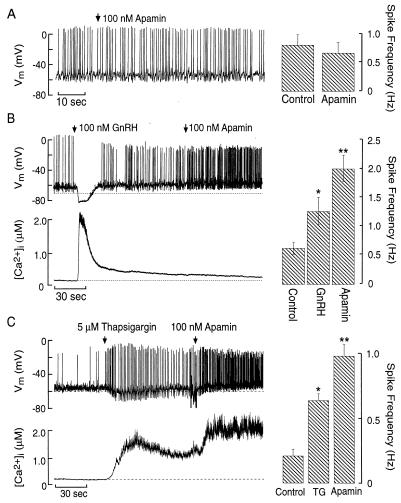
Figure 3.
Role of SK channels in unstimulated and store-depleted cells. (A) Lack of effect of apamin on AP firing in a spontaneously active cell. (B and C) Activation of SK channels during sustained store depletion by GnRH or TG. Simultaneous measurements of Vm and [Ca2+]i changes in response to GnRH (B Left) or TG (C Left) before and during apamin addition. (Right) Asterisk denotes significant difference (P < 0.05; n = 3) from control. Double asterisks denote significant difference (P < 0.05; n = 3) between responses to TG or GnRH and apamin.
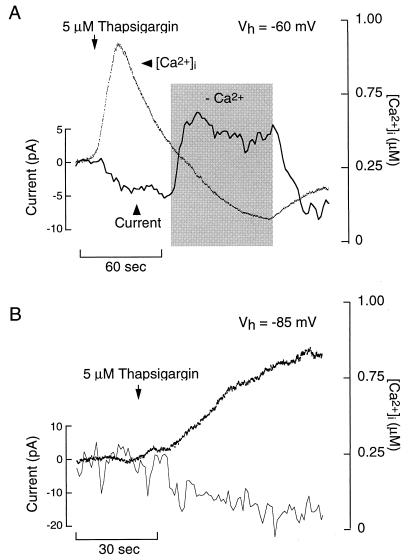
Figure 6.
Thapsigargin-induced Ca2+-conducting inward current in GT1 cells. Simultaneous measurements of current and [Ca2+]i were performed by using perforated-patch, voltage-clamp recording techniques. (A) Effects of extracellular Ca2+ depletion (shaded area) on thapsigargin-induced current and [Ca2+]i in a cell voltage-clamped at –60 mV. (B) Thapsigargin-induced inward current in cells voltage-clamped at –85 mV. In A and B, the bath contained 1 μM nifedipine, 50 μM Ni2+, and 100 nM apamin.
To determine whether the changes in membrane excitability were a result of an increase in [Ca2+]i, conventional whole-cell recording techniques were used to clamp [Ca2+]i around 20 nM (see Materials and Methods). Under these conditions, GnRH did not hyperpolarize the cell, but did induce a sustained depolarization and increase in spike frequency (Fig. 1C; n = 5). These results indicate that the transient hyperpolarization is dependent on the rise in [Ca2+]i because of store depletion, whereas the sustained depolarization and facilitation of firing are independent of [Ca2+]i.
Store Depletion and IK-Ca Activation.
