Abstract
Despite the importance and pervasiveness of marketing, almost nothing is known about the neural mechanisms through which it affects decisions made by individuals. We propose that marketing actions, such as changes in the price of a product, can affect neural representations of experienced pleasantness. We tested this hypothesis by scanning human subjects using functional MRI while they tasted wines that, contrary to reality, they believed to be different and sold at different prices. Our results show that increasing the price of a wine increases subjective reports of flavor pleasantness as well as blood-oxygen-level-dependent activity in medial orbitofrontal cortex, an area that is widely thought to encode for experienced pleasantness during experiential tasks. The paper provides evidence for the ability of marketing actions to modulate neural correlates of experienced pleasantness and for the mechanisms through which the effect operates.
Keywords: orbitofrontal cortex, modulation by marketing actions, neuroeconomics, taste
A basic assumption in economics is that the experienced pleasantness (EP) from consuming a good depends only on its intrinsic properties and on the state of the individual (1). Thus, the pleasure derived from consuming a soda should depend only on the molecular composition of the drink and the level of thirst of the individual. In opposition to this view, a sizable number of marketing actions attempt to influence EP by changing properties of commodities, such as prices, that are unrelated to their intrinsic qualities or to the consumer's state. This type of influence is valuable for companies, because EP serves as a learning signal that is used by the brain to guide future choices. For example, when facing the choice between previously experienced restaurants, one would tend to avoid locales where previously meals were unsavory. Contrary to the basic assumptions of economics, several studies have provided behavioral evidence that marketing actions can successfully affect EP by manipulating nonintrinsic attributes of goods. For example, knowledge of a beer's ingredients and brand can affect reported taste quality (2, 3), and the reported enjoyment of a film is influenced by expectations about its quality (4). Even more intriguingly, changing the price at which an energy drink is purchased can influence the ability to solve puzzles (5).
Despite the importance and pervasiveness of various marketing actions, very little is known about the neural mechanisms through which they affect decisions made by individuals. An exception is a previous study demonstrating that knowledge of the brand of a culturally familiar drink, such as Coke, increases activation in the hippocampus, parahipoccampus, midbrain, dorsolateral prefrontral cortex, and thalamus (6). The authors of the previous study interpreted such activity as evidence for retrieval of brand information during the consumption experience.
Here, we propose a mechanism through which marketing actions can affect decision making. We hypothesized that changes in the price of a product can influence neural computations associated with EP. This hypothesis is based on previous findings showing that affective expectations influence appraisals made about hedonic experiences and, through this, the actual quality of experiences (2, 7, 8). Consider, for example, the experience of an individual sampling a wine for which he or she has information about its retail price. Because perceptions of quality are known to be positively correlated with price (9), the individual is likely to believe that a more expensive wine will probably taste better. Our hypothesis goes beyond this by stipulating that higher taste expectations would lead to higher activity in the medial orbitofrontal cortex (mOFC), an area of the brain that is widely thought to encode for actual experienced pleasantness (6, 10–16). The results described below are consistent with this hypothesis. We found that the reported price of wines markedly affected reported EP and, more importantly, also modulated the blood-oxygen-level-dependent (BOLD) signal in mOFC.
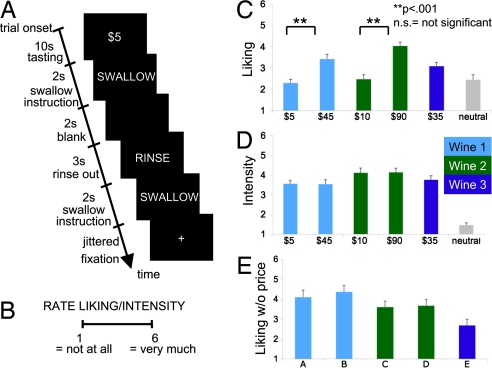
To investigate the impact of price on the neural computations associated with EP, we scanned human subjects (n = 20) using fMRI while they sampled different wines and an affectively neutral control solution, which consisted of the main ionic components of human saliva (17). We chose wine as a stimulus, because it is relatively easy to administer inside the scanner using computerized pumps, it induces a pleasurable flavor sensation in most subjects, and it varies widely in quality and retail price. Subjects were told they were sampling five different Cabernet Sauvignons, that the purpose of the experiment was to study the effect of degustation time on perceived flavors, and that the different wines would be identified by their retail prices (see Fig. 1A). Unbeknown to the subjects, the critical manipulation was that there were only three different wines, and two of them (wines 1 and 2) were administered twice, one identified at a high price and one at a low price. For example, wine 2 was presented half of the time at $90, its retail price, and half of the time at $10. Thus, the task consisted of six trial types: $5 wine (wine 1), $10 wine (wine 2), $35 wine (wine 3), $45 wine (wine 1), $90 wine (wine 2), and neutral solution. The wines were administered in random order, simultaneously with the appearance of the price cue. Subjects were asked to focus on the flavor of the wine during the degustation period and entered taste pleasantness or taste intensity ratings in every other trial (Fig. 1B).
