Abstract
Loose aggregations of fishes, or shoals, are a basal social organization of vertebrates and offer a valuable opportunity to determine how individual perceptions influence group formation. We used zebrafish, Danio rerio, to comprehensively investigate the preference space for shoaling related to adult pigment pattern variation, presented in the form of 17 zebrafish pigment pattern mutants or closely related species. We examined all combinations of these phenotypes in 2,920 initial and replicated preference tests, and used as subjects both domesticated laboratory stocks and wild-caught fish. By using multidimensional scaling and other approaches, we show that laboratory and wild zebrafish exhibit similar preferences, yet, unexpectedly, these preferences differ markedly between sexes, and also from how human observers perceive the same pigment patterns. Whereas zebrafish males respond to two traits (species and stripe patterning) in deciding whether to join a shoal, zebrafish female preferences do not correlate with a priori identifiable traits, and neither perceptual world is correlated with that of human observers. The observed zebrafish sex differences run counter to the most commonly accepted explanations for the individual selective advantages gained by shoaling. More generally, these data describe very different perceptual worlds between sexes and reveal the importance of sex differences in social group formation, as well as the critical importance of defining species specificity in visual signaling.
Keywords: perception, pigment pattern, shoaling, social behavior, zebrafish
Aristotle recognized that social behavior defines what it means to be human (1). Over two millennia later, the origins and maintenance of social behavior remain incompletely understood, yet better knowing why and how groups form will provide important insights into animal behavior, psychology, and human evolution. Most analyses of taxonomic variation in social behavior have focused on its fitness consequences and the ecological and evolutionary correlates for particular social structures. Less attention has been given to the mechanisms by which groups form and, particularly, the signals between group members and prospective members that influence individual decisions whether or not to join (2).
One approach to elucidating why and how social structures form is to focus on transitional groups at the interstices of social and solitary behavior, of which shoals of fish are a classic example. Defined as a loose aggregative behavior, shoaling can be viewed as the forerunner to all vertebrate social groups; shoaling is engaged in by the majority of fishes as well as amphibian larvae, representing a broad swath of vertebrate diversity (3–9). Although shoaling provides benefits to individuals via enhanced predator avoidance and foraging efficiency, individuals constantly assess the costs and benefits of joining or remaining in a shoal relative to acting alone (2, 5, 10).
A convenient species for studying shoaling is the zebrafish. These fish shoal as mixed sex groups in the field and in the laboratory (11–14) and their tendency to shoal is heritable (15, 16). Zebrafish respond to visual signals when deciding between prospective shoals, and early life history plays a critical role in the formation of shoaling preferences (13, 14, 17, 18). Nevertheless, the salient features of these visual signals and how they are interpreted remains unknown. As a major component of the visual phenotype is the adult pigment pattern, we reasoned that pigment pattern variation could play a critical role in determining whether individuals elect to join a shoal.
Results
Diverse Visual Signals Exhibited by Zebrafish Mutants and Closely Related Species.
To assay the perceptual space of zebrafish, we used a panel of 17 phenotypes representing an array of pigment patterns including the wild type, “simple” variants in the form of zebrafish mutants, and “complex” variants in the form of closely related species (Fig. 1). The wild-type zebrafish (WT) exhibits dark stripes comprising black melanophores and silver iridophores, light interstripes of yellow xanthophores and iridophores, and dorsal scale melanophores (26). Zebrafish single-locus mutant phenotypes have changes in pigment cell organization, missing pigment cell classes, reduced pigment within cells, or multiple alterations. By using fish that are singly or doubly mutant, we can examine the attractiveness of signals that are one or two mutational steps from the wild type. Other species are within Danio or the closely related Devario; some resemble zebrafish wild-type or mutant phenotypes, whereas others introduce novel pigment pattern elements (e.g., vertical bars), pigment cell classes (e.g., red erythrophores), or both (23). None cooccur with zebrafish (11).
Fig. 1.
