Abstract
Fruit ripening is characterized by processes that modify texture and flavor but also by a dramatic increase in susceptibility to necrotrophic pathogens, such as Botrytis cinerea. Disassembly of the major structural polysaccharides of the cell wall (CW) is a significant process associated with ripening and contributes to fruit softening. In tomato, polygalacturonase (PG) and expansin (Exp) are among the CW proteins that cooperatively participate in ripening-associated CW disassembly. To determine whether endogenous CW disassembly influences the ripening-regulated increase in necrotropic pathogen susceptibility, B. cinerea susceptibility was assessed in transgenic fruit with suppressed polygalacturonase (LePG) and expansin (LeExp1) expression. Suppression of either LePG or LeExp1 alone did not reduce susceptibility but simultaneous suppression of both dramatically reduced the susceptibility of ripening fruit to B. cinerea, as measured by fungal biomass accumulation and by macerating lesion development. These results demonstrate that altering endogenous plant CW disassembly during ripening influences the course of infection by B. cinerea, perhaps by changing the structure or the accessibility of CW substrates to pathogen CW-degrading enzymes. Recognition of the role of ripening-associated CW metabolism in postharvest pathogen susceptibility may be useful in the design and development of strategies to limit pathogen losses during fruit storage, handling, and distribution.
Keywords: expansin, polygalacturonase, tomato, plant pathogen
Plant cells are surrounded by complex composite walls that are important preformed barriers to pathogen infection (1, 2). Many saprophytic and plant pathogenic organisms secrete cell wall-degrading enzymes (CWDEs) that specifically target the components of the plant cell wall (CW) so that the pathogen can penetrate the host and acquire nutrients from the digested wall material and cellular contents (3). Some CWDEs have been shown to be virulence factors (4–9).
During ripening, many fruit, including tomato, disassemble components of the CW, resulting in changes in the CW rheological properties and softening of the ripe fruit (10). The tomato fruit CW is comprised of two interacting polysaccharide networks, the cellulose-hemicellulose (Cel-Hem) network containing the primary strength-conferring elements embedded in a coextensive pectin network (11). During ripening, CW modifying proteins, including polygalacturonases (PGs) and expansins (Exps), act cooperatively to disassemble the polymer networks and thereby contribute to fruit softening. PGs hydrolyze the homogalacturonan polymers that are major components of the pectin matrix, and Exps are thought to influence the interactions within the Cel-Hem network, although no enzymatic functions have been definitively associated with Exps (12, 13). While the participation of PG and Exps in ripening-associated wall metabolism is clear (10, 14), little is known about the ways these CWDEs interact to disassemble the CW.
Fruit ripening is characterized by a dramatic increase in susceptibility to necrotrophic pathogens, resulting in large economic losses of perishable horticultural products (14, 15). CW metabolism of many edible fruit has been studied extensively in the context of ripening (10), but the contribution of ripening-associated CW disassembly to pathogen susceptibility has not been experimentally defined. It is generally assumed that self-disassembly of the fruit wall contributes to susceptibility because many of the fruit CWDEs that participate in fruit wall metabolism also are produced by the invading pathogens and secreted into fruit tissues (16). Ripening-impaired tomato fruit with the Nr or nor mutations show reduced ripening-associated softening and decreased susceptibility to pathogens (17, 18). However, in these genotypes, the absence of the normal ripening-associated CW metabolism is just one of many processes affected, so it is not possible to conclude that CW metabolism of the mutants per se contributes to reduced pathogen susceptibility (18). Fruit in which the expression of genes encoding CWDEs such as PG and pectin methyl esterase has been suppressed apparently have increased tissue integrity at fully ripe or overripe stages (18–21). The suppression of the tomato fruit ripening-associated Exp gene, LeExp1, resulted in firmer fruit with prolonged shelf-life (22), but did not reduce the susceptibility to Botrytis cinerea and Alternaria alternata (4).
The CW's complexity and structural plasticity complicate approaches designed to understand the relationship between ripening-associated wall metabolism, fruit softening, and increased pathogen susceptibility. This may explain why studies based on modified expression of individual CWDEs in ripening fruit have not resulted in significant beneficial impacts on either susceptibility or softening. Here, we have investigated the impact of simultaneous suppression of LePG and LeExp1 expression in ripening tomato fruit on susceptibility to B. cinerea and fruit firmness. The results demonstrate that the cooperative action of these two CW proteins is an important determinant of fruit ripening-associated increases in necrotrophic pathogen susceptibility.
Results
Characterization of Transgenic Tomato Lines.