Fig. 1.
Experimental design and behavioral results. (A) Time course for a typical trial. (B) Reported pleasantness and intensity rating scales. (C) Reported pleasantness for the wines during the cued price trials. (D) Taste intensity ratings for the wines during the cued price trials. (E) Reported pleasantness for the wines obtained during a postexperimental session without price cues.
Results
Modulation of Reported Pleasantness and Taste Intensity by Price.
We measured the impact of price information on EP by comparing the mean reported liking rating for wines 1 and 2 when administered at a high vs. a low price. We found significant differences for both wines (P < 0.001, Fig. 1C). In addition, reported pleasantness was correlated with wine prices (r = 0.59, P < 0.000). We could not find a similar behavioral effect for intensity ratings (Fig. 1D). To explore further the role of prices on experienced pleasantness, we administered a follow-up behavioral session 8 weeks after the main experiment, during which wines were presented without price information. As expected, in this case, there were no reported differences among the wines (Fig. 1E). Interestingly, while the pleasantness ratings were increasing in price in the main experimental task (Fig. 1C), they were not in the postscanning blind test (Fig. 1D). A potential concern with these behavioral results is they might exhibit “experimenter demand” effects. In particular, some subjects might deem it inappropriate to report to the experimenter that a cheaper wine tastes better.
Modulation of Brain Correlates of Experienced Pleasantness by Price.
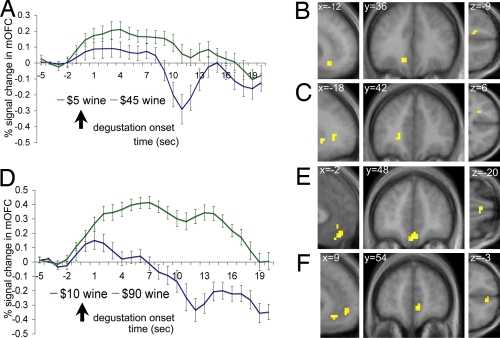
We analyzed brain imaging data using two different general linear models [see Materials and Methods and supporting information (SI) Text for details]. First, we looked for brain areas whose activity increased with the price of wine. More concretely, we estimated the BOLD response to each of the liquids at degustation and swallowing and then analyzed the contrasts “high–low price” at degustation separately for wines 1 and 2. Fig. 2 B and C describe the results of this contrast for wine 1. We found increased activation in the left mOFC and the left ventromedial prefrontal cortex (vmPFC). Another cluster was found in a superior part of the vmPFC adjoining the rostral anterior cingulate cortex (rACC). We also found increased activation in the dorsolateral prefrontal cortex, visual cortex, middle temporal gyrus, and cingulate gyrus (see SI Table 2, upper). As shown in Fig. 2 E and F, the contrast generated similar results for wine 2: increased activation was observed in the bilateral mOFC, vmPFC, and rACC. In addition, for the wine 2, we also found activation changes in the amygdala, lateral parts of the OFC, dorsolateral prefrontal cortex, inferior and middle temporal gyrus, and posterior cingulate cortex (see SI Table 3, upper).
Fig. 2.