Diverse pigment patterns of zebrafish (Left and Center) and closely related species (Right). Abbreviations: A, albino mutant; C, csf1r mutant; CE, csf1r, ednrb1 double mutant; CK, csf1r, kit double mutant; DI, dali/+ mutant; DU, duchamp/+ mutant; E, ednrb1 mutant; K, kit mutant; M, mitfa mutant; O, oberon mutant; S, seurat mutant; Da, Danio albolineatus; Dc, D. choprae; Dk, D. aff. kyathit; Dn, D. nigrofasciatus; Ds, Devario shanensis. For simplicity, only phenotype abbreviations are used in the text and figures. For additional information on the genetic bases of mutant phenotypes and species differences, see refs. 19–25.
We used this panel of variant phenotypes in binary preference tests in which single subject fish were presented with all possible pairs of the 17 phenotypes exhibited by shoals of stimulus fish (each shoal comprising two males and two females), a total of 136 tests for each subject fish. We assayed the duration of time spent with each shoal [for detailed methods, see supporting information (SI)]. Subject fish were 10 male and 10 female wild-type zebrafish of the inbred mapping strain ABwp that had experienced only their own phenotype throughout development. We used a within-individual design both to control for potential individual differences in shoaling tendencies or preferences, and because multidimensional scaling analyses (see below) require each subject to judge between all possible pairs of stimuli. We thus conducted 2,720 initial tests; subject fish were actively shoaling in 2,443 tests, which were used for analyses. We subsequently conducted an additional 200 tests for particular stimulus pairs, using naïve subjects, to assess the generalizability of observed preferences and to control for effects of presentation order in the initial analyses (see below). Stimulus and subject fish were separated by Plexiglas transparent to visible and UV light, allowing the transmission of broad spectrum visual cues while attenuating any potential nonvisual cues.
Male and Female Zebrafish Perceive Variant Pigment Patterns Differently When Compared with the Wild Type.
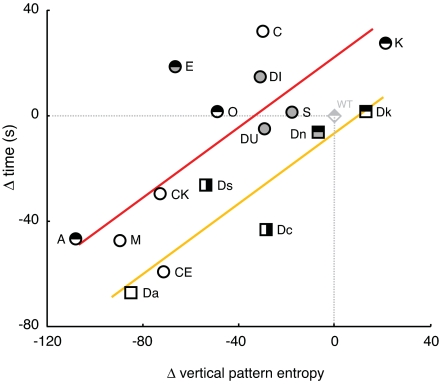
We first examined the preference of subject fish for variant stimulus shoals when they were paired with wild-type stimulus shoals. These comparisons are the simplest and most ecologically relevant, because zebrafish shoal with other zebrafish in the wild, although they encounter fishes exhibiting a range of phenotypes and sometimes can be found shoaling with other species [e.g., Esomus danricus (11, 12)]. We found a strong positive relationship (F(1,13) = 22.5, P < 0.0005; multivariate R2 = 0.58) between shoaling preference of male subjects and one aspect of stimulus shoal pigment patterns, vertical pattern entropy, defined most simply as the average magnitude of shading difference between adjacent pixels along the dorsal–ventral axis (Fig. 2; see SI). At one extreme, phenotype K has two to three melanophore stripes and lacks scale melanophores that normally give a dark cast to the dorsum; K has larger vertical pattern entropy than WT and was preferred when presented simultaneously with WT. At the other extreme, phenotype A lacks melanin and has a smaller vertical pattern entropy than WT, and subjects spent little time with A when it was presented with WT. Preferences for other species also were associated with vertical pattern entropy, ranging from the striped Dk to the uniform Da, although these preferences were lower than for zebrafish mutants (F(1,13) = 8.6, P < 0.05; multivariate R2 = 0.22).
Fig. 2.
Male shoaling preference increases with vertical pattern entropy of pigment pattern relative to WT when alternative phenotypes are presented simultaneously with WT. Shown are differences in the times spent with each phenotype (abbreviations in Fig. 1) compared with WT, plotted against differences in vertical pattern entropy compared with WT. Red line, regression for zebrafish mutants; yellow line, regression for other species; diamond, relative position for WT stimulus shoals; other symbols are alternative stimulus shoals. Circles, zebrafish mutants; squares, Other species; open symbols denote uniform pigment patterns; filled symbols indicate the presence of spots; symbols bisected horizontally denote horizontal stripes; symbols bisected vertically denote vertical bars.