A transgenic Solanum lycopersicon cv. Ailsa Craig (AC) line sense-suppressed for LeExp1 expression (−Exp) (23) and another line antisense-suppressed for LePG expression (−PG) (24) were crossed to obtain lines simultaneously suppressed for both LeExp1 and LePG (−PG−Exp) (22).PG protein (Fig. 1A) and activity (results not shown) are not detected in red ripe (RR) −PG or −PG−Exp fruit, and Exp protein is significantly reduced in RR −Exp or −PG−Exp fruit. None of the transgenic plants showed a modified phenotype or alterations in ripe fruit quality factors, including the contents of glucose, fructose, carotenoids, or titratable acidity (results not shown). However, the extent to which fruit from transgenic lines softened during ripening was altered significantly (Fig. 1B): −PG−Exp fruit were firmer than wild-type AC, −PG, or −Exp fruit, although −Exp fruit were firmer than AC at an early ripening stage, similar to what has been reported (22, 23).
Fig. 1.
LePG and LeExp1 expression influences the softening of ripening Ailsa Craig (AC) tomato fruit. (A) Western blots demonstrate that proteins recognized by antibodies to tomato PG and Exp are present in red ripe (RR) AC fruit, that PG is absent in −PG fruit, that Exp is absent in −Exp fruit, and that both proteins are missing from the −PG−Exp fruit. (B) Measuring the force to compress each fruit 2 mm shows that decreasing PG and Exp in fruit significantly reduces softening at all ripening stages. Firmness was measured at the mature green (MG) stage and in subsequent ripening stages [breaker (BR), light red (LR), and RR]. Letters correspond to significant differences between genotypes at each ripening stage; P < 0.05.
Fruit Susceptibility to B. cinerea.
The simultaneous suppression of LePG and LeExp1 led to a dramatic reduction in the susceptibility of light red (LR) or RR fruit to B. cinerea [P < 0.005 at 1, 2, and 3 days after inoculation (dpi)], whereas the suppression of either LePG or LeExp1 alone, did not result in a significant change in susceptibility (Figs. 2 and 3). To understand whether the engineered changes in fruit CW metabolism influenced fungal growth directly, the quantity of B. cinerea biomass at infection sites was assessed by using the monoclonal antibody BC12.CA4 that recognizes a mycelium-localized epitope (25). Fungal biomass accumulation was significantly reduced when LePG and LeExp1 were suppressed individually, and this effect was additive when expression of both genes was down-regulated (Fig. 4A).
Fig. 2.
Reduction of PG and Exp substantially decreases susceptibility to B. cinerea. LR and RR fruit were inoculated with B. cinerea spores at three of four puncture sites on each fruit (the fourth site was mock-inoculated with water), and disease symptoms were assessed at 1, 2, and 3 dpi. Fruit showed disease symptoms when an expanding macerating (water-soaked) lesion developed at any inoculated site, and the percentage of the fruit showing any symptoms of disease is shown. Approximately 50 fruit from each genotype were analyzed and the experiment was done at least twice.
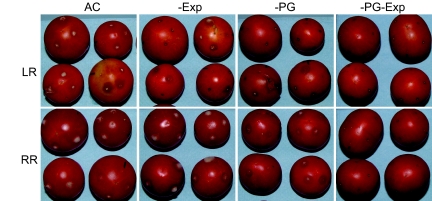
Fig. 3.
Reduction of PG and Exp substantially decreases gray mold symptoms in inoculated LR or RR fruit. The image is of fruit at 3 dpi.
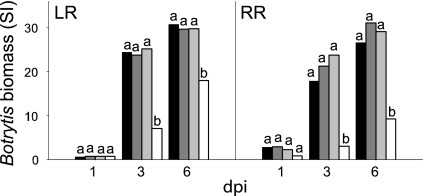
Fig. 4.
Reduction of PG and Exp decreases pathogen growth on fruit and the CW composition from infected tissue demonstrates that fungal biomass accumulation is reduced on −PG−Exp fruit. (A) Infected tissues were excised 36 and 72 h after inoculation (hpi), and the assessment of B. cinerea biomass in homogenates was based on signal intensity (SI) using the monoclonal antibody BC12.CA4. Letters correspond to significant differences between genotypes at each hpi; P < 0.001. (B) AIRs were prepared from infected tissues and analyzed colorimetrically for UA and NS contents, expressed as the UA/NS ratios. Block letters correspond to significant differences between genotypes at each hpi; italic letters correspond to significant differences between hpi for each genotype; P < 0.001. (C) AIR from RR fruit at 36 and 72 hpi were hydrolyzed and derivatized to determine the NS by GC-MS. GC-MS detector output is shown. Elution times of the alditol acetate derivative peaks are shown. Ara, arabinose; Xyl, xylose; Man, mannose; Gal, galactose; Glc, glucose; Ino, myo-inositol internal standard. The relative abundance of each sugar is expressed as the total ion count. (I) AC, 36 hpi. (II) AC, 72 hpi. (III) −PG−Exp, 36 hpi. (IV) −PG−Exp, 72 hpi. The elevated Man and Glc indicate the increased B. cinerea CW contents in AC fruit AIR. Identification of bars is as defined in Fig. 1B.