The effect of price on each wine. (A) Wine 1: averaged time courses in the medial OFC voxels shown in B (error bars denote standard errors). (B) Wine 1: activity in the mOFC was higher for the high- ($45) than the low-price condition ($5). Activation maps are shown at a threshold of P < 0.001 uncorrected and with an extend threshold of five voxels. (C) Wine 1: activity in the vmPFC was also selected by the same contrast. (D) Wine 2: averaged time courses in the medial OFC voxels shown in E. (E) Wine 2: activity in the mOFC was higher for the high- ($90) than for the low-price condition ($10). (F) Wine 2: activity in the vmPFC was higher for the same contrast.
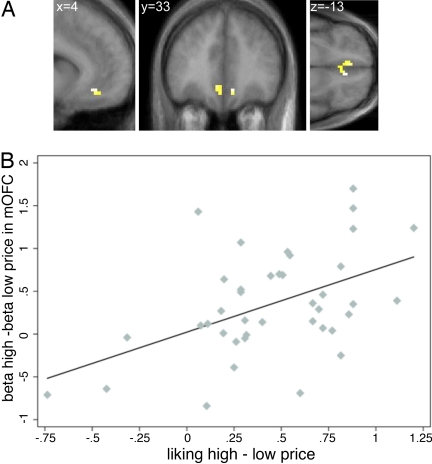
A comparison of SI Tables 2, upper, and 3, lower, or of the relevant figures, suggests there might be small differences on the areas of the medial prefrontal cortex activated by the two wines. SI Fig. 5 shows that, as the statistical threshold is lowered, these differences disappear. To investigate this further, we performed a conjunction analysis to identify areas in which brain activity was higher on the high price condition for both wines. As shown in Fig. 3A, bilateral mOFC and adjoining rACC exhibited this pattern.
Fig. 3.
The effect of price on both wines. (A) Conjunction analysis. Activity in the mOFC/rACC was higher in the high- than in the low-price condition for both wines 1 and 2. (B) Correlation of behavioral and BOLD responses (r = 0.49, P < 0.001). Each point denotes an individual wine pair. The horizontal axis measures the change in reported pleasantness between the high- and low-price conditions. The vertical axis computes an analogous measure using the betas from the general linear model in a 5-mm spherical volume surrounding the area depicted in A.
To investigate whether the increase in price had a differential effect on the two wines, we performed an interaction analysis (see Materials and Methods and SI Text for details). We found that the effect of price on mOFC activity was higher for the cheap $5 wine than for the expensive $90 wine. This suggests that the effect of a price increase on mOFC activity might be larger at low than at high prices.
Discussion
The main hypothesis of this study was that an increase in the perceived price of a wine should, through an increase in taste expectations, increase activity in the mOFC. The results described above provide evidence consistent with the hypothesis. The hypothesis was motivated by several previous studies, which have shown that activity in the mOFC is correlated with behavioral pleasantness ratings for odors (10–13), tastes (6, 14, and 15), and even music (16). This, together with our behavioral results and the additional imaging results described below, support the interpretation that, by modulating the activity in the mOFC, changes in the price of a wine might lead to a change in the actual EP derived from its consumption.
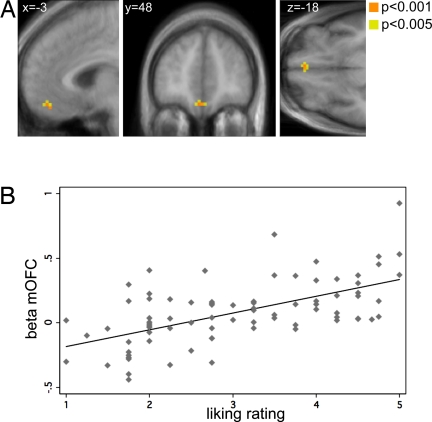
We performed two additional analyses to provide further support for this interpretation. First, for each individual and wine, we computed the change in reported EP between the high and low price conditions. We also computed the analogous difference in parameter estimates for the BOLD response from the general linear model in an area surrounding the mOFC. Fig. 3B shows that the neural and behavioral estimates were positively and highly correlated (r = 0.49, P < 0.001). Second, we verified that the results of the previous literature also held in our study by estimating a different general linear model and looking for brain regions whose activity was correlated with reported EP from sampling the different stimuli (see SI Text for details). The results replicated the findings of previous studies: activity in the mOFC was correlated with absolute reports of pleasantness (Fig. 4).