Despite the strong relationship between male shoaling preference and vertical pattern entropy, a similar relationship was not observed for females (P = 0.8; R2 = 0.007). We found no significant associations between shoaling preference and any other a priori quantitative phenotypic attributes including pattern entropy scores, reflectance measures, phylogenetic distance, or body size (SI). Thus, among males but not females, the attractiveness of shoals is closely associated with their vertical pattern entropy, presumably representing the visibility or regularity of a horizontal stripe pattern.
Sex-Specific Attractiveness of Pigment Pattern Variants.
To explore the zebrafish perceptual world more broadly, we examined subject responses to each of the 17 different stimulus phenotypes when presented in all possible combinations. We define the attractiveness of each stimulus phenotype to each sex as the average time that subjects spent with shoals of that phenotype across the entire dataset. Of the 17 phenotypes, DI had the highest attractiveness (far right columns in Tables 1 and 2). To see whether this was generalizable, we repeated preference tests with naïve ABwp subjects, by using DI paired against either of two less attractive phenotypes; males were tested with DI vs. WT and DI vs. CK, whereas females were tested with DI vs. WT and DI vs. M. In each of the four repeated comparisons, subject fish spent more time shoaling with DI than the alternative phenotypes (Table 3). In the original preference tests, males as a whole found some phenotypes significantly more attractive than others overall (Table 1; observed ranking value across all phenotypes, Dn = 68.8 > critical ranking value across all phenotypes, Dn,c = 26.3; least significant difference between phenotypes in ordered ranks, mc = 18.6; for statistical details, see SI and refs. 27 and 28). By contrast, females as a whole did not find particular phenotypes significantly more attractive overall (Dn = 23.7 < Dn,c = 26.3), despite the empirical repeatability of tests with DI. These data point to substantial differences between males and females in how prospective shoalmates are perceived in relation to WT: males exhibit robust preferences for particular phenotypes, whereas females exhibit preferences (e.g., DI and see below), but these preferences depend more strongly on the particular stimulus pair presented, with some phenotypes eliciting a strong preference and others not.
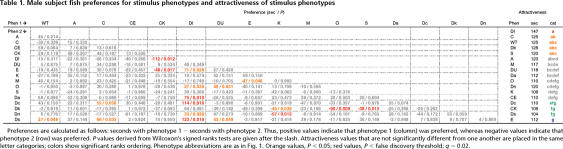
Table 1.
Male subject fish preferences for stimulus phenotypes and attractiveness of stimulus phenotypes
Preferences are calculated as follows: seconds with phenotype 1 − seconds with phenotype 2. Thus, positive values indicate that phenotype 1 (column) was preferred, whereas negative values indicate that phenotype 2 (row) was preferred. P values derived from Wilcoxon's signed ranks tests are given after the slash. Attractiveness values that are not significantly different from one another are placed in the same letter categories; colors show significant ranks ordering. Phenotype abbreviations are as in Fig. 1. Orange values, P < 0.05; red values, P < false discovery threshold; q = 0.02.
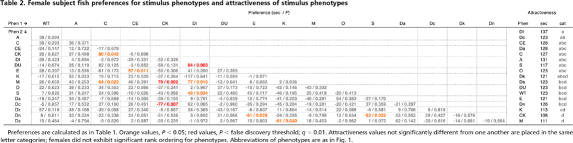
Table 2.
Female subject fish preferences for stimulus phenotypes and attractiveness of stimulus phenotypes
Preferences are calculated as in Table 1. Orange values, P < 0.05; red values, P < false discovery threshold; q = 0.01. Attractiveness values not significantly different from one another are placed in the same letter categories; females did not exhibit significant rank ordering for phenotypes. Abbreviations of phenotypes are as in Fig. 1.
Table 3.