To determine the proportion of fungal CW mass within the total wall mass isolated from infection sites, uronic acid (UA) and neutral sugars (NS) were measured and the distribution of sugars was assessed. B. cinerea CWs contain mostly NS and little UA (UA/NS ratio ≈1/12; data not shown). However, tomato fruit CWs contain 25–30% UA, and the remainder is NS. As B. cinerea infection of LR and RR AC fruit proceeds, the UA/NS ratio of the isolated infected site CW material significantly decreases, but this was not observed in CWs from infected −PG−Exp fruit inoculated at either ripening stage (Fig. 4B). The wall preparations from inoculated fruit contain both tomato fruit and B. cinerea wall polysaccharides and thus, the reduction of the UA/NS ratio is likely to reflect both the accumulation of fungal CW and the loss of fruit UA-rich pectins caused by the action of pathogen or fruit CWDEs. That the infected wall preparations contain fungal CWs was confirmed by the increased mannose and glucose contents of the wall preparations from infected fruits compared with the contents of these sugars in walls prepared from uninfected fruit (Fig. 4C). Glucose, coming from the oxidation of acetylglucosamine during sample preparation, and mannose are major components of B. cinerea CWs. Thus, the assessment of fungal biomass accumulation by using monoclonal antibody detection, together with the analysis of sugars in CW isolated from infected fruit, supports the conclusion that fungal mass accumulation or growth was substantially greater in AC than in −PG−Exp fruit.
B. cinerea Growth on Isolated Fruit CWs.
The differences in AC or −PG−Exp fruit CW composition or integrity could influence the pathogen's ability to metabolize or use CW components, and thus, may be, in part, responsible for the reduced susceptibility of −PG−Exp fruit. CWs isolated from uninfected fruit of the four genotypes at the LR and RR ripening stages that were used for evaluating fungal susceptibility were added as nutrient sources for liquid cultures of B. cinerea. Fig. 5 shows that fungal growth was 3-fold greater in cultures containing CWs isolated from AC, −PG, or −Exp LR or RR fruit than in cultures containing CWs isolated from LR or RR −PG−Exp fruit, suggesting that the LR and RR CW is a nutrient source for B. cinerea growth and that its utility for B. cinerea growth is influenced by the cooperative action of PG and Exp. No difference in fungal growth is observed on CWs prepared from mature green (MG) fruit from any of the genotypes (results not shown).
Fig. 5.
Pathogen growth is reduced in liquid cultures containing −PG−Exp CWs compared with cultures supplied with AC CWs. Mycelia were collected 1, 3, and 6 dpi from cultures containing AIR from uninfected LR and RR fruit of the four genotypes. Fungal biomass was measured as described in the legend of Fig. 4A. Identification of bars is as defined in Fig. 1B. Letters correspond to significant differences between genotypes at each dpi; P < 0.001.
Cooperative Action of PG and Exp Affects the Integrity of Fruit CW Polysaccharide Networks.
To determine which modifications in fruit CW fractions could account for different pathogen susceptibilities of the four genotypes, CWs were prepared from uninfected MG, breaker, LR, and RR fruit and sequentially fractionated to yield water-soluble, ionically and covalently bound pectins, base-soluble hemicelluloses, and an insoluble cellulose residue. The analyses of total CW carbohydrate composition revealed no differences among the genotypes (results not shown). When LePG or LeExp1 were suppressed in AC either alone or in combination, pectin solubilization, measured as the shift of less-soluble pectin polymers to water-soluble forms, was reduced, and this was most pronounced in walls from the RR fruit (Fig. 6A). Suppression of either LePG or LeExp1 alone in other tomato genotypes did not cause a reduction in solubilization of ionically or covalently bound pectin in RR fruit (23, 26). However, the simultaneous suppression of LePG and LeExp1 led to a dramatic reduction in depolymerization of the water-soluble fraction (Fig. 6B) and in the other pectin fractions [supporting information (SI) Fig. 8). However, no alterations of hemicellulosic polymer sugar composition, solubility, or size distribution were observed (data not shown), confirming that Exp has no effect on depolymerization of xyloglucans (23), although the Exp protein is thought to interact directly with the xyloglucan-rich Cel-Hem network (13)
Fig. 6.
Reduction of PG and Exp in ripening fruit decreases the solubilization and depolymerization of pectin polysaccharides. Pectins were extracted sequentially from LR and RR AIR from the four genotypes by using water, CDTA, and Na2CO3. (A) Histograms of the distribution of UAs in the pectin extracts. (B) Images show the size exclusion chromatographic fractionation of the water-soluble pectins (WSF) from AC and −PG−Exp fruit at the LR and RR ripening stages. The data show that neither the normal ripening-associated increase in fruit WSF content nor the decrease in polymer size, both observed in AC fruit, occurs in −PG−Exp fruit.