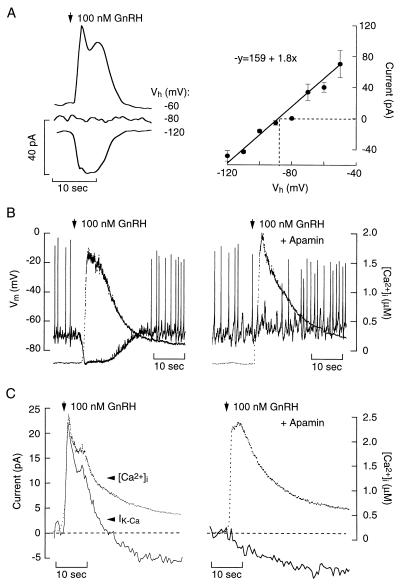
In neuronal and endocrine cells, Ca2+ release from the ER activates IK-Ca channels to modulate membrane excitability (5). Depending on the pattern of Ca2+ release, activation of IK-Ca channels generates transient or oscillatory membrane hyperpolarizations (17–19). In GT1 cells, the [Ca2+]i sensitivity of the GnRH-induced transient hyperpolarization (Fig. 1C) suggests the involvement of IK-Ca during store depletion. Consistent with this, GnRH activated a transient current with a reversal potential of –87 mV (Fig. 2A), which is the calculated K+ equilibrium potential in these experiments. From a holding potential of −60 mV, which is near the baseline Vm, the GnRH-induced outward current reached a peak amplitude of 40.0 ± 6.2 pA in 3.7 ± 1.3 sec (n = 8). The BK-type IK-Ca channel antagonist, charybdotoxin (5 nM; ref. 10), had no effect on the GnRH-induced membrane hyperpolarization (control = −84 ± 2 mV, n = 10, vs. charybdotoxin = −79 ± 5 mV; P > 0.05; n = 3). In contrast, the SK-type IK-Ca channel antagonist, apamin (100 nM), abolished the GnRH-induced hyperpolarization (Fig. 2B; n = 8) and outward current (Fig. 2C; n = 20), indicating the involvement of SK channels.
Figure 2.
Characterization of the current underlying GnRH-induced hyperpolarization in GT1 neurons. (A Left) Representative current traces of the initial GnRH-induced transient current at the indicated holding potentials (Vh). (Right) Current–voltage relation of the GnRH-induced current. Dotted line represents the reversal potential. (B) Simultaneous measurement of Vm (solid lines) and [Ca2+]i (dotted lines) in response to GnRH in the absence (Left) or presence (Right) of 100 nM apamin. (C) Characterization of currents activated by store depletion in GT1 neurons. Simultaneous measurement of current (solid lines) and [Ca2+]i (dotted lines) in response to GnRH in the absence (Left) or presence (Right) of 100 nM apamin.
Using a whole-cell conductance of 1.8 nS, calculated from the slope of the curve in Fig. 2A, and a single channel conductance for the SK channel between 9 and 14 pS (20, 21), an estimated 130–200 SK channels were activated by the GnRH-induced [Ca2+]i rise. Characterization of apamin-binding sites in GT1 cells yielded a similar number of channels per cell (150–250; n = 4). In addition, binding of 125I-apamin was inhibited by unlabeled apamin with an IC50 of 29 pM. Scatchard analysis of the binding data was consistent with the expression of a single class of apamin receptor channels, with a Kd of 20 pM (not shown), as observed in other cells (22, 23). Thus, GnRH activates most of the SK channels expressed in GT1 cells to cause the transient hyperpolarization and abolition of AP firing during the spike phase.
We next examined the impact of IK-Ca channels on AP firing under basal [Ca2+]i and sustained agonist-induced increases in [Ca2+]i. Voltage-gated Ca2+ influx during AP firing has been demonstrated to activate IK-Ca channels in many excitable cells (1). In GT1 cells, charybdotoxin (5 nM) did not alter the spontaneous firing pattern (before charybdotoxin, 1.37 ± 0.47 Hz vs. during charybdotoxin treatment, 1.21 ± 0.44 Hz, P > 0.05, n = 6). Similarly, 100 nM apamin had no effect on AP frequency or duration (Fig. 3A, n = 8). This is consistent with the ambient [Ca2+]i of 166 ± 27 nM (n = 21) in unstimulated GT1 cells, a concentration that is insufficient to activate IK-Ca channels (21, 24). In contrast, application of 100 nM apamin during sustained GnRH stimulation increased spike frequency (Fig. 3B) and duration (measured at one-half amplitude; GnRH = 10.3 ± 0.4 msec vs. GnRH + apamin = 15.6 ± 1.8 msec, P < 0.05, n = 3). Addition of apamin to TG-treated cells also increased spike frequency (Fig. 3C).