Table 3. Repeatable preferences of male and female subject fish
| Male subjects |
Female subjects |
||||
|---|---|---|---|---|---|
| Test 1 | Test 2 | Test 1 | Test 2 | ||
| DI | 126 ± 11 | 151 ± 28 | DI | 106 ± 17 | 110 ± 15 |
| WT | 111 ± 13 | 133 ± 29 | WT | 131 ± 14 | 134 ± 20 |
| DI | 181 ± 21 | 195 ± 25 | DI | 167 ± 12 | 151 ± 19 |
| CK | 69 ± 15 | 87 ± 22 | M | 90 ± 17 | 117 ± 18 |
| CK | 166 ± 11 | 169 ± 16 | |||
| M | 87 ± 6 | 88 ± 14 | |||
Shown are seconds (means ± SE) spent in preference areas by subject fish. Test 1, initial panel of 20 subject fish and all pairwise comparisons of 17 phenotypes. Test 2, naïve subject fish. In each phenotype pair, preferences in test 1 and test 2 were not significantly different from one another (all P > 0.2).
Multidimensional Scaling Reveals Sex-Specific and Species-Specific Perceptual Worlds.
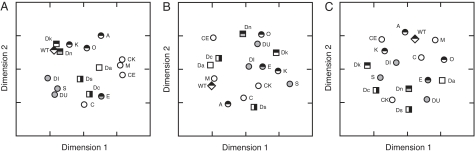
To depict graphically the perceptual space of zebrafish, we used multidimensional scaling (MDS) to represent the similarity (dissimilarity) of stimulus phenotypes. MDS is applicable to a wide variety of complex data sets and does not require an a priori model of the important explanatory factors or how they might be weighted (28–31). For this approach, each subject is presented with all possible pairs of the stimuli to construct a dissimilarity matrix. Analysis of this matrix generates a visual representation of preference space, without ascribing any particular units or values to the axes of the plots. Subject attributes then can be tested for correlations with the MDS axes. As such, an MDS solution and any subsequent phenotypic correlations identify hypotheses for additional, more directed testing. To illustrate how MDS can recover particular trends and groupings, we reconstructed a perceptual space for the 17 stimulus phenotypes by using human subjects who were presented with pairs of images and asked to rank their similarity from 1 to 10. Fig. 3A shows the MDS solution, which recovers groupings of phenotypes with distinct stripes (WT, Dk, Dn, K), uniform patterns (CK, M, CE, Da), and spots (DI, S, DU), in agreement with our subjective impressions.
Fig. 3.
MDS solutions for perceptual spaces of humans, male zebrafish, and female zebrafish. Abbreviations and symbols are as in Figs. 1 and 2, respectively. (A) MDS recovers intuitive groupings of stimulus phenotypes from similarity rankings by human subjects. MDS solution: R2 = 0.67; stress (a lack-of-fit measure; refs. 29 and 30), s = 0.22. (B and C) Shoaling preference spaces for male (B) and female (C) zebrafish differ dramatically from one another and from human perceptions of these phenotypes. (B) MDS solution for zebrafish males: R2 = 0.51; s = 0.27. (C) MDS solution for zebrafish females: R2 = 0.52; s = 0.26.
To assess the preference space of zebrafish, we determined for each possible pair of stimulus phenotypes the absolute difference in the times spent by subject fish with each shoal. We used these values to construct two 17 × 17 dissimilarity matrices, one for males and one for females (Tables 1 and 2). These matrices represent an averaged preference space for each sex and reveal the just meaningful differences between phenotypes (32). Our experiments do not address just noticeable differences between the phenotypes; that is, differences the subject fish perceive but do not act on.