Fig. 4.
Neural correlates of liking ratings. (A) Activity in the mOFC and the midbrain correlated with the reported pleasantness of the six liquids at degustation time. For illustration purposes, the contrast is shown both at P < 0.001 and P < 0.005 uncorrected and with an extend threshold of five voxels. (B) Correlation of pleasantness ratings and BOLD responses (r = 0.593, P < 0.000). Each point denotes a subject-price pair. The horizontal axis measures the reported pleasantness. The vertical axis computes the betas from the general linear model in a 5-mm spherical volume surrounding the area depicted in A.
Importantly, we did not find evidence for an effect of prices on areas of the primary taste areas such as the insula cortex, the ventroposterior medial nucleus of the thalamus, or the prabrachial nuclei of the pons. A natural interpretation is that the top-down cognitive processes that encode the flavor expectancies are integrated with the bottom-up sensory components of the wine in the mOFC, thus modulating the hedonic experience of flavor, but that the flavor expectancies generated by the change in prices do not impact more basic sensory representations. Interestingly, an analogous mechanism has been proposed for pain placebo effects (7).
Our results have implications for several disciplines. First, the EP signal plays a central role in neuroeconomics, because it serves as a teaching signal that guides future behavior. Unfortunately, very little is known about the factors that affect the neural computation of this signal. A natural starting hypothesis is the economic view, which states that EP depends only on the sensory properties of the item being consumed (i.e., its molecular properties) and the state of the consumer. Our results suggest that the brain might compute EP in a much more sophisticated manner that involves integrating the actual sensory properties of the substance being consumed with the expectations about how good it should be. It is important to emphasize that it might be adaptive for the brain to do this. To make good decisions in the future, the brain needs to carry out good measurements of the quality of current experiences. In a world of noisy measurements, the use of prior knowledge about the quality of an experience provides additional valuable information. A related study (13) provides additional supporting evidence for this point by showing that giving a cognitive label to an ambiguous odor (“cheddar cheese” or “body odor”) can affect both subjective pleasantness reports and neural activity related to EP. Unlike the current paper, however, de Araujo et al. (13) do not provide evidence that marketing actions, such as pricing, can affect neural correlates of EP.
Second, our findings also have implications for marketing. Whereas there is ample behavioral evidence that various marketing actions are successful in influencing the EP of individuals, that they can modulate neural representations of this signal had not been reported before. Furthermore, the neural findings also provide some clues about the mechanisms involved. In particular, it seems that price changes modulate the representations of experienced utility but not the encoding of the sensory properties of taste in the primary gustatory cortex.
Third, our results have implications for economics. EP is an important component of experienced utility, which is the economist's term for subjective well being. We show that, contrary to the standard economic view, EP depends on nonintrinsic properties of products, such as the price at which they are sold. It then follows that marketing manipulations might affect subjective perceptions of well being. This raises several difficult questions for the field. Should the effect of prices on experienced utility be counted as real economic well being or as a mistake made by individuals? To what extent are measurable differences in preferences based on intrinsic differences between products and price effects we have identified? What happens to the efficiency of competitive markets when firms can influence experienced utility by changing the price of items?
An important task for future research is to develop a more complete characterization of the range of marketing actions that can influence the neural computation of EP. We conjecture that any action affecting expectations of product quality, such as expert quality ratings; peer reviews; information about country of origin, store, and brand names (especially those associated with luxury products); and repeated exposure to advertisements might lead to effects similar to those identified here.
Materials and Methods
Subjects.
Twenty normal-weight subjects participated in the experiment (11 males, ages 21–30; mean age, 24.5 yr). One additional subject participated in the experiment but was excluded from the analysis, because he reported being confused about the task during a debriefing at the end of the experiment. All subjects were right-handed and healthy; had normal or corrected-to-normal vision and no history of alcohol abuse, psychiatric diagnoses, or neurological or metabolic illnesses; and were not taking any medications that interfere with the performance of fMRI. All subjects were screened for liking, and at least occasionally drinking, red wine. At the beginning of each experiment, subjects were required to show an official form of identification to provide evidence they were >21 yr old. Subjects were informed about the experiment and gave written consent before participating. California Institute of Technology's institutional review board approved the study.