These results demonstrate that apamin-sensitive IK-Ca channels are expressed in GT1 cells but do not participate in the control of spontaneous firing. However, the levels of [Ca2+]i reached during store depletion by GnRH and TG are sufficient to activate these SK-type channels during acute and prolonged stimulation. Such activation of SK channels during sustained GnRH stimulation does not abolish the agonist-induced depolarization and increase in spike frequency. The [Ca2+]i sensitivity of SK channels in GT1 neurons is in accord with observations in adrenal chromaffin cells (24) and skeletal muscle cells (21). Conversely, BK channels require [Ca2+]i levels more than 10 μM for their activation at the baseline potential (21), a concentration that is not reached in GnRH-stimulated GT1 cells.
Store Depletion-Activated Depolarizing Current.
Agonist-induced sustained depolarization of cells and increased spiking frequency is observed in many excitable cells (3). However, the mechanism(s) underlying increased spike frequency and the impact of IK-Ca channels have not been clarified. Our finding that GnRH causes cell depolarization and increased firing frequency despite the activation of SK channels indicates that GnRH modulates other current(s) that promote cell depolarization. Accordingly, the GnRH-stimulated transient outward current was followed by a sustained inward current (Fig. 2C Left) that was insensitive to apamin (Fig. 2C Right).
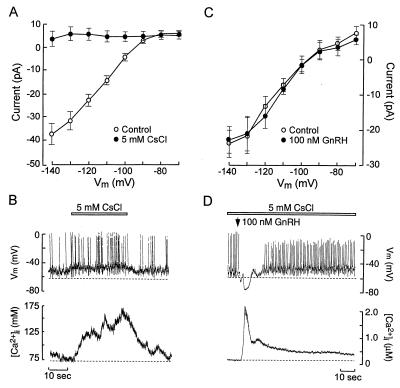
The GnRH-induced inward current observed from holding potentials more depolarized than Ek may reflect inhibition of a K+ channel that contributes to control of the interpulse interval or activation of an inward current. Within the K+ channel superfamily, Kir channels participate in the regulation of pacemaker activity (5). These channels are expressed in GT1 neurons (8) and are abolished by 5 mM Cs+ (Fig. 4A). In current-clamp experiments, abolition of Kir led to membrane depolarization (Fig. 4B Upper, dashed line) and a concomitant increase in spike frequency and [Ca2+]i (Table 1; Fig. 4B). Although this indicates that Kir channels are potential targets for agonist-induced modulation of membrane excitability, 100 nM GnRH did not alter the Kir current–voltage (I–V) relation (Fig. 4C). Moreover, in the presence of 5 mM Cs+, GnRH still induced membrane depolarization and increased firing frequency (Table 1), as in control cells (Fig. 1A vs. Fig. 4D). These results suggest that Kir channels participate in the control of pacemaker activity in spontaneously active GT1 cells, but do not mediate the actions of GnRH on membrane excitability.
Figure 4.
Effects of external Cs+ and GnRH on inwardly rectifying K+ current and membrane excitability in GT1 cells. (A) Current–voltage relation of the inwardly rectifying K+ current before (○) and during (●) the application of 5 mM CsCl. Under perforated-patch voltage-clamp recording conditions, Kir were activated by 1-sec hyperpolarizing voltage steps from –70 to –140 mV (holding potential = −40 mV). The mean current between 0.9 and 1 sec was measured and plotted against the Vm. (B) Simultaneous measurements of Vm and [Ca2+]i during application of 5 mM CsCl. (C) I–V characteristics of the Kir before (○) and during 100 nM GnRH application (●). (D) Simultaneous measurements of Vm and [Ca2+]i in response to 100 nM GnRH (arrow) in the presence of 5 mM CsCl (bar).
Table 1.
