Abstract
Angiogenesis and inflammation are central processes through which the tumor microenvironment influences tumor growth. We have demonstrated recently that peroxisome proliferator-activated receptor (PPAR)α deficiency in the host leads to overt inflammation that suppresses angiogenesis via excess production of thrombospondin (TSP)-1 and prevents tumor growth. Hence, we speculated that pharmacologic activation of PPARα would promote tumor growth. Surprisingly, the PPARα agonist fenofibrate potently suppressed primary tumor growth in mice. This effect was not mediated by cancer-cell-autonomous antiproliferative mechanisms but by the inhibition of angiogenesis and inflammation in the host tissue. Although PPARα-deficient tumors were still susceptible to fenofibrate, absence of PPARα in the host animal abrogated the potent antitumor effect of fenofibrate. In addition, fenofibrate suppressed endothelial cell proliferation and VEGF production, increased TSP-1 and endostatin, and inhibited corneal neovascularization. Thus, both genetic abrogation of PPARα as well as its activation by ligands cause tumor suppression via overlapping antiangiogenic pathways. These findings reveal the potential utility of the well tolerated PPARα agonists beyond their use as lipid-lowering drugs in anticancer therapy. Our results provide a mechanistic rationale for evaluating the clinical benefits of PPARα agonists in cancer treatment, alone and in combination with other therapies.
Keywords: stroma, inflammation, fibrates, microenvironment
Peroxisome proliferator-activated receptors (PPARs) are a family of nuclear receptors comprising three isoforms, PPARα, PPARδ, and PPARγ, which act as ligand-activated transcriptional factors. PPARs play key roles in energy homeostasis by modulating glucose and lipid metabolism and transport (1). PPARα is also critical in inflammation (2) and is the molecular target of the fibrate class of drugs, such as fenofibrate, which act as agonistic ligands of PPARα.
Long-term administration of certain PPARα agonists (clofibrate and WY14643) induces hepatocarcinogenesis in rodents but not in humans (3). Consequently, PPARα has not been established as a molecular target for cancer therapy by its agonistic ligands. In contrast, PPARγ and PPARδ agonists have been extensively studied to evaluate their anticancer effects because of their antiproliferative, proapoptotic, antiapoptotic, and differentiation-promoting activity (4). However, recent studies have revealed the expression of PPARα in tumor cells (5, 6), and PPARα ligands suppress the growth of several cancer lines, including colon, breast, endometrial and skin, in vitro (7–10). PPARα ligands also suppress the metastatic potential of melanoma cells in vitro and in vivo (11, 12). Furthermore, PPARα ligands decrease tumor development in murine colon carcinogenesis (7). Clofibric acid inhibits the growth of human ovarian cancer in mice (13). Most recently, the PPARα agonist WY14643 suppresses tumorigenesis in a PPARα-dependent manner (14).
Together, these data suggest that PPARα ligands may have an important role as antitumor agents, although the mechanism remains elusive. PPARα is expressed not only in tumor cells but also in endothelial and inflammatory cells (15, 16). Also, PPARα ligands can inhibit endothelial cell proliferation and migration and induce endothelial cell apoptosis in vitro (17–19). In addition, fenofibrate reduces adventitial angiogenesis and inflammation in a porcine model (20) and decreases VEGF levels in patients with hyperlipidemia and atherosclerosis (21). However, the relative role of PPARα in tumor angiogenesis and tumor inflammation has not been studied.
Here, we report that PPARα is expressed both in tumor cells and in tumor endothelium. We show that PPARα ligands have potent antitumor and antiangiogenic effects, both in vitro and in vivo. Our data demonstrate that PPARα expression in the host rather than in the tumor cell is critical for the antitumor, antiangiogenic, and antiinflammatory activity of PPARα ligands. This extends the repertoire of potential targets of PPARα ligands beyond cell-autonomous mechanisms of cancer. Our findings may be of clinical relevance because PPARα ligands such as fenofibrate are orally administered, Food and Drug Administration-approved drugs widely used for the treatment of hyperlipidemia with minimal side effects.
Results
PPARα Is Expressed in Tumor Cells in Vitro and in Tumor Endothelium in Vivo.
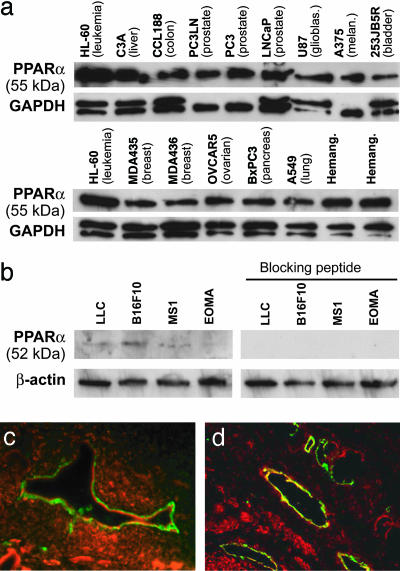
We first screened 19 human tumor cell lines for PPARα expression in vitro. In Western blot analysis of cell cultures, we found that all tumor cell lines examined expressed the PPARα protein, although at varying levels (Fig. 1a). We obtained similar results in murine tumor cell lines, albeit at a lower intensity. The signal could be specifically neutralized with a blocking peptide (Fig. 1b). Expression patterns in tumor tissues were assessed by immunofluorescent double staining for PPARα and the endothelial marker CD31. PPARα staining was examined in s.c. implanted human pancreatic cancer cells (BxPC3) grown in mice (Fig. 1c), as well as in clinical specimens from human prostate carcinoma (Fig. 1d). We found expression of PPARα in the tumor cells as well as in human and murine endothelial cells of microvessels (Fig. 1 c and d).
Fig. 1.