The ripening-associated shift of polymers rich in rhamnose (Rha) and arabinose (Ara) into the water-soluble wall fraction observed in ripening AC fruit was almost eliminated in the −PG−Exp transgenic fruit (Table 1). This suggests that the combined actions of PG and Exp enhance the accessibility of other fruit CWDEs to their wall-localized substrates, presumably including rhamnoglalacturonan-I (RG-I) and associated arabinan side chains. Interestingly, whereas RG-I is part of the CW pectin network, the RG-I backbone should not be a target of either PG or Exp.
Table 1.
Neutral sugar composition (mol %) of WSF from AC and −PG−Exp fruit at four ripening stages
| Genotype and stage | Rha | Fuc | Ara | Xyl | Man | Gal | Glc | |
|---|---|---|---|---|---|---|---|---|
| AC | Green | 1.4±1.0 | 4.1±0 | 33.5±0.1 | 9.4±0.3 | 6.8±0.2 | 31.3±0.1 | 13.5±1.1 |
| Breaker | 1.0±0.1 | 3.9±0 | 35.1±5.6 | 14.9±1.3 | 4.7±0.1 | 24.8±7.2 | 15.6±0.3 | |
| Light red | 4.1±0.7 | 0.2±0.1 | 42.5±1.2 | 7.9±0.2 | 4.3±0.2 | 28.3±0.3 | 12.7±0.1 | |
| Red ripe | 6.0±1.0 | ND | 51.5±9.9 | 7.6±0.3 | 3.2±0.3 | 23.6±6.9 | 8.1±2.1 | |
| −PG−Exp | Green | ND | ND | 5.4±3.0 | 2.0±0.7 | 2.9±0.8 | 72.1±3.8 | 17.6±0.9 |
| Breaker | ND | ND | 1.2±0.9 | 0.8±0.6 | 3.2±1.1 | 63.4±2.9 | 31.4±5.6 | |
| Light red | ND | ND | 1.3±0.7 | 0.5±0.1 | 2.9±0.5 | 66.1±2.0 | 29.2±0.6 | |
| Red ripe | ND | ND | 1.4±0.3 | 1.8±1.3 | 2.9±0 | 67.0±2.0 | 26.9±3.6 | |
ND, not detected. Values are expressed ±SDs.
Cooperative Action of PG and Exp Affects CW Relaxation.
Fruit that soften significantly at the fully ripe stage typically have CWs that appear swollen, both in planta and in vitro, and this ripening-associated wall swelling correlates with pectin solubilization (27, 28). CWs were isolated from LR and RR AC and transgenic fruit and suspended in water. The isolated CWs demonstrate that reduction of PG or Exp and, in particular, reduction of both PG and Exp results in walls that swell much less than walls from AC fruit (Fig. 7A) and only slightly more than walls from MG AC fruit (data not shown). Furthermore, when examined by electron microscopy, CWs from RR −PG−Exp fruit appear thinner than walls from RR AC fruit (Fig. 7 B and C)
Fig. 7.
The CW swells as AC tomato fruit ripen but not as −PG−Exp fruit ripen. (A) Electron micrographs show the increased thickness of walls (CW) from RR fruit pericarp cells and increased spacing of the wall's electron-dense fibrillar material in AC compared with −PG−Exp fruit. (Scale bars, 1 μm.) (B) The thickness of CW from 30 images as in A show that walls from RR AC (black bar) fruit are thicker than walls from RR −PG−Exp (white bar) fruit. Letters indicate significant differences between genotypes; P < 0.01. (C) By comparing the depth of the settled aqueous suspensions of AIR from LR and RR AC fruit, a typical ripening-associated AIR swelling is observed. No such change is seen when LR and RR AIR samples from −PG−Exp fruit are allowed to settle. (Scale bars, 1 cm.)
Discussion
The simultaneous suppression of the tomato fruit ripening-associated LePG and LeExp1 expression reduces susceptibility to B. cinerea infection during ripening, whereas suppression of LePG or LeExp1 alone does not reduce susceptibility, indicating that PG and Exp act cooperatively to support both softening (4, 22, 23, 29) and full pathogenicity of B. cinerea. The host CW is a primary target during B. cinerea growth on plant tissue (30). B. cinerea possesses a wide array of CWDEs (31, 32), including six PGs (33). B. cinerea mutants of either BcPG1 or BcPG2 resulted in significantly decreased virulence on multiple hosts, including tomato (5, 8). In addition, the ectopic expression of a potent PG-inhibiting protein (PGIP) from pear fruit (pPGIP) reduced the susceptibility of ripe tomato fruit to B. cinerea infection (6). Thus, the pathogen's ability to efficiently disassemble the tomato fruit CW appears to be critical for full virulence. Although the pathogen apparently possesses the required tools to carry out the targeted decomposition of plant CW components, we have shown that both its growth and the development of disease symptoms on tomato fruits depend on two endogenous plant CW proteins that are responsible, together with other fruit proteins, for some aspects of ripening-associated CW metabolism. In another pathosystems, the plant CWDE pectate lyase and full pectin methylesterase activity appear to be required for susceptibility of Arabidopsis to Erysiphe cichoracearum and B. cinerea, respectively (34, 35).