Stimuli.
During the course of the fMRI experiment, subjects sampled three different Cabernet Sauvignon wines and an affectively neutral tasteless control solution that consisted of the main ionic components of human saliva (25 mM KCl and 2.5 mM NaHCO3). The wines were administered in random order and simultaneously with the appearance of a price identifier. Two of the three wines were administered twice, once identified by their actual retail price and once by a 900% markup (wine 1: $5 real retail price, $45 fictitious price) or a 900% reduction (wine 2: $90 real retail price, $10 fictitious price). The third wine was used as a distracter and was identified by its retail price (wine 3: $35). We also carried out a follow-up behavioral tasting session 8 weeks after the main experiment. In this postexperimental session, the wines were presented without price information (see SI Table 1 for a complete description).
In each trial, 1 ml of each stimulus was delivered by a system of electronic syringe pumps (one for each stimulus) positioned in the scanning control room. These pumps transferred the stimuli to the subjects via ≈10-m polyethylene plastic tubes (6.4-mm diameter) and a perfusion manifold. The perfusion manifold allowed six incoming tubes to be connected to one output tube with a minimum of dead space to avoid the mixing of the wines. The subjects were instructed to hold the output tube between their lips like a straw while they lay in a supine position in the scanner. We made an effort to keep the tubes free of air bubbles to avoid wine oxygenation. Between experiments, the wines were preserved with a special corking system, which is typically used by wineries and wine shops to prevent oxidation.
The stimulus presentation and response recording was controlled by Cogent 2000 (Wellcome Department of Imaging Neuroscience) installed on a computer positioned in the control room that also received trigger pulses from the scanner to control timing. The visual stimuli were presented by using video goggles (Resonance Technologies).
Task.
The task consisted of six trial types: $5, $10, $35, $45, and $90 wines and a neutral liquid. Each trial type occurred 16 times during the course of the experiment, resulting on a total of 96 trials. Subjects were instructed to sample the liquid on each trial while it was on their mouth (for a period of 10 s), to evaluate its pleasantness during this time, and to swallow only when instructed. Between every wine administration, there was a rinse period in which the neutral solution was delivered. The rinse period was implemented to avoid taste spillovers across trials. Trials were separated by a random intertrial interval drawn from a Poisson distribution with a mean of 10 s (see Fig. 1A for a detailed description of the timing of each trial).
In every other trial, subjects were instructed to enter a rating of either flavor pleasantness or taste intensity. Thus, a total of four pleasantness ratings and four taste intensity ratings were sampled for each liquid. We used a six-point rating scale (1 = do not like it at all/not intense at all; 6 = like it very much/very intense) (see Fig. 1B). The timing of rating trials was identical to nonrating trials, except that, after swallowing the liquid and before rinsing, subjects were given 6 s to enter their ratings.
After an initial instruction period, subjects were trained on the use of the response boxes to enter the ratings. Given the large number of potential ratings, subjects entered ratings with both hands (three ratings for each hand). The assignment of ratings to buttons was counterbalanced across subjects to avoid motor artifacts.
Unbeknown to the subjects, the critical manipulation was that the $5 and $45 wines and the $10 and $90 wines were identical. This manipulation was not revealed to the subjects. Instead, the subjects were told they would be sampling five different Cabernet Sauvignons, that the purpose of the experiment was to study the effect of sampling time on perceived flavors, and that the different wines will be identified by their retail prices. Evidence for the success of our cover story was that all subjects reported at the end of the experiment being able to taste five different wines. Although the experiment contains an element of deception, subjects were not debriefed after the experiment to avoid contaminating California Institute of Technology's small subject pool.
Data Acquisition and Preprocessing.