PPARα is expressed in tumor cells and endothelium of neoplastic tissues. (a) Western blot analysis of PPARα expression in cultured human tumor cells and hemangioma specimens. Nuclear extract from leukemia cells (HL-60) was used as a control. (b) Western blot analysis of PPARα expression in cultured mouse tumor cells. The specificity of protein expression was confirmed by abrogation by a PPARα-blocking peptide. GAPDH and β-actin levels were measured to demonstrate equal loading of protein in each lane. (c and d) Immunofluorescent double staining for CD31 and PPARα demonstrates PPARα expression in endothelium of human pancreatic cancer (BxPC3) in SCID mice (c) and in patient prostate cancer tissue specimens (d). CD31-stained endothelial cells are shown in green, PPARα-positive cells are red, and colocalization of the two colors are yellow. Colocalization of red and green fluorescence (yellow) indicates PPARα expression in blood vessels.
PPARα Ligands Have Direct Antitumor and Antiendothelial Effects in Vitro.
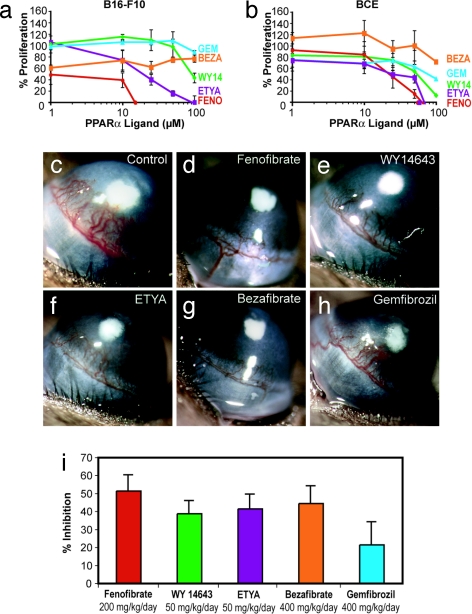
Given the presence of PPARα in multiple cell types, we next compared the effect of PPARα ligands for their ability to inhibit the proliferation of tumor cells, endothelial cells, and fibroblasts. PPARα ligands examined included fenofibrate, gemfibrozil, bezafibrate, WY14643, and 5,8,11,14-eicosatetraynoic acid (ETYA). Fenofibrate was most potent in suppressing the proliferation of all tumor cell lines tested, including melanoma (B16-F10), breast carcinoma (MDA436), and Lewis lung carcinoma (LLC) cells [Fig. 2a and supporting information (SI) Fig. 6]. Fenofibrate, WY14643, and ETYA inhibited FGF2-induced proliferation of bovine capillary endothelial cells up to 95% after 3 days (Fig. 2b). In addition, fenofibrate inhibited VEGF-stimulated proliferation of human umbilical vein endothelial cells (HUVECs) (data not shown). In contrast to tumor and endothelial cells, PPARα ligands failed to inhibit the proliferation of fibroblasts (foreskin) at doses <50 μM (SI Fig. 7a). Moreover, fenofibrate and WY14643 inhibited VEGF-stimulated endothelial cell migration (SI Fig. 7 b and c). These doses used here are clinically relevant because fibrates in humans readily achieve similar serum levels (22).
Fig. 2.
PPARα ligands have direct antitumor and antiendothelial effects in vitro and in vivo. (a) Percentage of proliferation of tumor cells (B16-F10 melanoma) is determined by comparing cells grown in media plus 10% FBS, and the PPARα ligands, to starved cells. FENO, fenofibrate; WY14, WY14643; BEZA, bezafibrate; GEM, gemfibrozil. (b) Percentage of proliferation of BCE cells is determined by comparing cells exposed to an angiogenic stimulus (FGF2) with those exposed to FGF2 and PPARα ligands (fenofibrate, WY14643, gemfibrozil, ETYA, and bezafibrate) relative to unstimulated cells. (c) FGF2-induced neovascularization in control cornea on day 6 in a mouse receiving vehicle. (d–h) Systemic treatment with fenofibrate at 200 mg/kg per day (d), WY14643 at 50 mg/kg per day (e), ETYA at 50 mg/kg per day (f), bezafibrate at 400 mg/kg per day (g), or gemfibrozil at 400 mg/kg per day (h). (i) Area of inhibition (percentage) by administration of various PPARα ligands: fenofibrate (200 mg/kg per day), 52% inhibition; WY14643 (50 mg/kg per day), 39% inhibition; ETYA (50 mg/kg per day), 42% inhibition; bezafibrate (400 mg/kg per day), 44% inhibition; and gemfibrozil (400 mg/kg per day), 22% inhibition. Inhibition was determined on day 6 by the following formula: pellet distance × 0.2π × neovessel length × clock hours of neovessels. (n = 6 eyes per group; the experiment was performed three times.)
To determine whether PPARα ligands could inhibit angiogenesis by down-regulating tumor-secreted growth factors and/or up-regulating endogenous angiogenesis inhibitors, such as thrombospondin (TSP)-1, we measured VEGF, FGF2, and TSP-1 levels in tumor-conditioned media. Fenofibrate and WY14643 at 50 μM inhibited VEGF secretion in glioblastoma (U87) cells by 43–55% and in LLC by 51–58% (SI Fig. 8 a and b). Both PPARα ligands also inhibited FGF2 secretion in K1000 cells (a tumor cell line that expresses and secretes high levels of FGF2) by up to 70% (SI Fig. 8c). In addition, fenofibrate also increased the expression of TSP-1 by 3- to 4-fold in HT-1080 fibrosarcoma and in LLC tumor cells (SI Fig. 8d and data not shown). Therefore, in addition to their direct antitumor and antiendothelial effects, PPARα ligands may suppress angiogenesis indirectly by inhibiting tumor cell production of VEGF and FGF2 and by increasing TSP-1.