The coevolution of fruits and their pathogens may be responsible for the relationship between complete softening and pathogen susceptibility of ripe fruit. LeExp1 and LePG are not expressed in fruit until the onset of ripening, a developmental transition that in tomato marks the completion of seed maturation. Once the seeds are mature, the fruit becomes an agent of seed dispersal, a process that may be facilitated by ripening-associated softening and ripening-associated pathogen susceptibility. The pathogen's dependence on the fruit's self-disassembly of its CWs assures that seeds mature before fruit decomposition is initiated by pathogen CWDEs. Thus, the activity of fungal virulence CWDEs and fruit ripening CWDEs combine to contribute to the completion of the life cycles of both plant and pathogen.
PG and Exp are thought to target structurally independent CW polymer systems; i.e., the wall's pectin and Cel-Hem networks (13, 36). Fruit softening requires the combined actions of PG and Exp for loosening the wall fabric, a process that is likely to reflect changes in intra- and interpolymer bonding that render the networks weaker and less well associated with one another. This may facilitate the action of other ripening-associated CWDEs as suggested by the reduced solubilization of Ara- and Rha-rich polymers in −PG−Exp ripe fruit. The observed cooperative effects of PG and Exp on CW swelling and thickness also suggest that the integrity of the wall fabric depends on the composition and interaction of the two major CW structural networks. Such an interaction, in turn, may alter substrate accessibility to both fruit and pathogen CWDEs. Reduced fungal growth in fruit when LePG and LeExp1 are suppressed may result directly from these alterations in substrate accessibility to pathogen CWDEs. However, there are several other possible mechanisms that may contribute to PG and Exp dependent alterations in pathogen susceptibility. For example, alterations in fruit CW structure resulting from reduced PG and Exp abundance may serve to retain inhibitors of pathogen function, such as PGIPs, whose loss of CW association during ripening has been correlated with elevated pathogen susceptibility (37–39). PG and Exp reduction also may alter pathogen-induced responses, a mechanism suggested by the demonstration that CW changes can activate novel defense pathways (40). These, or similar, mechanisms may operate alone or in conjunction with CW structural alterations that reduce the nutritional and/or signaling properties of the products of CW disassembly that are normally present in ripening fruit (41). Further investigations will be required to distinguish among these multiple and potentially overlapping mechanisms of PG- and Exp-mediated increases in pathogen susceptibility that accompany fruit ripening.
It now appears clear that simultaneous suppression of LePG and LeExp1 in ripening fruits concurrently reduces wall disassembly, slows fruit softening in vivo, and delays or decreases ripening-associated susceptibility to B. cinerea. Recognition of the role of ripening-associated CW metabolism in postharvest pathogen susceptibility may be useful in the design and development of strategies to limit pathogen losses during fruit storage, handling, and distribution.
Materials and Methods
Plant Material.
The tomato (S. lycopersicum) transgenic and control lines were in the cultivar Ailsa Craig (AC) and grown in fields in Davis, CA. The transgenic line with reduced PG (−PG) was the LePG antisense line Gr105 provided by D. Grierson (University of Nottingham, Nottingham, U.K.) (24), and the transgenic line with reduced Exp (−Exp) contained a sense-suppression construct of LeExp1, line 18–40 as described by ref. 23. The transgenic lines with suppressed expression of both LePG and LeExp1 were obtained by crossing the homozygous −PG and −Exp lines as described (22), and the homozygous progeny were confirmed by Southern blotting and PCR analysis (22). Fruit were tagged in the field 3–4 days after anthesis and harvested as MG fruit at 30–31 days, LR at 33–34 days, and RR at 35–36 days after anthesis. Ripening stages were confirmed by visual analysis of internal and external fruit color.
Measurement of Fruit Firmness.
Fruit firmness was measured by compressing the equator 2 mm by using a 2-mm-diameter flat stainless steel probe (test speed 0.5 mm/s) (TaXT2i Texture Analyzer; Texture Technologies). Fruit were measured at three sites, and the firmness observed was comparable to results in ref. 22.
Inoculation with B. cinerea.