Effects of cesium and GnRH on baseline potential and AP frequency in GT1 cells
| Controls | 5 mM Cs+ | 5 mM Cs+ + 100 mM GnRH | |
|---|---|---|---|
| Baseline Vm, mV | −62.4 ± 1.0 | −58.6 ± 1.3* | −51.1 ± 2.5** |
| Frequency, Hz | 0.4 ± 0.1 | 0.7 ± 0.1* | 1.4 ± 0.2** |
The results shown are mean ± SEM (n = 6).
P < 0.05 vs. controls; **P <0.05 vs. 5 mM Cs+.
Activation of Ca2+-mobilizing receptors in rat sympathetic neurons inhibits M currents because of the increase in [Ca2+]i (25). Suppression of such currents in GT1 neurons would induce membrane depolarization and may account for the GnRH-induced depolarization and increase in spike frequency. Such inhibition of M current has been proposed to account for the thyrotropin-releasing hormone-induced increase in firing frequency in pituitary lactotrophs (4). Also, GnRH inhibits M current in bullfrog sympathetic neurons (5) and superior cervical ganglion neurons transiently expressing human GnRH receptors (26). However, although our results do not exclude an action of GnRH on this current, two lines of evidence argue against its role in agonist-induced depolarization of GT1 neurons. First, GnRH increased the firing frequency of cells clamped at 20 nM Ca2+ (Fig. 1B). Because Ca2+ is responsible for the coupling of Ca2+-mobilizing receptors and M channels (25), it is unlikely that their inhibition would account for the sustained depolarization and increase in firing frequency.
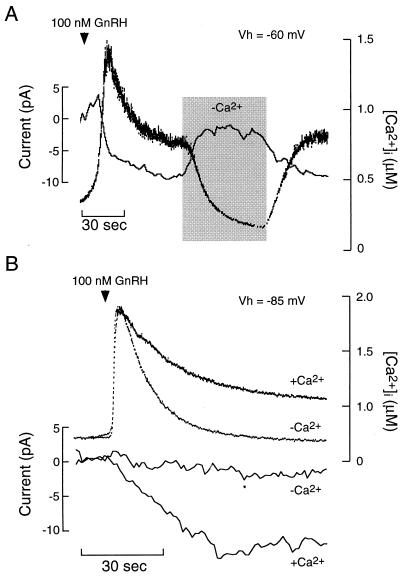
Second, the characteristics of the GnRH-induced inward current are not compatible with the inhibition of K+ channels. In GT1 cells held at −60 mV and in the presence of 1 μM nifedipine/50 μM Ni2+/100 nM apamin (to block voltage-gated calcium channels and SK channels), GnRH still induced a sustained inward current (Fig. 5A). This current activated slowly, reaching a peak amplitude of −9.2 ± 1.8 pA in 33.1 ± 6.7 sec (n = 8). Transient perfusion with Ca2+-deficient medium reversibly attenuated the inward current and abolished the [Ca2+]i plateau response. In cells held at –85 mV, near the Ek under these recording conditions, 100 nM GnRH also activated an inward current and caused a sustained plateau [Ca2+]i response (n = 5; Fig. 5B). As in cells held at –60 mV, both the GnRH-induced plateau [Ca2+]i response and the inward current in cells held at –85 mV were abolished in Ca2+-deficient medium (Fig. 5B). The ability of GnRH to induce an inward current at the Ek indicates further that its actions are not mediated by inhibition of a K+ channel. Moreover, the Ca2+ sensitivity of both the current and plateau [Ca2+]i responses at –60 and –85 mV indicates that the GnRH-induced inward current is a result of activation of a Ca2+-permeable channel.
Figure 5.
GnRH-induced activation of a Ca2+-conducting inward current in GT1 cells. (A) Effects of extracellular Ca2+ depletion (shaded area) on GnRH-induced current and [Ca2+]i in a cell voltage-clamped at –60 mV. (B) Application of 100 nM GnRH in the presence or absence of Ca2+-deficient medium in cells voltage-clamped at –85 mV. In A and B, the bath contained 1 μM nifedipine, 50 μM Ni2+, and 100 nM apamin. Simultaneous measurements of current and [Ca2+]i were performed by using perforated-patch, voltage-clamp recording techniques.