PPARα Ligands Inhibit FGF2-Induced Corneal Neovascularization.
To determine the optimal antiangiogenic doses of PPARα ligands for daily administration in mice, we performed the cornea angiogenesis assay in the presence of five different PPARα ligands (Fig. 2 c–h). Systemic oral administration of these PPARα agonists significantly inhibited FGF2-induced corneal angiogenesis by >50% compared with the control (depending on the compound) (Fig. 2i).
Systemic Therapy with PPARα Ligands Inhibits Primary Tumor Growth.
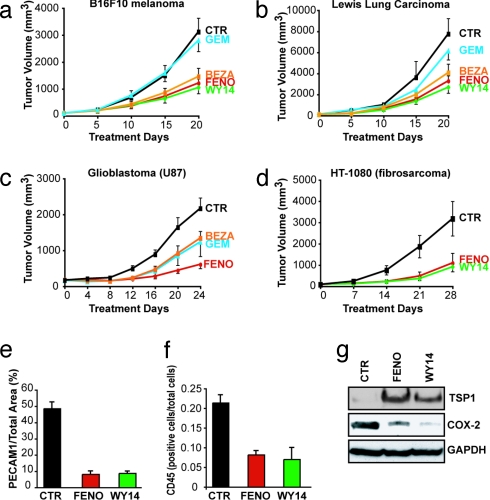
To determine whether these antiangiogenic effects of PPARα agonists translate to suppression of primary tumors, we treated established s.c. tumors of 100 mm3 with PPARα agonists. Oral fenofibrate (200 mg/kg per day) inhibited B16-F10 melanoma, LLC, glioblastoma (U87), and fibrosarcoma (HT1080) tumor growth by 61%, 58%, 72%, and 66%, respectively, and was more potent than other fibrates, such as bezafibrate and gemfibrozil (Fig. 3 a–d). Systemic therapy with WY14643 also inhibited the growth of B16-F10 melanoma, LLC, and fibrosarcoma (HT1080) by 66%, 65%, and 71%, respectively (Fig. 3 a, b, and d). No weight loss or evidence of other drug-related toxicity was observed. Furthermore, no signs of hepatocarcinogenesis were observed in mice treated with PPARα ligands.
Fig. 3.
Systemic therapy with PPARα ligands inhibits primary tumor growth. When tumors reached 100 mm3 in size, PPARα ligand treatment was initiated (day 0). On the last day of treatment, the statistical difference between control and treated group was determined by Student's t test. The most potent antitumor activity was obtained by fenofibrate and WY14643 at the following doses: fenofibrate, 200 mg/kg per day; WY14643, 50 mg/kg per day; bezafibrate, 200 mg/kg per day; and gemfibrozil, 200 mg/kg per day. (a) B16-F10 melanoma (P < 0.001). (b) LLC (P < 0.001). (c) Glioblastoma (U87) (P < 0.005). (d) Fibrosarcoma (HT-1080) (P < 0.0001). (e) Vessel density in fenofibrate-, WY14643-, and vehicle-treated B16-F10 tumors, as defined by the percentage of vessel area = PECAM1-positive area/tumor area in each field. (f) Leukocyte counts per total number of cells per field in fenofibrate-treated and WY14643-treated and vehicle-treated B16-F10 tumors, as determined by CD45 staining. (g) Western blot analysis of TSP-1 and COX-2 proteins in tumor lysates of fenofibrate-, WY14643-, and vehicle-treated B16-F10 melanomas on day 20.
Given the in vitro evidence for antiangiogenic activity and the known inflammation-modulatory role of PPARα stimulation, we analyzed the tissues of PPARα ligand treated B16-F10 tumors for antiangiogenic and antiinflammatory effects. Fenofibrate and WY14643 treatment reduced vessel density by 83% and 81%, respectively, relative to that in the control tumors (Fig. 3e and SI Fig. 9), consistent with the decrease of microvessel density in murine tumor models after treatment with PPARα ligands (13, 14). In addition, fenofibrate and WY14643 treated tumors exhibited a dramatic reduction in leukocytes by 62% and 67% (CD45) (Fig. 3f and SI Fig. 9). Treatment with PPARα ligands also led to an increase of TSP-1 in B16-F10 tumors (Fig. 3g). In contrast, the enzyme COX-2, which is an important mediator of inflammation and also regulates endothelial cell activity (23), was suppressed in both fenofibrate- and WY14643-treated B16-F10 tumors (Fig. 3g).
Antiangiogenic and Antitumor Effects of PPARα Ligands Are Specific to the Activation of PPARα.
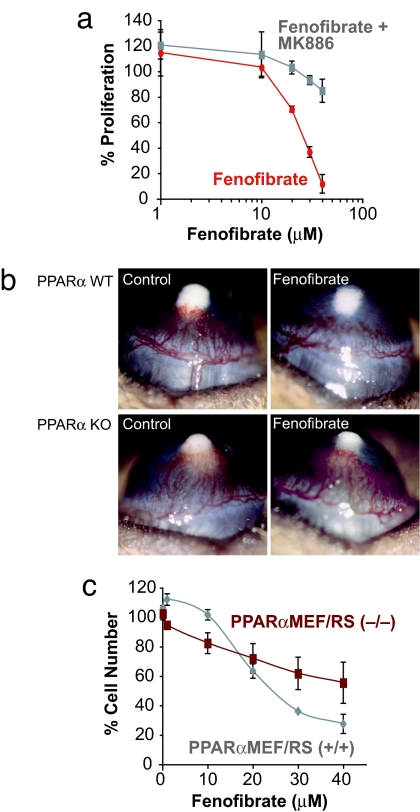
To demonstrate the activation of PPARα in endothelial cells, we measured the kinetics of induction of the medium chain acyl-dehydrogenase (MCAD), a target gene of PPARα, in HUVECs. After 12–24 h of fenofibrate treatment (25 μM), MCAD levels increased in a dose-dependent manner, indicating PPARα activation (data not shown). Furthermore, PPARα ligand-mediated inhibition of FGF2-induced proliferation of bovine capillary endothelial cells was reduced by 90% with the PPARα antagonist MK886 (10 μM; P < 0.001) (Fig. 4a). In addition, PPARα ligands inhibited corneal neovascularization in PPARα WT (52%) but not in PPARα KO mice (Fig. 4b). These findings indicate that the antiangiogenic activity of PPARα ligands specifically depends on activation of PPARα.