Preference spaces differed dramatically between males (Fig. 3B) and females (Fig. 3C), reflecting an overall lack of correlation between male and female dissimilarity matrices (Mantel test, r = 0.07, P = 0.4; 10,000 permutations). Neither MDS solution recovers anthropomorphically intuitive groupings (Fig. 3 A vs. B: r = −0.07, P = 0.3; Fig. 3 A vs. C: r = −0.05, P = 0.4); nor is either MDS solution correlated with any of the a priori quantified phenotypic attributes listed above (all P > 0.2; see SI). Nevertheless, inspection of all tests revealed pairs of phenotypes that exceed the false discovery thresholds (33) for significance (q = 0.02, 0.01 for males and females, respectively) (Tables 1 and 2). For example, both CK and M have uniform pigment patterns and lack melanophores; CK also lacks xanthophores (26). Despite the apparent similarity of these phenotypes, females strongly preferred CK over M (P < 0.005) in initial tests, and both the directionality and magnitude of this preference was confirmed by retesting with naïve ABwp female subjects (P < 0.05) (Table 3). These analyses show that overall preference spaces for male and female zebrafish differ markedly from one another (and from the perceptual space of human observers). They also demonstrate the context dependence of such preferences. For example, despite the strong association between male shoaling preference and vertical pattern entropy in tests with WT, a similar relationship was not observed across all pairwise phenotypic combinations.
Concordant Visual Preferences of Domesticated and Wild Zebrafish.
A critical question for studies that use laboratory strains is whether observed behaviors are concordant with those of wild populations. In the foregoing analyses, we used an inbred mapping strain, ABwp, to minimize genetic and behavioral variation. These fish were derived from the pet trade in the early 1970s and are >100 generations from the wild (12). Behavioral effects of domestication are well documented for many species including zebrafish (16, 34), so our findings might be specific to ABwp, owing to relaxed selection on traits important to wild fish or unintended selection in the laboratory. To test whether preferences of laboratory stocks are representative of wild fish, we obtained adult D. rerio from a natural population (CBR1) in the Cooch Bihar region of India. We selected 10 stimulus phenotype pairs to represent a range of preferences exhibited by ABwp, and presented them to CBR1 zebrafish (10 males, 10 females, or both; 150 tests total). Preferences of the wild fish CBR1 and laboratory strain ABwp were significantly positively correlated overall (R2 = 0.43, F(1,13) = 9.73, P < 0.01) (SI). These data suggest similar overall preference spaces for laboratory and wild zebrafish.
Discussion
Our analyses provide a unique window into the zebrafish perceptual world. The striking discordance between human and fish perceptual spaces highlights the importance of documenting organism-specific perception of environment, and how these perceptions are filtered, processed, and acted on (the Umwelt and Innenwelt of ref. 35). With the exception of two phenotypic attributes closely associated with male shoaling preferences (vertical pattern entropy and species identity), we found little correlation between a priori quantified components of the phenotype and zebrafish preference spaces, despite strong and repeatable individual preferences exhibited with particular pairs of phenotypes. For example, the preference of females for CK (which lacks melanophores and xanthophores) over M (which lacks only melanophores) shows a remarkable ability to differentiate between these apparently similar uniform pigment patterns. We speculate that, for some of these phenotypes, zebrafish attend to aspects unrelated to pigment pattern (although video analyses and other observations have not revealed gross behavioral differences apparent to the human observer). These results underscore the challenge of identifying the salient components of signals, a prerequisite for more fully understanding animal communication (28, 36, 37).
Previous studies showed that shoaling preferences are learned during development (13, 17) and are subsequently immutable (18), suggesting that individuals form a prototype for shoaling partners based on early experience. An interesting finding here is that putative prototypes of subject fish (here, WT), need not be the most attractive phenotype. In tests with WT, males preferred K. Across all 17 phenotypes, DI was most attractive and WT was only 3rd and 12th most attractive for males and females, respectively (although females did not display a significant ranking). The discordance between prototype and attractiveness differs from suggestions for human perception (38), but is consistent with mate choice preferences for phenotypes that are more extreme than the mean (39), and could reflect underlying biases of the visual system.
Perhaps our most striking finding is the profound and unexpected difference in shoaling preference spaces for males and females. Whereas males exhibited a clear preference for phenotypes with higher vertical pattern entropy, and significantly ranked absolute attractiveness, female preferences did not correlate with vertical pattern entropy, were of lower magnitude, and were stimulus pair-dependent. These differences could reflect sex differences in the acquisition or processing of visual stimuli, as has been documented for human subjects as well (40–42). Sex differences also could represent alternative motivations for joining shoals. Whereas shoaling has been mostly associated with benefits in predation avoidance and foraging efficiency (5, 10), selection on these factors would seem comparable between males and females. On the other hand, if the tendency to join a shoal rests on access to mates, or their avoidance, sex-specific preferences should evolve. These possibilities are testable and suggest a new emphasis on sex-specific costs and benefits during the formation of social groupings more generally.