The brain imaging was conducted in a 3-T Siemens Trio MRI scanner (Siemens). We acquired gradient echo T×2 weighted echoplanar images (EPI) with BOLD contrast and used a special sequence designed to optimize functional sensitivity in orbitofrontal cortex (18). This consisted of a tiled acquisition in an oblique orientation at 30° to the AC-PC line. In addition, we used an eight-channel phased array coil that yields a 40% signal increase in OFC over the standard head coil. The sequence enabled 32 axial slices of 3-mm thickness and 3 mm in-plane resolution that could be acquired with a TR of 2 s. A T1-weighted structural image was also acquired for each subject. Functional imaging data were acquired in four separate sessions of ≈13 min each.
To detect transient head movements due to swallowing, we attached a 1.5-cm-long copper coil with a radius of 0.5 cm to the neck of each subject. The setup was similar to those used by previous studies in which liquid food had been administered (14). Small movements of the coil induced a current in the magnetic field that could be detected after amplification using an EEG system positioned in the scanner room (Biopac Systems). This produced a time series of events reflecting transient larynx movement that was used in the general linear model (GLM) in two ways. First, the time series of the signal detected by the coil was added as an additional motion regressor in the GLM as regressor of no interest; this was done to take out the variance due to swallowing induced head movement. Second, swallowing events were either assigned to experimental conditions or classified as noninstructed swallowing. The former were used to correct the timing of swallowing onsets for each stimulus type that were entered in the GLM as a regressor of interest (see SI Text for details about the models). The latter were entered into the data analysis as a regressor of no interest.
fMRI data analysis was performed by using the Statistical Parametric Mapping software (SPM05; Wellcome Department of Imaging Neuroscience). We applied the following preprocessing steps to the imagining data: (i) slice-timing correction (centered at TR/2) (ii) realignment to the last volume, (iii) spatial normalization to a standard T2* template with a resampled voxel size of 3 mm3, (iv) spatial smoothing using a Gaussian kernel with full width at half maximum of 8 mm, and (5) intensity normalization and high-pass temporal filtering (filter width 128 s). The structural T1 images were coregistered to the mean functional EPI images for each subject and normalized using parameters derived from the EPI images.
fMRI Data Analysis 1: Influence of Price on Wine Sampling.
We estimated a general linear model in which the delivery of each of the six different liquids was entered as a regressor of interest. In addition, the swallows of each liquid type were also entered as separate regressors. Each of these regressors plus additional regressors of no interests were convolved with a canonical hemodynamic response function. We then calculated first-level single-subject contrasts to compare the administration of an identical wine at a high minus a low price. Finally, for each of these first-level contrasts, we calculated a second-level group contrast using a one-sample t test (see SI Text for details).
We also performed an interaction analysis to compare the effect of prices on the two wines. We calculated a first-level single-subject contrast to compare the administration of the low-quality wine at a high minus a low price minus the administration of the high quality wine at a high minus a low price. We also calculated a first-level single-subject contrast to compare the administration of the high-quality wine at a high minus a low price minus the administration of the low-quality wine at a high minus a low price. For each of these first-level contrasts, we calculated a second-level group contrast using a one-sample t test (see SI Text for details).
fMRI Data Analysis 2: Neural Representation of Experienced Pleasantness.
We estimated a second general linear model in which the delivery and swallow of all liquids (independently of type) were entered as regressors. In addition, we entered the reported pleasantness and intensity ratings as parametric modulators for both regressors. We then computed first-level single-subject contrasts for the parametric modulators at both liquid sampling and swallowing by reported EP and taste-intensity ratings. Finally, for each of these first-level contrasts, we calculated a second-level group contrast using a one-sample t test (see SI Text for details).
Supplementary Material
ACKNOWLEDGMENTS.
We thank Vivian Valentin, Jan Glaescher, and Axel Linder for their help with this project and Hackjin Kim, Sam Huang, and Shawn Wagner for developing the coil we used to detect swallowing movement and for providing support to process the swallowing signal. Financial support from the National Science Foundation (Grant SES-0134618) is gratefully acknowledged. This work was also supported by a grant from the Gordon and Betty Moore Foundation to the California Institute of Technology Brain Imaging Center.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0706929105/DC1.