Fig. 4.
PPARα ligands have PPARα-dependent and -independent effects. (a) Effect of fenofibrate with or without MK886 treatment on the percentage of proliferation on endothelial cells. (b) Corneal neovascularization (80 ng of FGF2 pellets) in fenofibrate- and vehicle-treated PPARα WT and PPARα KO mice. (c) Effect of fenofibrate treatment on proliferation of PPARα-positive tumor PPARα+/+MEF/RS and PPARα-negative tumor PPARα−/−MEF/RS on day 3.
To confirm that the suppression of tumor cell proliferation by PPARα agonists was specific to PPARα activation, we examined whether fenofibrate could inhibit the proliferation of PPARα-deficient tumor cells. Therefore, we created a PPARα-negative tumor cell line by transforming mouse embryonic fibroblasts (MEFs) derived from PPARα KO mice. Embryonic fibroblasts from PPARα KO and WT mice were transformed with SV40 large T antigen and H-ras, giving rise to two tumorigenic cell lines, PPARα−/−MEF/RS and PPARα+/+MEF/RS, respectively (SI Fig. 10). Although fenofibrate treatment for 3 days showed 44% dose-dependent inhibition of proliferation of the PPARα−/− cells, suggesting off-target effects, the inhibition in the PPARα+/+ was significantly higher (P < 0.02), with a maximal proliferation inhibition of 73% (Fig. 4c). Thus, whereas PPARα ligands may have PPARα-independent antiproliferative effects, pronounced inhibition of cell proliferation requires the presence of the nominal PPARα targets.
The Antitumor Activity of PPARα Ligands Depends on Host PPARα Receptors.
Our observations suggest a dual effect of PPARα ligands on both endothelial cells and tumor cells. To evaluate the relative importance of host cells versus tumor cells as targets of PPARα ligands, we treated PPARα-positive tumors (PPARα+/+MEF/RS) in PPARα WT and KO mice and PPARα-negative tumors (PPARα−/−MEF/RS) in PPARα WT mice. The reason we chose the MEF tumor to test the antitumor activity of fenofibrate was that it has shown sufficient growth in the PPARα KO mouse to reveal inhibition by a drug (24). The MEF tumors grew in the PPARα KO mice mainly because they were transfected with two oncogenes. This gave rise to MEF tumors that were capable of inducing angiogenesis over and above the antiangiogenic state imposed by the PPARα KO mice. In contrast, all other tumors remained viable but dormant and did not grow and therefore were not suitable for testing a drug that inhibits tumor growth.
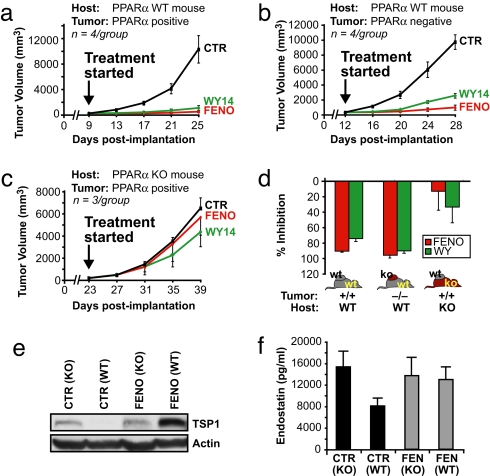
At the stage when the tumors were 100 mm3 (corresponding to 9 days postimplantation in PPARα WT mice and 23 days postimplantation in PPARα KO mice), mice were treated with PPARα ligands or vehicle for 16 days. In PPARα WT mice, fenofibrate and WY14643 inhibited the growth of PPARα-positive tumors by 90–95% (Fig. 5a) and of PPARα negative tumors by 75–90% by day 25 postimplantation (Fig. 5b). The near complete inhibition of tumor growth by PPARα ligands in PPARα-positive and PPARα-negative tumor cells indicates that PPARα in the tumor cell is not the major target of PPARα ligands. Conversely, in PPARα KO mice, fenofibrate and WY14643 failed to significantly inhibit the growth of PPARα+/+ tumors (13% and 33%, respectively; Fig. 5c) by day 39 postimplantation. Thus, expression of PPARα in the nontumor host tissue is essential for the antitumor activity of PPARα ligands and is sufficient to mediate the antitumor effects of PPARα agonists even if the tumors lack PPARα (Fig. 5d).
Fig. 5.
The antitumor activity of PPARα ligands is host PPARα receptor-dependent. After PPARα-positive (PPARα+/+MEF/RS) or PPARα-negative (PPARα−/−MEF/RS) tumors were 100 mm3 in size, PPARα ligand treatment was initiated (day 0). The doses were as follows: fenofibrate, 200 mg/kg per day; and WY14643, 50 mg/kg per day. (a) The effect of systemic therapy of PPARα ligands on PPARα-positive tumors (PPARα+/+MEF/RS) in PPARα WT mice (90–95% inhibition). (b) The effect of systemic therapy on PPARα-negative tumors (PPARα−/−MEF/RS) in PPARα WT (75–90% inhibition). (c) The effect of systemic therapy of PPARα-positive tumors (PPARα+/+MEF/RS) in PPARα (KO) mice (13–33% inhibition). (d) The columns summarize the effects of fenofibrate and WY14643 in host or tumor cells. (e) Western blot analysis of TSP-1 in plasma from fenofibrate and vehicle treated PPARα WT and PPARα KO mice. (f) Endostatin levels in plasma from fenofibrate- and vehicle-treated PPARα WT and PPARα KO mice.