Methods
Fish Preference Testing.
Preference tests were performed by using a large aquarium divided by transparent Plexiglas into center (subject) and side (stimulus) compartments. Subject fish were presented with alternative shoals of stimulus fish and times spent in proximity to each shoal were recorded for 5 min. Detailed testing procedures are described in SI.
Characterization of Fish Phenotypes by Human Observers.
Subjects were presented with all pairwise combination of stimulus phenotypes and asked to rank them for similarity on a scale of 1–10. Details are in SI.
Phenotype Quantification.
After acquiring digital images of subject pigment patterns, these were analyzed by using specially designed software to quantify the variation in pixel values along vertical and horizontal axes, providing vertical and horizontal pattern entropy scores. Color reflectance measures were additionally characterized at several locations. Details are in SI.
Statistical Analyses.
We examined effects of phenotype attributes on preference by using multiple linear regression, and we used multidimensional scaling to reconstruct perceptual spaces for zebrafish and humans. Preferences for all pairs of stimulus phenotypes were compared by using Wilcoxon's signed-ranks tests. Attractiveness measures were evaluated by nonparametric tests of overall equality to test the null hypothesis of equal attractiveness among phenotypes (rejected if Dn > critical value, Dn,c). Detailed statistical procedures are presented in SI.
Supplementary Material
ACKNOWLEDGMENTS.
For assistance with preference testing, thanks to Laura Alberici da Barbiano, Flavia Barbosa, Michelle Kierstead, Janian Kiger, Ashley McVeigh, and Krista Musser. Thanks to other members of the Parichy laboratory for fish rearing and helpful discussions, and three anonymous reviewers for their helpful comments. This work was supported by National Institutes of Health Grant R01 GM62182, National Science Foundation Grant IOB 0541733, and funds from the University of Washington (to D.M.P.). R.E.E. was supported for part of this work by a National Science Foundation Graduate Research Fellowship.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0708778105/DC1.
References
- 1.Aristotle . The Politics. London: Penguin Books; 1992. [Google Scholar]
- 2.Parrish JK, Edelstein-Keshet L. Complexity, pattern, and evolutionary trade-offs in animal aggregation. Science. 1999;284:99–118. doi: 10.1126/science.284.5411.99. [DOI] [PubMed] [Google Scholar]
- 3.Shaw E. The development of schooling behavior in fishes. Physiol Zool. 1960;33:79–86. [Google Scholar]
- 4.Breder CM. Studies on social groupings in fishes. Bull Amer Mus Nat Hist. 1959;117:393–482. [Google Scholar]
- 5.Krause J, Ruxton G. Living in Groups. Oxford: Oxford Univ Press; 2002. [Google Scholar]
- 6.Ruelle R, Hudson PL. Paddlefish (Polyodon spathula): Growth and food of young of the year and a suggested technique for measuring length. Trans Am Fish Soc. 1977;106:609–613. [Google Scholar]
- 7.Hevel KW. Tennessee Valley Authority, Knoxville TN, Tech Report TVA/ONR/WRF-83/4(b) Knoxville, TN: Tennessee Valley Authority; 1983. Trawling methodology for juvenile paddlefish. [Google Scholar]
- 8.Wassersug R, Hessler CM. Tadpole behaviour: Aggregation in larval Xenopus laevis. Anim Behav. 1971;19:386–389. doi: 10.1016/s0003-3472(71)80021-0. [DOI] [PubMed] [Google Scholar]