B. cinerea (Del 11) was grown on 1% potato dextrose agar, and conidia were harvested from sporulating colonies and filtered through glass wool. Fruit were sterilized in 10% bleach for 10 min, followed by deionized water rinses. Fruit wounded at four sites to a depth of 2 mm were inoculated with 10 μl containing 5,000 conidia of B. cinerea or 10 μl of water. Fruit were incubated at 20°C in high humidity, and susceptibility was determined as disease incidence (percentage of fruit showing soft rot symptoms expanding from any of the inoculation sites). Fungal biomass development was assessed by using the QuickStix kit for B. cinerea (EnviroLogix), which utilizes the monoclonal antibody BC12.CA4 (25). The test is quantitative in the range of signal intensity (SI) from 1 to ≈48 based on extracts of tomato tissue spiked with dry B. cinerea mycelium from cultures (r2 = 0.94; results not shown). Fruit tissue (0.3 g) was harvested at different times, pooled (nine lesions per pool, three pools per line), suspended and ground in the kit buffer (1:10, wt/vol). The antibody cross-reactive material was measured in 500 μl of the tissue suspension, and the intensity of the test line was determined by using the QuickStix reader (Envirologix).
Isolation of CWs.
CW preparation followed the protocols of ref. 42 to produce dry alcohol-insoluble residue (AIR). Approximately 15 g of tomato fruit tissue (exocarp and pericarp) for each fruit-ripening stage and Botrytis- and mock-inoculated treatments were used.
In Vitro Culture of B. cinerea on Isolated CW Material.
B. cinerea (three spores·μl−1) was grown in 10 ml of Pratt's medium, pH 4.5 (43), containing 10 mg of AIR from LR and RR fruit as the sole organic food source. Two experiments of three independent replicates per genotype and per dpi were carried out. At 1, 3, and 6 dpi, the cultures were centrifuged at 11,000 × g for 5 min, and 5 ml of supernatant were collected. The pellets were homogenized in the remaining 5 ml of medium by using an Ultraturrax T25 Basic homogenizer at 17,000 × g for 1 min. The homogenate was centrifuged at 11,000 × g for 5 min, and this supernatant was combined with the first supernatant to assess fungal biomass by using the QuickStix kit as described above, after 1:5 wt/vol dilution of the collected supernatant in the kit buffer.
CW Solubilization, UA, and NS Measurements.
Three mg of AIR were solubilized with H2SO4 (42), and the solubilized CW material (fruit or fruit plus B. cinerea walls) was assayed colorimetrically for total UA by using m-phenylphenol (44) and NS by using anthrone (45).
CW Fractionation.
Fractions enriched in CW pectic and hemicellulosic polymers were sequentially chemically extracted from AIR. From ≈200 mg of AIR, water-soluble (WSF), ionically bound (i.e., soluble in the chelator trans-1,2-diaminocyclohexane-N,N,N′,N′-tetraacetic acid, CSF) and covalently bound (i.e., soluble in 50 mM Na2CO3, NSF) pectins and hemicellulose (i.e., soluble in 4 M KOH, KSF) were separated (42). Two independent samples were extracted from each developmental stage and genotype. Samples were assayed in triplicate for NS and UA (44, 45).
Size-Exclusion Chromatography (SEC).
Aliquots of WSF, CSF, NSF, and KSF were dialyzed (Spectrapor, molecular mass cut-off 8 kDa) against distilled water for 1 d at 4°C and lyophilized. WSF, CSF, and NSF samples were dissolved in 200 mM ammonium acetate, pH 5.0, and analyzed by SEC on an HW65 (Tosoh Bioscience) column. The KSF samples were dissolved in 0.1 M NaOH and fractionated on a Sepharose CL-4B (1.0 × 90 cm) (Amersham Pharmacia) column eluted with 0.1 M NaOH (42).
GC-MS Analysis.
For analysis of the NS sugar compositions of the AIR or isolated wall polymers prepared from healthy and Botrytis-inoculated tissue, 2 mg of AIR or lyophilized samples of WSF, CSF, NSF, and KSF were hydrolyzed in 2 M trifluoroacetic acid (46) and converted to alditol acetates (47) for GC-MS analysis (42).
Transmission Electron Microscopy.
Pericarp excised from RR fruit was fixed in Karnovsky's fixative by using a vacuum-microwave (48). The samples were washed in 0.1 M sodium phosphate buffer, pH 7.2, microwaved under vacuum at 450 W for 40 seconds, postfixed for 2 h in 1% OsO4 buffered in 0.1 M sodium phosphate buffer and microwaved a second time at 450 W for 40 seconds. After 30 min in 0.1% tannic acid at 0°C, followed by 2% aqueous uranyl acetate for 1 h, samples were dehydrated in acetone and embedded in Epon/Araldite resin. Ultrathin sections were examined with a Philips CM120 Biotwin Lens transmission electron microscope (FEI).
CW Swelling in Vitro.
Ten milligrams of AIR were suspended in 8 ml of water and shaken horizontally for 24 h. Tubes were then placed vertically, and CW swelling was assessed based on the height of the sedimented AIR layer.
Statistical Analysis.
Differences between groups were investigated with the use of ANOVA, followed by post hoc testing (Tukey's honestly significant difference, Tukey HSD) by using SYSTAT 11.0 (Systat). For percentage values, statistical analysis was carried out after angular transformation.
Supplementary Material
ACKNOWLEDGMENTS.