The suppression of tumor growth in the absence of host PPARα has been associated with increased plasma levels of the antiangiogenic protein TSP-1 (24). TSP-1 was not detected in the plasma of WT mice but was present in PPARα KO mice (Fig. 5e). In WT but not PPARα KO mice, fenofibrate induced high levels of TSP-1, consistent with the strong tumor suppression in WT, ligand-treated animals (Fig. 5 a and b). Fenofibrate also induced high levels of endostatin in the plasma of non-tumor-bearing PPARα WT mice. Fenofibrate did not have an effect in PPARα KO mice, which already exhibited elevated basal levels of both TSP-1 and endostatin (Fig. 5 e and f), indicating that these antiangiogenic effects of fenofibrate were PPARα-mediated. In summary, PPARα agonists induced an antiangiogenic state characterized by elevated TSP-1 and endostatin, which is qualitatively similar to the effect of PPARα deficiency.
Discussion
The development of cancer is not simply attributable to the loss of growth control of a single cell clone but rather a developmental disease that involves the tumor cell as well as its interaction with the host tissue. This microenvironment includes endothelial cells, inflammatory cells, and other stromal elements. Therefore, targeting the noncancerous host tissue, mainly by antiangiogenesis mechanisms, has emerged as an important opportunity for tumor therapy (25). More recently, modulation of tumor-promoting inflammation in the tumor bed has been proposed as a target for cancer treatment (26).
Here, we report that expression of PPARα in the host tissue is required for PPARα agonists to exert their tumor-suppressing effect. The in vivo antitumor effect was not likely mediated by the in vitro observed direct antitumor cell activity of PPARα agonists, because in PPARα WT animals, the presence of PPARα in the tumor was not necessary to confer responsiveness to PPARα agonists. In summary, animal studies indicate that expression of PPARα in the nontumor host tissue is necessary and sufficient for the tumor-suppressive effect of PPARα agonists. The host tissue contribution may be local (tumor bed) or systemic. Our analysis suggests that this host-mediated effect of PPARα ligands may be attributable to the inhibition of angiogenesis.
Importantly, the doses of the pharmacological PPARα agonists required for tumor inhibition are in the same range as those used clinically to treat hyperlipidemia (22). PPARα ligands administered at continuous low doses in the diet can suppress tumor and metastatic growth in various experimental tumor models including melanoma, colon, and breast carcinogenesis (11, 27, 28). However, fenofibrate at daily low doses (25 mg/kg or 0.1–0.25%) lacked antitumor activity in primary hamster melanoma (11) and murine endometrial cancer (9). This finding is consistent with our observation that 25 mg/kg of fenofibrate had minimal antitumor and antiangiogenic effects (data not shown), whereas 200 mg/kg inhibited angiogenesis and tumor growth.
The antitumor activity of PPARα ligands is primarily PPARα-dependent but may also be mediated by PPARα-independent (“off-target”) pathways (22). Here we demonstrated specific PPARα-dependent effects of the nominal PPARα ligands: (i) direct activation of the target gene, MCAD, in endothelial cells; (ii) inhibition of tumor and endothelial cell proliferation at doses that selectively activate PPARα in vitro and that were reversed by a PPARα antagonist; and (iii) inhibition of corneal neovascularization in WT but not in PPARα KO mice. Conversely, the presence of PPARα-independent activity was evidenced in the moderate inhibition of proliferation of PPARα-negative cells. However, this effect may not contribute to in vivo tumor suppression because the antitumor effect of PPARα agonists was mediated by PPARα expressed in the nontumor host tissue.
In addition to the antiproliferative activity, PPARα ligands such as fenofibrate induce a dose-dependent increase in endothelial cell apoptosis and causes arrest in the cell cycle in the G0/G1 phase at higher doses (18). However, even higher PPARα concentrations also can activate PPARγ and/or PPARδ (22). Our studies also show that bezafibrate minimally suppressed the proliferation of endothelial cells yet strongly inhibited corneal neovascularization in vivo. Bezafibrate is a pan-PPAR agonist that activates all three nuclear receptors, PPARα, PPARγ, and PPARδ (22). We and others have found that ligand-induced activation of PPARγ inhibits endothelial proliferation and corneal angiogenesis (29). In contrast, activation of PPARδ promotes endothelial proliferation (30). Thus, one possibility to explain the weak antiendothelial activity of bezafibrate in vitro compared with its robust antiangiogenic activity in vivo is by the relative contribution of each activated PPAR to the overall angiogenic response.
Although our in vitro data revealed a potent role of PPARα ligands in the inhibition of tumor cells, endothelial cell proliferation, and angiogenesis, PPARα ligands also have antiinflammatory effects. The antiinflammatory effect of PPARα ligands is mediated notably through inhibition of inducible NOS, COX-2, and TNFα (31). Inflammatory cells present in the tumor play an important tumor-promoting role (26) by secretion of trophic cytokines for tumor cells as well as proangiogenic factors. Suppression of inflammation in tumors may correlate with improved prognosis and growth inhibition. In agreement with the role of inflammatory cells in tumors and the antiinflammatory activity of PPARα agonists, we show that tumor growth suppression caused by PPARα ligands significantly decreased leukocyte expression within the tumor. Moreover, expression of an inflammatory mediator, COX-2, was decreased in the PPARα ligand-treated tumors.