References
- 1.Kahneman D, Wakker PP, Sarin R. Back to Bentham? Explorations of Experienced Utility. Q J Econ. 1997;112:375–405. [Google Scholar]
- 2.Lee L, Frederick S, Ariely D. Try it, you'll like it: The influence of expectation, consumption, and revelation on preferences for beer. Psychol Sci. 2006;17:1054–1058. doi: 10.1111/j.1467-9280.2006.01829.x. [DOI] [PubMed] [Google Scholar]
- 3.Allison RI, Uhl KP. Influence of Beer Brand Identification on Taste Perception. J Market Res. 1964;1:36–39. [Google Scholar]
- 4.Klaaren KJ, Hodges SD, Wilson TD. The role of affective expectations in subjective experience and decision-making. Soc Cognit. 1994;12:77–101. [Google Scholar]
- 5.Shiv B, Carmon Z, Ariely D. Placebo Effects of Marketing Actions: Consumers May Get What They Pay For. J Market Res. 2005;XLII:383–393. [Google Scholar]
- 6.McClure SM, et al. Neural correlates of behavioral preference for culturally familiar drinks. Neuron. 2004;44:379–387. doi: 10.1016/j.neuron.2004.09.019. [DOI] [PubMed] [Google Scholar]
- 7.Wager T. The neural bases of placebo effects in pain. Curr Dir Psychol Sci. 2005;14:175–179. [Google Scholar]
- 8.Wilson TD, Klaaren KJ. In: Review of Personality and Social Psychology. Clark MS, editor. Vol 14. Newbury Park, CA: Sage; 1992. pp. 1–31. [Google Scholar]
- 9.Rao A, Monroe KB. The effect of price, brand name, and store name on buyers' perceptions of product quality: An integrative review. J Market Res. 1989;26:351–357. [Google Scholar]
- 10.de Araujo IE, Kringelbach ML, Rolls ET, McGlone F. Human cortical responses to water in the mouth, and the effects of thirst. J Neurophysiol. 2003;90:1865–1876. doi: 10.1152/jn.00297.2003. [DOI] [PubMed] [Google Scholar]
- 11.Anderson AK, et al. Dissociated neural representations of intensity and valence in human olfaction. Nat Neurosci. 2003;6:196–202. doi: 10.1038/nn1001. [DOI] [PubMed] [Google Scholar]
- 12.de Araujo IE, Rolls ET, Kringelbach ML, McGlone F, Phillips N. Taste-olfactory convergence, and the representation of the pleasantness of flavour, in the human brain. Eur J Neurosci. 2003;18:2059–2068. doi: 10.1046/j.1460-9568.2003.02915.x. [DOI] [PubMed] [Google Scholar]
- 13.de Araujo IE, Rolls ET, Velazco MI, Margot C, Cayeux I. Cognitive modulation of olfactory processing. Neuron. 2005;46:671–679. doi: 10.1016/j.neuron.2005.04.021. [DOI] [PubMed] [Google Scholar]
- 14.Kringelbach ML, O'Doherty J, Rolls ET, Andrews C. Activation of the human orbitofrontal cortex to a liquid food stimulus is correlated with its subjective pleasantness. Cereb Cortex. 2003;13:1064–1071. doi: 10.1093/cercor/13.10.1064. [DOI] [PubMed] [Google Scholar]
- 15.Small DM, et al. Dissociation of neural representation of intensity and affective valuation in human gustation. Neuron. 2003;39:701–711. doi: 10.1016/s0896-6273(03)00467-7. [DOI] [PubMed] [Google Scholar]
- 16.Blood AJ, Zatorre RJ. Intensely pleasurable responses to music correlate with activity in brain regions implicated in reward and emotion. Proc Natl Acad Sci USA. 2001;98:11818–11823. doi: 10.1073/pnas.191355898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Francis S, et al. The representation of pleasant touch in the brain and its relationship with taste and olfactory areas. NeuroReport. 1999;10:453–459. doi: 10.1097/00001756-199902250-00003. [DOI] [PubMed] [Google Scholar]
- 18.Deichmann R, Gottfried JA, Hutton C, Turner R. Optimized EPI for fMRI studies of the orbitofrontal cortex. NeuroImage. 2003;19:430–441. doi: 10.1016/s1053-8119(03)00073-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.