- 9.Lefcort H. Chemically mediated fright response in southern toad (Bufo terrestris) tadpoles. Copeia. 1998:445–450. [Google Scholar]
- 10.Pitcher TJ, Parrish JK. In: Behaviour of Teleost Fishes. Pitcher TJ, editor. New York: Chapman & Hall; 1993. pp. 363–439. [Google Scholar]
- 11.Engeszer RE, Patterson LB, Rao AA, Parichy DM. Zebrafish in the wild: A review of natural history and new notes from the field. Zebrafish. 2007;4:21–40. doi: 10.1089/zeb.2006.9997. [DOI] [PubMed] [Google Scholar]
- 12.Spence R, Gerlach G, Lawrence C, Smith C. The behaviour and ecology of the zebrafish, Danio rerio. Biol Rev Camb Philos Soc. 2007 doi: 10.111/j.1469-185x.2007.00030.x. [DOI] [PubMed] [Google Scholar]
- 13.McCann LI, Carlson CC. Effect of cross-rearing on species identification in zebra fish and pearl danios. Dev Psychobiol. 1982;15:71–74. doi: 10.1002/dev.420150110. [DOI] [PubMed] [Google Scholar]
- 14.Moretz JA, Martins EP, Robison BD. The effects of early and adult social environment on zebrafish (Danio rerio) behavior. Environ Biol Fishes. 2007;80:91–101. [Google Scholar]
- 15.Wright D, Rimmer LB, Pritchard VL, Butlin RK, Krause J. Inter and intra-population variation in shoaling and boldness in the zebrafish (Danio rerio). J Fish Biol. 2003;63:258–259. doi: 10.1007/s00114-003-0443-2. [DOI] [PubMed] [Google Scholar]
- 16.Wright D, Nakamichi R, Krause J, Butlin RK. QTL analysis of behavioral and morphological differentiation between wild and laboratory zebrafish (Danio rerio). Behav Genet. 2006;36:271–284. doi: 10.1007/s10519-005-9029-4. [DOI] [PubMed] [Google Scholar]
- 17.Engeszer RE, Ryan MJ, Parichy DM. Learned social preference in zebrafish. Curr Biol. 2004;14:881–884. doi: 10.1016/j.cub.2004.04.042. [DOI] [PubMed] [Google Scholar]
- 18.Engeszer RE, Alberici da Barbiano L, Ryan MJ, Parichy DM. Timing and plasticity of shoaling behaviour in the zebrafish, Danio rerio. Anim Behav. 2007;74:1269–1275. doi: 10.1016/j.anbehav.2007.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Parichy DM, et al. Mutational analysis of endothelin receptor b1 (rose) during neural crest and pigment pattern development in the zebrafish Danio rerio. Dev Biol. 2000;227:294–306. doi: 10.1006/dbio.2000.9899. [DOI] [PubMed] [Google Scholar]
- 20.Parichy DM, Ransom DG, Paw B, Zon LI, Johnson SL. An orthologue of the kit-related gene fms is required for development of neural crest-derived xanthophores and a subpopulation of adult melanocytes in the zebrafish, Danio rerio. Development. 2000;127:3031–3044. doi: 10.1242/dev.127.14.3031. [DOI] [PubMed] [Google Scholar]
- 21.Parichy DM, Rawls JF, Pratt SJ, Whitfield TT, Johnson SL. Zebrafish sparse corresponds to an orthologue of c-kit and is required for the morphogenesis of a subpopulation of melanocytes, but is not essential for hematopoiesis or primordial germ cell development. Development. 1999;126:3425–3436. doi: 10.1242/dev.126.15.3425. [DOI] [PubMed] [Google Scholar]
- 22.Lister JA, Robertson CP, Lepage T, Johnson SL, Raible DW. nacre encodes a zebrafish microphthalmia-related protein that regulates neural-crest-derived pigment cell fate. Development. 1999;126:3757–3767. doi: 10.1242/dev.126.17.3757. [DOI] [PubMed] [Google Scholar]
- 23.Quigley IK, et al. Evolutionary diversification of pigment pattern in Danio fishes: Differential fms dependence and stripe loss in D. albolineatus. Development. 2005;132:89–104. doi: 10.1242/dev.01547. [DOI] [PubMed] [Google Scholar]