Although the role of PPARα in inflammation has been well characterized, the modulating effect of PPARα on inflammatory processes in the context of tumor growth remains unclear. We recently reported that PPARα KO mice exhibited a significant increase in inflammatory infiltrates in tumors (24), consistent with the role of PPARα in the negative modulation of inflammation (2). However, contrary to the recent notion of inflammation as a tumor promoter (26), this overt inflammation in PPARα KO mice not only failed to support tumor growth but also appeared to actively suppress it. Specifically, the lack of PPARα resulted in an increase of TSP-1 and endostatin levels in plasma and/or tumors, which may explain the observed tumor suppression.
It is counterintuitive that PPARα activation by agonists and genetic abrogation of PPARα in the host would both lead to tumor inhibition. Treatment of PPARα WT mice with a PPARα agonist led to an increase in TSP-1 and endostatin in tumors and/or plasma, producing an antiangiogenic state similar to the PPARα KO mice (24). Two perturbations of PPARα activity in opposite directions both inhibit tumor growth and increase TSP-1 and endostatin levels. This underscores the central role of these angiogenesis inhibitors in the control of tumor growth. Endostatin induces TSP-1 expression (32). This raises the possibility that these two angiogenesis inhibitors are coordinated.
An analogous paradox of PPARα effect has been described in atherosclerosis. Plaque growth depends on angiogenesis (33). Atherosclerosis is suppressed not only in PPARα KO mice (34) but also in mice treated with fenofibrate (35). In more general terms, these counterintuitive results suggest a biphasic (U-shaped) dose–response curve of host tissue to PPARα activity, as is also observed with PPARγ agonists (29). In other words, very high concentrations or “very low” concentrations of PPARα in the host yield the same outcome: maximal suppression of tumor angiogenesis.
Of interest, clinical evidence suggests that long-term administration of fibrates may reduce melanoma progression. Gemfibrozil-treated patients had a 9-fold decrease in melanoma compared with placebo-treated controls, whereas statin-treated patients had a 1.9-fold reduced incidence of melanoma compared with placebo treated controls (36). Fenofibrate also increased the response rate to retinoids in a human clinical trial for cutaneous T cell lymphoma (37), suggesting that PPARα ligands may potentiate the effect of other anticancer agents.
In conclusion, we provide a mechanistic rationale for extending the clinical use of the well tolerated PPARα agonists to anticancer therapy, and we show their efficacy in tumor treatment in animal models. The antitumor properties of PPARα ligands appear to be mediated primarily by their direct and indirect antiangiogenic effects and their antiinflammatory activity but also by direct antitumor effects. This provides another example for the paradigm of achieving antitumor efficacy through synergistic attack on multiple targets that encompass cell autonomous and non-cell-autonomous mechanisms of cancer growth. Because of their multifaceted effects and excellent safety and tolerability profile after chronic and prolonged exposure, PPARα ligands may be potential tumor-preventative agents. They may be used for maintenance of long-term angiogenesis suppression. Furthermore, our findings support recent studies (11–14) that suggest that PPARα ligands may be ideally suited to complement conventional modalities for cancer treatment. Specifically, because fenofilerate is commercially available, it could be evaluated as an extension of existing multidrug regimens, notably in metronomic (antiangiogenic) chemotherapy schemes (38, 39). However, further research into the pathophysiological role of PPARα and their pharmacological regulators will be paramount to unravel all mechanisms for the antitumor effects of PPARα agonists.
Materials and Methods
Cells and Reagents.
Endothelial cells, fibroblasts, and tumor cells were maintained as described (29) in SI Methods. Fenofibrate, bezafibrate, and gemfibrozil were obtained from Sigma; WY14643 and ETYA were from Chem-Syn.
Western Blot Analysis.
Western blots were performed by using tumor cell lysates collected from plated cells that were 60% confluent. Total protein extracts (30 μg) were analyzed on PVDF membrane blots incubated overnight with rabbit anti-mouse PPARα (Affinity Bioreagents) or rabbit anti-human PPARα (Active Motif). All blots were incubated for 1 h with their corresponding HRP-conjugated secondary antibodies (Amersham Biosciences) and developed with ECL (Pierce). For immunoblotting of TSP and COX-2 (Labvision), the primary antibody was incubated at room temperature for 2 h.
Immunohistochemistry.
Sections of tumors were treated with 40 μg/ml proteinase K (Roche Diagnostics) for 25 min at 37°C for PECAM1. PECAM1 was amplified by using tyramide signal amplification direct and indirect kits (NEN Life Science Products). CD45 (BD Biosicences) was detected by using a rat-on-mouse kit (InnoGenex).
Proliferation Assays.
Endothelial, fibroblast, and tumor cell proliferation were assayed as described (29). For PPARα antagonist studies, MK886 (Alexis Biochemicals) was used. For proliferation of PPARα-negative and PPARα-positive cells, percentage cell number = 100 × (cellsligand)/(cellsstimluated).
Angiogenesis Assays.
Corneal neovascularization assays were performed as described (29). After implantation of 80 ng of FGF-2 into C57Bl6, PPARα WT, and PPARα KO mice, PPARα ligands were administered over 6 days by gavage in an aqueous solution of 10% DMSO in 0.5% methylcellulose, whereas control mice received vehicle. Tumor cells were injected s.c. (1 × 106 cells in 0.1 ml of PBS) into C57BL/6, SCID, PPARα WT, or PPARα KO mice (The Jackson Laboratory). Once tumors reached 100 mm3, PPARα ligands were administered by daily gavage for 20–28 days. Tumor volume was calculated as width squared × length × 0.52.
Statistical Analysis.
The Student's paired t test was used to analyze the difference between the two groups. Values were considered significant at P < 0.05.