We thank the Electron Microscopy Laboratory, School of Medicine, University of California, Davis, and Tsokyi Choera, William Sinko, and Heidi Sher for assistance. This work was supported by National Science Foundation Grant IOB 0544504, the Fulbright Commission, and the Consejo Nacional de Investigaciones Cientificas y Tecnícas (CONICET) Argentina.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. H.K. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/cgi/content/full/0709813105/DC1.
References
- 1.Vorwerk S, Somerville S, Somerville C. Trends Plant Sci. 2004;9:203–209. doi: 10.1016/j.tplants.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 2.Hückelhoven R, editor. Annu Rev Phytopath. 2007;45:101–127. doi: 10.1146/annurev.phyto.45.062806.094325. [DOI] [PubMed] [Google Scholar]
- 3.Collmer A, Keen NT. Annu Rev Phytopath. 1986;24:383–409. [Google Scholar]
- 4.Brummell DA, Howie WJ, Ma C, Dunsmuir P. Postharvest Biol Tech. 2002;25:209–220. [Google Scholar]
- 5.ten Have A, Mulder W, Visser J, van Kan JAL. Mol Plant–Microbe Interact. 1998;11:1009–1016. doi: 10.1094/MPMI.1998.11.10.1009. [DOI] [PubMed] [Google Scholar]
- 6.Powell ALT, van Kan J, ten Have A, Visser J, Greve LC, Bennett AB, Labavitch JM. Mol Plant–Microbe Interact. 2000;13:942–950. doi: 10.1094/MPMI.2000.13.9.942. [DOI] [PubMed] [Google Scholar]
- 7.Oeser B, Heidrich PM, Muller U, Tudzynski P, Tenberge KB. Fungal Genet Biol. 2002;36:176–186. doi: 10.1016/s1087-1845(02)00020-8. [DOI] [PubMed] [Google Scholar]
- 8.Kars I, Krooshof GH, Wagemakers L, Joosten R, Benen JAE, van Kan JAL. Plant J. 2005;43:213–225. doi: 10.1111/j.1365-313X.2005.02436.x. [DOI] [PubMed] [Google Scholar]
- 9.Roper MC, Greve LC, Warren JG, Labavitch JM, Kirkpatrick BC. Mol Plant–Microbe Interact. 2007;20:411–419. doi: 10.1094/MPMI-20-4-0411. [DOI] [PubMed] [Google Scholar]
- 10.Brummell DA. Funct Plant Biol. 2006;33:103–119. doi: 10.1071/FP05234. [DOI] [PubMed] [Google Scholar]
- 11.Carpita N, McCann M. In: Biochemistry and Molecular Biology of Plants. Buchanan B, Gruissem W, Jones R, editors. 2000. pp. 52–108. Am Soc Plant Physiol. [Google Scholar]
- 12.Cosgrove DJ. Nature. 2000;407:321–326. doi: 10.1038/35030000. [DOI] [PubMed] [Google Scholar]
- 13.Rose JKC, Bennett AB. Trends Plants Sci. 1999;4:176–183. doi: 10.1016/s1360-1385(99)01405-3. [DOI] [PubMed] [Google Scholar]
- 14.Giovannoni J. Annu Rev Plant Physiol Plant Mol Biol. 2001;52:725–749. doi: 10.1146/annurev.arplant.52.1.725. [DOI] [PubMed] [Google Scholar]
- 15.Narayanasamy P. Postharvest Pathogens and Disease Management. Hoboken, NJ: Wiley; 2006. [Google Scholar]
- 16.Cantu D, Vicente AR, Greve LC, Labavitch JM, Powell ALT. Fresh Produce. 2007;1:101–110. [Google Scholar]
- 17.Lavy-Meir G, Barkai-Golan R, Kopeliovitch E. Plant Dis. 1989;73:976–978. [Google Scholar]
- 18.Kramer M, Sanders R, Bolkan H, Waters C, Sheehy RE, Hiatt WR. Postharvest Biol Tech. 1992;1:241–255. [Google Scholar]
- 19.Carrington CMS, Greve LC, Labavitch JM. Plant Physiol. 1993;103:429–434. doi: 10.1104/pp.103.2.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tieman DM, Harriman RW, Ramamohan G, Handa AK. Plant Cell. 1992;4:667–679. doi: 10.1105/tpc.4.6.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hall LN, Tucker GA, Smith CJS, Watson CF, Seymour GB, Bundick Y, Boniwell JM, Fletcher JD, Ray JA, Schuch W, et al. Plant J. 1993;3:121–129. [Google Scholar]
- 22.Powell ALT, Kalamaki MS, Kurien PA, Gurrieri S, Bennett AB. J Agric Food Chem. 2003;51:7450–7455. doi: 10.1021/jf034165d. [DOI] [PubMed] [Google Scholar]
- 23.Brummell DA, Harpster MH, Civello PM, Palys JM, Bennett AB, Dunsmuir P. Plant Cell. 1999;11:2203–2216. doi: 10.1105/tpc.11.11.2203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smith CJS, Watson CF, Bird CR, Ray J, Schuch W, Grierson D. Mol Gen Genet. 1990;224:1432–1874. doi: 10.1007/BF00262443. [DOI] [PubMed] [Google Scholar]