- 24.Quigley IK, et al. Pigment pattern evolution by differential deployment of neural crest and post-embryonic melanophore lineages in Danio fishes. Development. 2004;131:6053–6069. doi: 10.1242/dev.01526. [DOI] [PubMed] [Google Scholar]
- 25.Mills MG, Nuckels RJ, Parichy DM. Deconstructing evolution of adult phenotypes: genetic analyses of kit reveal homology and evolutionary novelty during adult pigment pattern development of Danio fishes. Development. 2007;134:1081–1090. doi: 10.1242/dev.02799. [DOI] [PubMed] [Google Scholar]
- 26.Parichy DM. Evolution of danio pigment pattern development. Heredity. 2006;97:200–210. doi: 10.1038/sj.hdy.6800867. [DOI] [PubMed] [Google Scholar]
- 27.David HA. The Method of Paired Comparisons. London: Charles Griffin; 1988. [Google Scholar]
- 28.Ryan MJ, Rand AS. Sexual selection in female perceptual space: How female tungara frogs perceive and respond to complex population variation in acoustic mating signals. Evol Int J Org Evol. 2003;57:2608–2618. doi: 10.1111/j.0014-3820.2003.tb01503.x. [DOI] [PubMed] [Google Scholar]
- 29.Ingwer B, Groenen PJF. Modern Multidimensional Scaling: Theory and Applications. New York: Springer; 2005. [Google Scholar]
- 30.Kruskal JB, Wish M. Multidimensional Scaling. Newbury Park, CA: SAGE; 1978. [Google Scholar]
- 31.Kemmler G, et al. Multidimensional scaling as a tool for analysing quality of life data. Qual Life Res. 2002;11:223–233. doi: 10.1023/a:1015207400490. [DOI] [PubMed] [Google Scholar]
- 32.Nelson DA, Marler P. In: Comparative Perception: Complex Signals. Stebbins WC, Berkeley MA, editors. New York: Wiley; 1990. pp. 443–478. [Google Scholar]
- 33.Storey JD, Tibshirani R. Statistical significance for genomewide studies. Proc Natl Acad Sci USA. 2003;100:9440–9445. doi: 10.1073/pnas.1530509100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Robison BD, Rowland W. A potential model system for studying the genetics of domestication: Behavioral variation among wild and domesticated strains of zebra danio (Danio rerio). Can J Fish Aquat Sci. 2005;62:2046–2054. [Google Scholar]
- 35.von Uexkull J. In: Foundations of Comparative Ethology. Burghardt GM, editor. New York: Van Nostrand Reinhold; 1909. [Google Scholar]
- 36.Maynard-Smith J. Animal Signals. Oxford: Oxford Univ Press; 2003. [Google Scholar]
- 37.Lin DY, Zhang SZ, Block E, Katz LC. Encoding social signals in the mouse main olfactory bulb. Nature. 2005;434:470–477. doi: 10.1038/nature03414. [DOI] [PubMed] [Google Scholar]
- 38.Halberstadt J. The generality and ultimate origins of the attractiveness of prototypes. Pers Soc Psychol Rev. 2006;10:166–183. doi: 10.1207/s15327957pspr1002_5. [DOI] [PubMed] [Google Scholar]
- 39.Ryan MJ, Keddy-Hector A. Directional patterns of female mate choice and the role of sensory biases. Am Nat. 1992;139:S4–S35. [Google Scholar]
- 40.Hamann S, Herman RA, Nolan CL, Wallen K. Men and women differ in amygdala response to visual sexual stimuli. Nat Neurosci. 2004;7:411–416. doi: 10.1038/nn1208. [DOI] [PubMed] [Google Scholar]
- 41.Barkley CL, Gabriel KI. Sex differences in cue perception in a visual scene: investigation of cue type. Behav Neurosci. 2007;121:291–300. doi: 10.1037/0735-7044.121.2.291. [DOI] [PubMed] [Google Scholar]
- 42.Hurlbert AC, Ling Y. Biological components of sex differences in color preferences. Curr Biol. 2007;17:R623–R625. doi: 10.1016/j.cub.2007.06.022. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.