Supplementary Material
ACKNOWLEDGMENTS.
We thank Lars Akslen for helpful discussions regarding pathology; Deborah Freedman-Cass (Fox Chase Cancer Center, Philadelphia) and Michael Rogers for suggestions in preparing the manuscript; William Hahn (Dana–Farber Cancer Institute) for Large T and ras constructs; Kristin Johnson for photography; and Giorgio Pietramaggiori and Ricky Sanchez for excellent technical assistance. This work was supported by the Stop and Shop Pediatric Brain Tumor Fund, the C. J. Buckley Pediatric Brain Tumor Research Fund (M.W.K., D.P., A.K.), Department of Defense Innovator Award W81XWH-04-1-0316 (to J.F.), the Breast Cancer Research Foundation (J.F.), National Cancer Institute Grant R01CA064481 (to J.F.), and private philanthropic funds.
Note.
During the finalization of this article, Pozzi et al. (14) published a report in which they showed PPARα-mediated inhibition of angiogenesis and tumor growth. Their findings, although by using a different tumor model and focusing on a single agonist, WY14643, are consistent with ours and confirm the important role of PPARα in tumor suppression.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0711281105/DC1.
References
- 1.Lefebvre P, Chinetti G, Fruchart JC, Staels B. Sorting out the roles of PPARα in energy metabolism and vascular homeostasis. J Clin Invest. 2006;116:571–580. doi: 10.1172/JCI27989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Devchand PR, et al. The PPARα-leukotriene B4 pathway to inflammation control. Nature. 1996;384:39–43. doi: 10.1038/384039a0. [DOI] [PubMed] [Google Scholar]
- 3.Peters JM, Cattley RC, Gonzalez FJ. Role of PPARα in the mechanism of action of the nongenotoxic carcinogen and peroxisome proliferator Wy 14,643. Carcinogenesis. 1997;18:2029–2033. doi: 10.1093/carcin/18.11.2029. [DOI] [PubMed] [Google Scholar]
- 4.Michalik L, Desvergne B, Wahli W. Peroxisome-proliferator-activated receptors and cancers: complex stories. Nat Rev Cancer. 2004;4:61–70. doi: 10.1038/nrc1254. [DOI] [PubMed] [Google Scholar]
- 5.Collett GP, et al. Peroxisome proliferator-activated receptorα is an androgen-responsive gene in human prostate and is highly expressed in prostatic adenocarcinoma. Clin Cancer Res. 2000;6:3241–3248. [PubMed] [Google Scholar]
- 6.Suchanek KM, et al. Peroxisome proliferator-activated receptorα and the human breast cancer cell lines MCF-7 and MDA-MB-231. Mol Carcinog. 2002;34:165–171. doi: 10.1002/mc.10061. [DOI] [PubMed] [Google Scholar]
- 7.Tanaka T, et al. Ligands for peroxisome proliferator-activated receptorsα and γ inhibit chemically induced colitis and formation of aberrant crypt foci in rats. Cancer Res. 2001;61:2424–2428. [PubMed] [Google Scholar]
- 8.Maggiora M, et al. An overview of the effect of linoleic and conjugated-linoleic acids on the growth of several human tumor cell lines. Int J Cancer. 2004;112:909–919. doi: 10.1002/ijc.20519. [DOI] [PubMed] [Google Scholar]
- 9.Saidi SA, Holland CM, Charnock-Jones DS, Smith SK. In vitro and in vivo effects of the PPARα agonists fenofibrate and retinoic acid in endometrial cancer. Mol Cancer. 2006;5:13. doi: 10.1186/1476-4598-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thuillier P, et al. Activators of peroxisome proliferator-activated-α partially inhibit mouse skin tumor promotion. Mol Carcinog. 2000;29:134–142. doi: 10.1002/1098-2744(200011)29:3<134::aid-mc2>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 11.Grabacka M, et al. Inhibition of melanoma metastases by fenofibrate. Arch Dermatol Res. 2004;296:54–58. doi: 10.1007/s00403-004-0479-y. [DOI] [PubMed] [Google Scholar]
- 12.Grabacka M, Plonka PM, Urbanska K, Reiss K. Peroxisome proliferator-activated receptorα activation decreases metastatic potential of melanoma cells in vitro via down-regulation of Akt. Clin Cancer Res. 2006;12:3028–3036. doi: 10.1158/1078-0432.CCR-05-2556. [DOI] [PubMed] [Google Scholar]
- 13.Yokoyama Y, et al. Clofibric acid, a peroxisome proliferator-activated receptorα ligand, inhibits growth of human ovarian cancer. Mol Cancer Ther. 2007;6:1379–1386. doi: 10.1158/1535-7163.MCT-06-0722. [DOI] [PubMed] [Google Scholar]
- 14.Pozzi A, et al. Peroxisomal proliferator-activated receptor-α-dependent inhibition of endothelial cell proliferation and tumorigenesis. J Biol Chem. 2007;282:17685–17695. doi: 10.1074/jbc.M701429200. [DOI] [PubMed] [Google Scholar]
- 15.Marx N, Sukhova GK, Collins T, Libby P, Plutzky J. PPARα activators inhibit cytokine-induced vascular cell adhesion molecule-1 expression in human endothelial cells. Circulation. 1999;99:3125–3131. doi: 10.1161/01.cir.99.24.3125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Berger J, Moller DE. Mechanism of action of PPARs. Annu Rev Med. 2002;53:409–435. doi: 10.1146/annurev.med.53.082901.104018. [DOI] [PubMed] [Google Scholar]
- 17.Goetze S, et al. PPAR activators inhibit endothelial cell migration by targeting Akt. Biochem Biophys Res Commun. 2002;293:1431–1437. doi: 10.1016/S0006-291X(02)00385-6. [DOI] [PubMed] [Google Scholar]