Depletion of the ER Ca2+ store by the inhibition of (Ca2+)ATPase also increases membrane excitability (Fig. 1B). Like GnRH, 5 μM TG activated a sustained inward current in cells held at –60 mV with a peak amplitude of 7.8 ± 1.5 pA, n = 4 (Fig. 6A). Transient application of Ca2+-deficient medium reversibly abolished the inward current and reduced [Ca2+]i. In cells held near EK (Vh = −85 mV), TG also induced an inward current and increased [Ca2+]i (Fig. 6B), indicating that K+ channels do not mediate the action of TG on current or [Ca2+]i. In addition, the ability of TG to increase [Ca2+]i and activate an inward current in cells held at –60 mV or –85 mV negates the involvement of voltage-gated calcium channels. Thus, both receptor- and non-receptor-mediated store depletion activate a Ca2+-permeable, inward current that increases membrane excitability. The profile of this current, its voltage independence and dependence on extracellular Ca2+, and its role in sustained Ca2+ influx are comparable to those observed in nonexcitable and some excitable cells stimulated by Ca2+-mobilizing agonists or inositol 1,4,5-trisphosphate (27–30).
Conclusions.
These results indicate that store depletion by GnRH and TG modifies the pattern of spontaneous firing because of activation of two currents: SK outward current and a Ca2+-conducting and voltage-insensitive inward current. Whereas the rise in [Ca2+]i to the submicromolar or low-micromolar levels signals for activation of SK current, store depletion activates inward current in a [Ca2+]i-independent manner. The nature of SK channels, their [Ca2+]i-sensitivity, and their role in electrical activity of GT1 cells are similar to those observed in other excitable cells (19–24). On the other hand, the store depletion-activated inward current resembles that observed in nonexcitable cells (27–30). Activation of these two opposite currents by GnRH calcium-mobilizing receptors leads to bidirectional control of firing in GT1 neurons. When [Ca2+]i is high, transient hyperpolarization of cells occurs and firing is abolished. At lower [Ca2+]i, inward current overcomes the hyperpolarizing action of SK current, leading to cell depolarization and facilitation of pacemaking. Thus, the store depletion-activated inward current in GT1 neurons drives Ca2+ per se and also facilitates voltage-gated Ca2+ influx by depolarizing the cell. Such dual actions of this current may provide a common mechanism for the integration of voltage-gated Ca2+ influx into inositol 1,4,5-trisphosphate-controlled Ca2+ mobilization in excitable cells.
ABBREVIATIONS
- GnRH
gonadotropin-releasing hormone
- ER
endoplasmic reticulum
- SK channels
calcium-controlled apamin-sensitive potassium channels
- TG
thapsigargin
- AP
action potential
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
References
- 1.Berridge M J. Neuron. 1998;21:13–26. doi: 10.1016/s0896-6273(00)80510-3. [DOI] [PubMed] [Google Scholar]
- 2.Putney J W, Jr, Bird G, St J. Endocr Rev. 1993;14:610–631. doi: 10.1210/edrv-14-5-610. [DOI] [PubMed] [Google Scholar]
- 3.Stojilkovic S S, Catt K J. Endocr Rev. 1992;13:256–280. doi: 10.1210/edrv-13-2-256. [DOI] [PubMed] [Google Scholar]
- 4.Sankaranarayanan S, Simasko S M. J Neurosci. 1996;16:1668–1678. doi: 10.1523/JNEUROSCI.16-05-01668.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hille B. Ionic Channels of Excitable Cells. Sunderland, MA: Sinauer; 1991. [Google Scholar]