- 25.Meyer UM, Dewey FM. Mycol Res. 2000;104:979–987. [Google Scholar]
- 26.Brummell DA, Labavitch JM. Plant Physiol. 1997;115:717–725. doi: 10.1104/pp.115.2.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ben-Arie R, Kislev N, Frenkel C. Plant Physiol. 1979;64:197–202. doi: 10.1104/pp.64.2.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Redgwell RJ, MacRae E, Hallett I, Fischer M, Perry J, Harker R. Planta. 1997;203:162–172. [Google Scholar]
- 29.Kalamaki MS, Harpster MH, Palys JM, Labavitch JM, Reid DS, Brummell DA. J Agric Food Chem. 2003;51:7459–7464. doi: 10.1021/jf034164l. [DOI] [PubMed] [Google Scholar]
- 30.van Kan JAL. Trends Plants Sci. 2006;11:247–253. doi: 10.1016/j.tplants.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 31.Brito N, Espino JJ, González C. Mol Plant–Microbe Interact. 2006;19:25–32. doi: 10.1094/MPMI-19-0025. [DOI] [PubMed] [Google Scholar]
- 32.Espino JJ, Brito N, Noda J, Gonzalez C. Physiol Mol Plant Path. 2005;66:213–221. [Google Scholar]
- 33.Wubben JP, Mulder W, ten Have A, van Kan JAL, Visser J. Appl Environ Microbiol. 1999;65:1596–1602. doi: 10.1128/aem.65.4.1596-1602.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vogel JP, Raab TK, Schiff C, Somerville SC. Plant Cell. 2002;14:2095–2106. doi: 10.1105/tpc.003509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lionetti V, Raiola A, Camardella L, Giovane A, Obel N, Pauly M, Favaron F, Cervone F, Bellincampi D. Plant Physiol. 2007;143:1871–1880. doi: 10.1104/pp.106.090803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hadfield KA, Bennett AB. Plant Physiol. 1998;117:337–343. doi: 10.1104/pp.117.2.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bergmann CW, Ito Y, Singer D, Albersheim P, Darvill AG, Benhamou N, Nuss L, Salvi G, Cervone F, De Lorenzo G. Plant J. 1994;5:625–634. doi: 10.1111/j.1365-313x.1994.00625.x. [DOI] [PubMed] [Google Scholar]
- 38.Spadoni S, Zabotina O, Di Matteo A, Mikkelsen JD, Cervone F, De Lorenzo G, Mattei B, Bellincampi D. Plant Physiol. 2006;141:557–564. doi: 10.1104/pp.106.076950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Abu-Goukh AA, Greve LC, Labavitch JM. Physiol Plant Path. 1983;23:111. [Google Scholar]
- 40.Hernandez-Blanco C, Feng DX, Hu J, Sanchez-Vallet A, Deslandes L, Llorente F, Berrocal-Lobo M, Keller H, Barlet X, Sanchez-Rodriguez C, et al. Plant Cell. 2007;19:890–903. doi: 10.1105/tpc.106.048058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Melotto E, Greve LC, Labavitch JM. Plant Physiol. 1994;106:575–581. doi: 10.1104/pp.106.2.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vicente AR, Powell A, Greve LC, Labavitch JM. Funct Plant Biol. 2007;34:614–623. doi: 10.1071/FP07002. [DOI] [PubMed] [Google Scholar]
- 43.Aguero CB, Uratsu SL, Greve C, Powell ALT, Labavitch JM, Meredith CP, Dandekar AM. Mol Plant Path. 2005;6:43–51. doi: 10.1111/j.1364-3703.2004.00262.x. [DOI] [PubMed] [Google Scholar]
- 44.Blumenkrantz N, Asboe-Hansen G. Anal Biochem. 1973;54:484–489. doi: 10.1016/0003-2697(73)90377-1. [DOI] [PubMed] [Google Scholar]
- 45.Yemm EW, Willis AJ. Biochem J. 1954;57:508–514. doi: 10.1042/bj0570508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Albersheim P, Nevins DJ, English PD, Karr A. Carbohydr Res. 1967;5:340–345. [Google Scholar]
- 47.Blakeney AB, Harris PJ, Henry RJ, Stone BA. Carbohydr Res. 1983;113:291–299. doi: 10.1016/0008-6215(84)85106-x. [DOI] [PubMed] [Google Scholar]
- 48.Russin WA, Trivett CL. In: Microwave: Techniques and Protocols. Giberson RT, Demaree RS, editors. Totowa, NJ: Humana; 2001. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.