- 18.Varet J, et al. Fenofibrate inhibits angiogenesis in vitro and in vivo. Cell Mol Life Sci. 2003;60:810–819. doi: 10.1007/s00018-003-2322-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meissner M, et al. PPARα activators inhibit vascular endothelial growth factor receptor-2 expression by repressing Sp1-dependent DNA binding and transactivation. Circ Res. 2004;94:324–332. doi: 10.1161/01.RES.0000113781.08139.81. [DOI] [PubMed] [Google Scholar]
- 20.Kasai T, Miyauchi K, Yokoyama T, Aihara K, Daida H. Efficacy of peroxisome proliferative activated receptor (PPAR)-α ligands, fenofibrate, on intimal hyperplasia and constrictive remodeling after coronary angioplasty in porcine models. Atherosclerosis. 2006;188:274–280. doi: 10.1016/j.atherosclerosis.2005.10.047. [DOI] [PubMed] [Google Scholar]
- 21.Blann AD, Belgore FM, Constans J, Conri C, Lip GY. Plasma vascular endothelial growth factor and its receptor Flt-1 in patients with hyperlipidemia and atherosclerosis and the effects of fluvastatin or fenofibrate. Am J Cardiol. 2001;87:1160–1163. doi: 10.1016/s0002-9149(01)01486-2. [DOI] [PubMed] [Google Scholar]
- 22.Willson TM, Brown PJ, Sternbach DD, Henke BR. The PPARs: From orphan receptors to drug discovery. J Med Chem. 2000;43:527–550. doi: 10.1021/jm990554g. [DOI] [PubMed] [Google Scholar]
- 23.Grau R, Punzon C, Fresno M, Iniguez MA. Peroxisome-proliferator-activated receptorα agonists inhibit cyclo-oxygenase 2 and vascular endothelial growth factor transcriptional activation in human colorectal carcinoma cells via inhibition of activator protein-1. Biochem J. 2006;395:81–88. doi: 10.1042/BJ20050964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kaipainen A, et al. PPARα deficiency in inflammatory cells suppresses tumor growth. PLoS ONE. 2007;2:e260. doi: 10.1371/journal.pone.0000260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Folkman J. Tumor angiogenesis: Therapeutic implications. N Engl J Med. 1971;285:1182–1186. doi: 10.1056/NEJM197111182852108. [DOI] [PubMed] [Google Scholar]
- 26.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Niho N, et al. Concomitant suppression of hyperlipidemia and intestinal polyp formation in Apc-deficient mice by peroxisome proliferator-activated receptor ligands. Cancer Res. 2003;63:6090–6095. [PubMed] [Google Scholar]
- 28.Pighetti GM, et al. Therapeutic treatment of DMBA-induced mammary tumors with PPAR ligands. Anticancer Res. 2001;21:825–829. [PubMed] [Google Scholar]
- 29.Panigrahy D, et al. PPARγ ligands inhibit primary tumor growth and metastasis by inhibiting angiogenesis. J Clin Invest. 2002;110:923–932. doi: 10.1172/JCI15634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Piqueras L, et al. Activation of PPARβ/δ induces endothelial cell proliferation and angiogenesis. Arterioscler Thromb Vasc Biol. 2007;27:63–69. doi: 10.1161/01.ATV.0000250972.83623.61. [DOI] [PubMed] [Google Scholar]
- 31.Moraes LA, Piqueras L, Bishop-Bailey D. Peroxisome proliferator-activated receptors and inflammation. Pharmacol Ther. 2006;110:371–385. doi: 10.1016/j.pharmthera.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 32.Abdollahi A, et al. Endostatin's antiangiogenic signaling network. Mol Cell. 2004;13:649–663. doi: 10.1016/s1097-2765(04)00102-9. [DOI] [PubMed] [Google Scholar]
- 33.Moulton KS, et al. Angiogenesis inhibitors endostatin or TNP-470 reduce intimal neovascularization and plaque growth in apolipoprotein E-deficient mice. Circulation. 1999;99:1726–1732. doi: 10.1161/01.cir.99.13.1726. [DOI] [PubMed] [Google Scholar]
- 34.Tordjman K, et al. PPARα deficiency reduces insulin resistance and atherosclerosis in apoE-null mice. J Clin Invest. 2001;107:1025–1034. doi: 10.1172/JCI11497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Duez H, et al. Reduction of atherosclerosis by the peroxisome proliferator-activated receptorα agonist fenofibrate in mice. J Biol Chem. 2002;277:48051–48057. doi: 10.1074/jbc.M206966200. [DOI] [PubMed] [Google Scholar]
- 36.Dellavalle RP, Nicholas MK, Schilling LM. Melanoma chemoprevention: A role for statins or fibrates? Am J Ther. 2003;10:203–210. doi: 10.1097/00045391-200305000-00007. [DOI] [PubMed] [Google Scholar]
- 37.Talpur R, Ward S, Apisarnthanarax N, Breuer-Mcham J, Duvic M. Optimizing bexarotene therapy for cutaneous T-cell lymphoma. J Am Acad Dermatol. 2002;47:672–684. doi: 10.1067/mjd.2002.124607. [DOI] [PubMed] [Google Scholar]
- 38.Kieran MW, et al. A feasibility trial of antiangiogenic (metronomic) chemotherapy in pediatric patients with recurrent or progressive cancer. J Pediatr Hematol Oncol. 2005;27:573–581. doi: 10.1097/01.mph.0000183863.10792.d4. [DOI] [PubMed] [Google Scholar]
- 39.Kerbel RS, Kamen BA. The anti-angiogenic basis of metronomic chemotherapy. Nat Rev Cancer. 2004;4:423–436. doi: 10.1038/nrc1369. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.