- 6.Charles A C, Hales T G. J Neurophysiol. 1994;73:56–64. doi: 10.1152/jn.1995.73.1.56. [DOI] [PubMed] [Google Scholar]
- 7.Van Goor, F., Krsmanovic, L. Z., Catt, K. J. & Stojilkovic, S. S. (1999) Mol. Endocrinol., in press. [DOI] [PubMed]
- 8.Bosma M M. J Membr Biol. 1993;136:85–96. doi: 10.1007/BF00241492. [DOI] [PubMed] [Google Scholar]
- 9.Hales T G, Sanderson M J, Charles A C. Neuroendocrinology. 1994;59:297–308. doi: 10.1159/000126671. [DOI] [PubMed] [Google Scholar]
- 10.Spergel D J, Catt K J, Rojas E. Neuroendocrinology. 1996;63:101–111. doi: 10.1159/000126946. [DOI] [PubMed] [Google Scholar]
- 11.Krsmanovic L Z, Stojilkovic S S, Mertz L M, Tomic M, Catt K J. Proc Natl Acad Sci USA. 1993;90:3908–3912. doi: 10.1073/pnas.90.9.3908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rae J, Cooper K, Gates P, Watsky M. J Neurosci Methods. 1991;37:15–26. doi: 10.1016/0165-0270(91)90017-t. [DOI] [PubMed] [Google Scholar]
- 13.Kao J P Y. Methods Cell Biol. 1994;40:155–181. doi: 10.1016/s0091-679x(08)61114-0. [DOI] [PubMed] [Google Scholar]
- 14.Barry P H. J Neurosci Methods. 1994;51:107–116. doi: 10.1016/0165-0270(94)90031-0. [DOI] [PubMed] [Google Scholar]
- 15.Hamill O P, Marty A, Neher E, Sakmann B, Sigworth F J. Pflügers Arch. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- 16.Takemura H, Hughes A R, Thastrup O, Putney J W. J Biol Chem. 1989;264:12266–12271. [PubMed] [Google Scholar]
- 17.Tse A, Hille B. Science. 1992;255:462–464. doi: 10.1126/science.1734523. [DOI] [PubMed] [Google Scholar]
- 18.Kukuljan M, Stojilkovic S S, Rojas E, Catt K J. FEBS Lett. 1992;301:19–22. doi: 10.1016/0014-5793(92)80201-q. [DOI] [PubMed] [Google Scholar]
- 19.Ritchie A K. J Physiol (London) 1987;385:611–625. doi: 10.1113/jphysiol.1987.sp016510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lang D G, Ritchie A K. J Physiol (London) 1990;425:117–132. doi: 10.1113/jphysiol.1990.sp018095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Blatz A L, Magleby K L. Nature (London) 1986;323:718–720. doi: 10.1038/323718a0. [DOI] [PubMed] [Google Scholar]
- 22.Hugues M, Duval D, Kitabgi P, Lazdunski M, Vincent J P. J Biol Chem. 1982;257:2762–2769. [PubMed] [Google Scholar]
- 23.Hugues M, Romey G, Duval D, Vincent J P, Lazdunski M. Proc Natl Acad Sci USA. 1982;79:1308–1312. doi: 10.1073/pnas.79.4.1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Park Y B. J Physiol (London) 1994;481:555–570. doi: 10.1113/jphysiol.1994.sp020463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cruzblanca H, Koh D-S, Hille B. Proc Natl Acad Sci USA. 1998;95:7151–7156. doi: 10.1073/pnas.95.12.7151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lewis D L, Ikeda S R. Neuroendocrinology. 1997;66:235–245. doi: 10.1159/000127244. [DOI] [PubMed] [Google Scholar]
- 27.Hoth M, Penner R. Nature (London) 1992;355:353–356. doi: 10.1038/355353a0. [DOI] [PubMed] [Google Scholar]
- 28.Fasolato C, Hoth M, Penner R. J Biol Chem. 1993;268:20737–20740. [PubMed] [Google Scholar]
- 29.Mathes C, Thompson S H. J Gen Physiol. 1994;104:107–121. doi: 10.1085/jgp.104.1.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Parekh A B, Fleig A, Penner R. Cell. 1997;89:973–980. doi: 10.1016/s0092-8674(00)80282-2. [DOI] [PubMed] [Google Scholar]